About two-thirds of the products utilized in the pharmaceutical industry are in the form of particulate solids.[1] Controlled release as an effective means of delivery of these products to target tissues remains a significant challenge. Herein, we describe the use of supercritical carbon dioxide as an antisolvent for the formation of nanoparticles that comprise biologically active therapeutics dispersed in a biodegradable polymer. Release of a model compound, luciferin, from poly(lactic acid) (PLA) particles (approximately 250–350 nm in size) was observed for up to 40 days with up to 90% drug recovery. In vivo bioluminescence imaging of transgenic mice,[2] which were genetically engineered to universally express luciferase, provides a rapid readout for luciferin release and demonstrates a slow and sustained release of luciferin upon subcutaneous injection of these particles. This work demonstrates that our process, which is readily scaled to kilogram quantities, represents a new drug delivery approach with great potential, particularly if particles can be targeted for site-specific delivery.[3]
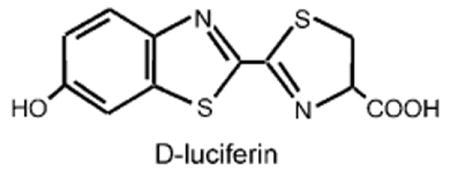
We used d-luciferin as a model system to mimic therapeutic molecules. d-Luciferin is a substrate for the enzyme luciferase that produces a bioluminescent signal at approximately 610 nm at 37°C which can be measured in vivo. Luciferase oxidizes luciferin in a process that involves the simultaneous emission of bioluminescence over a wide dynamic range. Using such a model drug provided us with a rapid method to evaluate in vivo data noninvasively, which in turn allowed us to optimize the process parameters. We used transgenic mice that were engineered to express luciferase in many, if not all, cells of the body by using a modified β-actin promoter.[4] As the majority of luciferin is cleared from the body of the mouse in 30–60 min, it represents a drug model with a rapid pharmacokinetic profile.[5]
In order to prolong the biodistribution kinetics, a drug is usually dispersed in a biodegradable polymer that provides a time-delayed release and protects the therapeutic from degradation. Moreover, the polymeric nanoparticle could be engineered to target different cells.[3, 6] PLA is an Food and Drug Administration (FDA) approved polymer that is known to release drugs in a controlled manner, and is compatible with supercritical carbon dioxide (sc-CO2). PLA can also serve as a representative material for our process which can then be adopted for other polymers.[7]
The use of supercritical fluids (SCFs) as antisolvents is key to the fabrication process. Carbon dioxide is the most popular SCF because of its biocompatibility, nontoxicity, low critical parameters (Tc=31°C, Pc=75.8 bar), low cost and environmental friendliness; it is also considered safe by the FDA. Figure 1 shows the experimental setup, which is a version of the apparatus derived from solution-enhanced dispersion by supercritical fluids (SEDS).[8] A high-pressure pump controls the CO2 flow into the particle chamber, with a regulator maintaining the preset pressure. The system is first conditioned with sc-CO2 to obtain the desired temperature and pressure in the particle chamber. The cosolvent mixed with the solute is then injected through 250 μm internal diameter (i.d.) tubing and premixed with the sc-CO2 flow before it enters the particle chamber. The antisolvent effect of sc-CO2 causes nucleation and particle growth of the solute.[7] Dry particles are collected on a 0.5 μm stainless-steel filter, allowing the SCF to pass through and expand into the coalescer. The low surface tension of the SCF combined with the high mass-transfer rate allows for the formation of nanosized particles, which are much smaller than those prepared by the liquid–liquid antisolvent process.[9, 10]
Figure 1.
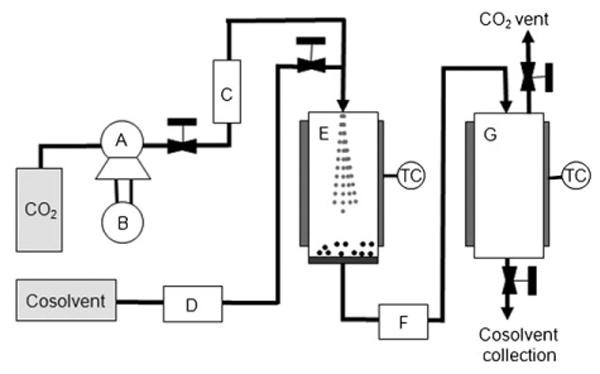
Schematic of the SEDS setup: A) CO2 pump, B) cooling bath, C) heat exchanger, D) cosolvent pump, E) particle vessel, F) automatic back-pressure regulator, G) coalescer. TC=temperature controller.
The SEDS process was first optimized to form pure luciferin-containing particles. Both methanol and DMSO were studied as cosolvents. The temperature and pressure were varied from 40 to 80°C and from 80 to 300 bar. The cosolvent injection flow rate was maintained at 1 mL min−1 while the sc-CO2 flow rate was varied between 50 and 150 g min−1. The temperature had no significant effect on the particle size, but a higher sc-CO2 flow rate and increased pressure favored reduction of the particle size and particle size distribution. Figure 2A shows SEM images of luciferin after SEDS. Dynamic light scattering (DLS) data showed a reduction in size to 68 nm when the nanoparticles were dispersed in solution.
Figure 2.
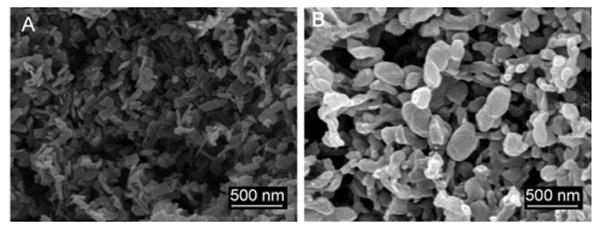
SEM images of A) luciferin particles (20 mg) obtained from methanol (20 mL) and B) luciferin/PLA nanoparticles after SEDS (2 mg luciferin in 2 mL methanol, 100 mg PLA in 8 mL DCM, which gives a total of 10 mgmL−1 of polymer in the cosolvent with 2 wt% luciferin). Operating conditions: T=40°C, P=100 bar, CO2 flow rate 150 g min−1, and injection flow rate 1 mL min−1.
To develop this process to include drug dispersion within a polymer, we optimized the luciferin process at 40°C and 100 bar. These conditions would allow biologically active compounds (such as proteins and nucleic acids) to be processed at mild temperatures. The lower pressure is important to ensure that the process is run below the glass transition temperature (Tg) of the polymers to avoid plasticization. The sorption of CO2 into the polymer also reduces the Tg of the polymer.[11] PLA was dissolved in dichloromethane (DCM) and luciferin in methanol. A homogeneous solution of the luciferin/PLA cosolvent mixture was sprayed into the SEDS system, whilst the same process parameters as for pure luciferin were varied. SEM images of 2 wt% luciferin dispersed in PLA (MW 40–70 kDa) are shown in Figure 2B. The size of the PLA particles as determined by SEM was in the range of 250–350 nm.
The amount of luciferin encapsulated in the PLA particle was determined by completely dissolving the dry particles in DCM and then extracting the luciferin into water. The concentration of luciferin was determined by fluorescence measurements. Initial ratios of 2.0 and 5.0 wt% luciferin/PLA resulted in a final composition of 2.15 and 4.2 wt% luciferin, respectively, in the particles after SEDS.
The cumulative release of luciferin in phosphate-buffered saline (PBS, pH 7.4) containing the surfactant Pluronic F68 (0.5 wt%) at 37°C is shown in Figure 3. The surfactant was added to help disperse the PLA particles as well as to reduce the zeta potential. The zeta potential of the PLA particles in PBS was −21 mV, but dropped to −3.3 mV in the presence of Pluronic F68, a value which is more suitable for in vivo injections.[12] The PSD distribution of PLA particles in 0.5 wt% Pluronic F68 was measured using DLS. Some agglomeration of the particles occurs leading to a slightly larger particle size of (460 ± 47) nm which is slightly larger than the values obtained by the SEM measurement of dry particles. The first release data point was obtained after 5 min and showed that free luciferin was present in the solution. Subsequently, a slow release of luciferin from the particles was observed over 1–8 weeks and was dependent on the on the molecular weight of the polymer.[13] The last data point was followed by extraction of the remaining luciferin. The PLA particles were dissolved by adding DCM and then the luciferin was extracted into H2O. The remaining luciferin extracted from the particles is noted on the graph as a significant increase in the last data point. This result shows that approximately 90% of the luciferin encapsulated in the particle could be accounted for. There is a slight degradation of luciferin by oxidation over time when it is dissolved in the PBS buffer; this is thought to account for the largest part of the 10% loss of luciferin.
Figure 3.
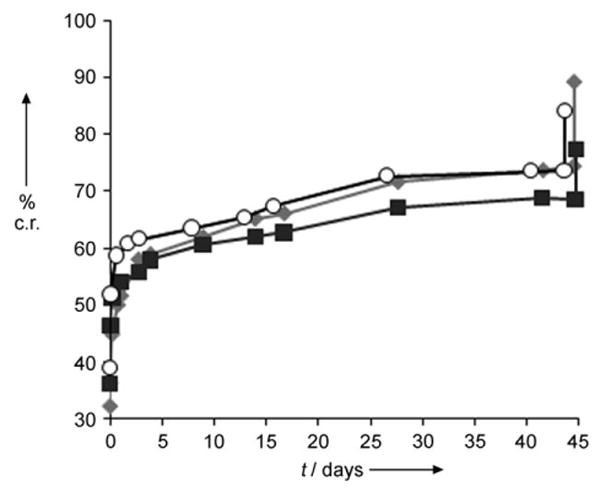
Cumulative release (c.r.) of luciferin from luciferin-dispersed PLA nanoparticles (mean value, n=3). ■: 0.1 mg of particles mL−1/0.5wt% Pluronic F68,
 : 0.1 mg particles mL−1/PBS, and ○: 0.3 mg particles mL−1/0.5wt% Pluronic F68. The last data point in each run was taken after addition of DCM to dissolve the remaining PLA particles which freed all unreleased luciferin.
: 0.1 mg particles mL−1/PBS, and ○: 0.3 mg particles mL−1/0.5wt% Pluronic F68. The last data point in each run was taken after addition of DCM to dissolve the remaining PLA particles which freed all unreleased luciferin.
PLA particles formed by SEDS containing 2 wt% luciferin were implanted intradermally into three mice (strain L2G85) and monitored by in vivo bioluminescence imaging over a period of 34 days (Figure 4). Each mouse was injected with 4.7 mg of particles (containing a total of 100 μg of luciferin) dispersed in 250 μL of 0.5 wt% Pluronic F68 in PBS. After the initial release of unbound or surface-bound luciferin, a slow and sustained release was observed, which mimics the in vitro data. As a comparison, pure luciferin was cleared within 2 days. Rapid readouts of the luminescence arising from the dissolution of the luciferin-dispersed nanoparticles provide a unique platform to visualize and optimize the development of nanoparticle drug delivery systems. This gives an opportunity for rapid testing of therapeutic strategies in complex environments and to use new approaches to address the challenges of achieving sufficient concentrations of therapeutic agents at a specific target site.
Figure 4.
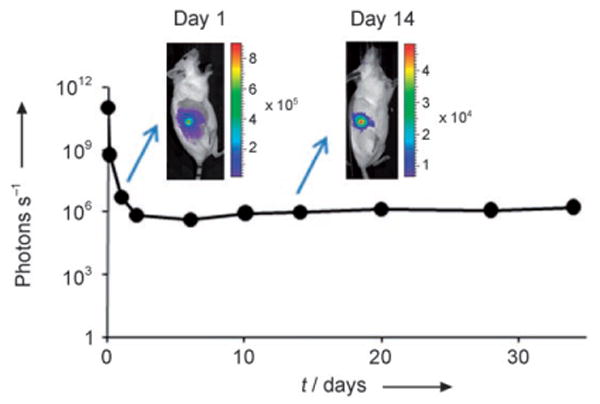
Sustained release of luciferin after subcutaneous injection of PLA particles containing 2 wt% luciferin. Scale bar units are photons per second.
Experimental Section
d-Luciferin was purchased from Biosynth International. PLA (MW=40000–70000 Da) was purchased from Polysciences Inc. and used as received. PBS 1X solution and analytical grade methanol, DCM, and DMSO and were purchased from Sigma–Aldrich and used as received.
The SEDS equipment was based on a modified instrument (SAS50, Thar Technologies) originally set up for running the supercritical antisolvent (SAS) process. The CO2 inlet tubing to the particle vessel was reduced to a 1/6″ tubing (250 μm i.d.) and connected to a tee to allow premixing with the cosolvent before exiting the nozzle into the particle vessel (see Figure 1).
Scanning electron microscope (SEM) images were acquired using an FEI XL30 Sirion SEM with FEG source and EDX detector. Dry samples on carbon sticky tape were sputter-coated for 45 s at 40 mA with Pd/Au. DLS and zeta potential measurements were performed on a Malvern Zetasizer Nano ZS90. Fluorescence measurements of luciferin were performed on a microplate spectrofluorometer (SpectraMAX GeminiEM, Molecular Devices) with excitation at 330 nm and detection at 525 nm.
Transgenic mice that express firefly luciferase (FVB-luc +) were used to evaluate delivery of luciferin from the PLA particles.[14] A patch of hair was removed from each animal with hair clippers; Nair depilatory cream (Church and Dwight) was applied for 60 s and then wiped and washed off. Bioluminescence imaging was performed on a Xenogen IVIS 200 using a cooled charge-coupled device camera.[15] Data was analyzed with LivingImage software (Xenogen) and expressed in photons per steradian per second for each region of interest such that the data are not dependent on camera settings, chamber geometry, or integration time.
Footnotes
We acknowledge financial support from the National Institutes of Health (1 R21 CA125467), the Bio-X IIP Program at Stanford, and VINNOVA (The Swedish Agency for Innovation Systems).
Contributor Information
Gunilla B. Jacobson, Department of Chemistry, Stanford University, 333 Campus Drive, Stanford, CA 94305-5080 (USA)
Rajesh Shinde, Departments of Pediatrics, Radiology, and Microbiology & Immunology, Stanford University, School of Medicine.
Christopher H. Contag, Departments of Pediatrics, Radiology, and Microbiology & Immunology, Stanford University, School of Medicine
Richard N. Zare, Department of Chemistry, Stanford University, 333 Campus Drive, Stanford, CA 94305-5080 (USA), Fax : (+1) 650-725-0259, E-mail: zare@stanford.edu
References
- 1.Roberts CJ, Debenedetti PG. AIChE J. 2002;48:1140–1144. [Google Scholar]
- 2.Lee SY, Wang Z, Lin CK, Contag CH, Olds LC, Cooper AD, Sibley E. J Biol Chem. 2002;277:13099–13105. doi: 10.1074/jbc.M112152200. [DOI] [PubMed] [Google Scholar]
- 3.Hughes GA. Nanomed: Nanotechnol Biol Med. 2005;1:22–30. [Google Scholar]
- 4.Zhang WS, Contag PR, Hardy J, Zhao H, Verman HJ, Hajdena-Dawson M, Wong RJ, Stevenson DK, Contag CH. J Mol Med. 2002;80:655–664. doi: 10.1007/s00109-002-0375-x. [DOI] [PubMed] [Google Scholar]
- 5.Wender PA, Guon EA, Jones LR, Pillow TH, Rothbard JB, Shinde R, Contag CH. Proc Natl Acad Sci USA. 2007;104:10340–10345. doi: 10.1073/pnas.0703919104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen TM, Cullis PR. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 7.Ulrich KE, Cannizzaro SM, Langer RS, Shakesheff K. Chem Rev. 1999;99:3181–3198. doi: 10.1021/cr940351u. [DOI] [PubMed] [Google Scholar]
- 8.Hanna MH, York P. US Patent No 5851453. 1998
- 9.Reverchon E, Caputo G, De Marco I. Ind Eng Chem Res. 2003;42:6406–6414. [Google Scholar]
- 10.Martín A, Cocero MJ. Adv Drug Delivery Rev. 2007;59:339–350. doi: 10.1016/j.addr.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 11.Kokes RJ, Long FA. J Am Chem Soc. 1953;75:6142. [Google Scholar]
- 12.Gref R, Domb A, Quellec P, Blunk T, Muller RH, Verbavatz JM, Langer R. Adv Drug Delivery Rev. 1995;16:215–233. doi: 10.1016/0169-409X(95)00026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panyam J, Labhasetwar V. Adv Drug Delivery Rev. 2003;55:329–347. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 14.Cao YA, Wagers AJ, Beilhack A, Dusich J, Bachmann MH, Negrin RS, Weissman IL, Contag CH. Proc Natl Acad Sci USA. 2003;101:221–226. doi: 10.1073/pnas.2637010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Contag CH, Bachmann MH. Annu Rev Biomed Eng. 2002;4:235–260. doi: 10.1146/annurev.bioeng.4.111901.093336. [DOI] [PubMed] [Google Scholar]


