Summary
The receptor-evoked Ca2+ signal includes activation of the store-operated channels (SOCs) TRPC and Orai channels. Although both are gated by STIM1, it is not known how STIM1 gates the channels and whether STIM1 gates the TRPCs and Orais by the same mechanism. Here, we report the molecular mechanism by which STIM1 gates TRPC1, which involves interaction between two conserved, negatively charged aspartates in TRPC1(639DD640) with the positively charged STIM1(684KK685) in STIM1 polybasic domain. Charge swapping and functional analysis revealed that exact orientation of the charges on TRPC1 and STIM1 are required, but all positive-negative charge combinations on TRPC1 and STIM1, except STIM1(684EE685)+TRPC1(639RR640), are functional as long as they are reciprocal, indicating that STIM1 gates TRPC1 by intermolecular electrostatic interaction. Similar gating was observe with TRPC3(697DD698). STIM1 gates Orai1 by a different mechanism since the polybasic and S/P domains of STIM1 are not required for activation of Orai1 by STIM1.
Introduction
The receptor-evoked Ca2+ signal starts with Ca2+ release from internal stores and is followed by activation of store-operated Ca2+ influx channels (SOCs) at the plasma membrane (PM) (Parekh and Putney, 2005). Ca2+ influx through SOCs mediates numerous physiological functions and loads the stores with Ca2+ (Berridge et al., 2003). Recent advances have defined the molecular identity of the SOCs and how they are regulated by Ca2+ content in the ER. The two major SOCs are the TRPC (Nilius et al., 2007; Worley et al., 2007) and the Orai channels (Feske et al., 2006; Vig et al., 2006b; Zhang et al., 2006). The Orais mediate the highly Ca2+-selective, inward rectifying Ca2+ release-activated Ca2+ current Icrac (Prakriya et al., 2006; Vig et al., 2006a; Yeromin et al., 2006), while TRPCs mediate a non-selective, Ca2+ permeable current Isoc (Ambudkar et al., 2007). The Orais and TRPCs are gated by the endoplasmic reticulum Ca2+ sensor STIM1 that signals the Ca2+ load of the endoplasmic reticulum (ER) to the SOCs (Liou et al., 2005; Roos et al., 2005). STIM1 has an N terminal EF hand and SAM domains that reside in the ER lumen (Liou et al., 2005; Roos et al., 2005). In response to Ca2+ release from the ER, Ca2+ dissociates from the EF hand, and STIM1 clusters next to the plasma membrane to activate Orai1 and TRPC channels (Huang et al., 2006; Liou et al., 2005; Roos et al., 2005; Wu et al., 2006). Very little is known on how STIM1 regulates the Orais, except that STIM1 is obligatory for the Orais to function as channels (Mercer et al., 2006; Peinelt et al., 2006; Zhang et al., 2006).
Regulation of TRPCs by STIM1 is understood somewhat better. The STIM1 N terminus, which includes the STIM1 single transmembrane domain, is not required for activation of TRPCs, while the STIM1 C terminus that includes the ERM, serine/proline (S/P) and polybasic lysine- (K-) rich domains is sufficient to fully activate the TRPCs (Huang et al., 2006; Yuan et al., 2007). STIM1 binds TRPCs via its ERM domain, but the binding is not sufficient to activate the channels. Gating of TRPCs by STIM1 requires the K-domain, although the K-domain does not participate in binding of STIM1 to TRPCs (Huang et al., 2006). Thus, the simplest model that explains gating of TRPCs by STIM1 is that the ERM domain binds to the TRPCs to present the K-domain to a regulatory domain in the channels in a manner that the K-domain opens the channels. Considering the meager information available on the molecular mechanism of the gating of TRPC and Orai1 channels by STIM1, fundamental questions are how STIM1 gates the two SOCs and whether STIM1 gates both TRPC and Orai1 by the same mechanism.
In the present work, we found that STIM1 gates Orai1 and TRPC channels by different mechanisms. Gating of TRPC1 by STIM1 is mediated by intermolecular electrostatic interaction between the conserved, negatively charged aspartate residues in TRPC1(639DD640) that interact with positively charged lysines of STIM1(684KK685). Mutation of 639DD640 or 684KK685 to the electroneutral AA showed that the charges are required, while mutations of TRPC1(639DD640) to KK or RR inhibit TRPC1 activity, but remarkably, channel activity of TRPC1(639KK640) is rescued by reverse charged STIM1(684EE685) and STIM1(684DD685). Interestingly, the single mutants TRPC1(D639K) and TRPC1(D640K) are not active and the single mutants STIM1(K684E) and STIM1(K685E) act as dominant negatives, indicating that the exact orientation of STIM1(684KK685) and TRPC1(639DD640) is required for gating of TRPC1 by STIM1. A C-terminal fragment of STIM1CT(684EE685) is sufficient to rescue reverse charge TRPC1(639KK640), indicating that gating does not require signals from the ER. These findings suggest a model of gating in which channel opening is linked to intermolecular electrostatic interactions that remove channel inhibition, rather than regulation of the TRPC channels pore by STIM1. Structure-function analysis revealed that both the polybasic- and S/P- domains of STIM1 are essential for activation of TRPC1 but are not required for activation of Orai1. These findings reveal how STIM1 gates TRPC1 and rationalize how TRPC1 and Orai1 may be independently gated.
Results and Discussion
The STIM1 polybasic, K-domain is predicted to include a central, amphipathic α-helical region that is flanked by globular positively charged regions in a configuration like a dumbbell. Based on these predictions, we searched for regions of TRPCs that might participate in both charged and amphipathic helical interactions and noted that the TRP box is predicted to form an α-helix and is bounded by negative charges; a negative dumbbell. This general sequence is conserved across all TRPC family members (Fig. 1a). Accordingly, we asked if this region of TRPC1 is critical for gating of TRPC1 by STIM1 and whether the STIM1 K-domain mediates this form of gating.
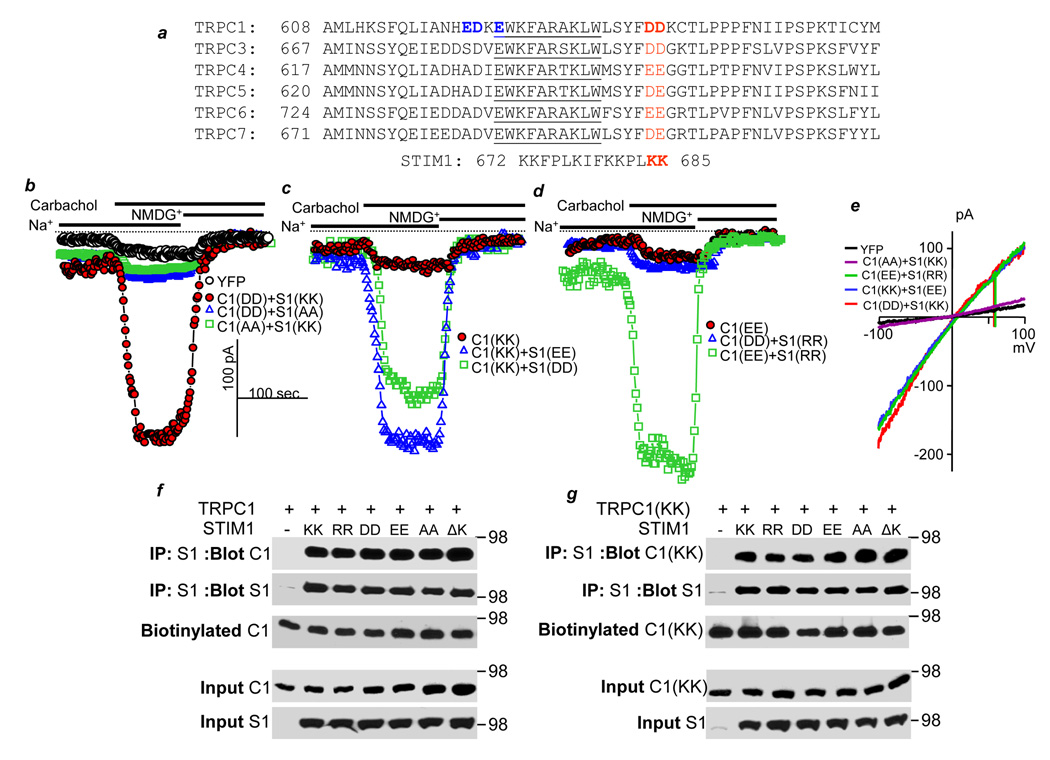
Fig. 1. Gating of TRPC1 by STIM1 is mediated by electrostatic interaction of TRPC1(639DD640) with STIM1(684KK685).
a. Alignment of the indicated C terminal sequences of TRPC channels. Highlighted in blue are mutations that have no effect of TRPC1 activity or its regulation by STIM1. Highlighted in red are the conserved negative charges (DD, EE or DE) in TRPCs. The TRP box is underlined. Also shown is the STIM1 K-rich domain. In this Fig., in Table 1 and in Fig. 2, as indicated by the bars, the cells were incubated in Na+-containing media, then stimulated with 100 µM carbachol and finally incubated in Na+-free, NMDG+-containing media to determine the zero current. b-e: cells were transfected with HA-TRPC1 and myc-STIM1 constructs. (b) shows the current of control cell transfected with YFP (black symbols) and cells transfected with wild type TRPC1(639DD640) and STIM1(684KK685) (red symbols), inhibition of TRPC1(639DD640) by STIM1(684AA685) (blue symbols) and a lack of current by TRPC1(639AA640) (green symbols). (c) shows the lack of current of TRPC1(639KK640) (red symbols) and its rescue by STIM1(684EE685) (blue symbols) and STIM1(684DD685) (green symbols). (d) shows the lack of current of TRPC1(639EE640) (red symbols), inhibition of wild-type TRPC1(639DD640) by STIM1(684RR685) (blue symbols) and rescue of TRPC1(639EE640) by STIM1(684RR685) (green symbols). (e) shows representative I/Vs of the indicated TRPC1+STIM1 combinations. In (f), HEK cells were co-transfected with wild-type HA-TRPC1 and the indicated myc-STIM1 mutants, biotinylated and used to determine effect of the mutants on co-IP of STIM1 and TRPC1 and total and surface expression of TRPC1. TRPC1 was detected with anti-HA, and STIM1 was detected with anti-myc. (g) the same experiment as in (e) except that effect of the STIM1 mutants was measured on expression of TRPC1(639KK640).
In all Figs. except Figs. 4g–4i, the spontaneous TRPC1 current was estimated from the Na+ current recorded about 1 min after break-in. The cells were then stimulated with 100 µM carbachol to measure the receptor-stimulated portion of the current. Finally, external Na+ was replaced with NMDG+ to record the leak current. The leak current was subtracted from the maximal current recorded at −100 mV, and cell capacitance was used to determine current density as pA/pF. To begin to test the role of the STIM1 K-domain and the TRPC1 dumbbell in gating of TRPC1 by STIM1, we mutated lysines STIM1(684KK685) to different charged and uncharged residues and found that they are essential for activation of TRPC1 (Table 1). Next, we found that mutation of the TRPC1 blue residues in Fig. 1a to positively charged residues had no effect on channel activity. On the other hand, mutation of 639DD640 on TRPC1 (marked in red) discloses how STIM1 gates TRPC channels. We mutated TRPC1(639DD640) and STIM1(684KK685) to the residues listed in Table 1 and measured the effect of the mutations on TRPC1 channel activity and its gating by STIM1. Productive and partial rescues are in bold and underlined and lack of rescue in bold letters. The control is with wild-types TRPC1+STIM1. Figs. 1b–1d show example traces of key findings and Fig. 1e shows example I/Vs. Importantly, deletion of the STIM1 K-domain and the K-domain point mutations have no affect on total or surface expression of wild-type TRPC1, TRPC1(639KK640) or on the coimmunoprecipitation of STIM1 and the TRPC1s (Figs. 1g and 1f). Similarly, the TRPC1(639RR640) and TRPC1(639AA640) mutations hd no effect on surface expression of TRPC1 or its interaction with STIM1 (Supplement Fig. S1c). Controls for surface expression and full blots are shown in supplementary Fig. S1a. In addition, all STIM1 mutants cluster in response to store depletion (not shown), and Fig. S1c shows that the STIM1 mutants co-cluster with TRPC1(639KK640). Hence, the effects of the STIM1 and TRPC1 mutations are not due to inhibition of STIM1 or TRPC1 clustering.
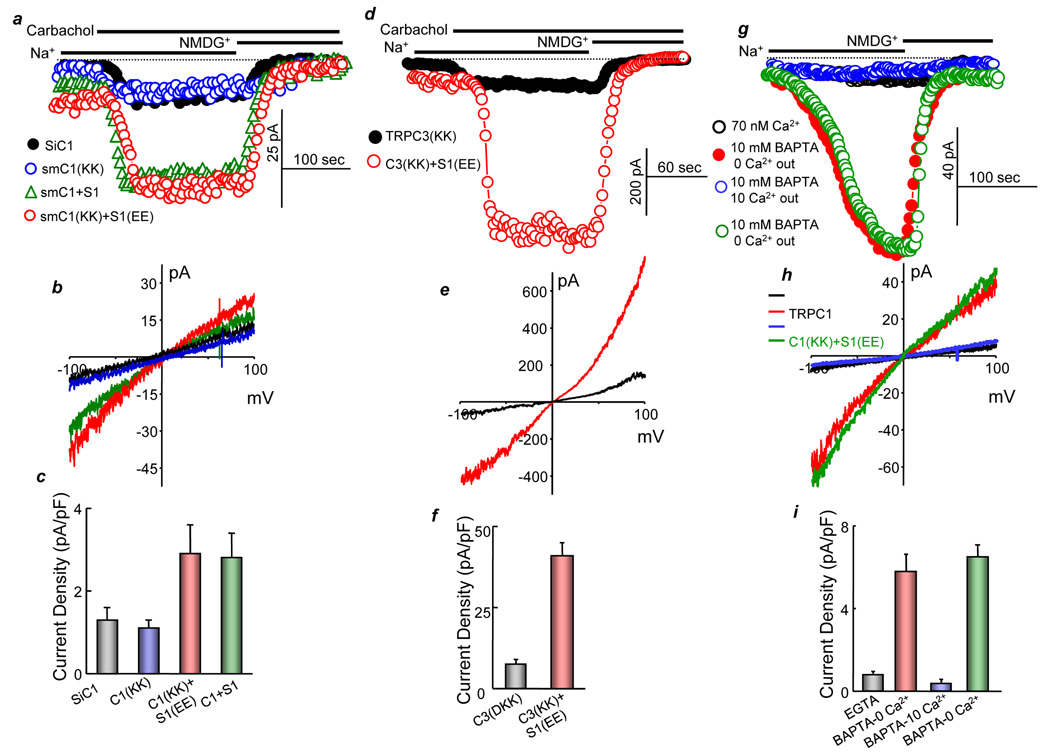
Fig. 4. Electrostatic gating is independent of TRPC1-STIM1 expression levels, observed with TRPC3 and activation of TRPC1 by store depletion.
In (a–c) HEK cells were treated with TRPC1 siRNA (siC1) and transfected with empty vector (black symbols), or 50 ng of siRNA protected (sm) TRPC1(639KK640) (blue symbols), smTRPC1+STIM1 (green symbols) or smTRPC1(639KK640)+ STIM1(684EE685) (red symbols). Note the low current level. (d–f) Current was measured in HEK cells transfected with the mutants TRPC3(697KK698) (black symbols) or TRPC3(697KK698) and STIM1(684EE685) (red symbols). (g–i) HEK cells transfected with TRPC1 (black, blue, red symbols) or TRPC1(639KK640)+STIM1(684EE685) (green symbols) were dialyzed with pipette solutions containing 70 nM Ca2+ buffered with 5 mM EGTA (black symbols) or 10 mM BAPTA with no added Ca2+ (blue, red and green symbols) and bathed in solutions containing 2 mM EGTA (black, green and red symbols) or 10 mM Ca2+ (blue symbols). (b, e, h) are the corresponding I/Vs, and the columns in (c, f, i) show the mean±s.s.m. of 4–5 experiments.
Table 1. Effect of mutation of STIM1(684KK685) and of TRPC1(639DD640) on TRPC1 channel activity.
HEK cells were transfected with the TRPC1 mutants alone (first column) or with the indicated STIM1 mutants. The protocol of Fig. 1 was used to measure TRPC1 current. The results are the mean±s.e.m. (n≥4 for each condition) of the current in pA recorded at −100 mV and extracted from the −100 to +100 RAMPs. Highlighted in bold are combinations that were expected to have current but showed no current. Highlighted in bold and underlined are partial or full rescues. ND=not determined.
| Native | KK | RR | DD | EE | AA | ||
|---|---|---|---|---|---|---|---|
 |
DD | 115±16 | 135±24 | 31±5 | 22±3 | 13±4 | 26±4 |
| EE | 21±4 | 57±14 * | 149±11 | ND | ND | ND | |
| KK | 18±3 | 23±7 | ND | 79±6 * | 98±17 * | ND | |
| RR | 16±5 | 21±3 | ND | 17±6 | 11±2 | ND | |
| AA | 11±3 | 19±3 | ND | ND | ND | 14±3 | |
P<0.05 relative to wild-type TRPC1+STIM1
ND = Not determined
To determine the importance of the charge, 684KK685 and 639DD640 were mutated to alanines. TRPC1(639AA640) was inactive, and STIM1(684AA685) acted as a dominant negative that inhibited TRPC1 activity. Moreover, neither wild-type STIM1 nor STIM1(684AA685) rescued TRPC1(639AA640) activity. In addition, STIM1(684DD685) and STIM1(684EE685), in which the charges are reversed from positive to negative, acted as dominant negatives that inhibited TRPC1 current. Finally, TRPC1(639KK640) and TRPC1(639RR640), in which the charges are reversed from negative to positive, were not active. The combined findings indicate that the charges in TRPC1 and STIM1 are essential for gating of TRPC1 by STIM1.
Next, we asked whether the identity of the charge on each protein is essential for the gating. This was tested by determining whether reverse charge STIM1 mutants can rescue reverse charge TRPC1 mutants. Remarkably, as shown in Fig. 1c and Table 1, the answer is yes. The negatively charged STIM1(684DD685) and STIM1(684EE685) that inhibit the activity of wild-type TRPC1 activated TRPC1(639KK640). Hence, the identity of the charge is not important as long as a negative charge is matched with a positive charge in either protein. These findings provide compelling evidence for the direct gating of TRPC1 by STIM1 and that the gating is not mediated by an intermediary protein.
Another notable finding in Fig. 1 and Table 1 is the behavior of TRPC1(639RR640) and TRPC1(639EE640). TRPC1(639RR640) is not active and could not be rescued either by STIM1(684DD685), STIM1(684EE685) or STIM1CT mutants (see below). This is not because the RR-EE pair is not productive since the conserved charge mutant TRPC1(639EE640) is not active and is poorly rescued by STIM1(684KK685), but maximally by STIM1(684RR685). Note that STIM1(684RR685) inhibited wild-type TRPC1(639DD640). Hence, 684KK685 of STIM1 matched best with 639DD640 of TRPC1, while 684RR685 of STIM1 matched best with 639EE640 of TRPC1. These findings are consistent with requirement for an electrostatic interaction between STIM1 and TRPC1 and a match of positive and negative residues. The lack of rescue of TRPC1(639RR640) by any STIM1 mutant and inhibition of wild-type TRPC1(639DD640) by STIM1(684RR685) may suggest steric incompatibility of the RR mutants, or that the interaction between TRPC1 and STIM1 may involve interactions in addition to the size and shape of the pairs of positive and negative charges.
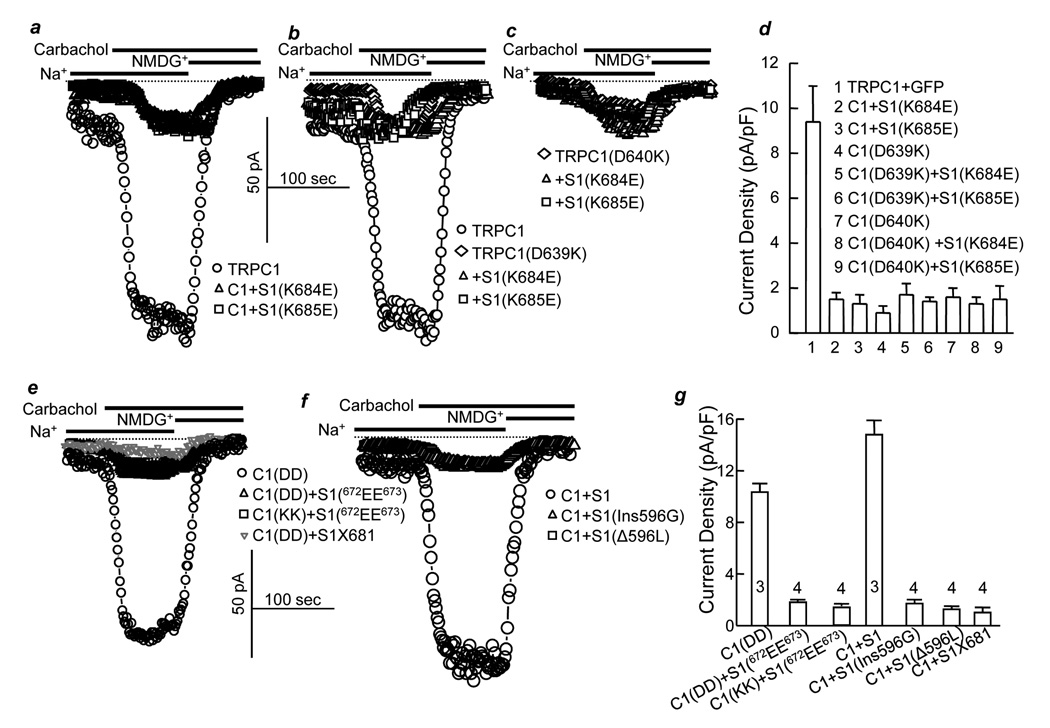
To further probe the importance of the orientation of the two negative and two positive charges, we first examined the behavior of the individually mutated charges. Fig. 2a–d show that STIM1(K684E) and STIM1(K685E) act as dominant negatives, and TRPC1(D639K) and TRPC1(D640K) are inactive. Notably, STIM1(K684E) and STIM1(K685E) are not able to rescue the activity of either TRPC1(D639K) or TRPC1(D640K), indicating that only the 684KK685 and 639DD640 orientation is productive. The specificity of 684KK685 and 639DD640 interaction is further demonstrated by the findings that STIM1(672EE673) also inhibits the activity of wild-type TRPC1(639DD640) but is not able to rescue the activity of TRPC1(639KK640) (Fig. 2e, g). Generation of new KK end by truncation of STIM1 after position 681 (STIM1X681) also resulted in a dominate negative that inhibited TRPC1 (Fig. 2e). Moreover, the 684KK685 - 639DD640 interaction does not appear to tolerate any disruption, since deletion of leucine 596, which is located 89 amino acids upstream of 684KK685, or insertion of glycine between serine 595 and leucine 596 resulted in dominant negative STIM1 (Fig. 2f, g).
Fig. 2. Orientation of both STIM1(K684) and STIM1(K685) with TRPC1(D639) and TRPC1(D640) is required for TRPC1 channel activity.
TRPC1 current was measured in HEK cells transfected with wild-type TRPC1 (○), TRPC1+STIM1(K684E) (Δ) or TRPC1+STIM1(K685E) (□) (a), TRPC1(D639K) alone (◊;), TRPC1(D639K)+STIM1(K684E) (Δ) or TRPC1(D639K)+STIM1(K685E) (□) (b), TRPC1(D640K) alone (◊), TRPC1(D640K)+STIM1(K684E) (Δ) or TRPC1(D640K)+STIM1(K685E) (□) (c). Panel (d) shows the mean±s.e.m. of 7 experiments with TRPC1 and 5 experiments with all other conditions. Current was also measured in HEK cells transfected with TRPC1 (○), TRPC1+STIM1(672EE673) (Δ), TRPC1(639KK640)+STIM1(672EE673) (□) or TRPC1 with STIM1X681 (▽) (e), TRPC1+STIM1 (○), TRPC1+STIM1(Ins596G) (Δ) or TRPC1+S1(Δ596L) (□) (f). The columns in (g) are the mean±sem of the indicated number of experiments.
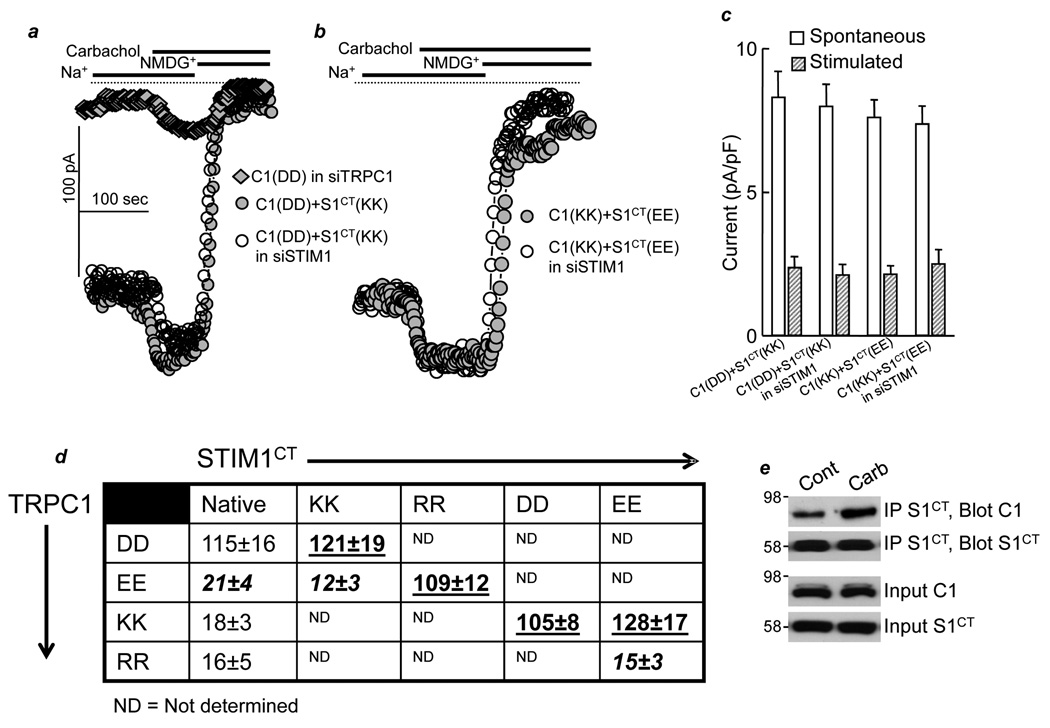
The next question we addressed is whether the electrostatic interaction between STIM1 and TRPC1 requires agonist stimulation. For this, we took advantage of the finding that the cytoplasmic C terminus domain of STIM1 (STIM1CT) activates TRPC1 independent of ER Ca2+ store depletion and receptor stimulation (Huang et al., 2006). Fig. 3 shows that receptor stimulation is not obligatory for gating by electrostatic interaction. Thus, in the presence of the cytoplasmic STIM1CT(684KK685), wild-type TRPC1(639DD640) is largely spontaneously active (Fig. 3a), and stimulation with carbachol only slightly further increased the current, similar to the current increase observed in cells treated with STIM1 siRNA and transfected with TRPC1. The STIM1CT(684EE685) mutant rescues the activity of TRPC1(639KK640) and makes it spontaneously active (Fig. 3b). The summary table in Fig. 3d further shows that STIM1CT(684RR685) rescues TRPC1(639EE640) and that STIM1CT(684DD685) and STIM1CT(684EE685) rescue TRPC1(639KK640). Moreover, the native STIM1 is not required for the electrostatic interaction between TRPC1 and STIM1CT, since knockdown of native STIM1 by siRNA has no effect on the activation of TRPC1 by STIM1CT (Fig. 3a–c).
Fig. 3. Electrostatic interaction between TRPC1(639DD640) and STIM1(684KK685) is independent of receptor stimulation.
a-b. HEK cells treated with TRPC1 siRNA (filled diamonds) or HEK cells treated with STIM1 siRNA (siSTIM1) (○) were transfected with 0.25 µg/ml cDNA of TRPC1 (C1(DD), a), TRPC1(639KK640) (C1(KK), b), STIM1CT (S1CT(KK), a) and STIM1CT(639EE640) (S1CT(EE), b). (c) shows the mean±s.e.m of 3 experiments at each condition; doted columns depict the spontaneous current and striped columns depict the current stimulated by 100 µM carbachol. (d) HEK cells were transfected with the TRPC1 mutants alone (first column) or with the indicated STIM1CT mutants. The protocol of panel (a) was used to measure the maximal TRPC1 current after carbachol stimulation. The results are the mean±s.e.m. of at least 4 experiments for each condition. Highlighted in black bold are combinations that were expected to have current but showed no current. Highlighted in bold and underlined are near maximal or maximal rescues. Panel (e) shows that carbachol enhances the interaction between TRPC1 and STIM1CT.
We noted that even in cells treated with siSTIM1 and transfected with the mutants TRPC1(639KK640) and STIM1CT(684EE685), where the current can be mediated only by the mutants, stimulation with carbachol further increases the current. This suggests that agonist stimulation can further increase the activity of channels associated with STIM1. Support for this notion was obtained by measuring the effect of cell stimulation on the interaction of the soluble, cytoplasmic STIM1CT with TRPC1. Fig. 3e shows that carbachol stimulation enhances the interaction of STIM1CT with TRPC1. Similar enhanced interaction was observed with wild-type STIM1 and TRPC1 (not shown). Since the cytoplasmic STIM1CT should have full access to TRPC1, the enhanced interaction with TRPC1 may account for the further increase in the current by carbachol stimulation.
The gating by electrostatic interaction of TRPC1-STIM1 is not affected by the expression level of the proteins or the presence of endogenous TRPC1. Figs. 4a–4c show that low level of siRNA-protected smTRPC1(639KK640) expressed in cells treated with TRPC1 siRNA is rescued by STIM1(684EE685). The efficiency of the siRNA and the protection by silent mutations (sm) is shown in supplementary Fig. S1b.
Conservation of the negative charges in TRPCs raised the question of whether gating by electrostatic interaction mediates activation of other TRPCs by STIM1, including TRPCs that are indirectly regulated by STIM1, such as TRPC3 (Yuan et al., 2007). The two critical negative charges in TRPC3(697DD698) were mutated to TRPC3(697KK698) Figs. 4d–4f show that TRPC3(697KK698) is not active. Significantly, the activity of TRPC3(697KK698) was completely rescued by STIM1(684EE685), suggesting that gating by electrostatic interaction is a general mechanism for gating of TRPCs by STIM1.
Another channel gated by STIM1 is Orai1. STIM1 is obligatory for the function of Orai1 as a CRAC channel (Mercer et al., 2006; Peinelt et al., 2006; Zhang et al., 2006); however, unlike the case with TRP channels, there are no obvious negatively charged clusters in Orai1. To compare regulation of Orai1 and TRPCs by STIM1, it is necessary to measure the TRPC1 and Orai1 current in the same cells. To do so, we tested whether TRPC1 can be activated by passive store depletion and the effect of high extracellular Ca2+ on the activity of TRPC1. Figs. 4g–4i show that dialyzing cells with pipette solution buffered to contain 70 nM free Ca2+ to prevent store depletion and incubation in Ca2+-free bath solution did not result in activation of TRPC1. Interestingly, passive store depletion by including 10 mM BAPTA in the pipette solution resulted in slow activation of TRPC1 current. Activation by store depletion is also mediated by electrostatic interaction, as shown by store depletion-mediated activation of TRPC1(639KK640)+STIM1(684EE685). Activation of TRPC1 by store depletion together with the results in Fig. 3 provide further support for the notion that TRPC1 functions as a SOC when activated by agonist stimulation of passive store depletion. Figs. 4g–4i show that including 10 mM external Ca2+ completely inhibited TRPC1 current activated by passive store depletion, conditions that are used to record Orai1 Ca2+ current. Similar inhibition by external Ca2+ was observed when TRPC1 was activated by receptor stimulation (not shown).
The inhibition of TRPC1 current by external Ca2+ and the lack of activation of Orai1 under the conditions used to isolate TRPC1 current (70 nM free pipette Ca2+ buffered with 5 mM EGTA and absence of mitochondrial substrates (Parekh and Putney, 2005)), allow us to isolate the Orai1 and TRPC1 currents in cells co-expressing TRPC1, Orai1 and STIM1 (Fig. 5). Supplementary Figs. S2a–c show that expression of TRPC1 does not affect activation, current or the I/V characteristics of Orai1. Supplementary Figs. S2d–f show that Orai1 does not affect activation, current or the I/V characteristics of TRPC1. Finally, supplementary Figs. 2Sg–I show that knock-down of native Orai1 by siRNA which reduces SOC activity by about 80% (Supplemental Fig. S3a) has no effect on the activity or properties of TRPC1. Therefore, the findings in Supplementary Fig. 2 indicate that expressed TRPC1 and Orai1 can function independently of each other and can be independently activated by STIM1.
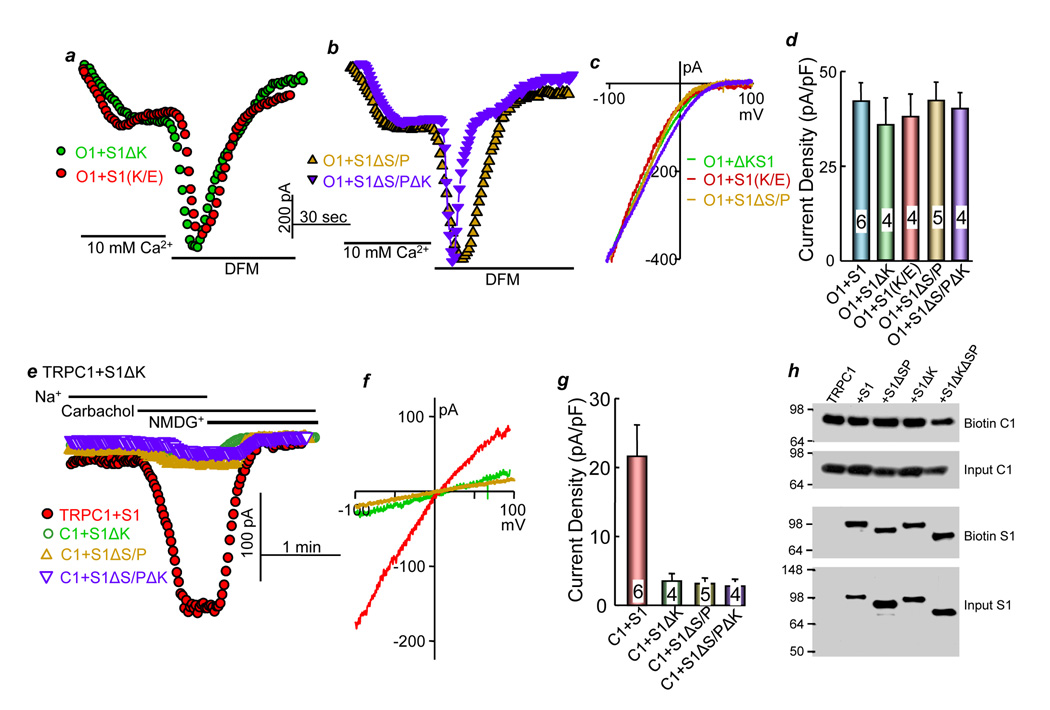
Fig. 5. STIM1 lysine (K) and serine/proline (S/P) domains are not required for activation of Orai1.
(a–d) HEK cells were transfected with Orai1 (O1) and STIM1(ΔK) (S1ΔK, green symbols), STIM1(684EE685) (S1(K/E), red symbols), STIM1(ΔS/P) (S1ΔS/P, yellow symbols) or STIM1(ΔS/PΔK) (S1ΔS/PΔK, blue symbols), and Orai1 current was measured in the presence of 10 mM Ca2+ or divalent-free media (DFM). (c) shows typical Icrac current I/V curves for Orai1 and all STIM1 constructs and (d) shows the mean±s.e.m currents from the indicated number of experiments. (e–g) HEK cells were transfected with 0.25 µg/ml TRPC1 and 0.25 μg/ml STIM1 (red symbols), S1ΔK (green symbols), S1ΔS/P (yellow symbols) or S1ΔS/PΔK (blue symbols), and the TRPC1 current was measured. (g) shows the mean±s.e.m currents in the indicated number of experiments. h) shows that the STIM1 constructs do not affect total or surface expression of TRPC1 or STIM1. TRPC1 was detected with anti-HA, and STIM1 was detected with anti-myc.
To further examine the role of STIM1 in the regulation of Orai1 and TRPC1, we analyzed the requirement of the STIM1 K- and S/P- domains for the activation of each channel type. It was reported that deletion of the K-domain did not prevent activation of Orai1 (Li et al., 2007). Figs. 5a–d extend these findings to show that deletion of STIM1 K domain and the mutant STIM1(684EE685) had no effect of the activation of Orai1 by STIM1. Similarly, supplementary Figs. S3b and S3c show that the STIM1 mutants in Table 1, the insertion of 596G or the deletion of 596L have no effect on activation of Orai1 by STIM1. It was reported that the combined deletion of the S/P and the K-domains resulted in STIM1 that failed to activate Orai1 (Li et al., 2007). However, the truncation was made at position 425 that disrupts the ERM domain of STIM1, and we have already shown that the ERM domain is essential for activation of Icrac by STIM1 (Huang et al., 2006). Therefore, to further examine the role of the S/P and K-domains, we truncated STIM1 at residue 535, which deletes both domains but maintains an intact ERM domain. Deletion of the S/P and K-domains does not prevent activation of Orai1 by STIM1 (Figs. 5b and 5d).
STIM1(684EE685) and deletion of K and S/P domains do not affect the ability of Orai1 to function as a SOC. SOC was activated by depleting ER Ca2+ by incubating the cells in Ca2+-free media and inhibiting the SERCA pumps with cyclopiazonic acid (CPA). Supplementary Fig. 3a shows that, as reported before (Mercer et al., 2006; Soboloff et al., 2006), expression of Orai1 alone markedly inhibited the native SOC and provided a clean background to test the effect of the STIM1 mutants on Orai1 activity. Expression of wild-type and all STIM1 mutants with Orai1 did not affect ER Ca2+ content but restored prominent Ca2+ influx in response to store depletion.
In contrast with the findings with Orai1, STIM1(ΔK), STIM1(ΔS/P) and STIM1(ΔS/PΔK) prevented activation of TRPC1 (Figs. 5e–g). Native STIM1 is sufficient for full activation of TRPC1, as evident from elimination of TRPC1 current by knockdown of STIM1 with siRNA (Fig. 3a) and by the dominant negative STIM1 mutants (Table1, Fig. 2). When expressed with TRPC1, STIM1(ΔS/P), STIM1(ΔK) and STIM1(ΔS/PΔK) inhibit TRPC1 activity, most likely by scavenging the native STIM1 and preventing it from activating TRPC1. Finally, Fig. 5h shows that inhibition of TRPC1 activity by the STIM1 constructs is not due to reduced plasma membrane expression of TRPC1. The combined results in Figs. 5 indicate that STIM1 gates Orai1 and TRPC channels by different mechanisms.
The present work reveals that STIM1 gates Orai1 and TRPC channels by two distinct mechanisms. These findings may have several implications. First, Orai1 and TRPC channels can form two independent SOCs. In this manner, discrete intracellular Ca2+ signals can be generated depending on whether STIM1 couples to TRPCs or Orai1 or form a complex with both TRPCs and Orai1. As local Ca2+ influx is a key determinant of a range of cellular responses (Chang et al., 2006), specific Ca2+ influx pathways may regulate specific cellular responses (Chang et al., 2008; Chang and Parekh, 2004). Second, receptor-stimulated Ca2+ influx may be mediated by different SOCs, depending on the combination of TRPC and Orai channels in a given cell. Third, receptors may independently activate the TRPC and Orai channels to tune their Ca2+ signals and perhaps generate receptor-specific Ca2+ signals. Binding of STIM1 to TRPCs may reduce its binding to Orai1 and vice versa to determine which type of Ca2+ influx pathway mediate Ca2+ entry. This may be suggested by the findings that TRPCs and Orai1 may exist in the same complex with STIM1 (Ambudkar et al., 2007; Liao et al., 2008; Liao et al., 2007; Ong et al., 2007).
Our data supports a novel molecular mechanism for TRPC channel gating that involves a negatively charged region that is conserved in all TRPC channels and that lies downstream of the TRP box (Fig. 1a). TRPC1 gating by STIM1 is mediated by restricted physical and electrostatic interactions that do not require association with ER stores, since C-terminal cytosolic fragments of STIM1 can rescue gating in charge swap experiments. While the physical interaction of TRPC and STIM1 is mediated by the ERM domain of STIM1, the charge coupling of TRPC appears essential to transduce the high-local density of STIM1 molecules, which results from store depletion (Liou et al., 2005; Wu et al., 2006), to channel opening. It is possible that electrostatic interactions between TRPC1 and STIM1 are required to complete the assembly of a functional channel, where positive-negative and negative-positive charges are required for activation and positive-positive and negative-negative charges always inhibit the channels. This model is different than models of gating such as the “ball and chain” model of K+ channels gating. In the case of a “ball and chain” gating, the identity of the residues are not important, but the precise charge is important to promote the approach of the inactivation ball towards its binding site through long-range electrostatic interactions (Hoshi et al., 1990; Murrell-Lagnado and Aldrich, 1993). The negatively charged motif is absent from Orai1, consistent with the lack of gating of Orai1 by the K- and S/P domains of STIM1. These findings provide precedent for understanding the molecular basis of gating of TRPC channels by conformational coupling.
Experimental Procedures
Solutions, reagents and clones
The TRPC1, TRPC3, and STIM1 clones were described previously (Huang et al., 2006; Yuan et al., 2007). TRPC3-YFP and YFP-STIM1 clones were generously provided by Dr. Thomas Gudermann (University of Marburg) and Dr. T. Meyer (Stanford University), respectively. The human Orai1 clone was obtained from Open Biosystems (clone #: BC013386.1). YFP-STIM1 was cloned into the pcDNA3.1(+) vector using EcoRI(5’) and NotI(3’). The TRPC1 and Orai1 cDNAs were cloned into an HA-tagged pRK5 mammalian expression vector using SalI(5’) and NotI(3’), while STIM1 cDNA was cloned into myc-tagged pRK5. mCherry-Red was cloned into the p3XFLAG-CMV vector using XbaI, and TRPC1 was subsequently cloned into this Red vector using NotI(5’) and SalI(3’). STIM1ΔK was generated by introducing a STOP codon after amino acid 671. The STIM1(1-535) deletion mutant was generated by introducing a STOP codon after amino acid 535 and, thus, eliminates the S/P and K domains. STIM1X681 was generated by introducing a STOP codon after amino acid 681. All point mutations on STIM1 and TRPC1 were generated using the site-directed mutagenesis kit (Stratagene). The antibodies used were monoclonal anti-myc and HRP-conjugated anti-myc and anti-HA (all from Santa Cruz Biotech). Anti-myc antibodies were used for co-IP, while HRP-conjugated anti-myc and anti-HA antibodies were used for Western blotting. The siRNA sequence used to knockdown STIM1 was the same as described before (Yuan et al., 2007), and its effectiveness was confirmed in the present work. The siRNA sequence used to knockdown TRPC1 is: 5’-CCACCUGUAAGAAGAUAAUGACUGT-3’. smTRPC1, the silent mutant TRPC1 that is protected from siTRPC1, was generated by introducing point mutations in the third (wobble) position of the 2 consecutive codons encoding lysines-315 and -316 that are within the siTRPC1 sequence. Thus, each of the 2 AAGs were mutated to AAA. siRNA transfection of HEK293 cells was done using the Qiagen TransMessenger Transfection kit. The amount of siRNA used was 0.8 µg per 12-well with HEK cells at 80–90% confluency. Wells were coated with 0.5 mg/ml poly-L-ornithine in 0.15M borate buffer, pH8.6. After 6 hours of siRNA transfection time, 70% of cells were re-plated into fresh wells so that confluency will again be 80–90% the following day. Next, plasmid transfection was done using Lipofectamine 2000 reagent for 6 hours. The total amount of cDNA used per 12-well was 0.5 µg/0.5 ml. Thus, for a typical transfection, 0.13 µg of TRPC channel was used for low expression channel level, along with 0.13 µg of M3 receptor, 0.13 µg of STIM1, and 0.1 µg of GFP. For Orai1, M3 receptor was replaced with empty vector in the transfection mix. Current was measured or cells were harvested and extracted for co-IP analysis or biotinylation assay the following day.
Western blot and co-IP
Transfected cells were harvested and lysed using 500 µL of binding buffer: 1× PBS buffer containing 1 mM NaVO3, 10 mM NaPyrophosphate, 50 mM NaF [pH 7.4], and 1% Triton X-100. The cell extracts were sonicated, and insoluble material was spun down at 30,000 × g for 20 min. For the co-IP experiments, 1 µg of myc antibody was added to 100 µL of cell extract and incubated for 1 hr at 4 C. Then, 50 µL of 1:1 slurry of protein G sepharose 4B beads were added to the antibody-extract mix and incubated for an additional hr at 4 C. Beads were washed 3 × 10 min with binding buffer, proteins were released from the beads with 50 µL of SDS-loading buffer. 25 µL was loaded onto 8% tris-glycine SDS-PAGE gels. Gels were transferred onto PVDF membrane, and Western blot analysis was done.
Biotinylation
Transfected cells were washed once with 1X PBS on ice. 0.5 mg/mL of EZ-Link Sulfo-NHS-SS-Biotin (Pierce) was added to the cells for 30 min on ice. Afterwards, the biotin was quenched with 50 mM glycine on ice for 10–15 min. The cells were then processed as described above to make cell extract. 50 µL of 1:1 slurry of immobilized avidin beads(Pierce) were added to 100 µL of cell extract and incubated for 2 hrs at 4 C. Beads were washed 3 × 10 min with binding buffer, proteins were released from the beads with 50 µL of SDS-loading buffer. 25 µL was loaded onto 8% tris-glycine SDS-PAGE gels. Gels were transferred onto PVDF membrane, and Western blot analysis was done.
Current measurement
TRPC1 current was measured in transiently transfected HEK cells by whole current recording, as described previously (Yuan et al., 2003). Briefly, the pipette solution contained (in mM) 140 CsCl, 2 MgCl2, 1 ATP, 5 EGTA, 1.5 CaCl2 (free Ca2+ 70 nM) and 10 HEPES at pH 7.2 with CsOH, to eliminate K+ current and prevent inhibition of the channel by high cytoplasmic Ca2+. The bath solution contained (in mM) 140 NaCl or 140 NMDG-Cl, 5 KCl, 0.5 EGTA and 10 HEPES at pH 7.4 with NaOH or NMDG-OH−). Cells were transfected with TRPC1 and empty vector or TRPC1 and the indicated STIM1 mutants. The current recorded at −100 mV was used to calculate current density as pA/pF and current recorded in multiple experiments was used to obtain the mean ±s.e.m. and calculate significance by Student's t-test.
Orai1 current was measured by recording the whole cell current in HEK cells co-transfected with Orai1 and STIM1 or its mutants (Huang et al., 2006). The standard pipette solution contained (in mM): 140 Cs aspartate, 6 MgCl2, 10 BAPTA, and 10 Hepes (pH 7.2 with CsOH). The standard bath solution contained (in mM): 130 NaCl, 5 KCl, 10 CaCl2, 1 MgCl2, and 10 Hepes (pH 7.4 with NaOH). The divalent-free (DVF) solution contained (in mM): 150 NaCl, 10 EDTA, and 10 Hepes (pH 7.4 with NaOH). The current was recorded by 400 ms rapid alterations of membrane potential (RAMPs) from −100 to +100 mV from a holding potential of 0 mV. The current recorded at −100 mV was used to calculate current density as pA/pF and current recorded in multiple experiments was used to obtain the mean ±s.e.m.
Measurement of [Ca2+]i
[Ca2+]i was measured about 24 hrs post transfection. [Ca2+]i was measured by loading the cells with Fura2 and recording fura2 fluorescence at excitation wavelengths of 340 and 380 nm and collecting the light emitted at wavelength above 500 nm. [Ca2+]i is expressed as the 340/380 ratio.
Supplementary Material
Acknowledgments
This work was supported in part by a grant from the American Heart Association, Inc., Texas Affiliate BG1A 0665192Y to W. Z., National Institutes of Health Grants DE12309 and DK38938 and the Ruth S. Harrell Professorship in Medical Research to S. M. and by the National Institute on Drug Abuse (NIDA; DA00266, DA10309) and the National Institute of Mental Health (NIMH; MH068830) to P. F. W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambudkar IS, Ong HL, Liu X, Bandyopadhyay B, Cheng KT. TRPC1: the link between functionally distinct store-operated calcium channels. Cell Calcium. 2007;42:213–223. doi: 10.1016/j.ceca.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Chang WC, Di Capite J, Singaravelu K, Nelson C, Halse V, Parekh AB. Local Ca2+ influx through Ca2+ release-activated Ca2+ (CRAC) channels stimulates production of an intracellular messenger and an intercellular pro-inflammatory signal. The Journal of biological chemistry. 2008;283:4622–4631. doi: 10.1074/jbc.M705002200. [DOI] [PubMed] [Google Scholar]
- Chang WC, Nelson C, Parekh AB. Ca2+ influx through CRAC channels activates cytosolic phospholipase A2, leukotriene C4 secretion, and expression of c-fos through ERK-dependent and -independent pathways in mast cells. Faseb J. 2006;20:2381–2383. doi: 10.1096/fj.06-6016fje. [DOI] [PubMed] [Google Scholar]
- Chang WC, Parekh AB. Close functional coupling between Ca2+ release-activated Ca2+ channels, arachidonic acid release, and leukotriene C4 secretion. The Journal of biological chemistry. 2004;279:29994–29999. doi: 10.1074/jbc.M403969200. [DOI] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- Hoshi T, Zagotta WN, Aldrich RW. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science (New York, N.Y. 1990;250:533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- Li Z, Lu J, Xu P, Xie X, Chen L, Xu T. Mapping the interacting domains of STIM1 and Orai1 in CRAC channel activation. The Journal of biological chemistry. 2007 doi: 10.1074/jbc.M703573200. [DOI] [PubMed] [Google Scholar]
- Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, Birnbaumer L. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2895–2900. doi: 10.1073/pnas.0712288105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong DL, Birnbaumer L. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4682–4687. doi: 10.1073/pnas.0611692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer JC, Dehaven WI, Smyth JT, Wedel B, Boyles RR, Bird GS, Putney JW., Jr Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. The Journal of biological chemistry. 2006;281:24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell-Lagnado RD, Aldrich RW. Interactions of amino terminal domains of Shaker K channels with a pore blocking site studied with synthetic peptides. The Journal of general physiology. 1993;102:949–975. doi: 10.1085/jgp.102.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, et al. Dynamic assembly of TRPC1/STIM1/Orai1 ternary complex is involved in store operated calcium influx: Evidence for similarities in SOC and CRAC channel components. The Journal of biological chemistry. 2007 doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM reconstitute store-operated calcium channel function. The Journal of biological chemistry. 2006;281:20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A, et al. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006a;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science (New York, N.Y. 2006b;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley PF, Zeng W, Huang GN, Yuan JP, Kim JY, Lee MG, Muallem S. TRPC channels as STIM1-regulated store-operated channels. Cell Calcium. 2007;42:205–211. doi: 10.1016/j.ceca.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, Dehoff MH, Schwarz MK, Seeburg PH, Muallem S, Worley PF. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell. 2003;114:777–789. doi: 10.1016/s0092-8674(03)00716-5. [DOI] [PubMed] [Google Scholar]
- Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol. 2007;9:636–645. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.