Abstract
Nasal solitary chemoreceptor cells (SCCs) are a population of specialized chemosensory epithelial cells presumed to broaden trigeminal chemoreceptivity in mammals (Finger et al., 2003). SCCs are innervated by peptidergic trigeminal nerve fibers (Finger et al., 2003) but it is currently unknown if intact innervation is necessary for SCC development or survival. We tested the dependence of SCCs on innervation by eliminating trigeminal nerve fibers during development with neurogenin-1 knockout mice, during early postnatal development with capsaicin desensitization, and during adulthood with trigeminal lesioning. Our results demonstrate that elimination of innervation at any of these times does not result in decreased SCC numbers. In conclusion, neither SCC development nor mature cell maintenance is dependent on intact trigeminal innervation.
Keywords: chemoreceptor, trigeminal, neurogenin1, capsaicin, nasal, development
Introduction
For many epithelial sensory cells intact innervation is necessary for survival. Taste buds degenerate when their nerve supply is removed and do not regenerate until innervation to gustatory papillae is restored (el-Eishi and State, 1974; Farbman, 1969; State and Bowden, 1974; Zalewski, 1981). Likewise, cutaneous mechanoreceptive Merkel cells atrophy when their nerve supply is removed (Barber and Raisman, 1978; English, 1977; Harding et al., 1977). However, in other populations of epithelial receptor cells, e.g. auditory hair cells, survival is autonomous of innervation (Ma et al., 2000).
The nasal epithelium of mice and rats contains a population of modified epithelial sensory cells called solitary chemoreceptor cells (Finger et al., 2003) or solitary chemosensory cells (Tizzano et al. 2006), both abbreviated SCC. In mammals, SCCs are distributed throughout the nasal respiratory epithelium with the highest number positioned in the anterior regions. Populations of similar chemoreceptor cells extend throughout the respiratory and digestive tracts (Bezencon et al., 2006; Finger et al., 2003; Hofer et al., 1996; Kaske et al., 2007; Sbarbati et al., 2004; Sbarbati and Osculati, 2005; Wu et al., 2002). Many nasal SCCs express T2R “bitter taste” receptors, the G-protein alpha-gustducin, phospholipase C isoform β2 (PLCβ2), and the transient receptor potential melastatin channel subtype 5 (TRPM5) suggesting that their molecular transduction machinery is similar to type II taste receptor cells (Finger et al., 2003; Gulbransen and Finger, 2005). Given that SCCs express elements of a chemosensory transduction cascade, it is likely that SCCs function to expand the chemical detection capabilities of the trigeminal nerve which innervates them.
Although SCCs share many of the morphological characteristics of type II taste cells, they are different in that SCCs form identifiable synapses with afferent nerve fibers whereas type II taste cells do not (Finger et al., 2003). Many of the fibers innervating SCCs contain the neuropeptide calcitonin gene related peptide (CGRP) and are presumably c-fibers involved in nociception (Finger et al., 2003).
It is currently not known if SCCs, like taste buds and other populations of innervated epithelial sensory cells, depend on intact innervation for survival in adult animals. Taking into account their similarities to taste receptor cells, we hypothesized that SCCs would rapidly degenerate following removal of their afferent nerve supply. We tested this hypothesis using several models. First, we studied development of SCCs in the absence of trigeminal innervation in neurogenin-1−/− (KO) mice, which never form a trigeminal ganglion due to the failure of precursor populations to migrate (Ma et al. 2000). Second, we used capsaicin desensitization in rats to remove peptidergic innervation during early postnatal development. Finally, we eliminated all trigeminal innervation by lesioning the Gasserian ganglion in adult mice. We then analyzed tissue immunocytochemically from these animals to assay for the presence of SCCs and nerve fibers in the nasal epithelium. The results of these experiments indicate that SCC generation and survival are autonomous of trigeminal innervation.
Methods
All experiments referred to in this report were in accordance with approved UCDHSC or Wake Forest IACUC animal protocols.
Collection of embryos
Wild type C57/B6 embryos were taken at various stages from embryonic day (E)14.5 to E18.5, decapitated, and immersion fixed in 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB) (pH 7.2) for 2 hours. Tissue was then stored in methanol at −20°C. Before sectioning, tissue was rehydrated in a graded Methanol series and sunk in 0.1 M PB containing 20% sucrose. Cryostat sections (16 μM) through the nasal cavity were adhered to Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA), and stored at −20°C before being analyzed by immunocytochemistry.
Neurogenin-1 knockout mice
Neurogenin-1−/+ heterozygous breeding pairs were obtained from the David J. Anderson lab at the California Institute of Technology, Pasadena, CA. Heterozygous pairs were bred to obtain neurogenin-1 null mutant mice as described by Ma et al. (Ma et al., 1998). Pups were collected at birth, anaesthetized with chloral hydrate and perfused with 4% PFA in 0.1 M PB (pH 7.2). Genotyping of pups was performed by polymerase chain reaction (PCR) as described by Ma et al. (Ma et al., 2000). Following a 2 hr post fix, the heads were sunk overnight in PB with 20% sucrose, sectioned at 16 μM on a cryostat, and stored at −20°C until use in immunocytochemical experiments.
Capsaicin desensitization
Capsaicin desensitization of rats was carried out as described in Silver et al. (1991). Briefly, Sprague-Dawley rat pups were wrapped in gauze, placed on ice, and injected with capsaicin (50 mg/kg) the day after birth. After 40 days, rats were examined electrophysiologically for ethmoid nerve responses to chemical and mechanical stimulation and then perfused with 4% PFA. Heads were then briefly post-fixed and stored overnight in 0.1 M PB. The following day, heads were decalcified with RDO (Apex Engineering, Produts, Aurora IL) for 6 hours, rinsed in 0.1 M PB, and sunk overnight in 0.1 M PB with 20% sucrose. Rat noses were cut at 16 μm on a cryostat and stored at −20°C before being analyzed by immunocytochemistry.
Trigeminal lesioning
Adult wild type C57Bl6J mice of both sexes were deeply anaesthetized with a 100mg/kg Ketamine - 10mg/kg Xylazine mix, shaved and cleaned with 70% EtOH, and secured with a stereotaxic head holder. A small incision parallel to the midline exposed the surface of the skull and allowed Bregma and Lambda to be located and leveled. A small hole was then drilled through the skull over the trigeminal ganglion. A lesioning electrode (FHC Inc. Bowdoin, ME) was positioned 1.5 mm anterior and 0.8 mm lateral to Bregma and lowered 6.9–7.0 mm from the top of the skull into the ophthalmic division of the right trigeminal ganglion. 2.0 mA of current was then passed for 20 seconds to create a lesion. In all, five such lesions were made in the right trigeminal ganglion (1.5 mm anterior – 0.8 mm lateral, 1.5 mm anterior – 1.5 mm lateral, 1.0 mm anterior – 1.1 mm lateral, 1.0 mm anterior – 1.8 mm lateral, and 1.0 mm anterior – 1.4 mm lateral). The hole in skull was then sealed with bone wax and the incision sutured closed. Mice were then given peanut butter and water with children’s Tylenol ad libitum to aid in recovery and pain.
Mice were sacrificed 5–6 days (n = 3), 10–12 days (n = 3), and 18–22 days (n = 4) post-lesion and perfused with 4% PFA in 0.1 M PB (pH 7.2). Tissue was then post-fixed in PFA for 2 hours and rinsed in PB overnight. The following day, tissue was decalcified for 3.5 hours with RDO rapid decalcifier (Apex, Plainsfield, IL), rinsed well with PB, and then sunk overnight in PB with 20% sucrose. 16 μm thick sections were cut on a cryostat, adhered to Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA), and stored at −20°C before being analyzed by immunocytochemistry.
Antibodies and immunocytochemistry
Dual-label immunocytochemistry
A modification of the protocol described in Bartel et al. (2006) for immunocytochemistry using two antibodies raised in the same host was used to identify SCCs and nerve fibers in the nasal epithelium. Sections were treated with 3% H2O2 in 0.1M PB for 10 min to quench endogenous peroxidases, rinsed in PBS, blocked for 1 hr with 10% normal goat serum (NGS) in PBS with 0.4% Triton X-100 and 1% BSA (Blocking solution), and then incubated overnight with the 1st primary antibody: rabbit anti-α-gustducin (directed against a peptide fragment containing amino acids 93–112 of rat α-gustducin origin; cat. # sc-395; Santa Cruz Biotechnology Inc., Santa Cruz, CA) diluted 1:10,000 in blocking solution. The next day sections were washed in PBS, incubated for 2 hours with biotin F(ab)2 anti-rabbit (1:1,000, Jackson ImmunoResearch Laboratories Inc., West Grove, PA), rinsed again in PBS, and then incubated for 2 hours with avidin-biotin-complex (ABC, Vector Labs, Burlingame, CA). Slides were then rinsed in PBS and reacted with Tyramide Signal Amplification (TSA conjugated to Alexa 568, Molecular Probes, Invitrogen Corp.) for 10 minutes before being rinsed well with PBS containing 0.1% triton X-100. IgG binding sites were then blocked by an overnight incubation with unconjugated F(ab) goat anti-rabbit (1:50, Jackson ImmunoResearch Laboratories Inc., West Grove, PA). The following day, sections were rinsed in PBS, blocked for 1 hr with 10% NGS, and incubated overnight with the 2nd primary antibody (either rabbit anti-protein gene product 9.5 (PGP), a 27kDa ubiquitin-protein hydrolase (used at 1:1,000) (generated against native human PGP9.5 from brain; detects a western blot band of approximately 27 kDa in rat brain cell lysates; AbD Serotec, Raleigh, NC) or rabbit anti-calcitonin gene related peptide (CGRP) (used at 1:2000) (polyclonal antibody raised against the rat antigen SCNTATCVTHRLAGLLSRSGGVVLDNFVPTNVGSEAF; shows 100% radioimmunoassay crossreactivity to rat α-CGRP peptide; Peninsula Labs LLC, San Carlos, CA)). The following day tissues were rinsed in PBS, incubated with goat anti-rabbit Alexa Fluor 488 (1:400, Molecular Probes, Invitrogen Corporation, Carlsbad, CA) secondary for 2 hrs, rinsed in PBS, and coverslipped with Fluoromount G (Southern Biotech, Birmingham, AL). Omission of α-gustducin, the first primary, resulted in only PGP9.5 or CGRP staining and omission of the second primary, either PGP9.5 of CGRP, resulted in minimal inappropriate binding of the second secondary to the first primary.
Wholemount immunocytochemistry
Mice expressing GFP under control of the TRPM5 promoter (TRPM5-GFP mice developed by R.F. Margolskee contained 5′ to 3′: 11 kb of mouse TRPM5 5′ flanking sequence, TRPM5 Exon 1 (untranslated), Intron 1, and the untranslated part of Exon 2, and eGFP.) were perfused with 4% PFA as described above and the heads post-fixed for 30 minutes. The head was then split down the midline and the anterior nasal epithelium dissected in 0.1 M PB. Nasal tissue was then rinsed well with PBS, blocked for 45 minutes with 10% NGS, and incubated with the primary antibody (either Rb anti-PGP9.5 [1:1,000, AbD Serotec, Raleigh, NC] or Rb anti-CGRP [1:500, Peninsula Labs LLC, San Carlos, CA]) overnight at room temperature on a shaker table. The following day tissue was rinsed well with PBS and then incubated with the secondary antibody goat anti-Rb Alexa 568 (1:400, Molecular Probes, Invitrogen Corporation, Carlsbad, CA). Tissue was then rinsed well in PBS, spread flat on slides, and mounted with Fluoromount G (Southern Biotech, Birmingham, AL).
Confocal microscopy
Immunofluorescence of labeled SCCs was imaged on an Olympus Fluoview laser scanning confocal microscope (Olympus America Inc., Melville, NY) using 20X (0.80 n.a.) and 60X (1.4 n.a.) oil immersion lenses. Optical sections (0.8 μm) were acquired through each field of view and then compiled into a z-stack. Sequential scanning of the two separate fluorescence channels avoided “bleed through” of signal from the inappropriate channel.
Cell counting
SCC cell profiles were counted at 40X from approximately 6 sections, spaced 100 μm apart, per animal. These sections covered the area of nasal tissue from the most anterior regions of the nasal cavity to the posterior end of the vomeronasal organ. α-gustducin immunoreactive profiles were counted only when the non-reactive nucleus could be seen clearly. For lesioned mice, cells from control and lesioned sides of the same section were counted. Epithelial length was determined by imaging sections used for cell counts with a SPOT camera and SPOT software (Diagnostic instruments, Inc., Sterling Heights, MI) at either 16X or 32X and then measuring the length of the epithelium using Image J (NIH).
In capsaicin desensitized rat tissue SCC nuclear diameter was significantly different (P<0.05) than control rats (mean diameter ± SEM = 4.58 ± 0.22 μm in control and 5.42 ± 0.21 μm in capsaicin desensitized). Therefore we applied the Abercrombie correction to counts from control and desensitized rat tissue before statistical comparison to correct for this difference. Since we were not interested in exact cell number but rather an approximation to allow us to compare the two conditions, the Abercrombie correction was suitable for this analysis. Numbers of SCCs from capsaicin and control rats were then analyzed with ANOVA. Lesioned and control mice were compared using a paired t-test analysis. Counts are reported as mean number of SCC profiles with nuclei per mm nasal epithelium ± SEM. Statistical analysis of the cell counts was carried out using Origin Pro 7.5 (Northampton, Ma).
Results
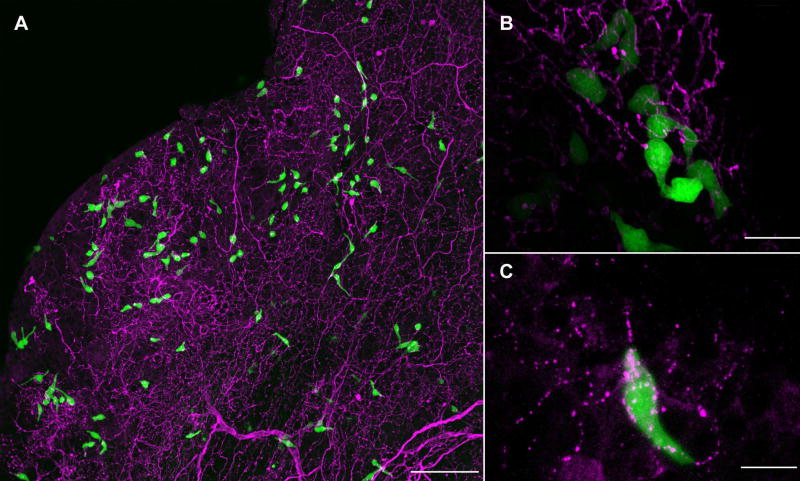
We find abundant scattered SCCs in the anterior respiratory epithelium of adult mice as described by Finger et al. (Finger et al., 2003) (Fig. 1A). While morphology varies from cell to cell, many adult SCCs display a slender, elongate or flask shaped profile. In agreement with previous studies (Finger et al., 2003; Lin et al 2007), we find that SCCs are heavily innervated by trigeminal nerve fibers and the bulk of that innervation is peptidergic (Fig 1.A–C).
Fig. 1.
A. SCCs in the nasal epithelium (identified by green GFP fluorescence in a wholemount of nasal tissue from a TRPM5-GFP mouse) receive dense trigeminal innervation (red PGP9.5-ir). Scale bar = 100 μm. B. Gustducin GFP SCCs are also densely innervated by PGP9.5-ir trigeminal nerve fibers. Scale bar = 50 μm. C. The bulk of the trigeminal innervation to SCCs is peptidergic as shown here with a SCC (green GFP fluorescence) densely innervated by CGRP-ir nerve fibers (red). Scale bar = 10 μm.
Neurogenin-1 knockouts
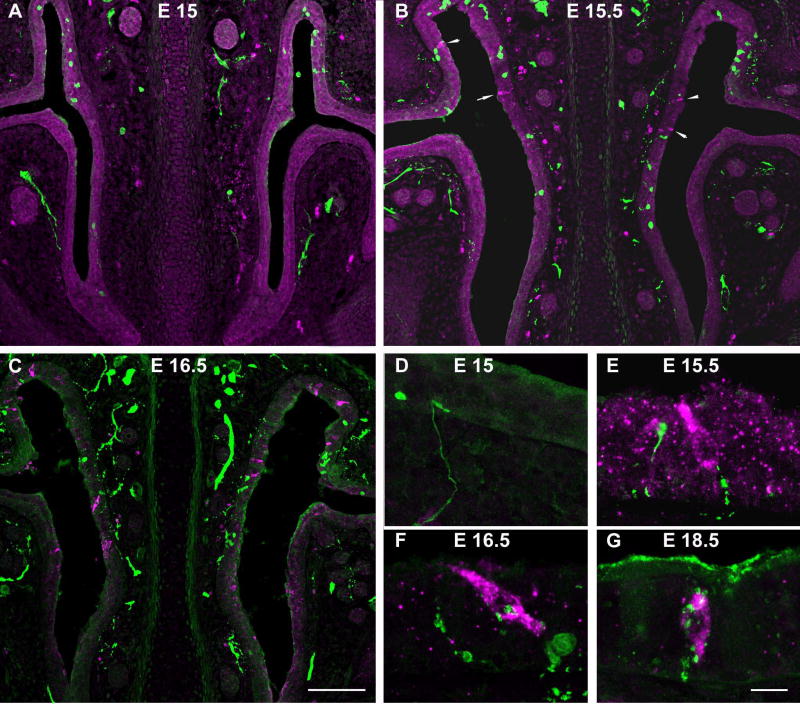
Since neurogenin-1−/− mice die shortly after birth, it was first necessary to determine when SCCs first appear in the nasal epithelium. We took embryos from timed pregnancy wildtype mice from E14.5 to E18.5 and processed the tissue for dual-label immunocytochemistry with α-gustducin to mark SCCs and protein gene product 9.5 (PGP9.5, ubiquitin carboxyl-terminal hydrolase) to identify nerve fibers. We used α-gustducin immunoreactivity as a marker for mature SCCs since this antibody reliably marks the cytoplasm of SCCs and α-gustducin is an integral component of the signal transduction cascade in mature cells. No gustducin immunoreactive (ir) SCCs were present in E14.5 or E15 embryos (Fig. 2A). Although PGP9.5-ir growth cones were observed at E14, individual fibers were not seen penetrating into the nasal epithelium until E15 (Fig. 2D). Gustducin-ir SCCs first appeared in the nasal epithelium at E15.5 and appeared to be morphologically similar to adult cells with a bipolar elongate or flask shape (Fig. 2E). At this stage, PGP9.5-ir nerve fibers innervated the nasal epithelium and occasional SCCs (Fig. 2E). One day later at E16.5, SCCs were more abundant and innervation to the epithelium was more extense (Fig. 2C and F). At E18.5, gustducin-ir SCCs were abundant and were frequently contacted by PGP9.5-ir nerve fibers (Fig. 2G).
Fig. 2.
Embryonic development of SCCs in the mouse nasal epithelium. A. At E15, no gustducin-ir SCCs (red) are present in the nasal epithelium. PGP9.5-ir growth cones (green) can be seen approaching the epithelium and some fibers start to penetrate into the epithelium (D) at this stage. B. Gustducin-ir SCCs (red) are first found in the epithelium at E15.5 (arrows). PGP9.5-ir fibers (green) are found near or contacting (E) some SCCs. C. By E16.5, there are many more SCCs and innervation to the epithelium is much more extense. Many of these cells are contacted by nerve fivers (F) and by E18.5 (G), innervation to SCCs is dense. 100 μm scale bar in C applies to A–C and 10 μm scale bar in G applies to D–G.
Having determined that SCCs are present prior at birth in wildtype animals, we were then able to assess if SCCs are affected by the absence of trigeminal innervation in neurogenin-1−/− animals that lack a trigeminal ganglion. Neurogenin-1 pups were taken at birth and genotyped by PCR. Knockout animals and wild type littermates were then processed for α-gustducin immunocytochemistry to determine if the embryological absence of trigeminal innervation affected SCC formation. In both wild type and neurogenin-1−/− pups, scattered α-gustducin-ir SCCs were present in the anterior respiratory nasal epithelium (Fig. 3). SCCs in knockout pups (n = 4) (Fig. 3D–F) did not differ morphologically or in their distribution in the nasal cavity from wild type littermates (n = 3) (Fig. 3A–C). These findings show that SCC formation is not induced by trigeminal fibers in the epithelium during development.
Fig. 3.

SCC differentiation is not induced by trigeminal nerve fibers. A–C In P0 wildtype littermates (Wt), gustducin-ir SCCs are innervated by PGP9.5-ir trigeminal nerve fibers (green). D–F While the nasal epithelium of neurogenin 1−/−(NGN1−/−) mice develops in the absence of a trigeminal ganglion (note the lack of PGP9.5-ir nerve fibers innervating SCCs), gustducin-ir SCCs (red) are still present at birth. Scale bar = 10 μm.
Capsaicin desensitization
We then asked if elimination of peptidergic trigeminal innervation to SCCs early in life would affect SCC number when the animal reaches adulthood. Adult capsaicin desensitized rats (40 days old) lacked ethmoid nerve responses to chemicals as reported previously (Silver et al., 1991) (data not shown). Immunocytocytochemical analysis of adult rat tissue from control rats (n = 3) revealed numerous α-gustducin-ir SCC profiles and dense peptidergic (calcitonin gene related peptide (CGRP)-ir) innervation of the SCCs (Fig. 4A). In capsaicin desensitized animals (n = 3), abundant α-gustducin positive SCC profiles were still present, although there was a marked decrease in the amount of CGRP-ir innervation to the nasal epithelium (Fig. 4B). Cell profile counts from control (0.52 ± 0.11 SCCs/mm epithelium) and capsaicin desensitized (0.83 ± 0.17 SCCs/mm epithelium) animals show no significant difference (p > 0.05) in the number of SCC profiles following removal of peptidergic innervation.
Fig. 4.
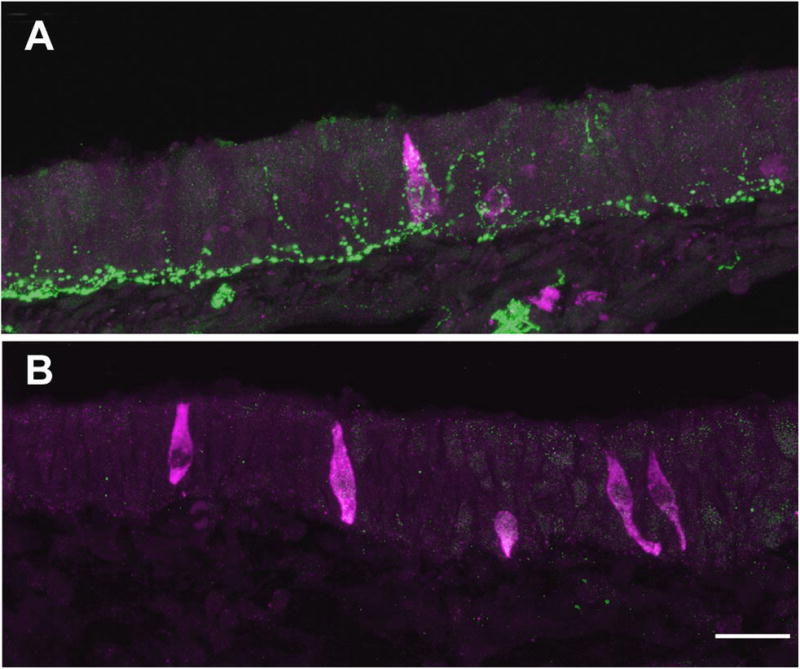
SCC survival is independent of peptidergic innervation. A. In control rats, gustducin-ir SCCs (red) are densely innervated by CGRP-ir (green) nerve fibers. B. When peptidergic innervation is removed by capsaicin desensitization, no CGRP-ir fibers are found in the nasal epithelium while gustducin positive SCCs remain. Scale bar = 20 μm.
Trigeminal denervation
Since capsaicin desensitization only removes the peptidergic component of trigeminal innervation to SCCs, we asked if removal of all trigeminal innervation to the nasal epithelium would affect SCC numbers. To eliminate trigeminal innervation to nasal SCCs, we stereotaxicly lesioned the right trigeminal ganglion. Tissue from these mice was immunocytochemically analyzed for the presence of SCCs (α-gustducin-ir) and nerve fibers (PGP9.5-ir). At short (5–6 days), medium (11–12 days), and long (18–22 days) time periods post-lesion, there was a marked decrease in the amount of PGP9.5-ir nerve fiber innervation to the nasal epithelium (green staining in Fig. 5). However, numerous SCC profiles were still present in the nasal epithelium in all cases (red staining in Fig. 5). Although the majority of SCCs on the lesioned side of the nasal cavity had no detectable remaining innervation, these cells were morphologically indistinguishable from SCCs on the control side of the nasal cavity (Fig. 6). Counts of SCC profiles from control and lesioned sides of the nasal cavity show no significant change in SCC number following lesion (see table 1).
Fig. 5.
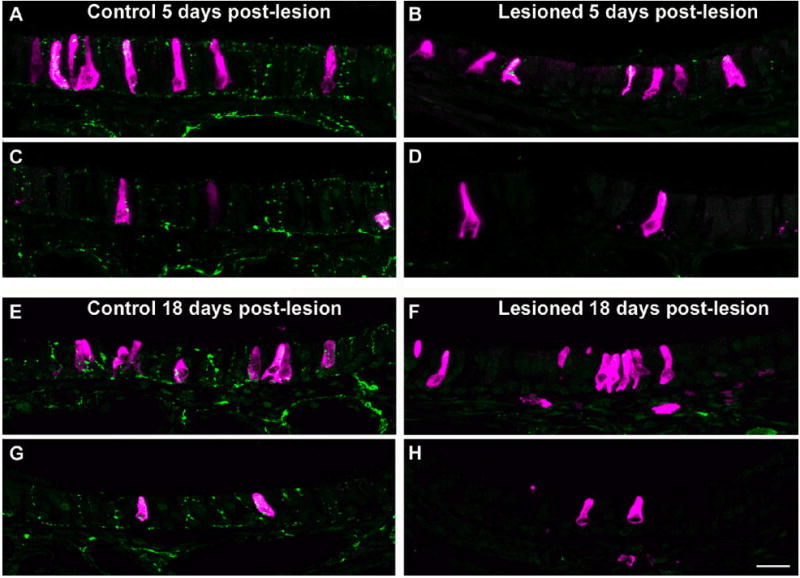
Mature SCC survival is independent of trigeminal nerve contact. Images A–D show the mouse nasal epithelium 5 days post-lesion. On the control side of the nasal cavity (A and C) there is extensive PGP9.5-ir innervation (green) to the epithelium and gustducin-ir SCCs (red). On the lesioned side (B and D), most of the innervation to the epithelium has been destroyed while SCCs are not affected. At this early time point, some remnant innervation to SCCs is still present as shown in B. At 18 days post-lesion (E–H), SCCs on the lesioned side of the nasal cavity appear completely denervated (F and H). Most SCCs on the control side of the nasal cavity (E and G) are contacted by PGP9.5-ir fibers while SCCs on the lesioned side (F and H) lack nerve contacts. Images of SCCs are from corresponding locations on left and right sides of the nasal cavity of the same section. A, B, E, and F show SCCs on the dorsal septum. C, D, G, and H show SCCs from the dorsal turbinate. Scale bar = 20 μm.
Fig. 6.
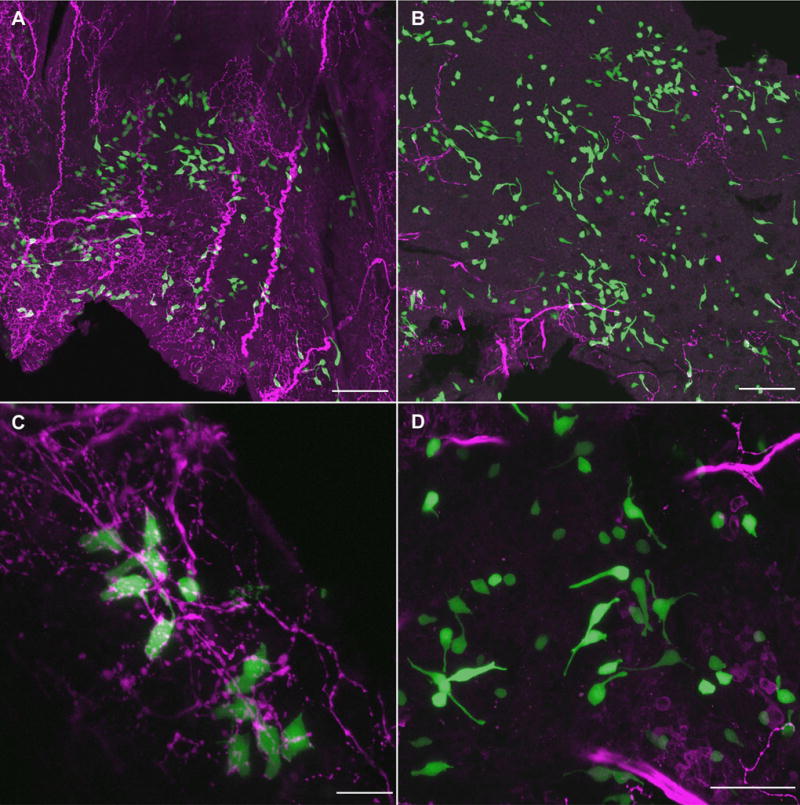
Mature TRPM5-GFP+ SCCs also do not depend on intact innervation. In wholemounts of nasal tissue from TRPM5-GFP mice at 18 days post-lesion, many SCCs (green) can be found on both control (A) and lesioned (B) sides of the nasal cavity. Note the greatly decreased amount of PGP9.5-ir innervation (red) to the lesioned side of the nasal cavity. SCCs on the control side of the nasal cavity have obvious contacts with PGP9.5-ir nerve fibers (C) while most SCCs on the lesioned side have no remaining nerve support (D). Scale bars in A and B = 100 μm, C = 20 μm, and D = 50 μm.
Table 1.
Counts of SCCs/mm are not statistically different between control and lesioned sides of the nasal cavity (paired t test p>0.05).
| Days post-lesion | Mean # SCCs/mm nasal epithelium ± SEM | |
|---|---|---|
| Left (control) | Right (lesioned) | |
| 5–6 (n = 3) | 0.60 ± 0.21 | 0.46 ± 0.20 |
| 10–12 (n = 3) | 0.94 ± 0.22 | 1.0 ± 0.56 |
| 18–22 (n = 4) | 1.2 ± 0.41 | 1.6 ± 0.93 |
Discussion
Nasal SCCs are a population of specialized sensory cells in the nasal respiratory epithelium that are innervated by afferent trigeminal nerve fibers (Finger et al., 2003). In the present study, we aimed to determine if nasal SCCs depend on intact trigeminal innervation for induction during embryonic development or survival in adult animals. To this end, we eliminated trigeminal innervation to the nasal epithelium and SCCs during development using neurogenin-1 null mice, during early postnatal development with capsaicin desensitization, and during adulthood by electrolytically lesioning the trigeminal ganglion. Our results demonstrate that SCCs not only develop in the absence of trigeminal innervation, but also do not depend on intact trigeminal innervation in the mature system.
SCCs are morphologically similar to individual taste receptor cells and contain much of the same molecular signaling machinery which suggests they may share functional similarities (Finger, 1997; Finger et al., 2003). In support of this idea, we previously described that SCCs, like taste cells, are continually replaced throughout adult life (Gulbransen and Finger, 2005). However, while taste buds develop around the time of parturition (Mistretta, 1991), our current data shows that nasal SCCs are identifiable by α-gustducin immunoreactivity several days prior to birth. This agrees with previous studies that have described SCCs in the oral cavity prior to the development of taste buds (El-Sharaby et al., 2001). These early nasal SCCs seem to appear concomitantly or slightly after nerve fibers begin to innervate the nasal epithelium. While this might initially suggest that the nerve fibers are responsible for the differentiation of nasal SCCs, our data suggest otherwise. In neurogenin-1 null mice, which lack a trigeminal ganglion, the nasal epithelium develops without trigeminal nerve influence (Ma et al., 1998). We show that SCCs are still present in the nasal epithelium of neurogenin-1 null mice and express markers of differentiated cells such as α-gustducin. This suggests that SCC differentiation is not induced by nerve contact and occurs independently of trigeminal influence.
Taste cells, on the other hand, are known to require contact by nerve fibers for differentiation. Hosley et al. (1987a; 1987b) denervated rat tongues during a critical period of postnatal development and found that the majority of taste buds fail to develop. While the lingual epithelium is able to produce the papillae within which taste buds develop without neuronal contact, taste buds only elaborate in papillae after the arrival of sensory nerves (Farbman and Mbiene, 1991; Whitehead and Kachele, 1994).
Our results also demonstrate that mature SCC survival is autonomous of nerve contact. Capsaicin desensitization has proved an effective method to remove peptidergic trigeminal innervation to the nasal cavity (Silver, 1992; Silver et al., 1991; Silver et al., 1985). While adult capsaicin treatment only temporarily desensitizes the fibers, neonatal desensitization results in the destruction of sensitive fibers and ganglion cells (Jancso et al., 1967; Szolcsanyi et al., 1975; Silver et al., 1991). Neonatal desensitization of rats in the current study resulted in a loss of responsiveness to chemical stimuli in the ethmoid branch of the trigeminal nerve in adulthood. We find that this treatment also resulted in the loss of peptidergic fibers in the nasal epithelium. While nasal SCCs in rats are known to be innervated by peptidergic fibers (Finger et al., 2003), we find that SCCs do not depend on this peptidergic innervation for survival.
An alternative explanation for this finding is that SCCs are innervated by another, nonpeptidergic population of trigeminal fibers that is not destroyed by capsaicin desensitization. PGP9.5 staining, which labels all nerve fibers, showed that in capsaicin desensitized rats, SCCs still had remnant innervation which was not peptidergic. To investigate the possibility that nonpeptidergic nerve fibers are able to sustain SCCs, we eliminated all innervation to SCCs in mice using trigeminal lesioning. Trigeminal lesioning does not discriminate between peptidergic and nonpeptidergic fibers but rather destroys all trigeminal innervation to the anterior nasal cavity. We placed our lesions in the ophthalmic branch of the trigeminal ganglion which is an area known to hold the cell bodies of chemosensitive fibers in the mouse anterior nasal epithelium (Damann et al., 2006). Lesioning this region of the trigeminal ganglion was effective at removing innervation to SCCs as evidenced by the loss of PGP9.5-ir fibers surrounding SCCs. Despite the loss of all innervation, SCCs seem to be relatively unaffected.
This is in contrast to similar populations of innervated epithelial sensory cells such as taste cells (el-Eishi and State, 1974; Farbman, 1969; State and Bowden, 1974; Torrey, 1934; Torrey, 1946; Zalewski, 1981) which degenerate following a lesion of their trophic nerve support. However, Pacinian corpuscles (Zelena, 1982), Merkel cells (English, 1977), muscle spindle receptors (Zelena, 1964), and hair cells (Ma et al., 2000) atrophy but remain identifiable following denervation. There is also evidence that taste buds vary in their dependence of innervation depending on the area of the tongue in which they reside. Whitehead et al. (1987) found that fungiform papillae persist for months following denervation while taste buds in denervated vallate papillae disappear within about a week (Yee et al., 2005). Similarly, (Guagliardo and Hill, 2007) show that almost all posterior fungiform taste buds disappear following nerve transaction while fungiform taste papillae on the anterior tip of the tongue retained nearly half of the regular number of taste buds. Our evidence suggests that SCCs are more similar to the latter group of epithelial receptor cells and persist following denervation.
In conclusion, we find that SCC induction and maintenance is not dependent on innervation by trigeminal fibers. Our neurogenin1−/− results show that SCCs do not require innervation for embryonic development and hence are not induced by nerve fibers. Capsaicin desensitization and trigeminal lesioning data demonstrates that SCCs do not require innervation in the adult system for normal differentiation.
Acknowledgments
Supported by NIDCD Grants NRSA DC008275-01, RO1 DC 006070 and P30 DC 04657
References
- Barber PC, Raisman G. Replacement of receptor neurones after section of the vomeronasal nerves in the adult mouse. Brain Res. 1978;147:297–313. doi: 10.1016/0006-8993(78)90841-7. [DOI] [PubMed] [Google Scholar]
- Bartel DL, Sullivan SL, Lavoie EG, Sevigny J, Finger TE. Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. J Comp Neurol. 2006;497:1–12. doi: 10.1002/cne.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezencon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses. 2006 doi: 10.1093/chemse/bjl034. [DOI] [PubMed] [Google Scholar]
- Damann N, Rothermel M, Klupp BG, Mettenleiter TC, Hatt H, Wetzel CH. Chemosensory properties of murine nasal and cutaneous trigeminal neurons identified by viral tracing. BMC Neurosci. 2006;7:46. doi: 10.1186/1471-2202-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Eishi HI, State FA. The role of the nerve in the formation and maintenance of taste buds. Acta Anat (Basel) 1974;89:599–609. doi: 10.1159/000144318. [DOI] [PubMed] [Google Scholar]
- El-Sharaby A, Ueda K, Wakisaka S. Differentiation of the lingual and palatal gustatory epithelium of the rat as revealed by immunohistochemistry of alpha-gustducin. Arch Histol Cytol. 2001;64:401–409. doi: 10.1679/aohc.64.401. [DOI] [PubMed] [Google Scholar]
- English KB. The ultrastructure of cutaneous type I mechanoreceptors (Haarscheiben) in cats following denervation. J Comp Neurol. 1977;172:137–163. doi: 10.1002/cne.901720107. [DOI] [PubMed] [Google Scholar]
- Farbman AI. Fine structure of degenerating tast buds after denervation. J Embryol Exp Morphol. 1969;22:55–68. [PubMed] [Google Scholar]
- Farbman AI, Mbiene JP. Early development and innervation of taste bud-bearing papillae on the rat tongue. J Comp Neurol. 1991;304:172–186. doi: 10.1002/cne.903040203. [DOI] [PubMed] [Google Scholar]
- Finger TE. Evolution of taste and solitary chemoreceptor cell systems. Brain Behav Evol. 1997;50:234–243. doi: 10.1159/000113337. [DOI] [PubMed] [Google Scholar]
- Finger TE, Bottger B, Hansen A, Anderson KT, Alimohammadi H, Silver WL. Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc Natl Acad Sci U S A. 2003;100:8981–8986. doi: 10.1073/pnas.1531172100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guagliardo NA, Hill DL. Fungiform taste bud degeneration in C57BL/6J mice following chorda-lingual nerve transection. J Comp Neurol. 2007;504:206–216. doi: 10.1002/cne.21436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbransen BD, Finger TE. Solitary chemoreceptor cell proliferation in adult nasal epithelium. J Neurocytol. 2005;34:117–122. doi: 10.1007/s11068-005-5051-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding J, Graziadei PP, Monti Graziadei GA, Margolis FL. Denervation in the primary olfactory pathway of mice. IV. Biochemical and morphological evidence for neuronal replacement following nerve section. Brain Res. 1977;132:11–28. doi: 10.1016/0006-8993(77)90703-x. [DOI] [PubMed] [Google Scholar]
- Hofer D, Puschel B, Drenckhahn D. Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:6631–6634. doi: 10.1073/pnas.93.13.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosley MA, Hughes SE, Morton LL, Oakley B. A sensitive period for the neural induction of taste buds. J Neurosci. 1987a;7:2075–2080. doi: 10.1523/JNEUROSCI.07-07-02075.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosley MA, Hughes SE, Oakley B. Neural induction of taste buds. J Comp Neurol. 1987b;260:224–232. doi: 10.1002/cne.902600206. [DOI] [PubMed] [Google Scholar]
- Jancso N, Jancso-Gabor A, Szolcsanyi J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br J Pharmacol Chemother. 1967;31:138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaske S, Krasteva G, Konig P, Kummer W, Hofmann T, Gudermann T, Chubanov V. TRPM5, a taste-signaling transient receptor potential ion-channel, is a ubiquitous signaling component in chemosensory cells. BMC Neurosci. 2007;8:49. doi: 10.1186/1471-2202-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Anderson DJ, Fritzsch B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J Assoc Res Otolaryngol. 2000;1:129–143. doi: 10.1007/s101620010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Mistretta C. In: Developmental neurobiology of the taste system. TV G, editor. New York: Raven Press; 1991. pp. 35–64. [Google Scholar]
- Sbarbati A, Merigo F, Benati D, Tizzano M, Bernardi P, Osculati F. Laryngeal chemosensory clusters. Chemical Senses. 2004;29:683–692. doi: 10.1093/chemse/bjh071. [DOI] [PubMed] [Google Scholar]
- Sbarbati A, Osculati F. The taste cell-related diffuse chemosensory system. Progress in Neurobiology. 2005;75:295–307. doi: 10.1016/j.pneurobio.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Silver WL. Neural and pharmacological basis for nasal irritation. Ann N Y Acad Sci. 1992;641:152–163. doi: 10.1111/j.1749-6632.1992.tb16540.x. [DOI] [PubMed] [Google Scholar]
- Silver WL, Farley LG, Finger TE. The effects of neonatal capsaicin administration on trigeminal nerve chemoreceptors in the rat nasal cavity. Brain Res. 1991;561:212–216. doi: 10.1016/0006-8993(91)91597-t. [DOI] [PubMed] [Google Scholar]
- Silver WL, Mason JR, Marshall DA, Maruniak JA. Rat trigeminal, olfactory and taste responses after capsaicin desensitization. Brain Res. 1985;333:45–54. doi: 10.1016/0006-8993(85)90122-2. [DOI] [PubMed] [Google Scholar]
- State FA, Bowden RE. The effect of transection of the glossopharyngeal nerve upon the structure, cholinesterase activity and innervation of taste buds in rabbits. J Anat. 1974;118(Pt 1):77–100. [PMC free article] [PubMed] [Google Scholar]
- Szolcsanyi J, Jancso-Gabor A, Joo F. Functional and fine structural characteristics of the sensory neuron blocking effect of capsaicin. Naunyn Schmiedebergs Arch Pharmacol. 1975;287:157–169. doi: 10.1007/BF00510447. [DOI] [PubMed] [Google Scholar]
- Tizzano M, Merigo F, Sbarbati A. Evidence of solitary chemosensory cells in a large mammal: the diffuse chemosensory system in Bos taurus airways. J Anat. 2006;209:333–7. doi: 10.1111/j.1469-7580.2006.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey TW. The relation of taste-buds to their nerve fibers. J Comp Neurol. 1934;59:203–220. [Google Scholar]
- Torrey TW. The influence of nerve fibers on taste-buds during embryonic development. Proc nat Acad Sci. 1946;26:627–634. doi: 10.1073/pnas.26.11.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead MC, Frank ME, Hettinger TP, Hou LT, Nah HD. Persistence of taste buds in denervated fungiform papillae. Brain Res. 1987;405:192–195. doi: 10.1016/0006-8993(87)91008-0. [DOI] [PubMed] [Google Scholar]
- Whitehead MC, Kachele DL. Development of fungiform papillae, taste buds, and their innervation in the hamster. J Comp Neurol. 1994;340:515–530. doi: 10.1002/cne.903400405. [DOI] [PubMed] [Google Scholar]
- Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:2392–2397. doi: 10.1073/pnas.042617699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee C, Bartel DL, Finger TE. Effects of glossopharyngeal nerve section on the expression of neurotrophins and their receptors in lingual taste buds of adult mice. J Comp Neurol. 2005;490:371–390. doi: 10.1002/cne.20670. [DOI] [PubMed] [Google Scholar]
- Zalewski AA. Regeneration of taste buds after reinnervation of a denervated tongue papilla by a normally nongustatory nerve. J Comp Neurol. 1981;200:309–314. doi: 10.1002/cne.902000302. [DOI] [PubMed] [Google Scholar]
- Zelena J. Development, Degeneration And Regeneration Of Receptor Organs. Prog Brain Res. 1964;13:175–213. [PubMed] [Google Scholar]
- Zelena J. Survival of Pacinian corpuscles after denervation in adult rats. Cell Tissue Res. 1982;224:673–683. doi: 10.1007/BF00213762. [DOI] [PubMed] [Google Scholar]