Abstract
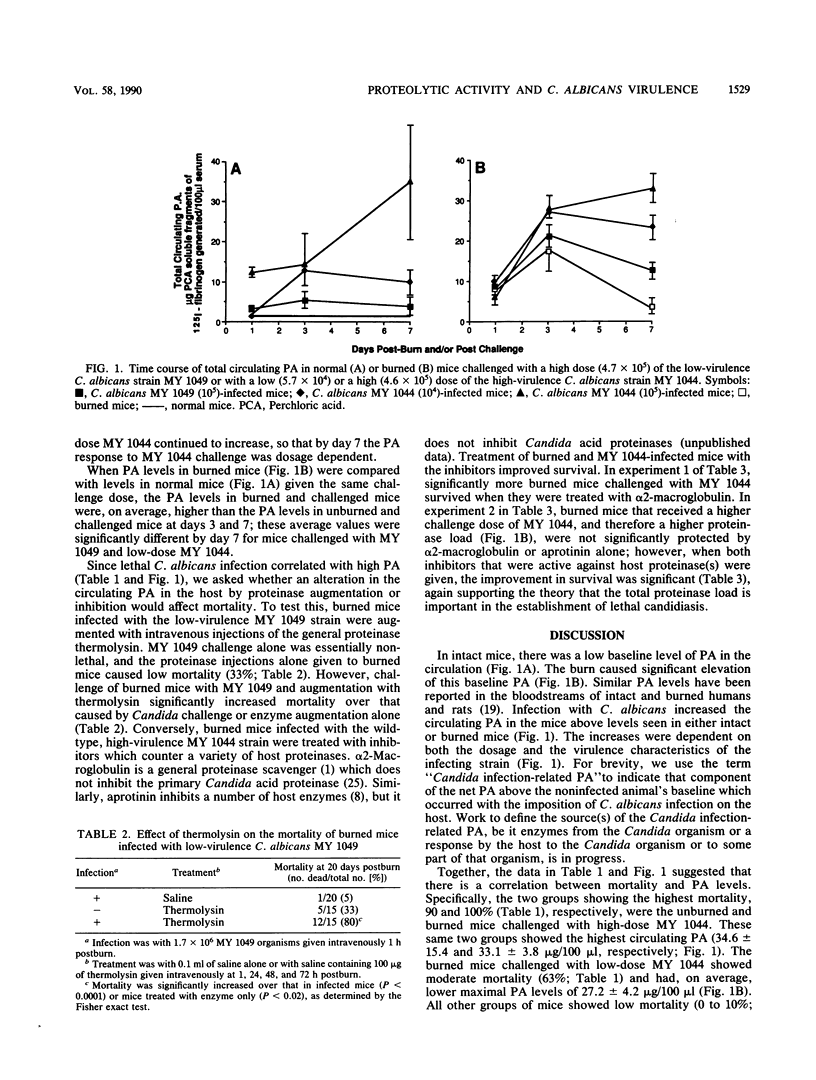
Total circulating proteolytic activity (PA) was determined by measuring the acid-soluble 125I-protein fragments generated per 100 microliters of serum incubated with 125I-protein at 37 degrees C for 15 min. Normal mice had low circulating PA (1.3 +/- 0.2 micrograms/100 microliters), and burned mice had a higher average PA; the actual value depended on the time of measurement postburn. We measured the effect on mortality and on circulating PA of challenging normal and burned mice with high-virulence strain Candida albicans MY 1044 and its less virulent mutant MY 1049. Burned and normal mice challenged with a high dose (10(5)) of MY 1044 had high mortality (greater than 90%) and high circulating PA (greater than 33 micrograms generated per 100 microliters). Burned mice challenged with a lower dose (10(4] of MY 1044 had moderate mortality (63%) and lower PA (27.2 +/- 4.2 micrograms/100 microliters). All other groups of mice, including burned mice challenged with 10(5) MY 1049, had low mortality (less than 10%), and PAs were less than 22 micrograms/100 microliters. Augmentation of burned mice challenged with 10(5) MY 1049 with proteinase significantly increased mortality; with treatment of burned mice challenged with 10(5) MY 1044 with proteinase inhibitor significantly decreased mortality. We conclude that mortality correlated with total circulating PA; that the contribution to this net PA was the background PA level in the normal mice, the PA associated with the burn, and the PA caused by infection with a C. albicans strain with a particular virulence; that most deaths caused by C. albicans occurred past a PA threshold of 25 micrograms/100 microliters in the host; and that the number of burned and infected mice that died of candidiasis could be modulated by the addition of proteinases or proteinase inhibitors to the host. This last finding may lead to some novel treatments for candidiasis in burned hosts.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J. Alpha 2-macroglobulin. Methods Enzymol. 1981;80(Pt 100):737–754. doi: 10.1016/s0076-6879(81)80056-0. [DOI] [PubMed] [Google Scholar]
- Bjornson A. B., Bjornson H. S. Theoretical interrelationships among immunologic and hematologic sequelae of thermal injury. Rev Infect Dis. 1984 Sep-Oct;6(5):704–714. doi: 10.1093/clinids/6.5.704. [DOI] [PubMed] [Google Scholar]
- Cassone A., De Bernardis F., Mondello F., Ceddia T., Agatensi L. Evidence for a correlation between proteinase secretion and vulvovaginal candidosis. J Infect Dis. 1987 Nov;156(5):777–783. doi: 10.1093/infdis/156.5.777. [DOI] [PubMed] [Google Scholar]
- Chattaway F. W., Odds F. C., Barlow A. J. An examination of the production of hydrolytic enzymes and toxins by pathogenic strains of Candida albicans. J Gen Microbiol. 1971 Aug;67(3):255–263. doi: 10.1099/00221287-67-3-255. [DOI] [PubMed] [Google Scholar]
- Edison A. M., Manning-Zweerink M. Comparison of the extracellular proteinase activity produced by a low-virulence mutant of Candida albicans and its wild-type parent. Infect Immun. 1988 May;56(5):1388–1390. doi: 10.1128/iai.56.5.1388-1390.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromtling R. A., Abruzzo G. K., Edison A., Manning-Zweerink M. Renal pathology and spleen cell chemiluminescence of mice infected with a wild-type and a low-virulence mutant of Candida albicans. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 May;268(3):405–415. doi: 10.1016/s0176-6724(88)80025-7. [DOI] [PubMed] [Google Scholar]
- Haberland G., McConn R. A rationale for the therapeutic action of aprotinin. Fed Proc. 1979 Dec;38(13):2760–2767. [PubMed] [Google Scholar]
- Highsmith R. F., Rosenberg R. D. A rapid and sensitive proteolytic assay for human plasminogen and plasmin using radioiodinated alpha-casein. Thromb Res. 1977 Aug;11(2):131–140. doi: 10.1016/0049-3848(77)90031-7. [DOI] [PubMed] [Google Scholar]
- Kaminishi H., Hagihara Y., Hayashi S., Cho T. Isolation and characteristics of collagenolytic enzyme produced by Candida albicans. Infect Immun. 1986 Aug;53(2):312–316. doi: 10.1128/iai.53.2.312-316.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komshian S. V., Uwaydah A. K., Sobel J. D., Crane L. R. Fungemia caused by Candida species and Torulopsis glabrata in the hospitalized patient: frequency, characteristics, and evaluation of factors influencing outcome. Rev Infect Dis. 1989 May-Jun;11(3):379–390. doi: 10.1093/clinids/11.3.379. [DOI] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Lehman D., Good C., Magee P. T. Genetic evidence for role of extracellular proteinase in virulence of Candida albicans. Infect Immun. 1985 Sep;49(3):571–575. doi: 10.1128/iai.49.3.571-575.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law E. J., Kim O. J., Stieritz D. D., MacMillan B. G. Experience with systemic candidiasis in the burned patient. J Trauma. 1972 Jul;12(7):543–552. doi: 10.1097/00005373-197207000-00001. [DOI] [PubMed] [Google Scholar]
- Law E. J. Patterns of infection over the past ten years. Mortality patterns. J Burn Care Rehabil. 1987 Jan-Feb;8(1):53–55. [PubMed] [Google Scholar]
- Macdonald F. Secretion of inducible proteinase by pathogenic Candida species. Sabouraudia. 1984;22(1):79–82. [PubMed] [Google Scholar]
- Manning M., Snoddy C. B., Fromtling R. A. Comparative pathogenicity of auxotrophic mutants of Candida albicans. Can J Microbiol. 1984 Jan;30(1):31–35. doi: 10.1139/m84-005. [DOI] [PubMed] [Google Scholar]
- Munster A. M., Hoagland H. C., Pruitt B. A., Jr The effect of thermal injury on serum immunoglobulins. Ann Surg. 1970 Dec;172(6):965–969. doi: 10.1097/00000658-197012000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely A. N., Law E. J., Holder I. A. Increased susceptibility to lethal Candida infections in burned mice preinfected with Pseudomonas aeruginosa or pretreated with proteolytic enzymes. Infect Immun. 1986 Apr;52(1):200–204. doi: 10.1128/iai.52.1.200-204.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely A. N., Nathan P., Highsmith R. F. Plasma proteolytic activity following burns. J Trauma. 1988 Mar;28(3):362–367. doi: 10.1097/00005373-198803000-00012. [DOI] [PubMed] [Google Scholar]
- Neely A. N., Odds F. C., Basatia B. K., Holder I. A. Characterization of Candida isolates from pediatric burn patients. J Clin Microbiol. 1988 Sep;26(9):1645–1649. doi: 10.1128/jcm.26.9.1645-1649.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensler J. M., Herndon D. N., Ptak H., Bonds E., Rutan T. C., Desai M. H., Abston S. Fungal sepsis: an increasing problem in major thermal injuries. J Burn Care Rehabil. 1986 Nov-Dec;7(6):488–491. [PubMed] [Google Scholar]
- Rüchel R. A variety of Candida proteinases and their possible targets of proteolytic attack in the host. Zentralbl Bakteriol Mikrobiol Hyg A. 1984 Jul;257(2):266–274. [PubMed] [Google Scholar]
- Rüchel R., Böning B., Jahn E. Identification and partial characterization of two proteinases from the cell envelope of Candida albicans blastospores. Zentralbl Bakteriol Mikrobiol Hyg A. 1985 Dec;260(4):523–538. doi: 10.1016/s0176-6724(85)80068-7. [DOI] [PubMed] [Google Scholar]
- Rüchel R. On the renin-like activity of Candida proteinases and activation of blood coagulation in vitro. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 Sep;255(2-3):368–379. [PubMed] [Google Scholar]
- Rüchel R. On the role of proteinases from Candida albicans in the pathogenesis of acronecrosis. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 Nov;255(4):524–536. [PubMed] [Google Scholar]
- Rüchel R., Uhlemann K., Böning B. Secretion of acid proteinases by different species of the genus Candida. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 Nov;255(4):537–548. [PubMed] [Google Scholar]
- Saltarelli C. G., Gentile K. A., Mancuso S. C. Lethality of Candida strains as influenced by the host. Can J Microbiol. 1975 May;21(5):648–654. doi: 10.1139/m75-093. [DOI] [PubMed] [Google Scholar]
- Schreiber B., Lyman C. A., Gurevich J., Needham C. A. Proteolytic activity of Candida albicans and other yeasts. Diagn Microbiol Infect Dis. 1985 Jan;3(1):1–5. doi: 10.1016/0732-8893(85)90060-4. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Kondoh Y., Tanaka K. Proteinase production and pathogenicity of Candida albicans. I. Invasion into chorioallantoic membrane by C. albicans strains of different proteinase activity. Microbiol Immunol. 1987;31(11):1045–1060. doi: 10.1111/j.1348-0421.1987.tb01337.x. [DOI] [PubMed] [Google Scholar]
- Spebar M. J., Pruitt B. A., Jr Candidiasis in the burned patient. J Trauma. 1981 Mar;21(3):237–239. doi: 10.1097/00005373-198103000-00007. [DOI] [PubMed] [Google Scholar]
- Stein D. K., Sugar A. M. Fungal infections in the immunocompromised host. Diagn Microbiol Infect Dis. 1989 Jul-Aug;12(4 Suppl):221S–228S. doi: 10.1016/0732-8893(89)90140-5. [DOI] [PubMed] [Google Scholar]
- Stieritz D. D., Holder I. A. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: description of a burned mouse model. J Infect Dis. 1975 Jun;131(6):688–691. doi: 10.1093/infdis/131.6.688. [DOI] [PubMed] [Google Scholar]
- Sundsmo J. S., Fair D. S. Relationships among the complement, kinin, coagulation, and fibrinolytic systems. Springer Semin Immunopathol. 1983;6(2-3):231–258. doi: 10.1007/BF00205875. [DOI] [PubMed] [Google Scholar]



