Abstract
The rd7 mouse, an animal model for hereditary retinal degeneration, has some characteristics similar to human flecked retinal disorders. Here we report the identification of a deletion in a photoreceptor-specific nuclear receptor (mPNR) mRNA that is responsible for hereditary retinal dysplasia and degeneration in the rd7 mouse. mPNR was isolated from a pool of photoreceptor-specific cDNAs originally created by subtractive hybridization of mRNAs from normal and photoreceptorless rd mouse retinas. Localization of the gene corresponding to mPNR to mouse Chr 9 near the rd7 locus made it a candidate for the site of the rd7 mutation. Northern analysis of total RNA isolated from rd7 mouse retinas revealed no detectable signal after hybridization with the mPNR cDNA probe. However, with reverse transcription–PCR, we were able to amplify different fragments of mPNR from rd7 retinal RNA and to sequence them directly. We found a 380-nt deletion in the coding region of the rd7 mPNR message that creates a frame shift and produces a premature stop codon. This deletion accounts for more than 32% of the normal protein and eliminates a portion of the DNA-binding domain. In addition, it may result in the rapid degradation of the rd7 mPNR message by the nonsense-mediated decay pathway, preventing the synthesis of the corresponding protein. Our findings demonstrate that mPNR expression is critical for the normal development and function of the photoreceptor cells.
Inherited retinal degenerations often lead to visual impairment and even blindness. Both syndromic and nonsyndromic forms of retinal degeneration have been reported in this heterogeneous group of diseases. The syndromic forms include pathology beyond the visual system, such as hearing loss in Usher's syndrome, and mental retardation, obesity, and polydactyly in Bardet–Biedl syndrome. The nonsyndromic retinal degenerations are divided according to the mode of inheritance and the type of retinal pathology observed. For example, retinitis pigmentosa (RP) is a disease that primarily affects the rods but also involves the cones and results in night blindness with a progressive loss of vision from the periphery to the center of the retina. On the other hand, cone dystrophies are associated with a panretinal loss of cones that often affects the macula and results in loss of central vision. Over 100 chromosomal loci for various inherited human retinal degenerations have been mapped, and the corresponding mutant genes have been identified in approximately 45 of them (Retnet, Retinal Information Network; www.sph.uth.tmc.edu/RetNet/home.htm). Invaluable information about the molecular and pathological basis of some of these diseases has been provided by the discoveries of gene mutations in several mouse retinal degeneration models. The finding of the retinal degeneration 1 (rd1, ref. 1) and tubby (tub, ref. 2) mouse genes led to the identification of mutations in the β-subunit of cGMP-phosphodiesterase (3–5) and tubby-like protein 1 (TULP1, refs. 6 and 7) in individuals affected with autosomal recessive RP. Similarly, the finding of the mutations in retinal degeneration slow (Rds, now Rd2, ref. 8) and shaker 1 (sh1, ref. 9) mouse genes paved the way for the identification of defects in the RDS-peripherin gene in patients with autosomal dominant RP and various cone, cone-rod, and macular dystrophies (for a review, see ref. 10), and in MYO7A in patients with Usher's syndrome type 1b (11).
The purpose of this work was to identify the defective gene responsible for the new autosomal recessive retinal degeneration of the rd7 mouse. rd7 occurred in a multiple recessive mutation stock at The Jackson Laboratory. It causes retinal folding associated with retinal spots and late-onset retinal degeneration. In this paper, we describe the mapping of the mutation to chromosome (Chr) 9 between the DNA markers D9Mit72 and D9Mit174 in a region homologous to human Chr 15q23-q25.
In studies of a pool of cDNAs generated by subtractive hybridization of mRNAs from normal and photoreceptorless rd mouse retinas (12), we identified several photoreceptor-specific clones and mapped them to their chromosomal loci. One of these cDNAs mapped near the rd7 locus. Here we report the identification of the photoreceptor-specific gene that is mutated in the rd7 mouse and the nature of the mutation.
Materials and Methods
Animals.
The mice in this study were bred and maintained in standardized conditions at The Jackson Laboratory. 77–2C2a-special-rd7/rd7, C57BL/6J, B6.Cg-rd7/rd7, and CAST/Ei mice were obtained from Jackson Laboratory production or research facilities. Initial phenotype characterization was done in the original 77–2 line; studies reported here were in the incipient C57BL/6J congenic mice that were characterized at the seventh backcross generation. All experiments were conducted in accordance with the Animal Care and Use Committee and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Clinical Examination.
Mice were clinically evaluated by indirect ophthalmoscopy with a 78-diopter lens to examine the fundus through pupils dilated with 1% atropine (13). Fundus photographs were taken by using a Kowa Genesis small animal camera (14).
Histology.
After the mice were euthanized with anesthetic overdose, eyes were immediately enucleated and prepared for light-microscope histology by immersing them in cold JBF-4 fixative (1% paraformaldehyde/2% glutaldehyde/0.1 M cacodylate buffer) for 24 h. Eyes were then transferred to a cold 0.1 M cacodylate buffer solution for 24 h, embedded in methacrylate historesin, and sectioned for staining with hematoxylin and eosin.
Electroretinography.
Mice were dark adapted for 2 h and anesthetized before testing with an i.p. injection of normal saline solution containing ketamine (15 mg/g body weight) and xylazine (7 mg/g body weight). The right eye of each mouse was dilated (0.5% cyclopentolate hydrochloride and 2.5% phenylephrine hydrochloride) to a maximum diameter of 2.0 mm. Electroretinograms (ERGs) were recorded by using a gold loop electrode placed on the corneal surface at the limbus and referenced to a gold wire in the mouth. A needle electrode inserted in the tail served as ground. Signals were amplified (×10,000, CP511 AC amplifier, Grass Instruments, Quincy, MA), sampled every 0.8 msec with the A/D board (PCI-1200 National Instruments, Austin, TX) in a personal computer, and averaged. Low- and high-frequency cutoffs were set at 1 and 1,000 Hz, respectively.
All stimuli were presented in a Ganzfeld dome painted with a highly reflective matte paint (no. 6080, Eastman Kodak). Rod-mediated ERGs were recorded to short wavelength (Wratten 47A; λmax = 470 nm) flashes that were varied over a 4.0 log unit range of intensities up to the maximum allowable by the photic stimulator (PS33 Plus, Grass Instruments). Cone-mediated responses were obtained with white flashes on a rod-saturating background after 10 min of light adaptation. Responses were computer averaged at all intensities with up to 50 records averaged for the weakest signals. A signal rejection window was used to eliminate electrical artifacts produced by blinking and eye movements.
Chromosomal Localization and Fine Mapping of the rd7 Locus.
77–2C2a-special-rd7/rd7 and CAST/Ei mice were crossed to obtain F1 mice that were subsequently backcrossed to 77–2C2a-special-rd7/rd7 for the initial linkage study. B6.Cg-rd7/rd7 and CAST/Ei mice were crossed and the F1 progeny intercrossed to refine the locus. Tail DNA was isolated according to Buffone and Darlington (15). The strategy of selective DNA pooling described by Taylor et al. (16) was used to map rd7. For the initial genome-wide screen, two pools of DNA with equal contribution from 10 affected or 10 unaffected backcross mice were tested with 57 SSLP markers (three per chromosome) polymorphic between the two parental strains. Additional markers were tested on individual animals around loci that showed significant skewing of alleles to confirm the linkage. Fine-structure genetic mapping of the region was carried out by testing DNA of F2 intercross mice with the flanking markers D9Mit72 and D9Mit163. DNAs from mice with chromosomes recombinant in the rd7 region were further tested with additional markers to identify the narrowest region containing rd7. Recombinant mice in the backcross also were progeny tested by crossing them to 77–2C2a-special-rd7/rd7 mice to confirm informative recombinants or to determine whether an uninformative recombinant carried the disease gene. A minimum of 20 offspring from each progeny test were genotyped and phenotyped. For PCR amplification, 25 ng DNA was used in a 10 μl volume containing 50 mM KCl, 10 mM Tris⋅Cl, pH 8.3, 2.5 mM MgCl2, 0.2 mM oligonucleotides, 200 μM dNTP, and 0.02 units AmpliTaq DNA polymerase. The reactions, which were initially denatured for 2 min at 95°C, were subjected to 49 cycles of 20 sec at 94°C, 20 sec at 50°C, 30 sec at 72°C, and a 7-min extension at 72°C. PCR products were separated by electrophoresis on a 4% MetaPhor (FMC) agarose gel and visualized under UV light after staining with ethidium bromide. Test cross progeny were phenotyped by clinical examination.
Chromosomal Localization of the Mouse PNR Gene.
Genes were mapped by analysis of the progeny of two sets of genetic crosses: (NFS/N or C58/J X M. m. musculus)F1 × M. m. musculus (17), and (NFS/N X M. spretus)F1 × M. spretus or C58/J (18). Loci were ordered by minimizing the number of recombinants, and recombination distances were determined according to Green (19).
Northern Blot Analysis.
Total RNA was extracted from retinas with TRIzol (GIBCO/BRL), separated by electrophoresis in 0.9% agarose gels containing 2.2 M formaldehyde, and transferred to Hybond-N+ membranes (Amersham Pharmacia). Fragments of mouse PNR cDNA were used as probes for hybridization with 5 mg of total RNA. The probes were labeled with [α-32P]-dCTP with the multiprime DNA-labeling kit (Amersham Pharmacia). After hybridization, the Northern blots were washed at a final stringency of 0.3 × SSC and 0.3% SDS at 60°C. Blots were exposed to x-ray film (Amersham Pharmacia) for 6 h at room temperature.
In Situ Hybridization.
Mouse PNR cDNA was digested with HindIII or XbaI to generate 5′ protruding ends. RNA riboprobes were synthesized with T3 (sense) or T7 (antisense) RNA polymerases in the presence of digoxigenin-labeled UTP. Preparation of tissue sections and in situ hybridization were performed as described by Reid et al. (20).
Reverse Transcription–PCR (RT-PCR) Amplification.
Fifty micrograms of total RNA was treated with 10 units of RNase-free DNase I (Boehringer Mannheim) in the presence of 10 units of placental ribonuclease inhibitor (Promega) to eliminate possible DNA contamination. RT-PCR was performed on 1 μg DNase-treated total RNA. First-strand cDNA was synthesized by using an oligo(dT) primer and MuLV Reverse Transcriptase (Perkin–Elmer), and PCR was performed with AmpliTaq DNA polymerase (Perkin–Elmer) and primers listed in Table 1. Gel-purified DNA fragments (Qiagen, Chatsworth, CA) were subjected to direct sequencing.
Table 1.
Primers used for RT-PCR
| Name | Sequence 5′ → 3′ | Position in cDNA | Product size, bp |
|---|---|---|---|
| MNR1 | TGAGAAGGAGGCTCATCTACAG | 398 -419 | |
| MNR4 | CTGGTCCCGGAAAGGCAGGTTG | 903 -882 | 506 |
| MNR3 | CTGTGCAGAATGAGCGCCAAC | 524 -544 | |
| MNR6 | CTCACAGGCTGGCTGGGGTGGT | 1250 -1229 | 727 |
| MNR5 | GTGATCTTGCTGGAAGAGGCA | 904 -924 | |
| MNR8 | CTGAATGTGGAATTACTGGCTG | 1479 -1458 | 576 |
| MNR7 | GTTTGGGAAATTGCTCCTCCTG | 1251 -1272 | |
| MNR19 | TTCACAGTTTTCTGGTTTTATT | 2001 -1980 | 751 |
| MNR1 | TGAGAAGGAGGCTCATCTACAG | 398 -419 | |
| MNR6 | CTCACAGGCTGGCTGGGGTGGT | 1250 -1229 | 853; 473* |
*, The size of the product amplified from the rd7 RNA.
5′ Rapid Amplification of cDNA Ends (RACE).
The 5′ end of mouse PNR cDNA was obtained by using the 5′ RACE System (GIBCO/BRL) with primers MNR6 (Table 1) and MNR2 (nucleotides 523–502; Fig. 4), 5′-CATCTTGGTTCATGCCTGCTTG-3′.
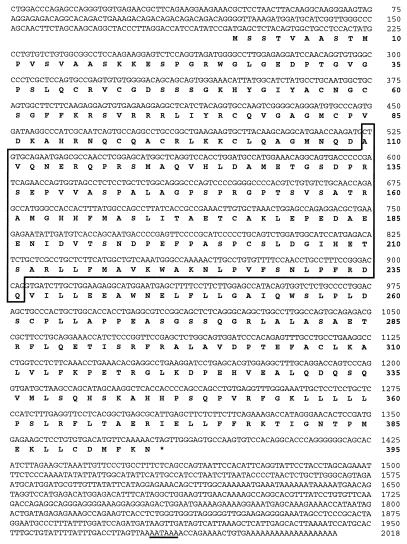
Figure 4.
Nucleotide sequence of the mouse PNR cDNA and its predicted amino acid sequence. The polyadenylation signal is underlined. The deleted region in the rd7 mouse PNR cDNA is boxed.
Sequence Analysis.
Nucleotide sequences were determined by the standard dideoxynucleotide chain termination method using the Sequenase kit (United States Biochemical) and the Taq Dye Deoxy Termination Cycle Sequencing kit (Applied Biosystems). All sequences shown in this paper have been determined on both DNA strands or at least twice on the same strand. blast analyses were performed with the use of the National Center for Biotechnology Database (www.ncbi.nlm.nih.gov/BLAST/) for homology searches. Sequence alignment, ORF determinations, and restriction mapping of DNA fragments were performed using sequencher software (Gene Codes, Ann Arbor, MI). Molecular weight of the predicted protein was calculated by using protean software (DNAstar, Madison, WI).
Results
Origin.
Strain “77–2C2a-special,” in which the rd7 mutation occurred, was among several strains constructed to contain as many pigment dilution genes as possible. Although this fully inbred strain was never crossed with all its original parental mutant lines to determine how many dilution genes it carries, it could potentially contain a (nonagouti black), Tyrp1b (brown), Myo5ad (dilute), ru1 (ruby 1), mr (maroon), and Tyrc-ch (the chinchilla allele of albino). The coat color of 77–2C2a-special is similar to albino, and the retina is lightly pigmented, similar to the appearance of the fundus background of a human Caucasian. Of the four 77–2 sublines, only 77–2C2a-special exhibited the rd7 phenotype, suggesting it was a new mutation. The rd7 mutation has been backcrossed to C57BL/6J to create a congenic strain with a standard genetic background (B6.Cg-rd7).
Phenotypic Characterization.
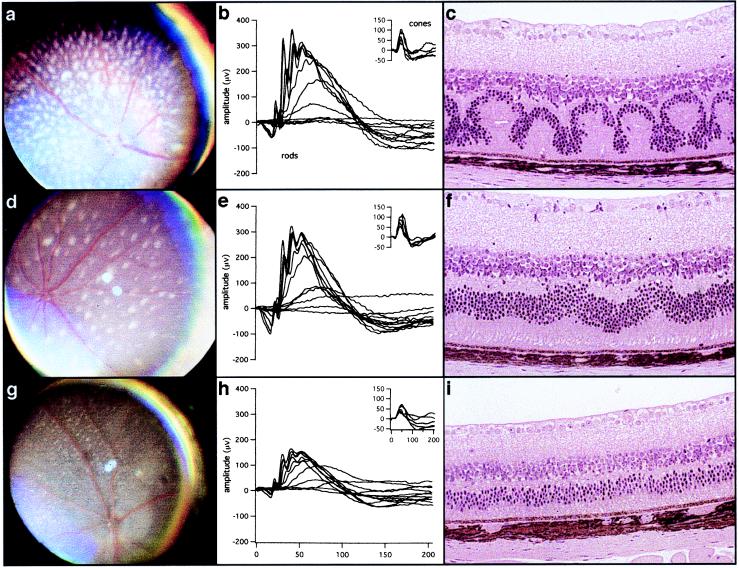
A large number of rd7 mice at different ages and on different backgrounds were characterized by indirect ophthalmoscopy, ERG, and histology. At least five B6.Cg-rd7/rd7 N7 mice were examined for retinal spotting, ERG response, and histological abnormalities at each of the three ages shown in Fig. 1.
Figure 1.
Fundus photographs, ERGs, and histology of eyes from rd7/rd7 mice at 1 mo (a–c), 5 mo (d–f), and 16 mo (g–i) of age. At 1 mo of age, rd7 mice have white spots over the entire retina (a), but the scotopic and photopic (Inset) ERGs are normal (b), and light micrographs show photoreceptor and outer nuclear layer waves, whorls, and rosettes in the retina (c). At about 5 mo of age, the number of retinal spots is decreased (d), ERGs are still normal (e), and histology shows few retinal waves (f). By 16 mo of age, attenuated retinal vessels and mottled retinal pigment indicate retinal degeneration (g), ERGs are now 50% of normal (h), and the outer nuclear layer and outer segment have degenerated to half normal (i).
Clinical Examination.
rd7 homozygous mutant mice have white spots over the entire retina at 1 mo postnatal (Fig. 1a); the spots disappear with age (Fig. 1 d and g). The mutation was initially detected by the histopathological anomalies described below as retinal spotting; these anomalies were not observed in the original 77–2C2a mice. The spotting appeared only when the mice were outcrossed to C57BL/6J for the purpose of genetic mapping. rd7 mice show clinical signs of retinal degeneration starting at 12 mo of age and mottled retinal pigment is observed at 16 mo.
Histology.
Light microscopic examination of histological preparations shows outer nuclear layer waves, whorls, and rosettes in the retinas of homozygous rd7 mutants by 1 mo of age (Fig. 1c). These waves flatten out by 5 mo and are gone by 16 mo (Fig. 1 f and i). Flattening of the waves is concordant with disappearance of the retinal spots. Late-onset progressive retinal degeneration is also observed. Photoreceptor cells degenerate as evidenced by the reduction of the outer nuclear layer from the normal thickness of 10 rows of nuclei to only 4 or 5 rows by 16 mo. Histological changes correlate directly with functional changes, as shown by electroretinography (Fig. 1 b, e, and h).
Electroretinography.
ERGs of homozygous rd7 mice are normal until 5 mo of age (Fig. 1 b and e); thereafter, there is a progressive reduction of both rod and cone signals (Fig. 1h). At 1 mo, when there is extensive disruption of retinal integrity by the waves of rosettes (Fig. 1c), the a- and b-wave amplitudes at maximal intensities are slightly smaller (Fig. 1b) than at 5 mo, when the waves have nearly disappeared (Fig. 1 e and f). By 16 mo, the ERG amplitudes are reduced by 50% of normal.
Chromosomal Localization of the rd7 Locus.
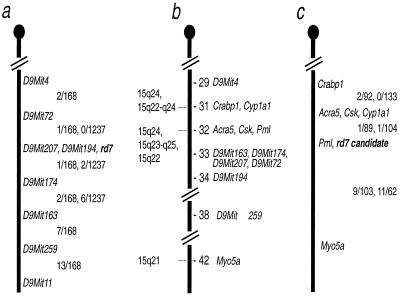
In the genome-wide screen of 57 markers using a selective DNA pooling approach (15), only marker D9Mit4 showed significant skewing of amplified products in affected and unaffected DNA pools. Testing of individual DNAs from the 20 animals that were originally pooled confirmed the linkage to Chr 9. Fine mapping of the region containing the rd7 gene mutation was carried out by using DNA from backcross (168 meioses) and intercross (1,240 meioses) mice. rd7 mapped to a 0.36 ± 0.16 cM region between markers D9Mit72 and D9Mit174. It did not recombine with markers D9Mit207 and D9Mit194 (Fig. 2a).
Figure 2.
Maps of the rd7 region of mouse Chr 9. (a) Mapping of the rd7 locus. The first fraction between the markers is the number of recombinants/total in the 77–2C2a-special-rd7/rd7 x CAST/Ei backcross, and the second fraction is the number of recombinants/total in the B6.Cg-rd7/rd7 X CAST/Ei intercross. (b) Composite map of mouse Chr 9 from the Encyclopedia of the Mouse Genome (www.informatics.jax.org/). The numbers to the right of the chromosome represent the cM distances from the centromere; the numbers to the left of the chromosome represent the chromosomal loci of the human orthologs of the mouse gene listed to the right. (c) Mapping of Pnr. The first fraction between the markers on the right is the number of recombinants/total from the cross (NFS/N X M. spretus) × M. spretus or C58/J, and the second fraction is the number of recombinants/total from the cross (NFS/N or C58/J X M. m. musculus) × M. m. musculus. Pml was typed only in the Mus spretus cross; Acra5 was typed only in the M. m. musculus cross.
In the first 450 F2 intercrossed animals examined, 108 mice were homozygous for 77–2C2a alleles in the region containing rd7, therefore presumably carrying the rd7 mutation. Of these animals, 14 were phenotypically unaffected, 17 showed a reduced number of spots across the retina, often confined to one quadrant of each eye, and 77 showed the characteristic spotting across the entire retina. The 31 phenotypically unaffected or mildly affected mice were shown to be homozygous for the rd7 mutant allele by progeny test crosses with rd7/rd7 mice.
Mapping of the Mouse PNR cDNA.
One of the clones (mPNR) that we isolated from a pool of cDNAs enriched in photoreceptor-specific messages was mapped to the region corresponding to the rd7 locus (Fig. 2c). Two loci were detected by the mPNR cDNA and mapped in the mouse by Southern blot analysis. In the Mus spretus crosses, BglII digestion produced fragments of 12.4 and 6.3 kb in Mus spretus and 12.6 and 5.2 kb in NFS/N. The 12.4- and 12.6-kb bands mapped to Chr 9 and the others to Chr 15. In the Mus m. musculus cross, we used a ScaI digest that produced NFS/N fragments of 4.2 and 3.2 kb and Mus m. musculus fragments of 4.3 and 3.2 kb. The polymorphic band mapped to Chr 9. The Chr 9 locus mapped in both crosses was located very near to the rd7 mutant gene. Fig. 2 shows the region of Chr 9 where the rd7 mutation and the candidate for the rd7 gene mapped in our crosses compared with the composite map of the corresponding region of Chr 9 from the Encyclopedia of the Mouse Genome, Rel. 3.0, Mouse Genome Informatics [(1994); www.informatics.jax.org/] and Chromosome 9 map from the Mouse Genome Database, Mouse Genome Informatics, The Jackson Laboratory [(1999); www.informatics.jax.org/]. The number of recombinants/total mice tested for each marker and cM distance between markers for the Chr 15 locus, which was identified only in the Mus spretus crosses, is as follows: Mlvi2 (13/87 = 14.9 ± 3.8) − Pnr-rs1 (3/90 = 3.3 ± 1.9) − Int6 (8/100 = 8.0 ± 2.7) − Hba-ps3. The Chr 15 gene is apparently a related gene because it is recognized by the same probe.
Characterization of the Mouse PNR cDNA.
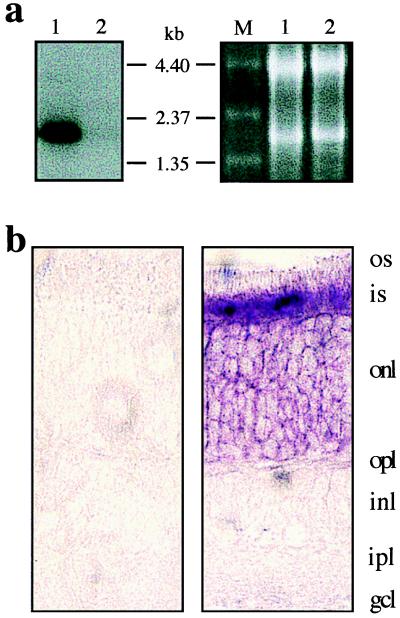
Northern blot analyses and in situ hybridization showed that the mPNR mRNA is expressed specifically and abundantly in retinal photoreceptor cells (Figs. 3 a and b) and is not present in cerebellum, cerebral cortex, heart, kidney, liver, lungs, or spleen (data not shown). The size of the mouse PNR mRNA is approximately 2.0 kb.
Figure 3.
Expression of mouse PNR mRNA. (a) Northern blot of adult mouse retinal RNAs hybridized with the mPNR cDNA probe. Lane 1, wild type; lane 2, (photoreceptorless) rd1/rd1; lane M, RNA molecular size standards (GIBCO/BRL). For loading control, ethidium bromide staining of the RNAs is shown (Right). (b) In situ hybridization of a mouse retina section with a mouse PNR probe. (Left) Sense probe; (Right) antisense probe.
Sequence analysis of the mPNR clone revealed a 1.625-kb cDNA fragment with an ORF of 987 nt and a complete 3′ untranslated region (UTR) with a poly(A) tail. Alignment of the ORF with GenBank sequences showed that this cDNA is an ortholog of the recently published human photoreceptor-specific nuclear receptor cDNA, PNR (21). However, the mouse cDNA clone was truncated at the 5′ end and lacked the region corresponding to the first 73 aa of the human protein. This region codes for part of the functionally important and highly conserved DNA-binding domain (DBD). The sequence corresponding to the missing N-terminal region of the protein as well as the 5′ UTR was obtained by 5′ RACE. The complete full-length mouse PNR clone is 2,018 nt in length and contains 195 nt, corresponding to the 5′ UTR, 1,188 nt of coding region, and 635 nt of 3′ UTR (Fig. 4). The predicted ORF encodes a 395-aa protein with an approximate molecular weight of 43 kDa.
Isolation and Analysis of the mPNR cDNA from rd7 Mouse.
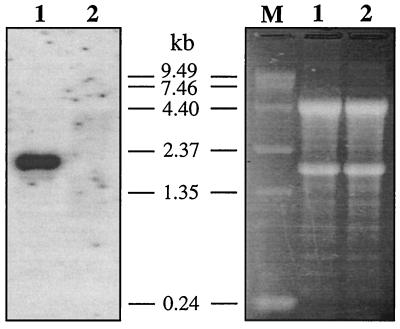
Chromosomal mapping of mPNR to the rd7 locus suggested its possible involvement in retinal degeneration. To determine the validity of this assumption, we analyzed the expression level and sequence integrity of the PNR mRNA from rd7/rd7 mice. These mice were C57BL/6J-rd7/rd7 congenic. Therefore, only a small region of Chr 9 including the rd7 locus remained from the original 77–2Ca-special −rd7/rd7 strain. Northern analysis of total retinal RNAs from 3- to 6-mo-old affected animals revealed no detectable hybridization signal with mPNR cDNA probe (Fig. 5). The absence of mPNR mRNA can be explained either by a dramatic reduction of transcriptional efficiency because of a mutation in the regulatory region of the gene or by rapid degradation of newly synthesized messages because of the presence of a nonsense mutation.
Figure 5.
Northern blot of rd7 mouse retinal RNA hybridized with a mouse PNR cDNA probe. For loading control, ethidium bromide staining of the RNAs is shown (Right). Lane 1, wild type; lane 2, rd7/rd7; lane M, RNA molecular size standards.
Using the more sensitive RT-PCR technique, we amplified different fragments of the mPNR cDNA and subjected them to direct sequencing. We found 380 nt missing from the coding region in the rd7 PNR cDNA (Fig. 4 and 6). This deletion was present in PNR mRNA of 10 rd7 mice and was not detected in corresponding mRNAs from more than 50 C57BL/6J controls. In the normal cDNA, this 380-nt fragment encodes 127 amino acids that encompass more than 32% of the total protein length and are part of the DBD. In addition, the deletion leads to a frame shift creating a premature stop codon. This change in the coding region of rd7 PNR mRNA may well be responsible for its rapid degradation (Fig. 5) through the nonsense-mediated decay pathway (22).
Figure 6.
Deletion in the rd7 mutant mouse PNR cDNA. (a) Representative sequence of a DNA fragment containing the site of the deletion in affected animals. The DNA fragment was obtained by RT-PCR by using primers MNR1 and MNR6. The arrow points to the site of the deletion. (b and c) Sequences of PNR fragments from unaffected animals obtained by RT-PCR by using primers MNR1 and MNR4 and MNR3 and MNR6, respectively. For primer sequences, see Table 1.
Discussion
The rd7 mouse is an animal model for hereditary retinal degeneration that has similarities to human flecked retinal disorders such as retinitis punctata albescens. The concordance of the appearance and disappearance of the discrete white spots observed clinically with the waves seen histologically suggests that the spots are a result of the pathologic changes in the retina. The fact that abnormal ERGs are detected only after the retinal waves have begun to flatten out suggests that the early retinal abnormalities do not interfere with gross retinal function. The inaccessibility of human ocular tissue makes it impossible to obtain histological preparations from retinal spotting conditions except at autopsy. Thus, the rd7 mouse may provide a model for studying some human retinal spotting diseases.
Our linkage backcross data demonstrate that rd7 segregates as a single recessive gene. All F1 mice studied were unaffected. The absence of spots in the original 77–2C2a-special inbred line is probably because of our inability to discern them against the very lightly pigmented retina of mice of this strain. However, evidence for reduced penetrance of the rd7 retinal spotting phenotype was observed in the intercross of B6.Cg-rd7/rd7 with the CAST/Ei strain. Therefore, progeny tests of phenotypically normal or mildly affected mice that were homozgyous for 77–2C2a-special alleles at flanking markers D9Mit72 and D9Mit163 were carried out. The resulting progeny confirmed that the mice in question were homozygous for the mutated allele. Although all offspring of these progeny tests showed spotting, a variation in the number of spots was observed. These two observations suggest that background genes can interact with the mutated rd7 gene to delay or prevent retinal degeneration. Because only 31 (28%) of the 108 rd7/rd7 mice showed the modified phenotype compared with 77 mice that showed the fully penetrant phenotype, it is possible that one or more gene(s) is necessary for protection from degeneration.
The chromosomal region to which the rd7 gene maps is homologous to human Chr 15q23-q25, which contains the loci of a recently identified autosomal recessive RP family (23), Bardet–Biedl 4 (24), as well as a syndrome characterized by retinal degeneration, mental retardation, and spasticity (25). This makes PNR a candidate for these forms of human retinal degeneration.
Results obtained by Northern blot hybridization and sequence analysis of the RT-PCR products indicate that the deletion in the mouse PNR mRNA will prevent the synthesis of the corresponding protein in the rd7 mouse retina. Therefore, the deletion in the mRNA encoding mouse PNR (Pnr) is responsible for the rd7 retinal degeneration. These results also indicate that Pnr expression is critical for the integrity and function of the photoreceptor cells.
PNR is a member of a nuclear receptor superfamily that includes over 150 different proteins (26–30). Nuclear receptors, which bind a complex array of extra- and intracellular signals, can activate a series of transcriptional responses involved in development, differentiation, metamorphosis, and physiology. The signaling molecules for these proteins are lipophilic and include steroids, retinoids, vitamin D, thyroid hormones, prostanoids, and farnesoids. Nuclear receptors are characterized by the presence of both DBDs and ligand-binding domains (LBDs). DBDs are responsible for the receptor specificity to DNA sequences (hormone response elements) and consist of two conserved zinc fingers. LBDs are the domains that selectively and specifically interact with ligands and turn the receptor into a transcriptionally active state. Comparison of the predicted mouse and human amino acid sequences of PNR shows that both have the characteristic DBDs and LBDs.
By gel-shift analysis, it has been determined that human PNR forms a stable complex with a direct dimeric DNA repeat containing two AAGTCA half-sites separated by one spacer nucleotide (21). This suggests that a functional unit of PNR could be a homodimer. However, whether PNR regulates transcription as a monomer, homodimer, or heterodimer, the sequence it recognizes in vivo and its ligand specificity have yet to be determined.
In summary, we have demonstrated that a deletion in the Pnr mRNA is responsible for retinal degeneration in the rd7 mouse. The Pnr gene, therefore, is a candidate for autosomal recessive RP and Bardet–Biedl 4 by virtue of its chromosomal localization, its expression in photoreceptors, and the fact that it carries a mutation that causes retinal degeneration in the mouse. In addition, the rd7 mouse offers the possibility for studying flecked retinal diseases in an animal model.
Acknowledgments
We thank Philip Rosensteil, Cindy Avery, Brian Coffman, and Ron Hurd for technical assistance. This research was supported by grants from the National Institutes of Health EY08285 (D.B.F.), EY11996 (P.M.N., B.C., N.L.H.), and EY07758 (J.R.H., B.C., M.T.D., N.L.H.). D.B.F. and J.R.H. are recipients of Senior Scientific Investigators Awards from Research to Prevent Blindness.
Abbreviations
- RP
retinitis pigmentosa
- rd
retinal degeneration
- mPNR
mouse photoreceptor-specific nuclear receptor
- RT-PCR
reverse transcription–PCR
- RACE
rapid amplification of cDNA ends
- DBD
DNA-binding domain
- Chr
chromosome
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF204053).
References
- 1.Bowes C, Li T, Danciger M, Baxter L C, Applebury M L, Farber D B. Nature (London) 1990;347:677–680. doi: 10.1038/347677a0. [DOI] [PubMed] [Google Scholar]
- 2.Noben-Trauth K, Naggert J K, North M A, Nishina P M. Nature (London) 1996;380:534–538. doi: 10.1038/380534a0. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin M E, Sandberg M A, Berson E L, Dryja T P. Nat Genet. 1993;4:130–134. doi: 10.1038/ng0693-130. [DOI] [PubMed] [Google Scholar]
- 4.Danciger M, Blaney J, Gao Y Q, Zhao D Y, Heckenlively J R, Jacobson S G, Farber D B. Genomics. 1995;30:1–7. doi: 10.1006/geno.1995.0001. [DOI] [PubMed] [Google Scholar]
- 5.Bayes M, Giordano M, Balcells S, Grinberg D, Vilageliu L, Martinez I, Ayuso C, Benitez J, Ramos-Arroyo M A, Chivelet P, et al. Hum Mutat. 1995;5:228–234. doi: 10.1002/humu.1380050307. [DOI] [PubMed] [Google Scholar]
- 6.Hagstrom S A, North M A, Nishina P L, Berson E L, Dryja T P. Nat Genet. 1998;18:174–176. doi: 10.1038/ng0298-174. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee P, Kleyn P W, Knowles J A, Lewis C A, Ross B M, Parano E, Kovats S G, Lee J J, Penchaszadeh G K, Ott J, et al. Nat Genet. 1998;18:177–179. doi: 10.1038/ng0298-177. [DOI] [PubMed] [Google Scholar]
- 8.Travis G H, Brennan M B, Danielson P E, Kozak C A, Sutcliffe J G. Nature (London) 1989;338:70–73. doi: 10.1038/338070a0. [DOI] [PubMed] [Google Scholar]
- 9.Gibson F, Walsh J, Mburu P, Varela A, Brown K A, Antonio M, Beisel K W, Steel K P, Brown S D. Nature (London) 1995;374:62–64. doi: 10.1038/374062a0. [DOI] [PubMed] [Google Scholar]
- 10.Keen T J, Inglehearn C F. Hum Mutat. 1996;8:297–303. doi: 10.1002/(SICI)1098-1004(1996)8:4<297::AID-HUMU1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Weil D, Blanchard S, Kaplan J, Guilford P, Gibson F, Walsh J, Mburu P, Varela A, Levilliers J, Weston M D, et al. Nature (London) 1995;374:60–61. doi: 10.1038/374060a0. [DOI] [PubMed] [Google Scholar]
- 12.Bowes C, Danciger M, Kozak C A, Farber D B. Proc Natl Acad Sci USA. 1989;86:9722–9726. doi: 10.1073/pnas.86.24.9722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heckenlively J R, Winston J V, Roderick T H. Doc Ophthalmol. 1989;71:229–239. doi: 10.1007/BF00170972. [DOI] [PubMed] [Google Scholar]
- 14.Hawes NL, Smith RS, Chang B, Davisson M, Heckenlively JR, John SW. Mol Vis. 1999;5:22. [PubMed] [Google Scholar]
- 15.Buffone G J, Darlington G J. Clin Chem. 1985;31:164–165. [PubMed] [Google Scholar]
- 16.Taylor B A, Navin A, Phillips S J. Genomics. 1996;21:626–632. doi: 10.1006/geno.1994.1323. [DOI] [PubMed] [Google Scholar]
- 17.Kozak C A, Peyser M, Krall M, Mariano T M, Kumar C S, Pestka S, Mock B A. Genomics. 1990;8:519–524. doi: 10.1016/0888-7543(90)90039-w. [DOI] [PubMed] [Google Scholar]
- 18.Adamson M C, Silver J, Kozak C A. Virology. 1991;183:778–781. doi: 10.1016/0042-6822(91)91010-e. [DOI] [PubMed] [Google Scholar]
- 19.Green M C. In: Methodology in Mammalian Genetics. Burdette W J, editor. San Francisco: Holden-Day; 1963. pp. 56–82. [Google Scholar]
- 20.Reid S N, Akhmedov N B, Piriev N I, Kozak C A, Danciger M, Farber D B. Gene. 1999;18:227. doi: 10.1016/s0378-1119(98)00578-2. , 257–266. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi M, Takezawa S, Hara K, Yu R T, Umesono Y, Agata K, Taniwaki M, Yasuda K, Umesono K. Proc Natl Acad Sci USA. 1999;96:4814–4819. doi: 10.1073/pnas.96.9.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Culbertson M R. Trends Genet. 1999;15:74–80. doi: 10.1016/s0168-9525(98)01658-8. [DOI] [PubMed] [Google Scholar]
- 23.Gerber S, Santos L, Lopez L, Gribouval O, Ferraz F, Kaplan J. Invest Ophthalmol Visual Sci. 1999;40:S604. [Google Scholar]
- 24.Bruford E A, Riise R, Teague P W, Porter K, Thomson K L, Moore A T, Jay M, Warburg M, Schinzel A, Tommerup N, et al. Genomics. 1997;41:93–99. doi: 10.1006/geno.1997.4613. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell S J, McHale D P, Campbell D A, Lench N J, Mueller R F, Bundey S E, Markham A F. Am J Hum Genet. 1998;62:1070–1076. doi: 10.1086/301821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mangelsdorf D J, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, et al. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mangelsdorf D J, Evans R M. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 28.Freedman L P. Cell. 1999;97:5–8. doi: 10.1016/s0092-8674(00)80708-4. [DOI] [PubMed] [Google Scholar]
- 29.Blumberg B, Evans R M. Genes Dev. 1998;12:3149–3155. doi: 10.1101/gad.12.20.3149. [DOI] [PubMed] [Google Scholar]
- 30.Leid M, Kastner P, Chambon P. Trends Biochem Sci. 1992;17:427–433. doi: 10.1016/0968-0004(92)90014-z. [DOI] [PubMed] [Google Scholar]