Abstract
Restrictions in the diversity of an adaptive immune repertoire can facilitate viral persistence. Because a host afflicted with an immune deficiency is not likely to purge a persistent infection using endogenous mechanisms, it is important to explore adoptive therapies to supplement the host with a functional immune defense. In this study we describe a virus carrier state that results from introducing lymphocytic choriomengitis virus (LCMV) into adult mice possessing a restricted T cell repertoire. Upon infection of these mice, LCMV establishes systemic persistence, and within the central nervous system (CNS), the virus infects astrocytes (and later oligodendrocytes) rather than its traditional parenchymal target - neurons. To determine whether LCMV could be purged from a novel target selection in the absence of an endogenous immune repertoire, we adoptively transferred virus-specific memory cells into adult carrier mice. The memory cells purged virus from the periphery as well as the CNS, but induced fatalities not typically associated with adoptive immunotherapy. When the repertoire of the recipient mice was examined, a deficiency in natural regulatory T cells was noted. We therefore supplemented carrier mice with regulatory T cells and simultaneously performed adoptive immunotherapy. Co-transfer of regulatory T cells significantly reduced mortality while still permitting the anti-viral memory cells to purge the persistent infection. These data indicate that regulatory T cells can be used therapeutically to lessen the pathogenicity of virus-specific T cells in an immunodeficient host. We also propose that the novel carrier state described herein will facilitate the study of immunotherapeutic regimens.
Keywords: viral, neuroimmunology, memory, cytotoxicity, immunodeficiency
Introduction
Pathogens such as cytomegalovirus (CMV), Epstein-Barr virus (EBV), herpes simplex virus (HSV), hepatitis B/C virus (HBV, HCV), and human immunodeficiency virus (HIV-1) can establish lifelong persistence, and each interact with their human host in unique ways to adversely affect health. The mechanisms that deteriorate human health during viral persistence are attributable to complex variables that include viral tropism, viral cytopathogenicity, and host immune responsiveness. Thus, from a therapeutic perspective it would appear that in order to thwart persistence each human pathogen should be dealt with individually to account for unique pathogen-host relationships. However, a series of seminal studies published in the LCMV model system revealed that systemic eradication of a persistent viral infection can be achieved using an approach referred to as adoptive immunotherapy (1-3). This approach has since been translated from bench to bedside and shown to be efficacious for the treatment of CMV (4, 5), HBV (6), EBV (7), and EBV-induced lymphoma (8). Thus, a single therapeutic approach can terminate several distinct states of persistence.
In the LCMV model, the traditional immunotherapeutic approach relies on the adoptive transfer of virus-specific memory splenocytes into mice persistently infected from birth with the virus (2, 3). Mice infected in utero or at birth with LCMV become lifelong carriers, with the virus persisting in every tissue compartment, including the CNS (9). Because carrier mice are tolerant to the virus at the T cell level (10), they provide an excellent model to explore cell based therapies to thwart persistence. In the LCMV model it was shown that adoptive transfer of memory splenocytes from a LCMV immune animal could purge virus from the entire host in 3-4 months (most peripheral tissues in 14 days and the CNS and kidneys in 100-200 days) (11, 12). The minimum number of anti-viral T cells required for clearance was determined to be ∼350,000 memory CD8+ T cells and ∼7,000 memory CD4+ T cells (13). Following successful adoptive immunotherapy, mice were no longer tolerant to LCMV (14) and maintained anti-viral immunity to the pathogen (14, 15).
It is presently not known how anti-viral memory cells respond to more complex states of persistence that exist in immunologically compromised individuals. We recently observed that ovalbumin TCR-tg mice (OT-I mice) are unable to mount an anti-LCMV T cell response because of their highly skewed repertoire, and thus become carrier mice when challenged with LCMV in adulthood (16). Interestingly, the distribution of LCMV in the CNS of OT-I mice appeared to be different than that observed in mice infected from birth or in utero (16). In OT-I carrier mice astrocytes (16) rather than neurons (11, 17) were the main cell population infected within the brain parenchyma.
Because infection of adult OT-I mice initiated a unique LCMV carrier state, we set out to address several unanswered questions. First, how do anti-viral memory cells perform in a host with a highly skewed T cell repertoire? This is of particular importance because repertoire skewing can give rise to defects in immune regulation (18). Second, can anti-viral memory cells achieve clearance of a persistent viral infection in the absence of endogenous T cell support? OT-I mice cannot mount a CD8 or CD4 T cell response to LCMV and thus provide an excellent model to determine whether exogenously derived memory T cells alone can purge a host. Finally, can memory T cells cleanse a brain inundated with persistently infected astrocytes? The altered LCMV tropism observed in OT-I mice exposes the adoptively transferred memory cells to a new CNS target (i.e., astrocytes), and it is presently not known whether astrocytes can be successfully purged of a persistent viral infection without the induction of fatal immunopathology
Materials and Methods
Mice
C57BL/6 (B6) (H-2b) and C57BL/6 OT-I TCR-tg (H-2b) mice (19, 20) were bred and maintained in a closed breeding facility at The Scripps Research Institute. The handling of all mice conformed to the requirements of the National Institutes of Health and The Scripps Research Institute animal research committee.
Virus
To establish a B6 LCMV carrier colony, newborn mice (1 day old) were infected intracerebrally (i.c.) with 1×103 plaque forming units (PFU) of LCMV Armstrong (Arm) clone 53b. The resultant carrier mice were then interbred to establish new carrier mice through vertical transmission (i.e., passage of virus from mother to offspring). Persistent infections were established in OT-I mice by infecting them i.c. at 6-8 weeks of age with 1×103 PFU LCMV Arm. Memory donor mice were generated by infecting B6 mice intraperitoneally (i.p.) with 1×105 PFU LCMV Arm. Stocks were prepared by a single passage on BHK-21 cells, and viral titers were determined by plaque formation on Vero cells.
Mononuclear cell isolations and tissue processing
To obtain cell suspensions for immunocytotherapy, spleens were harvested from donor mice, and, afterward, single-cell suspensions were then prepared by mechanical disruption through a 100-μm filter. Spleen cells were treated with red blood cell lysis buffer (0.14 M NH4Cl and 0.017 M Tris-HCl, pH 7.2), washed twice, and resuspended in PBS. For lymph node analyses, the axial, mesenteric, inguinal, paraaortic, and brachial lymph nodes were harvested, combined, mechanically disrupted into a single cell suspension, washed, and resuspended in PBS. Immunohistochemical studies were performed on fresh, unfixed tissues frozen on dry ice in Optimal Cutting Temperature (OCT; Tissue-Tek). For the detection of infectious virus in the CNS, brains were cut sagittally and then half was homogenized using a Mini Beadbeater (BioSpec Products). Homogenates were analyzed using a standard plaque assay on Vero cells. Viral titers in the blood were determined by performing plaque assays on sera.
Immunocytotherapy
Immunocytotherapy was performed by adoptively transferring the denoted number of memory splenocytes i.p. into B6 or OT-I LCMV carrier mice. Splenocytes were harvested from donor B6 mice 45 days following an i.p. infection with LCMV Arm – a time point when the spleen is replete with LCMV-specific memory T cells (21).
Immunohistochemistry
To visualize LCMV, astrocytes, and oligodendrocytes, 6-μm frozen sections were cut, fixed with 2% formaldehyde, blocked with an avidin/biotin-blocking kit (Vector Laboratories), and stained for 1 h at room temperature with guinea pig anti-LCMV (1:1500), rabbit anti-glial fibrillary acidic protein (anti-GFAP; 1:800; DakoCytomation), or mouse ascites anti-2′,3′-Cyclic Nucleotide 3′-Phosphodiesterase (CNPase; clone 11-5b; 1:2000; Sigma-Aldrich Incorporated), respectively. After the primary antibody incubation, sections were washed, stained for 1 h at room temperature with a biotinylated secondary antibody (1:400; Jackson ImmunoResearch Laboratories), washed, and stained for 1 h at room temperature with streptavidin–Rhodamine Red-X (1:400; Jackson ImmunoResearch Laboratories). For co-labeling of LCMV and GFAP or LCMV and CNPase (Fig. 2), frozen sections were stained as described above except that the anti-LCMV antibody was detected with an anti–guinea pig secondary antibody directly conjugated to FITC (1:750; for 1 h at room temperature). All sections were co-stained for 5 min at room temperature with 1 μg/ml DAPI (Sigma-Aldrich) to visualize cell nuclei. All working stocks of primary and secondary reagents were diluted in PBS containing 2% fetal bovine serum (FBS).
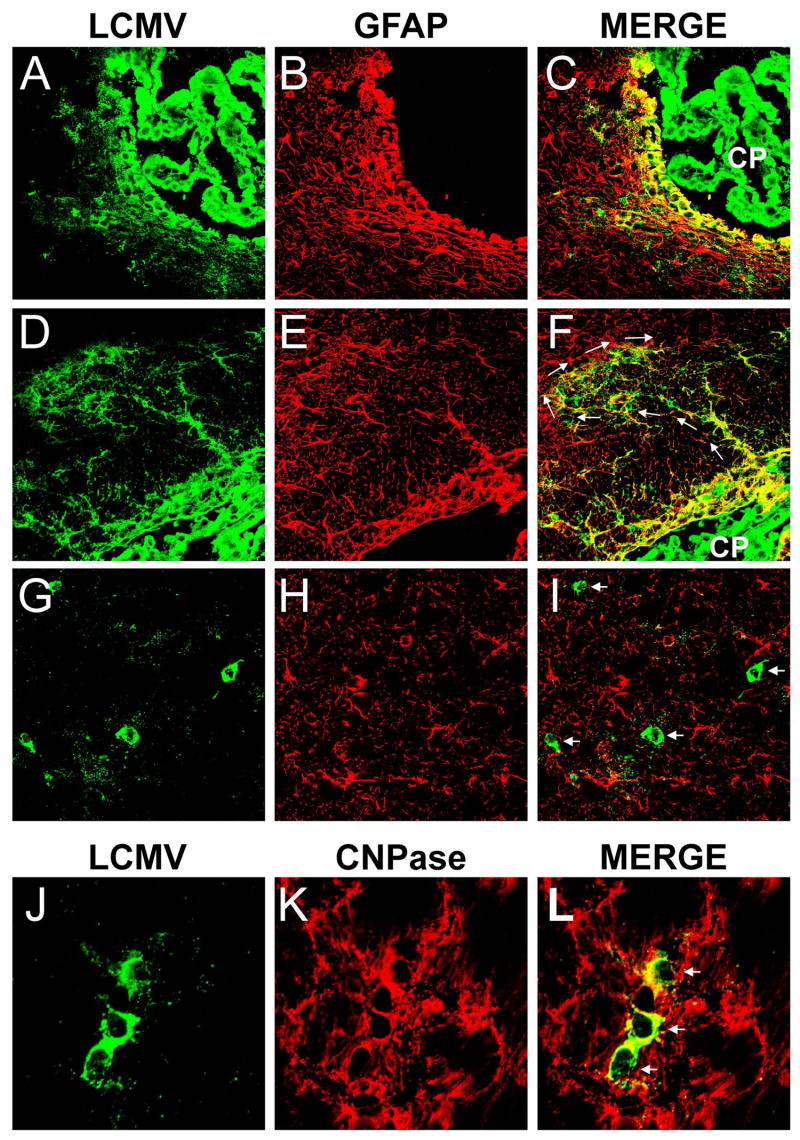
Figure 2. Tropism of LCMV in the CNS at early and late time points post-infection.
The tropism of LCMV was examined by confocal microscopy at the time points shown in Figure 1. An early (day 16; panels A-F) and late (day 154; panels G-L) time point post-infection are illustrated in this figure. Note the localization of LCMV antigen (green) in GFAP+ (red) astrocytes at early time points post-infection. After infection of primary structures such as the choroid plexus (CP) and ependymal cells lining the ventricular system (panels A-C), LCMV moved into the parenchyma by infecting astrocytes. Note the likely pattern of viral spread from astrocyte to astrocyte (panels D-F; white arrows) and the associated astrocyte activation (i.e., elevated GFAP expression). Astrocytes were the main cells infected in the parenchyma for the first 90 days post-infection (data not shown). At late time points post-infection (>90 days), LCMV was found in oligodendrocytes. Note the circular LCMV+ cell bodies at 154 days post-infection that do not co-localize with GFAP+ astrocytes (panels G-I; white arrows). LCMV antigen at this time co-localized with CNPase+ oligodendrocytes (panels J-L; white arrows).
Flow Cytometry and intracellular staining
The following antibodies were purchased from BD Biosciences and were used to stain lymphocytes isolated from spleens and lymph nodes: anti-Vβ5.1-PE, anti-CD4-PerCP, anti-CD25-Biotin (clone 7DA), and anti-CD45-FITC. Streptavidin-APC to detect biotinylated antibodies was purchased from Invitrogen, and anti-CD8-Pacific Blue was purchased from Caltag. Cells were stained with antibodies in PBS containing 1% FBS and 0.5% sodium azide on ice for 30 minutes. For intracellular staining, cell suspensions were simultaneously fixed and permeabilized with a paraformaldehyde-saponin solution for 10 minutes at room temperature. Cells were then washed twice with a PBS-saponin solution and stained with anti-FoxP3-FITC (eBioscience). Cells were acquired using a digital flow cytometer (Digital LSR II; Becton Dickinson) and flow cytometric data were analyzed with FlowJo software (Tree Star Incorporated).
Isolation of Regulatory T cells
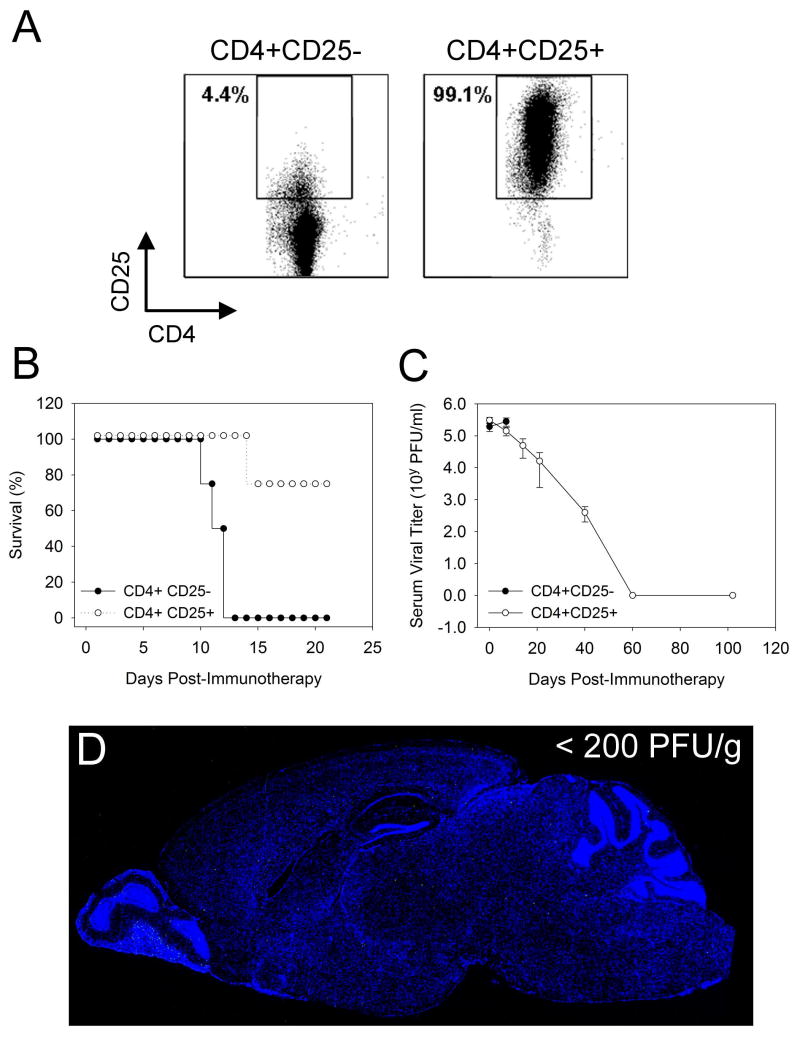
To isolate regulatory T cells, 20 spleens were harvested from B6 mice. Single-cell suspensions were then prepared by mechanical disruption and treated with red blood cell lysis buffer. Afterward, the cells were washed twice and resuspended in pre-cooled PBS containing 1% FBS. Regulatory T cells were isolated using a mouse CD4+CD25+ regulatory T cell isolation kit (Miltenyi Biotec). The purity of the CD4+CD25+ regulatory T cells was determined flow cytometrically using CD25-PE and CD4-APC antibodies (BD Pharmingen) to be greater than 99% (Fig. 5A). The column flow through of CD4+CD25- T cells (Fig. 5A) was used as a negative control for all regulatory T cell experiments. CD4+CD25- T cells and CD4+CD25+ T cells were resuspended in PBS and 4×106 of each cell population were injected i.p. into separate groups of persistently infected OT-I mice that simultaneously received 2×107 memory splenocytes.
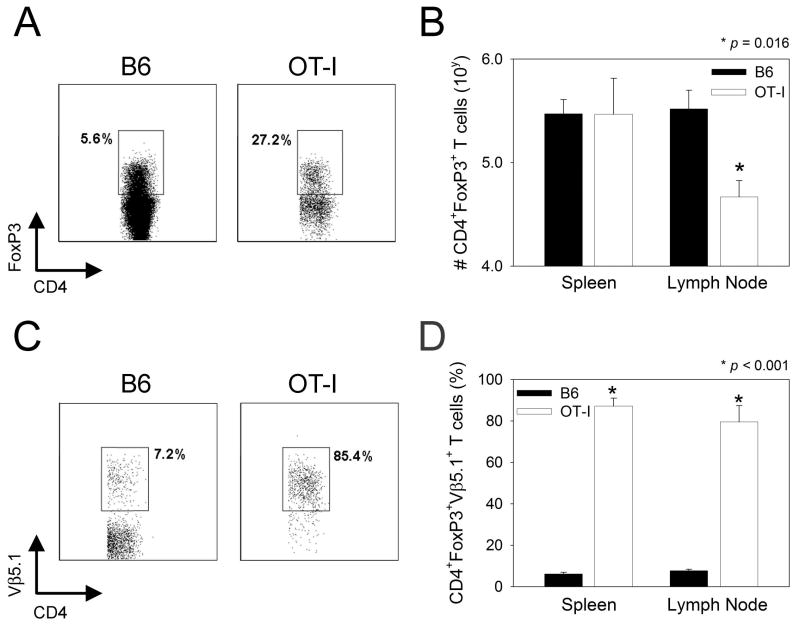
Figure 5. Analysis of regulatory T cells in OT-I mice.
A-B) Regulatory CD4+ T cells (CD45+CD4+FoxP3+) were quantified flow cytometrically in the spleens and lymph nodes of naïve OT-I versus B6 mice (n=4 per group). Note the increased frequency of regulatory T cells in the lymph nodes of OT-I mice (27.2 ± 6.9%) when compared to B6 mice (5.6% ± 0.5%) (A). The plots are gated on CD45+CD4+ T cells. A similar increase was observed in the spleen (17.4 ± 3.5% in OT-I vs. 4.4% ± 0.7% in B6) (plots not shown). When the absolute number of regulatory cells was enumerated (B), no reduction was observed in the spleen and statistically significant reduction (p = 0.016) was observed in the lymph nodes of OT-I mice when compared to B6 controls. C-D) The frequency of regulatory T cells (CD45+CD4+FoxP3+) expressing Vβ5.1 (the transgenic TCR beta chain) was quantified in the spleens and lymph nodes of OT-I versus B6 mice. Note the significantly (p < 0.001) elevated frequency of regulatory T cells expressing Vβ5.1 in spleens and lymph nodes of OT-I mice. Plots are shown for lymph nodes, but not spleens. Cells are gated on CD45+CD4+FoxP3+ cells.
Microscopy
Two-color organ reconstructions (Fig. 1) to visualize the distribution of LCMV on 6-μm frozen sections were obtained using an immunofluorescence microscope (Axiovert S100; Carl Zeiss MicroImaging, Inc.) fitted with an automated xy stage, a color digital camera (Axiocam, Carl Zeiss MicroImaging, Inc.), and a 5× objective. Registered images were captured for each field on the tissue section, and reconstructions were performed using the MosaiX function in KS300 image analysis software (Carl Zeiss MicroImaging, Inc.). Higher resolution images of LCMV-infected astrocytes or oligodendrocytes (Fig. 2) were captured with a confocal microscope (MRC1024; Bio-Rad Laboratories) fitted with a krypton/argon mixed gas laser (excitation at 488, 568, and 647 nm) and a 40× oil objective (Carl Zeiss MicroImaging, Inc.).
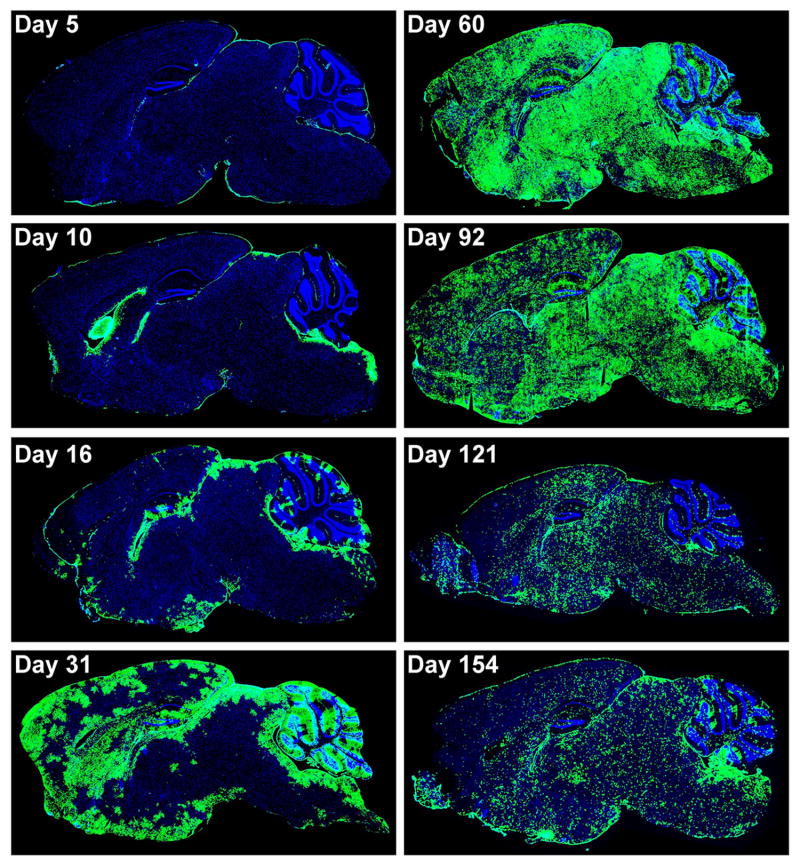
Figure 1. Distribution of LCMV in the CNS of OT-I mice infected intracerebrally.
Sagittal brain reconstructions were generated to examine the distribution of LCMV (green) in the CNS of mice (n=3-4 per group) infected i.c. with 103 PFU LCMV. Note that the virus replicated in the meninges, ependyma, and choroid plexus at early time points post-infection (days 5 and 10), but eventually moved from these primary targets into the brain parenchyma over time. The brain parenchyma was completely inundated with viral antigen by day 60 post-infection, and a distribution change was observed between days 92 and 154. Note that the pattern of viral antigen expression became more punctate at the later time points post-infection. Cell nuclei are shown in blue for anatomical purposes.
Image analysis
The percentage of CNS tissue occupied by LCMV staining was calculated from two-color (DAPI, LCMV) sagittal brain reconstructions using a program written for the KS300 image analysis software (Carl Zeiss MicroImaging, Inc.). Briefly, the total area of each cross section was determined using the DAPI channel in the two-color image. The gray scale values corresponding LCMV signal were then segmented from each image to obtain the total tissue area occupied by LCMV. The LCMV area was then divided by the DAPI area and multiplied by 100% to obtain the percentage of CNS tissue occupied by LCMV staining.
Statistical Analyses
Statistical differences were determined using a Student's t test (p < 0.05) using SigmaStat 3.1.
Results
LCMV establishes persistence in the CNS and periphery of OT-I mice inoculated intracerebrally
Intracebral (i.c.) inoculation of immunocompetent mice (e.g., B6 mice) with LCMV induces fatal choriomeningitis within 6-7 days (16). This process is known to be dependent on LCMV-specific CD8+ T cells (22), and we have shown previously that transgenic mice with a CD8 repertoire specific for ovalbumin (OT-I mice) (19, 20) do not succumb to this fatal disorder (or even develop symptoms) upon i.c. inoculation (16). Interestingly, expression of the tg TCR specific to KbOVA257-264 skews both the CD8 as well as the CD4 repertoire, resulting in no LCMV-specific CD8 or CD4 T cells following viral challenge (16). Thus, LCMV is able to establish a systemic, asymptomatic carrier state upon infection of OT-I mice. The advantage of studying persistent infection of adult OT-I mice is that they have a complete immune system replete with T and B lymphocytes, but are simply unable to recognize LCMV at the T cell level. With this model we intend to mirror scenarios in which viruses gain access to humans with restricted or skewed repertoires.
To further define the pattern of viral replication in this model following i.c. challenge, we assembled a time course of sagittal brain reconstructions (Fig. 1). At early time points post-infection (days 5-16), LCMV adhered to its well-described pattern of tropism, replicating in the meninges, choroid plexus, and ependyma (Fig. 2A-C). Because OT-I mice are devoid of virus-specific T cells, LCMV was permitted to move from primary to secondary sites of replication within the brain parenchyma at days 30 and 60 post-infection (Fig. 1). The virus appeared to move from brain lining to parenchyma in a cell-to-cell manner following juxtaposed astrocytes (Fig. 2D-F), which served as the main reservoir for viral antigen between days 30 and 90 post-infection. Interestingly, despite the absence of LCMV-specific T cells, the tropism of LCMV shifted at chronic time points post-infection (days 120 and 150) and the virus gain access to a new target – oligodendrocytes (Fig. 1G-L). The tropism of LCMV in the brains of OT-I mice is entirely different from that observed in mice persistently infected from birth, where virus is found solely in CNS neurons (11, 17). OT-I mice infected as adults therefore provide a novel LCMV paradigm to explore the establishment of viral persistence as well as perturbations in astrocytes and oligodendrocytes.
In addition to establishing persistence in the brain, LCMV also gained access to the periphery, achieving a steady state titer of 105 to 106 PFU/ml in sera by day 45 post-infection (Fig. 3A). Viremia is indicative of LCMV distributed systemically. In fact, the serum titer observed in OT-I carrier mice was one log higher than what is typically observed in B6 mice persistently infected from birth with LCMV (Fig. 3C). Thus, the state of persistence in OT-I mice differs both quantitatively and qualitatively from B6 carrier mice.
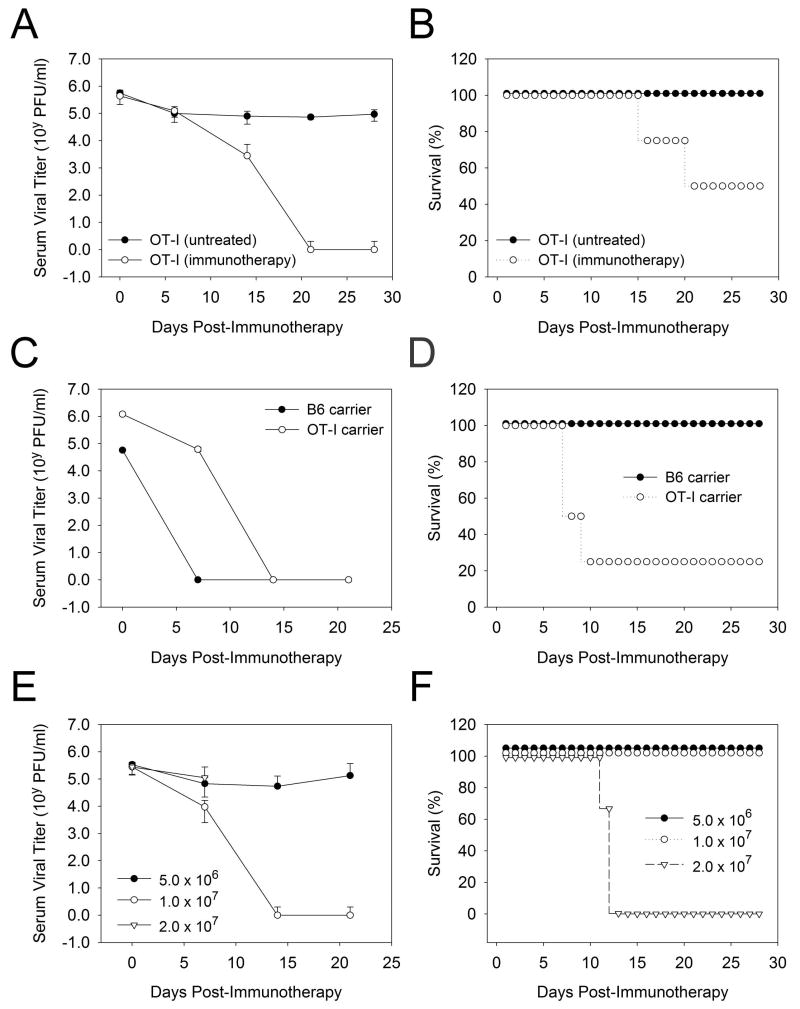
Figure 3. Administration of adoptive immunotherapy to OT-I carrier mice.
A-B) Serum viral titers (A) and survival (B) were quantified in OT-I carrier mice (45 days following i.c. inoculation with 103 PFU LCMV) that received no treatment (n=4) or 2×107 memory splenocytes (n=4). Only 50% of the OT-I carrier mice survived following adoptive immunotherapy, and this was associated with a reduction in serum viral titers to undetectable levels by 21 days post-treatment. No fatalities or reductions in serum viral titers were observed in control mice. C-D) Serum viral titers (C) and survival (D) were compared in B6 carrier mice persistently infected from birth with LCMV (n=4) and OT-I carrier mice (45 days post-infection; n=4). Both strains received 2×107 memory splenocytes. Note that 100% of the B6 carrier survived, whereas only 25% of the OT-I carrier survived. Serum viral titers were reduced to undetectable levels in both strains, although delayed clearance kinetics were observed in OT-I mice. E-F) Serum viral titers (E) and viral loads (F) were quantified in OT-I carrier mice (45 days post-infection) that received 5×106, 1×107, and 2×107 memory splenocytes (n=4 per group). 0% of the mice receiving 2×107 memory splenocytes survived, whereas all of the mice survived in the two lower dosage groups. Serum viral titers were reduced to undetectable levels in the mice that received 1×107 cells, but not 5×106 cells. Serum viral titers could not be followed in the 2×107 group because of the 100% mortality rate.
Adoptive immunotherapy purges persistently infected OT-I mice but is associated with increased mortality
Because the tropism of LCMV infection in persistently infected OT-I mice differed from that observed in B6 carriers, we initially set out to examine whether adoptive immunotherapy could be used to purge parenchymal astrocytes bearing a high antigenic burden. Such a burden can be found in the brains of OT-I mice between days 30 and 60 post-infection. Adoptive immunotherapy relies on the transfer of virus-specific memory cells from an LCMV immune animal into a persistently infected carrier (3). Traditionally, adoptive immunotherapeutic regimens have been studied in B6 mice persistently infected from birth, which harbor viral antigen in all peripheral tissues as well as CNS neurons (9). Using the B6 model it was shown that adoptively transferred LCMV-specific CD8 and CD4 memory T cells can eradicate LCMV systemically (13).
To gain insights into how LCMV memory cells perform upon encountering a different CNS target (astrocytes instead of neurons) and a higher viral burden, we first adoptively transferred 2×107 memory splenocytes from LCMV immune mice (the standard immunotherapeutic regimen) into OT-I mice challenged 45 days earlier with LCMV i.c. A secondary aim in this study was to test whether memory cells could achieve clearance in the absence of endogenous T cell support. OT-I mice provide an ideal model to address this question, because they cannot mount either a CD8 or CD4 T cell response to LCMV (16). When 2×107 memory splenocytes were transferred i.p. into persistently infected OT-I mice, serum viral loads were reduced to an undetectable level within 20 days (Fig. 3A). However, the therapeutic regimen, surprisingly, was associated with increased mortality (Fig. 3B), suggesting that the memory cells induced an intolerable level of pathogenesis. To ensure that this elevated mortality rate was unique to the OT-I system, we generated a colony of B6 carrier mice by persistently infecting them from birth. When 2×107 memory splenocytes were transferred into B6 carriers and compared to persistently infected OT-I mice (at day 45), it was again observed that the majority of the OT-I mice succumbed to the therapy, whereas all of the B6 carrier mice survived (Fig. 3D). Serum viral loads were reduced to an undetectable level in surviving OT-I mice as well as B6 carriers (Fig. 3C). These data demonstrate that the elevated mortality rate was unique to the treatment of the OT-I carrier state and that the memory T cells purge a persistent infection in the absence of endogenous T cell support.
Having observed elevated mortality rates in treated OT-I carrier mice, we next addressed whether the pathogenesis was a quantitative issue. To address this we titrated out the dose of memory splenocytes and measured the impact this had on viral clearance and fatalities. Administration of 2×107 memory cells to persistently infected OT-I mice resulted in 0% survival, whereas 100% of the mice survived that received 1.0×107 (a 2-fold reduction) and 5.0×106 (a 4-fold reduction) cells (Fig. 3F). Interestingly, only mice receiving the intermediate dose (1×107 memory cells) survived and purged the virus (Fig. 3E). Administration of 5×106 memory cells had no impact on serum viral titers. These data indicate that the quantity of memory cells administered can dictate the degree of pathogenesis and the efficacy of the immunotherapeutic regimen, which is an important variable to consider when performing adoptive immunotherapy in humans.
Another important endpoint of this experiment was to determine whether memory splenocytes could purge a brain inundated with persistently infected astrocytes (Fig. 1,2). Despite the fact that memory cells can purge LCMV-infected neurons (11), cleansing astrocytes in OT-I mice seemed improbable given the high CNS antigenic burden (Fig. 1, d60). Surprisingly, when the brains of untreated OT-I carriers (Fig. 4A) were compared to the surviving treated carriers (Fig. 4B) at 100 days post-immunotherapy, it was revealed that virus was completely purged from the latter. To prove this quantitatively, we measured CNS viral titers by plaque assay (Fig. 4C) and antigenic loads on sagittal brain cross sections by image analysis (Fig. 4D). Whereas virus was readily detectable by both assays in untreated OT-I mice, LCMV dropped below the level of detection in surviving OT-I mice that received memory splenocytes (Fig. 4C-D). These data demonstrate for the first time that astrocytes can be purged of persistent infection using adoptive immunotherapy.
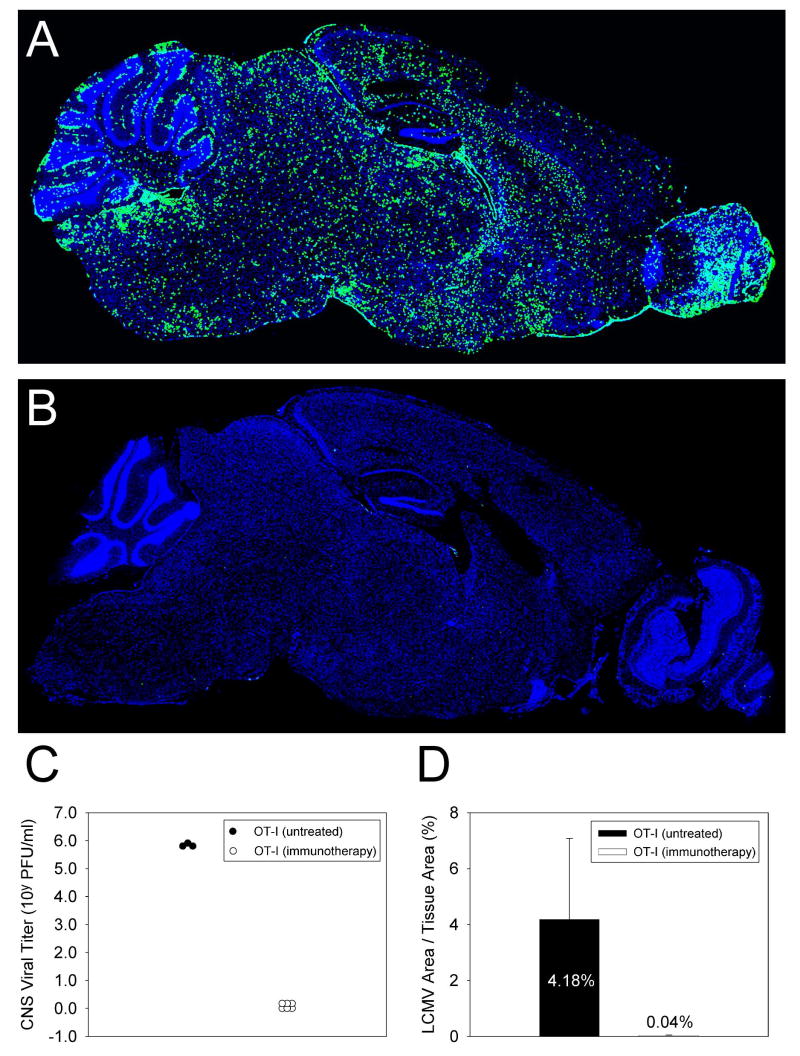
Figure 4. Quantification of CNS viral loads surviving OT-I carrier that received adoptive immunotherapy.
Sagittal brain reconstructions were generated (panels A-B) and quantified (panel D) to determine whether LCMV (green) was purged from the brains of OT-I carrier mice that survived adoptive immunotherapy. Note that when compared to untreated control mice (A), no LCMV antigen was observed in the brains of immunotherapy recipients at >100 days post-immunotherapy (B). This observation was confirmed by quantitative image analysis (D). Cell nuclei are shown in blue. Infectious viral titers in the CNS as determined by plaque assay were also reduced to undetectable levels in OT-I mice that survived immunotherapy, whereas 5-6 logs of virus remained in untreated mice (C).
OT-I mice have a reduced and highly restricted repertoire of FoxP3-expressing regulatory CD4 T cells
TCR-tg mice deficient in recombinase activating gene-1 (RAG-1) are known to have problems regulating T cells responses (18), but this can be accounted for by the absence of all T cells besides those expressing the tg TCR. We opted to conduct studies in RAG-1 expressing OT-I mice for several reasons. First, adoptive transferred T lymphocytes undergo significant “empty space” induced clonal expansion in RAG-1 knockout mice, and we intended to minimize this expansion in OT-I mice replete with T and B lymphocytes. Second, OT-I mice express a TCR that selects CD8 T cells which recognize ovalbumin (KbOVA257-264). We had hoped that this would minimize the impact on the quantity and quality of CD4 regulatory cells in the repertoire. In the spleen and draining lymph nodes of naïve OT-I mice versus wild type B6 mice, we next quantified FoxP3-expressing CD4 T cells – a marker known to be expressed by natural T regulatory cells in mice (23-25). A higher percentage of regulatory cells was observed in both tissues obtained from OT-I mice (Fig. S1A). This translated into no difference in the absolute number of FoxP3-expressing cells in the spleen and a significant 7.1-fold reduction (p = 0.016) in the number of cells in the lymph nodes (Fig. S1B). The most striking difference, however, was observed when the frequency of FoxP3+ CD4 cells expressing Vβ5.1 (the beta chain transgenically expressed in OT-I mice) was compared between OT-I and B6 mice. Approximately 85% of FoxP3+ CD4 T cells expressed Vβ5.1 in the lymph nodes of OT-I mice compared to 7% in B6 mice (Fig. S1C,D). A similar profile was observed in the spleen (Fig. S1D). These data indicate that the regulatory T cell repertoire is not only quantitatively reduced but also highly skewed in OT-I mice.
Regulatory T cells dampen the pathogenicity of adoptively transferred memory T cells in OT-I mice
Because OT-I mice harbored a highly skewed repertoire of regulatory T cells, we theorized that these mice would provide an excellent paradigm to test whether supplementation with regulatory cells from a normal repertoire could control a pathogenic regimen of anti-viral memory cells while permitting them to successfully purge a systemic persistent infection. The latter issue is of great significance from a therapeutic standpoint, as it is important that the anti-viral memory cells retain their ability to cleanse the host despite being regulated. To determine whether regulatory T cells could control the pathogenicity of therapeutically administered anti-viral memory cells, we purified CD4+CD25+ regulatory cells from the spleens of B6 mice (Fig. 5A). As a control for this experiment we used CD4+CD25- T cells (Fig. 5A). OT-I carrier mice at 45 days post-infection were injected i.p. with a dose of anti-viral memory cells (2×107) previously shown to induce fatalities within 10-15 days (Fig. 3F). These mice were simultaneously injected i.p. with 4×106 CD4+CD25+ or CD4+CD25- T cells. Importantly, the CD4+CD25+ regulatory T cells dramatically lessened the mortality rate associated with the pathogenic immunotherapy regimen. A 75% survival rate was observed in mice receiving CD4+CD25+ T cells, whereas no mice survived in the CD4+CD25- control group.
In the surviving mice that received regulatory T cells, we next evaluated whether the virus was purged from the periphery as well as the CNS over time. Quantification of serum viral titers (a measure of peripheral viral clearance) revealed that LCMV was reduced to undetectable levels albeit with delayed kinetics (Fig. 5C). Clearance in mice receiving regulatory cells was observed by day 60 post-immunotherapy instead of within 10-15 days, which occurred when OT-I mice were injected with a non-pathogenic dose (1×107) of memory cells (Fig. 3F). Thus, the regulatory cells did control pathogenicity but also delayed the clearance of the virus. To determine whether the regulated memory cells also purged the heavily inundated CNS, we quantified infectious virus and antigenic loads at day 125 post-immunotherapy. No infectious virus (measured by plaque assay) or LCMV antigen (measured by immunohistochemistry) was observed in the CNS at this time (Fig. 5D). This finding is particularly important because the CNS is known to be more difficult to purge of a persistent viral infection than the periphery (11).
Discussion
At this the outset of this study it was not known how anti-viral memory cells perform in a host with a restricted T cell repertoire or whether transferred memory cells require endogenous, pathogen-specific T cells to achieve clearance. Furthermore, it was not known how memory cells would respond to a brain inundated with persistently infected astrocytes instead of neurons. Our results demonstrate that anti-viral memory cells can operate within a restricted endogenous repertoire to achieve systemic clearance of a persistent infection, and, importantly, can even eradicate virus from CNS astrocytes. The downside of performing immunotherapy in OT-I carrier mice was that elevated mortality rates were observed and were directly linked to the number of cells transferred. We postulate that this could be due to an immune regulation deficiency in OT-I mice. Importantly, and in support of this supposition, we demonstrated that the elevated mortality rate was dramatically lessened by including regulatory T cells in the therapeutic regimen. These data indicate that regulatory T cells can mitigate the pathogenicity of anti-viral memory cells. Nevertheless, one should proceed with caution when performing adoptive therapies in immunologically deficient humans, as the potential for fatal immunopathology does exist.
Much of our knowledge about immunotherapeutic regimens stems from pioneering work done in the LCMV system (1, 26-28). The traditional LCMV model used to evaluate immunotherapeutic regimens relies on the establishment of a carrier colony by infecting mice in utero or at birth (29, 30). The resultant mice are asymptomatic and grow to adulthood with virus persistently in every tissue compartment (9), including CNS neurons (17, 31). These carrier mice are immunologically tolerant to the pathogen at the T cell level (32) and thus are incapable of mounting a successful endogenous anti-viral immune response to the pathogen. OT-I mice infected as adults are also unable to mount an anti-viral T cell response, but the reason for this does not involve immunological tolerance. Rather, the T cell repertoire in OT-I mice is so heavily restricted in diversity (20) that no naïve LCMV-specific T cells likely exist. Despite the absence of virus-specific T cells, LCMV establishes an entirely distinct pattern of tropism within the brains of OT-I mice. Whereas neurons are the only parenchymal cell population infected in carriers established from birth, astrocytes and ultimately oligodendrocytes were infected in the brains of adult OT-I mice. OT-I mice therefore provide a novel CNS carrier state to examine interactions between LCMV-specific T cells and persistently infected astrocytes / oligodendrocytes.
To date the only successful therapeutic approach described for LCMV carrier mice infected from birth has been the adoptive transfer of virus-specific memory T cells (2). Attempts to stimulate an endogenous T cell response through vaccination have been entirely unsuccessful (33). Studies have shown that successful adoptive immunotherapy requires the activities of virus-specific memory T cells (13) and depends on cooperation with endogenous, non-specific bone marrow derived cells (e.g., dendritic cells) (17, 34). However, it was not known whether endogenous T cells also contributed to the success of the therapy. Our studies in OT-I carrier mice provide convincing evidence that endogenous LCMV-specific T cells are not required to purge a persistent infection. The adoptively transferred virus-specific T cells alone are capable of achieving systemic viral clearance. However, the high mortality rate observed in OT-I mice suggested another role for endogenous T cells in determining the success of adoptive immunotherapy - to control the pathogenicity of the incoming anti-viral memory T cells.
Regulatory T cells (both natural and adaptive) have a remarkable ability to modulate various facets of immunity and thus hold great promise for the treatment of inflammatory disorders in humans (35). Importantly, our studies demonstrate for the first time in the LCMV system that regulatory T cells can be used therapeutically to mitigate the pathogenicity of anti-viral memory cells while still permitting them to achieve systemic viral clearance. Regulatory T cells are known to control self-reactive T cells (35), and emerging data now indicate that they are also important in modulating pathogen-specific immunity (36, 37). In OT-I carrier mice, a surprisingly high mortality rate was observed in mice treated with a normally non-pathogenic dose of cells (2×107 memory cells). Because mice with restricted T cell repertoires have deficiencies in T regulatory cell activity (18), we hypothesized that the mortality observed in treated OT-I mice was due to a deficiency in this compartment. Indeed, OT-I mice had a highly restricted repertoire of regulatory T cells and addition of normal regulatory T cells to the therapeutic regimen dramatically improved survival. It is presently unknown whether natural or adaptive regulatory T cells are responsible for controlling anti-viral memory cells in the OT-I system, but studies are underway to address this issue as well as to identify the mechanism by which the regulation occurs.
In conclusion, immunotherapeutic treatment of OT-I carrier mice should provide an excellent new experimental paradigm to examine relationships between regulatory and anti-viral T cells cooperating to achieve clearance of persistent infection without causing death of the host. Supplementation of a host with anti-viral memory T cells can achieve systemic viral eradication in the absence of endogenous T cell support; however, when these cells are not properly regulated, the outcome can be fatal. Therefore, it is of importance to examine the regulatory T cell compartment of a persistently infected patient before proceeding with an adoptive immunotherapeutic treatment. Another advantage of the OT-I model system is that LCMV establishes a novel pattern of CNS tropism not observed in carriers infected from birth. This opens the possibility of studying the mechanisms by which interactions between anti-viral T cells and persistently infected astrocytes / oligodendrocytes lead to CNS viral clearance. Such insights can then be applied to the task of eradicating viruses that target the same CNS cell populations in humans.
Figure 6. Therapeutic administration of regulatory T cells to counterbalance the pathogenicity of anti-viral memory cells.
Regulatory T cells (CD4+CD25+) were purified from the spleens of naïve B6 mice. The purity of the regulatory T cells is shown in panel A. The CD4+CD25- flow through was used as a control for this experiment. Survival (B), serum viral titers (C) and CNS viral loads (D) were quantified in OT-I carrier mice (day 45 post-infection) that received 2×107 memory splenocytes combined with 4×106 CD4+CD25+ or CD4+CD25- T cells (n=5 mice per group). Note that no mice receiving CD4+CD25- T cells survived, whereas 75% of the mice survived that received CD4+CD25+ regulatory T cells. Serum (C) and CNS (D) viral titers were reduced to undetectable levels in surviving OT-I mice that received regulatory T cells. CNS viral loads were quantified by sagittal brain reconstructions and plaque assay at 102 days post-immunotherapy. A representative sagittal brain reconstruction devoid of LCMV antigen (green) is shown in panel D. All mice had less than 200 PFU/g by plaque assay, which is the limit of detection for the assay. Cell nuclei are shown in blue.
Acknowledgments
This work was supported by National Institutes of Health grants NS048866-01, MH062261-06, AI070967-01 and a grant from The Burroughs Wellcome Fund (all to D.B. McGavern).
References
- 1.Volkert M. Studies on immunological tolerance to LCM virus. A preliminary report on adoptive immunization of virus carrier mice. Acta Pathol Microbiol Scand. 1962;56:305. [PubMed] [Google Scholar]
- 2.Oldstone MB. Immunotherapy for virus infection. Curr Top Microbiol Immunol. 1987;134:211. doi: 10.1007/978-3-642-71726-0_9. [DOI] [PubMed] [Google Scholar]
- 3.Homann D. Immunocytotherapy. Curr Top Microbiol Immunol. 2002;263:43. doi: 10.1007/978-3-642-56055-2_4. [DOI] [PubMed] [Google Scholar]
- 4.Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257:238. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 5.Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, Riddell SR. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 6.Lau GK, Suri D, Liang R, Rigopoulou EI, Thomas MG, Mullerova I, Nanji A, Yuen ST, Williams R, Naoumov NV. Resolution of chronic hepatitis B and anti-HBs seroconversion in humans by adoptive transfer of immunity to hepatitis B core antigen. Gastroenterology. 2002;122:614. doi: 10.1053/gast.2002.31887. [DOI] [PubMed] [Google Scholar]
- 7.Heslop HE, Ng CY, Li C, Smith CA, Loftin SK, Krance RA, Brenner MK, Rooney CM. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat Med. 1996;2:551. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- 8.Smith CA, Ng CY, Loftin SK, Li C, Heslop HE, Brenner MK, Rooney CM. Adoptive immunotherapy for Epstein-Barr virus-related lymphoma. Leuk Lymphoma. 1996;23:213. doi: 10.3109/10428199609054823. [DOI] [PubMed] [Google Scholar]
- 9.Fazakerley JK, Southern P, Bloom F, Buchmeier MJ. High resolution in situ hybridization to determine the cellular distribution of lymphocytic choriomeningitis virus RNA in the tissues of persistently infected mice: relevance to arenavirus disease and mechanisms of viral persistence. J Gen Virol. 1991;72:1611. doi: 10.1099/0022-1317-72-7-1611. [DOI] [PubMed] [Google Scholar]
- 10.Pircher H, Rohrer UH, Moskophidis D, Zinkernagel RM, Hengartner H. Lower receptor avidity required for thymic clonal deletion than for effector T-cell function. Nature. 1991;351:482. doi: 10.1038/351482a0. [DOI] [PubMed] [Google Scholar]
- 11.Oldstone MB, Blount P, Southern PJ, Lampert PW. Cytoimmunotherapy for persistent virus infection reveals a unique clearance pattern from the central nervous system. Nature. 1986;321:239. doi: 10.1038/321239a0. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed R, Jamieson BD, Porter DD. Immune therapy of a persistent and disseminated viral infection. J Virol. 1987;61:3920. doi: 10.1128/jvi.61.12.3920-3929.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berger DP, Homann D, Oldstone MB. Defining parameters for successful immunocytotherapy of persistent viral infection. Virology. 2000;266:257. doi: 10.1006/viro.1999.0074. [DOI] [PubMed] [Google Scholar]
- 14.Jamieson BD, Ahmed R. T-cell tolerance: exposure to virus in utero does not cause a permanent deletion of specific T cells. Proc Natl Acad Sci U S A. 1988;85:2265. doi: 10.1073/pnas.85.7.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jamieson BD, Somasundaram T, Ahmed R. Abrogation of tolerance to a chronic viral infection. J Immunol. 1991;147:3521. [PubMed] [Google Scholar]
- 16.McGavern DB, Truong P. Rebuilding an immune-mediated central nervous system disease: weighing the pathogenicity of antigen-specific versus bystander T cells. J Immunol. 2004;173:4779. doi: 10.4049/jimmunol.173.8.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lauterbach H, Zuniga EI, Truong P, Oldstone MB, McGavern DB. Adoptive immunotherapy induces CNS dendritic cell recruitment and antigen presentation during clearance of a persistent viral infection. J Exp Med. 2006;203:1963. doi: 10.1084/jem.20060039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hori S, Haury M, Coutinho A, Demengeot J. Specificity requirements for selection and effector functions of CD25+4+ regulatory T cells in anti-myelin basic protein T cell receptor transgenic mice. Proc Nat Acad Sci USA. 2002;99:8213. doi: 10.1073/pnas.122224799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 20.Clarke SR, Barnden M, Kurts C, Carbone FR, Miller JF, Heath WR. Characterization of the ovalbumin-specific TCR transgenic line OT-I: MHC elements for positive and negative selection. Immunol Cell Biol. 2000;78:110. doi: 10.1046/j.1440-1711.2000.00889.x. [DOI] [PubMed] [Google Scholar]
- 21.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 22.Fung-Leung WP, Kundig TM, Zinkernagel RM, Mak TW. Immune response against lymphocytic choriomeningitis virus infection in mice without CD8 expression. J Exp Med. 1991;174:1425. doi: 10.1084/jem.174.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 24.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 25.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 26.Volkert M. Studies on immunological tolerance to LCM virus. Treatment of virus carrier mice by adoptive immunization. Acta Path et Microbiol Scand. 1962;56:305. [PubMed] [Google Scholar]
- 27.Volkert M. Studies On Immunological Tolerance To Lcm Virus. 2. Treatment Of Virus Carrier Mice By Adoptive Immunization. Acta Pathol Microbiol Scand. 1963;57:465. [PubMed] [Google Scholar]
- 28.Volkert M, Larsen JH. Studies On Immunological Tolerance To Lcm Virus. 3. Duration And Maximal Effect Of Adoptive Immunization Of Virus Carriers. Acta Pathol Microbiol Scand. 1964;60:577. doi: 10.1111/apm.1964.60.4.577. [DOI] [PubMed] [Google Scholar]
- 29.Hotchin JE, Sintis M. Lymphocytic choriomeningitis infection of mice as a model for the study of latent virus infection. Can J Microbiol. 1958;4:149. doi: 10.1139/m58-016. [DOI] [PubMed] [Google Scholar]
- 30.King CC, Jamieson BD, Reddy K, Bali N, Concepcion RJ, Ahmed R. Viral infection of the thymus. J Virol. 1992;66:3155. doi: 10.1128/jvi.66.5.3155-3160.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez M, Buchmeier MJ, Oldstone MB, Lampert PW. Ultrastructural localization of viral antigens in the CNS of mice persistently infected with lymphocytic choriomeningitis virus (LCMV) Am J Pathol. 1983;110:95. [PMC free article] [PubMed] [Google Scholar]
- 32.Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342:559. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 33.von Herrath MG, Berger DP, Homann D, Tishon T, Sette A, Oldstone MB. Vaccination to treat persistent viral infection. Virology. 2000;268:411. doi: 10.1006/viro.1999.0130. [DOI] [PubMed] [Google Scholar]
- 34.Jamieson BD, Butler LD, Ahmed R. Effective clearance of a persistent viral infection requires cooperation between virus-specific Lyt2+ T cells and nonspecific bone marrow-derived cells. J Virol. 1987;61:3930. doi: 10.1128/jvi.61.12.3930-3937.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rouse BT. Regulatory T cells in health and disease. J Intern Med. 2007;262:78. doi: 10.1111/j.1365-2796.2007.01836.x. [DOI] [PubMed] [Google Scholar]
- 36.Rouse BT, Sarangi PP, Suvas S. Regulatory T cells in virus infections. Immunol Rev. 2006;212:272. doi: 10.1111/j.0105-2896.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- 37.Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol. 2007;7:875. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]