Abstract
Members of the order Coleoptera are sometimes referred to as ‘living jewels’, in allusion to the strikingly diverse array of iridescence mechanisms and optical effects that have arisen in beetles. A number of novel and sophisticated reflectance mechanisms have been discovered in recent years, including three-dimensional photonic crystals and quasi-ordered coherent scattering arrays. However, the literature on beetle structural coloration is often redundant and lacks synthesis, with little interchange between the entomological and optical research communities. Here, an overview is provided for all iridescence mechanisms observed in Coleoptera. Types of iridescence are illustrated and classified into three mechanistic groups: multilayer reflectors, three-dimensional photonic crystals and diffraction gratings. Taxonomic and phylogenetic distributions are provided, along with discussion of the putative functions and evolutionary pathways by which iridescence has repeatedly arisen in beetles.
Keywords: iridescence, structural colors, Coleoptera, multilayer reflectors, diffraction gratings, photonics
1. Introduction
Optical mechanisms in the natural world have fascinated researchers since the science of optics began; Newton, Young, Michelson and Rayleigh all used their increased understanding of light rays to explain iridescence in the feathers of birds and the scales of butterflies and moths (Rayleigh 1930; Greenewalt et al. 1960; Kinoshita et al. 2008). Butterflies have long been the focus of iridescence research and industrial biomimicry, probably due to their conspicuous structural colours, large body size and diurnal activity. However, an equally important array of optical mechanisms exists in another group of insects, the enormously diverse order Coleoptera (figure 1). Beetles comprise an estimated 5–30 million species, over 350 000 of which have been formally described (Erwin 1982; Lawrence & Britton 1994). In addition to the striking iridescence, beetles have also evolved facultative luminescence, ultraviolet signals, polarized reflectance and complex photonic crystals analogous to the fibre-optic technology used to deliver high-speed Internet connections (Parker 1998; Galusha et al. 2008). Although iridescent beetles have long been collected and used in human ornamentation, we still do not know what purpose iridescence may serve to the beetles themselves. This question cannot be approached without a coherent grasp of both iridescence (as an optical phenomenon) and beetles (as organisms within an evolutionary context).
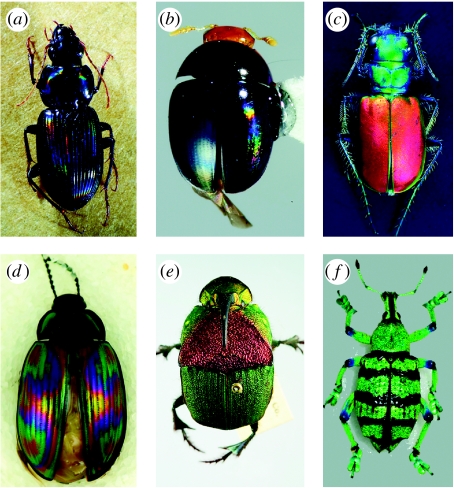
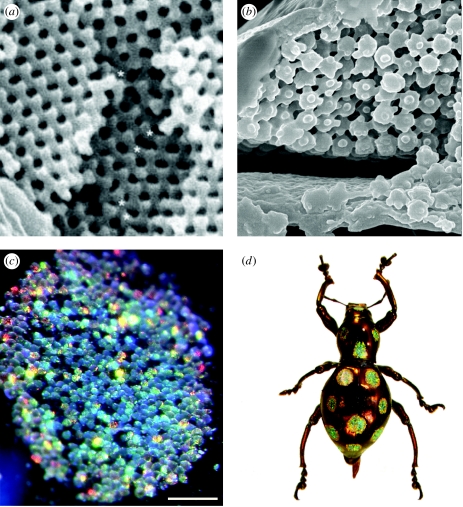
Figure 1.
Examples of beetle iridescence. (a) Loxandrus rectus (Carabidae: Harpalinae), (b) Phalacridae gen. sp., (c) Cicindela scutellaris scutellaris (Carabidae: Cicindelinae), (d) Amarygminae gen. sp. (Tenebrionidae), (e) Phanaeus vindex (Scarabaeidae: Phanainae), (f) Eupholus sp. (Curculionidae: Entiminae).
In recent years, researchers in both the physics and biology fields have discovered and characterized wholly novel iridescence mechanisms in insects (e.g. Parker et al. 2003; Prum et al. 2006). Unfortunately, these disciplines have a bimodal distribution of interests and rarely intersect: publications on beetle iridescence have appeared either in entomological literature without optical context (e.g. Pope & Hinton 1977; Robertson et al. 2004; Doberski & Walmesley 2007) or in the physics literature without a strong organismal context (e.g. Parker 2000, 2002; Goldstein 2005; Parker & Martini 2006; Jewell et al. 2007b; Vukusic et al. 2007; Kinoshita et al. 2008; Liu et al. 2008). The optical literature on beetle iridescence displays a lack of synthesis, with repeated ‘discovery’ or description of virtually identical structures, misidentification of study organisms, outdated nomenclature and occasional misunderstandings of insect anatomy (e.g. Hariyama et al. 2002; Parker et al. 2003; De Silva et al. 2005; Hegedüs et al. 2006b; Jewell et al. 2007a,b; Liu et al. 2008).
The entomological literature on iridescence suffers from both misunderstandings of optical mechanisms and a severe terminological confusion. Although methods and terms have been proposed for accurate diagnosis of pigment colours (Paclt 1983; Aguiar 2005), there is no consistent vocabulary for structural colours; ‘iridescence’ is used to describe a number of dissimilar phenomena, including metallic colours, spectral iridescence and opal-like effects. (As a point of reference, the standard entomological vocabulary contains approx. 30 terms for various shades of brown, but no way to distinguish more than one type of iridescence (Smith 1909; Torre-Bueno 1989)). This long-standing confusion can be extremely detrimental when the word iridescence is used to connote a diagnostic character in taxonomic works or treated as a single homologous character state in cladistic analyses (e.g. Robertson et al. 2004).
We provide a much-needed comprehensive review of known iridescence mechanisms in Coleoptera, synthesizing the entomological and optical perspectives. Our aims are threefold: (i) to provide a comprehensive overview of beetle iridescence mechanisms, (ii) to provide taxonomic, phylogenetic and functional context to these optical phenomena, and (iii) to create a robust, accurate and accessible vocabulary with which to diagnose and discuss iridescence in beetles and other insects.
1.1 Conventions
We adopt a definition of iridescence based on that of Mason (1927): ‘iridescence has for its main characteristic a change in the hue of the object exhibiting it as the angle of vision is varied’. Although the ‘entomological’ definition of iridescence implies strictly spectral (rainbow-like) reflectance (Torre-Bueno 1989), the term has been so widely and variously applied that a broad interpretation is preferable in order to avoid confusion. We have designated three main classes of iridescence mechanism observable in beetles (multilayer reflectors, three-dimensional photonic crystals and diffraction gratings), and propose that these terms be considered in future descriptive and experimental work.
Taxonomic groups are discussed in the narrowest sense applicable: we attempt to list the known distribution of structural colour mechanisms as thoroughly as possible, but (space being limited) do not list every species known or observed to possess a particular mechanism. Names and classification used for weevils (Curculionidae) follow Oberprieler et al. (2007); names for ruteline scarabs follow Hawks (2001).
We recognize that some of the anatomical terms used here may not be familiar to readers without an entomological background; however, we believe that incorporating the correct vocabulary is necessary to improve communication throughout a disparate literature. Thus, we present a brief review of relevant structures and terminology in appendix B.
2. Multilayer reflectors
2.1 Mechanism
Multilayer reflectors are without question the most common and the best understood iridescence mechanism in beetles (Parker et al. 1998; Noyes et al. 2007; Kinoshita et al. 2008). During the formation of insect integument, thin parallel layers of chitin (sometimes interspersed with other materials) that differ in refractive index are secreted by the epidermis and later harden during sclerotization. If the spacing of these layers approaches one-quarter the wavelength of visible light (approx. 380–750 nm), one or more colours will be produced by constructive interference (Land 1972; figures 1c–e, 2–6).
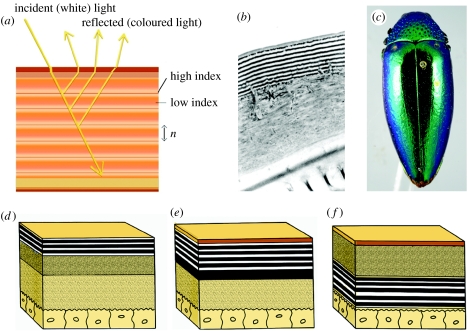
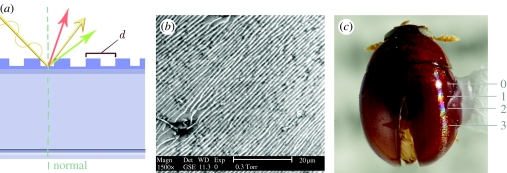
Figure 2.
Multilayer reflectors. (a) A schematic of simple cuticular multilayer reflector, (b) Cicindela scutellaris, TEM cross section of cuticular reflector, (c) simple multilayer colour in a buprestid; schematic of (d) epicuticular reflector, (e) exocuticular reflector and (f) endocuticular reflector.
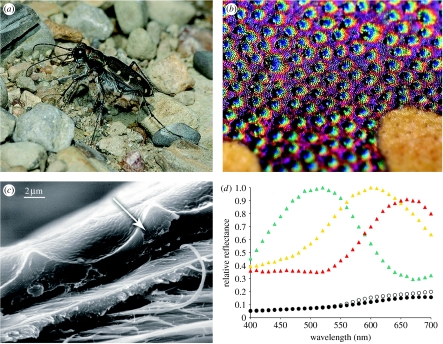
Figure 3.
Additive coloration systems in Cicindelinae. (a) Cicindela repanda, habitus view, (b) Cicindela repanda elytral surface with punctae, and (c) close-up view, SEM of elytron showing epicuticular reflector (arrow) and surface microsculpture, (d) reflectance spectra of punctae and surrounding areas (5 m2) of the elytron, the entire elytron and sand substrate (T. D. Schultz 1994, unpublished data). Green triangles, puncta; yellow triangles, perimeter; red triangles, field; open circles, wet sand; filled circles, elytron.
Figure 4.
Circularly polarized multilayer reflectors. (a) Schematic of helical multilayer reflector, (b) Chrysina boucardi viewed through quarter wave plate rotated 0°, and (c) C. boucardi viewed through quarter wave plate rotated 90° clockwise.
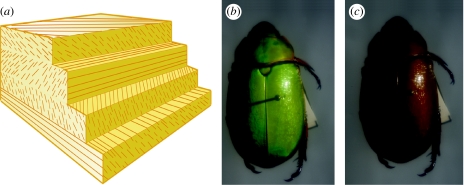
Figure 5.
Broadband multilayer reflectors. (a) Schematic of chirped reflector and (b) broadband reflectance in Chrysina chrysagyrea (Scarabaeidae: Rutelinae).
Figure 6.

Phanolinus sp. (Staphylinidae: Staphylininae) specimen exposed to sunlight (including UV) on left side for 20+ years.
Contrary to the reports in some textbooks and articles (e.g. Daly et al. 1998), multilayer reflectors of beetles can be located at different layers within the integument (figure 2d–f). Mason (1927) noted that beetle iridescence arose from different depths within the cuticle and could be removed from meloid, carabid and buprestid beetles by scraping the (epicuticular) surface; transmission electron microscopy (TEM) later revealed multilayer reflectors in the outer 1–2 μm of the exoskeleton of buprestids and carabids (Durrer & Villiger 1972; Mossakowski 1979, 1982). Using electron microscopy and chemical analysis, Schultz & Rankin (1985a) identified the reflectors of tiger beetles (Cicindelinae) as non-chitinous epicuticle laminated with ultra-thin layers of melanin. A similar epicuticular reflector occurs in the iridescent chrysomelid Plateumaris sericea (Kurachi et al. 2002). Layers of chitin fibrils and protein in the exocuticle form the interference reflectors of many scarabs (Neville & Caveney 1969), while the facultative, ‘switchable’ iridescence of some tortoise beetles (Chrysomelidae: Cassidinae; see discussion in ‘Other mechanisms’ section) appears to arise from thin layers in the endocuticle adjacent to the epidermis (Hinton 1973a).
The colour reflected by a multilayer structure depends on the refractive index of the component layers and their periodicity. Layers with a greater optical thickness reflect longer wavelengths than thinner layers and the peak wavelengthmax is equal to 2 (nada+nbdb), where n is the refractive index; d is the actual layer thickness; and a and b are the alternating layers in the reflector (Land 1972). When reflector thickness varies between body regions, multiple colours of different hue may be reflected (e.g. figure 1c,d). Polymorphism and geographical variations in metallic colour within species have been shown to result from differences in the periodicity of reflecting layers (Knisley & Schultz 1997; Kurachi et al. 2002). Estimates for the average refractive index of individual reflecting layers in beetles range between 1.4 and 1.73 (Caveney 1971; Parker et al. 1998; Kurachi et al. 2002; Noyes et al. 2007).
The apparent colour of a simple multilayer reflector also varies with the angle of observation; given a constant angle of illumination, as the angle of observation increases (i.e. deviates from normal), the reflected colour will undergo a ‘blue shift’ to a shorter wavelength, i.e. towards the blue end of the spectrum (see Vigneron et al. (2006) and Kinoshita et al. (2008) for a photographic illustration). The colour shift occurs because reflected light rays are travelling a shorter distance through each layer, which means that constructive interference occurs for shorter wavelengths. This relationship between viewing angle and apparent hue results in a multicoloured appearance in convex beetles: when viewed from above, the lateral regions of the pronotum and elytra will be blue shifted with respect to the top of the segment (figure 2c). Deparis et al. (2008) have proposed a novel measure by which to directly quantify iridescence in structurally coloured objects; this metric, termed ‘spectral richness’, describes the change in apparent wavelength as the viewing angle departs from normal.
2.2 Modifications
2.2.1 Additive or ‘pointillistic’ colour mixing
An interesting modification of multilayer reflectors appears in tiger beetles (Carabidae: Cicindelinae). These predatory beetles are often found running on exposed soil or sand, where bright metallic colours would render them conspicuous to any natural enemy reliant on visual cues. In an apparent adaptation for camouflage, the elytra of many cicindeline species are covered with an array of closely packed, minute dimples or punctae (figure 3a–c). Each dimple constructively reflects a narrow band of wavelengths that differ from the surrounding field owing to differences in periodicity within the epicuticular reflector (Schultz & Rankin 1985a). Generally, the reflecting layers within the punctae are thinner and reflect shorter wavelengths than the layers around the perimeter. When viewed at a distance, these ‘pixels’ of bright iridescent colour blend to create a diffuse, matte brown, green or similarly unsaturated hue, often matching the colour of natural backgrounds (figure 3d; Schultz 1986; Schultz & Bernard 1990; Acorn 1992). In some tiger beetles, a ‘structural black’ is produced by the additive mixing of magenta and green iridescence. In addition to the punctae, the surface microsculpture of the elytron is composed of smaller hexagonal pits or alveoli, 10 μm in diameter (figure 3c) that enhance the matte-like reflectance. Owing to the effect of angle on interference colour (see above), the walls of the alveoli reflect shorter wavelengths than the floor (Berthier 2007) contributing to the additive mixture of colours. Similar colour mixing systems have recently been documented in lagriine tenebrionids (Chlorophila; Liu et al. 2008) and melolonthine and ruteline scarabs (see following discussion of circular polarization). Unlike tiger beetles, these mechanisms use identically bicoloured dimples (e.g. Jewell et al. 2007b), instead of the ‘pointillism’ of differently hued punctae.
2.2.2 Circularly polarizing reflectors
Reflectance of circularly polarized light by beetle cuticle was first reported in beetles by Michelson (1911) in Chrysina resplendens (see Goldstein (2005) for an in-depth analysis of reflectance and polarization properties); this phenomenon appears to be restricted to Scarabaeidae, occurring predominantly in Rutelinae, Scarabaeinae and Cetoniinae.
Whereas linearly polarized light is light in which the electric vectors (e-vectors) are all propagating in the same plane, the e-vectors in circularly polarized light all propagate with the same clockwise or anticlockwise rotation. Unlike linearly polarized light, the detection of circular polarization is not angle dependent. Circularly polarized light is also rare in nature; among insects, it has been documented only among scarabs and in the photic organs of certain lampyrid larvae (Hegedüs et al. 2006b). In adult beetles, a circularly polarizing reflector is formed when birefringent chitin layers are deposited helically (i.e. with successive rotation), with a periodicity (one rotation of the helix) equal to a wavelength of visible light (figure 4a). According to Neville & Caveney (1969) this ‘helical stack’ of chitin microfibrils is optically analogous to a cholesteric liquid crystal. Under incident unpolarized white light (i.e. sunlight), polarized coloured light is reflected; the majority of green ruteline scarabs reflect polarized light with a left-handed (anticlockwise) rotation (Neville & Caveney 1969; Caveney 1971; Kattawar 1994).
Circular polarization appears in combination with additive colour mixing in certain scarabs (Rutelinae: Chrysina boucardi, Melolonthinae: Pyronota festiva; De Silva et al. 2005; Jewell et al. 2007a,b). In these beetles, polarizing multilayer reflectors take the shape of closely packed, bowl-shaped ‘micromirrors’. Under normal microscopy, these reflectors resemble the tiger beetle structures described above; however, the colours they reflect are circularly polarized, and the surface of the dimples is sometimes covered by a smooth, transparent wax layer (Chrysina, Pyronota). In wax-covered reflectors, the overall chromatic effect is deeper in colour (e.g. C. boucardi, figure 4b), evocative of the ‘metal flake’ paint used on automobiles.
The human eye cannot readily discern circular polarization in ordinary light, but polarized colours in beetles can be easily identified by viewing specimens through a ‘circular analyser’, essentially a linear polarizer placed over a quarter wave plate (Kattawar 1994). This tool can be used to block out the polarized green reflectance, while allowing the unpolarized specular and pigment reflectance to pass through, which causes the normally virescent Chrysina to appear an ordinary ‘beetle brown’ (figure 4c).
2.2.3 Broadband reflectors
A broadband reflector is any multilayer structure that reflects most or all wavelengths of light simultaneously, hence a ‘broadband’ of the visible spectrum. A multilayer reflector with many layers of different thicknesses (regardless of the order in which they are arranged) will produce broadband reflectance; however, only chirped broadband reflectors (those in which cuticle layers gradually increase or decrease in thickness; figure 5a) have been reported in beetles (Parker 1998). The colour of broadband reflectors is also less directionally dependent, because the full range of wavelengths is reflected (as opposed to the ‘narrow-band’ reflectance of chromatic multilayers, wherein the apparent hue changes dramatically with a change in viewing angle). The broader the range of bandwidths, the closer to pure gold or silver (mirror-like) the cuticle appears (figure 5b).
2.2.4 Colour change in multilayers
The colours of beetles produced by multilayer reflectors may change during development or as the result of environmental conditions, which should be considered when examining and comparing the colours of specimens. After eclosion, some beetles will exhibit a gradual increase in reflected wavelengths as layers attain their maximal optical thickness; dehydration of the cuticle during this period may limit or reduce the wavelengths (Schultz & Rankin 1985b). In some beetle multilayer reflectors with porous regions, air or fluid can be introduced in order to change the colour reflected (see §4, ‘Other structural colours’ for an in-depth discussion of this phenomenon, which is unique to polyphagan beetles). Permanent colour change in multilayers can be induced only under extreme stress of sufficient force to alter the thickness or refractive index of the cuticular chitin layers. Adachi (2007) found that the metallic colour of buprestid (Chrysochroa) elytra could be changed by heating the specimen to 200°C or by soaking it in bromoform for one month. A staphylinid specimen partially exposed to sunlight for 20+ years lost the multilayer colours on one side of its body, presumably due to long-term exposure to UV (figure 6; S. Chatzimanolis 2008, personal communication). Under normal museum conditions, most beetle multilayer reflectors retain their colours in perpetuity; the oldest known beetle reflector is that described by Parker & McKenzie (2003), still reflecting metallic blue after an estimated 49 million years.
2.3 Taxonomic distribution
Multilayer colours occur in many families of adephagan and polyphagan beetles, and in too many species to count. In the majority of beetle families where they occur, multilayer reflectors are restricted to a few lineages (e.g. within Trogossitidae, Tenebrionidae, Silphidae, Meloidae, Staphylinidae); they are most commonly encountered in the metallic wood-boring beetles (Buprestidae, figure 2c), scarabs (Scarabaeidae, particularly Phanaeinae, figure 1e; and Rutelinae, figures 4b and 5b), ground beetles (Carabidae, figure 1c), leaf beetles (Chrysomelidae), some longhorn beetles (Cerambycidae) and the weevil tribe Baridinae. In many families, there is a distinct phylogenetic pattern to the distribution of multilayer reflectors: some clades are almost entirely metallic, e.g. the scarab tribe Rutelini and the carabid subfamily Cicindelinae.
Additive colour mixing is widespread in the ground beetle subfamilies Cicindelinae and Elaphrinae; colour mixing with polarized reflectors has been documented only in scarabs and tenebrionids, but may be more widespread.
2.4 Terminology and diagnosis
Multilayer reflectors in beetles have also been described as ‘thin-layer stacks’, ‘one-dimensional photonic crystals’ and ‘thin-film reflectors’ (e.g. Parker 1998, 2002; Vigneron et al. 2006). The vocabulary used to describe these structures is somewhat dispersive, as the variously intersecting disciplines of entomology, physics and applied optics (e.g. laser technology, fibre-optic data transmission, telescopes and microscopy) have all developed slightly different suites of terminology. Other synonyms for ‘multilayer reflector’ include multilayer stack, quarter wave stack, interference reflector and dielectric mirror.
We propose that the term multilayer reflector be applied to such structures in Coleoptera; this describes the multilayered nature of cuticular chitin lamellae (which are not true films) and the reflective mechanism by which colour is produced.
The terms ‘metallic colours’ or ‘metallic iridescence’ can be used to distinguish multilayer effects from those produced by other optical structures. Multilayer reflectance can typically be diagnosed as such by its limited palette (usually one or two apparent hues per reflector), blue shift with decreased observation angle and fixed position on the cuticle surface.
3. Three-dimensional photonic crystals (including opal analogues)
3.1 Mechanism
Three-dimensional crystalline structures producing scintillating, gem-like reflectance were described by Parker et al. (2003) in the entimine weevil Metapocyrtus sp. (initially misidentified as Pachyrrhynchus argus); by Welch et al. (2007) in Pachyrrhynchus congestus, and recently in another entimine weevil, Lamprocyphus augustus, by Galusha et al. (2008). The photonic crystals found in the scales of pachyrrhynchine weevils (Pachyrrhynchus and Metapocyrtus) have a close-packed hexagonal arrangement analogous to (mineral) opal, while the photonic crystal of Lamprocyphus has a diamond-based lattice (i.e. a face-centred cubic system rather than a hexagonal one).
Similar to the broadband multilayer reflectors, the three-dimensional photonic crystals found in beetles are an ‘iridescence-reducing’ colour mechanism: that is, they reflect vivid, saturated interference colours but reduce the angle dependency of the chromatic effect. Colour is produced by a highly ordered lattice of nanoscale spheres in the interior of flattened scales (figures 1f and 7a–d). The inverse of this structure, spherical lacunae in a chitin matrix, produces the same optical effect; Galusha et al. (2008) have interpreted the latter as ‘hexagonally ordered air cylinders’. Parker & Martini (2006) described the scales in Metapocyrtus as filled with ‘transparent spheres, each 250 nm in diameter … arranged in flat layers, [with] a precise, hexagonal-close-packing order’. On a higher level of organization, each scale contains a dense array of differently oriented micrometre-scale single-crystalline ‘domains’ (Galusha et al. 2008).
Figure 7.
Three-dimensional photonic crystals in weevils and longhorn beetles. (a) Pachyrrhynchus congestus pavonius (Curculionidae: Entiminae), SEM of crystal structure from scale interior (from Welch & Vigneron 2007), (b) SEM of the interior structure of Prosopocerus lactator (Cerambycidae: Lamiinae), (c) Pachyrrhynchus gemmatus, light photograph of opalescent scale patch, (d) P. gemmatus, habitus view.
Although they are frequently compared with highly regular mineral structures, photonic crystals found in living organisms are not ‘perfect’ from a mathematical or engineering perspective. However, if we assume that these optical mechanisms have been selected for, their inherent imperfections may be considered to be part of an optimized diffuse reflection device. In weevils, the three-dimensional photonic structures are usually divided into an irregular assemblage of regular domains (e.g. figure 7a). Thus, the interior of the scale is better described as a photonic polycrystal than as a monocrystal: each grain in the polycrystal is cut from a highly ordered photonic crystal (short-range order), but the orientation of the different grains varies across the scale (long-range disorder). The size of the domains ranges from the full size of the scale (monocrystal) to the size of the photonic crystal period in the grain (amorphous structure). These various levels of disorder can be quantified by the grain size, i.e. the coherence length of the structure. The visual effects produced by structures with different coherence lengths include iridescence (e.g. figures 1f and 7c,d), dull metallic colours and whites.
3.2 Taxonomic distribution
Most members of the ‘higher weevils’ (Curculionidae sensu stricto, Brentidae and Attelabidae) bear pigmented or structurally coloured scales; species with opalescent green scales appear in most of the tribes of Curculionidae, albeit most commonly in the subfamilies Curculioninae and Entiminae. The most striking iridescence occurs in various members of Entiminae, the tribes Leptopiini and Pachyrrhynchini in particular. Entiminae is a large, tremendously diverse and taxonomically problematic weevil clade, comprising over 12 000 species (Anderson & Lanteri 2000). Entimine adults (many of which are flightless) feed on flowers or green foliage, which suggests that their coloration may be important in mimicry or crypsis.
In cerambycids, the African lamiine species Prosopocera lactator has slender, pearlescent scales with an internal ‘ball-and-stick’ crystal structure (figure 7b). Another African lamiine, Sternotomis virescens (as well as some other Sternotomis), has elytral scale patterns with a greenish, opalescent appearance, strongly reminiscent of the reflectance in entimine weevils; the structure of the scale interior has yet to be investigated for this genus.
3.3 Terminology and diagnosis
Although the term ‘photonic crystal’ applies to any ordered subwavelength structure that affects the propagation of specific wavelengths of light (Parker & Townley 2007), it is the three-dimensionally ordered structures to which the term is most commonly applied. We recommend use of the term ‘three-dimensional photonic crystal’, which distinguishes these structures from the one-dimensional periodicity of multilayer reflectors or Bragg gratings. The terms ‘opal’ and ‘diamond based’ have been used to describe iridescence in weevil scales, but refer to phenomena that are relatively similar from an organismal perspective; it is important to note that these terms refer to crystalline lattice morphology and not the appearance of the scales themselves. Maldovan & Thomas (2004) provided an excellent overview of diamond-based lattice morphology (as observed in Lamprocyphus) in photonic crystals; Yablonovitch (1993) provided a thorough introduction to the photonic band-gap mechanism by which colours are produced in three-dimensional photonic crystals.
4. Diffraction gratings
4.1 Mechanism
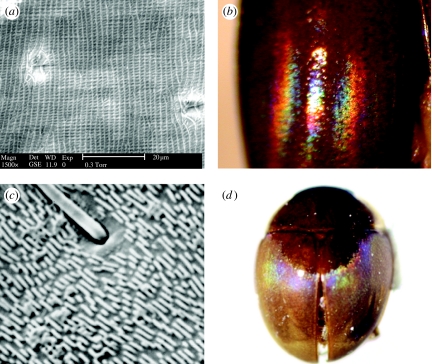
Diffraction gratings were first described in a scarab beetle by Anderson & Richards (1942) and in six other beetle families by Hinton (1969, 1973b) and by Hinton & Gibbs (1969a,b, 1971; see Hinton (1973a) for a complete list of beetle taxa with cuticular diffraction gratings). They have recently been discovered in five additional families, all within the suborder Polyphaga (A. E. Seago 2008, unpublished data; e.g. figures 1a,b, 8a–c and 9a–d).
Figure 8.
Diffraction gratings. (a) Schematic of cuticular grating, (b) SEM of diffraction grating, Sphaeridiinae gen. sp. (Hydrophilidae), and (c) Sphaeridiinae gen. sp., habitus view with zero, first, second and third spectral orders labelled.
Figure 9.
Modified diffraction gratings. (a) Pallodes sp. (Nitidulidae), SEM of bidirectional grating, (b) Pallodes sp. (Nitidulidae), ‘double’ spectral reflectance, (c) Aglyptinus tumerus (Leiodidae: Leiodinae), SEM of ‘interrupted’ grating, and (d) A. tumerus, habitus view.
A diffraction grating is any nanoscale array of parallel ridges or slits that disperses white light into its constituent wavelengths (figure 8a shows a grating in cross section). Because white light consists of many different wavelengths, it diffracts into full spectra, creating the rainbow-like reflectance shown in figures 1a,b, 8c and 9b,d. While man-made diffraction gratings can disperse light via reflection or transmission, all beetle gratings are strictly reflection mechanisms. Diffraction gratings in beetles can be derived from strigulose microsculpture, microtrichiae or modified setae (see Harris (1979) for useful basic classification of surface microsculpture in insects). Iridescence arising from diffraction gratings always takes the form of one or more ordered spectra—that is, colours are ordered in the same sequence as the colours in the spectrum of visible light, red–orange–yellow–green–blue–violet. This distinguishes diffraction colours from the unordered ‘faux spectra’ produced in some beetles by variable thickness multilayer reflectors (figure 1d). The spectra produced by diffraction gratings are also ‘ordered’ in a different sense: when compared with a standard birefringence chart used in mineral analysis, they follow the same sequence of spectral orders (labelled in figure 8), from zero-order (pure specular reflectance), first-order (saturated red/yellow/blue) to high-order spectra of secondary and tertiary colours.
Diffraction grating iridescence can easily be characterized in terms of spectral order: for example, loxandrine carabids (figure 1a) display predominantly first-order spectra, while most phalacrid gratings (figure 1b) display long series of spectra, usually up to the third order or higher. This difference is less likely to be due to taxonomic differences and more likely to be due to differences in grating morphology and curvature of the elytron itself; basic literature on the effects of convexity on diffraction gratings is sparse.
4.2 Modifications
Modification refers here to deviations from a ‘standard’ diffraction grating, not to evolutionary modification from a shared ancestral morphology. Diffraction gratings in beetles occur in a wide variety of forms, from weakly organized arrays of parallel ridges (e.g. Aglyptinus, figure 9c,d) to dense strigulae (figure 8b); see Hinton (1976a,b) and Ball & Shpeley (1983) for additional examples of grating morphology, including the ‘fringed’ gratings of sericine scarabs and the stretched sculpticells found in carabids, respectively.
Neotropical members of Nitidulidae (e.g. some Pallodes) have evolved a particularly curious grating microsculpture not hitherto observed in nature: in these species, iridescence on the pronotum arises from two intersecting, perpendicular diffraction gratings; this results in the reflectance of ordered spectra along both longitudinal and lateral axes, forming a spectral ‘halo’ around the point of specular reflectance (figure 9a,b).
4.3 Taxonomic distribution
Diffraction gratings are remarkably widespread throughout Coleoptera; families in which gratings have been observed include Torridincolidae (Ytu, Reichardtia, Iapir and Hintonia), Carabidae (Loxandrus, Seleophrus, many Loxandrini and Oodini), Noteridae, Gyrinidae (Gyretes), Dytiscidae, Hydrophilidae (Sphaeridiinae), Scarabaeidae (Serica), Staphylinidae (Staphylininae and Scaphidiinae), Silphidae, Leiodidae (Aglyptinus and Agathidium), Mordellidae (Boatia), Phalacridae (many genera), Nitidulidae (Pallodes), Bothrideridae (Ogmoderes, Prolyctus and others) and Erotylidae (Mycotretus) (Hinton 1969; Hinton & Gibbs 1969a,b; N. Lord 2008, personal communication; A. E. Seago 2008, unpublished data). In several of these families diffraction gratings have a polyphyletic distribution, i.e. are present in two or more non-sister lineages. The families Carabidae, Phalacridae and Staphylinidae appear to contain the most independent origins of diffraction gratings, although explicit phylogenetic and evolutionary hypotheses are available only for Carabidae (e.g. Lindroth 1974; Allen & Ball 1980).
4.4 Terminology and diagnosis
Diffraction gratings give rise to iridescence in its strictest sense, literally rainbow like (from the Greek goddess Iris, associated with the rainbow). We use the narrower term ‘spectral iridescence’ (in reference to ordered spectra) to distinguish diffraction colours from the metallic or jewel-like colours produced by other structures. Diffraction gratings can be recognized by the reflectance of one or more ordered spectra, running parallel or perpendicular to the longitudinal body axis and appearing to change position with the angle of observation.
5. Other structural colours in Coleoptera
Although iridescence is the most familiar mode of structural coloration in beetles, several other notable structural colour mechanisms exist in this group. The optical mechanisms discussed below produce colours that do not vary significantly with viewing angle or give rise to spectral reflectance.
5.1 Whites and UV reflectance
All whites in beetles are structural, arising from non-ordered, broadband or Mie scattering of incident light by nanoscale particles. White light may be scattered by unpigmented cuticle, setae, scales and surface waxes. The white maculations of tiger beetles arise from broadband scattering by areas of the cuticle that lack melanin (Schultz & Rankin 1985a). Vukusic et al. (2007) described particularly bright white reflectance in the melolonthine scarab Cyphochilus; this whiteness arises from fine (5 nm diameter) unordered cuticular filaments on the interior of scales, which in cross section form a slender but extremely effective array of scattering structures. Several desert tenebrionid species are white or pale blue due to scattering from wax filaments that prevent water loss, reduce radiation absorption and provide crypsis (Hadley 1979; McClain et al. 1985).
UV reflectance in beetles is a special case of ‘insect white’ that also results from broadband scattering (contra Pope & Hinton (1977), who wrongly attributed UV reflectance to pigments). Both white and UV wavelengths may be reflected by microtrichiae (Hinton 1973b); however, species that appear similar in white light may produce dramatically different reflectance patterns in the ultraviolet. Pope & Hinton (1977) documented several cases of species that are superficially similar in this way in Carabidae, Scarabaeidae, Tenebrionidae, Cerambycidae, Curculionidae and Chrysomelidae; such ‘UV-cryptic’ signals are relatively widespread in insects (Silberglied 1979). Ultraviolet vision (or at least sensitivity) is well documented in beetles; it is therefore plausible that UV reflectance functions in visual recognition of conspecifics or mate location (Frantsevich et al. 1977; Dacke et al. 2002).
5.2 Quasi-ordered scattering
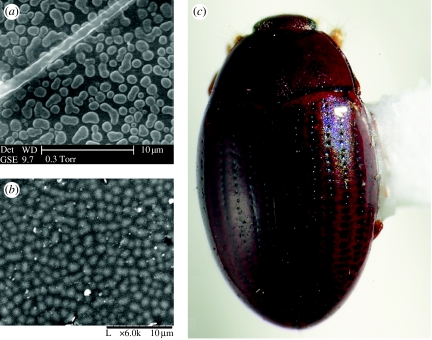
Quasi-ordered two- or three-dimensional microstructures can give rise to vivid, non-iridescent colours, most frequently blues and greens. Colours are produced when identically sized nanoscale light-scattering objects (particles or lacunae) are evenly spaced in a transparent or translucent matrix; the wavelength that will be constructively reflected is determined by the size and spacing of the particles (Prum & Torres 2003). The semi-ordered nature of this phenomenon distinguishes it from Tyndall or Rayleigh scattering, which arises from a completely unordered distribution of particles (Prum & Torres 2003). This effect has been most extensively documented in birds, Lepidoptera (Prum et al. 2006, see also Prum & Torres 2003), Odonota (Prum et al. 2004) and Hemiptera (Miyamoto & Kosaku 2002), but has not hitherto been described in Coleoptera. Among beetles, quasi-ordered arrays of surface tubercles (figure 10a,b) produce a blue or purple sheen in some chrysomelids, scaphidiine staphylinids and cerylonids (e.g. Mychocerus, figure 10c). The function of this mechanism in beetles is unknown; the faint, diffuse reflectance is unlikely to act as a strong visual signal.
Figure 10.
Quasi-coherent scattering. (a) Heikertingerella sp. (Chrysomelidae: Alticinae), SEM of scattering structure, (b) Mychocerus sp. (Cerylonidae), SEM of scattering structure, and (c) Mychocerus sp. (Cerylonidae), habitus view showing diffuse reflectance.
5.3 Reversible colour change
In a few species of beetles, reversible colour change is effected by partially hydrating or dehydrating multilayer structures, thus changing the refractive index of porous layers; this mechanism is also known as ‘hygrochromic colour’ (Mason 1929; Hinton 1973a,b; Rassart et al. 2008). Hygrochromic colours were first noted in Dynastes hercules by Beebe (1947), and later described in detail by Hinton & Jarman (1972) in the same species. In D. hercules, the ‘resting state’ elytral coloration of greenish-grey changes to black when hydrated; this change is purported to occur only in males (Rassart et al. 2008) and can also be induced by strong compression or mechanical stress to the elytra. The hygrochromic structure itself is a light-scattering, weakly ordered three-dimensional lattice with air-filled porous regions. As moisture infiltrates the spaces, the difference in refractive index between chitin and lacunae—and thus the amount of scattering/reflection—is decreased dramatically, more light is absorbed, and the elytron appears black in colour (Hinton & Jarman 1972; Hinton 1973a; Rassart et al. 2008).
Dramatic reversible colour change has also been documented in the cassidine chrysomelids Aspidomorpha tecta (Hinton (1976a), Charidotella egregia (Vigneron et al. 2007), Deloyala and Metriona (=Charidotella; Barrows 1979), all of which change from ‘resting’ gold colour to red or reddish when disturbed. Vigneron et al. (2007) performed an extremely thorough study of the colour change mechanism in C. egregia, concluding that the ‘default’ gold colour of this species is produced in the exocuticle by a broadband reflector comprising high-index chitin layers interspersed with irregular, porous patches. Unlike the colour-change mechanism in D. hercules (in which reflectance is disrupted by the addition of moisture to the exocuticle) Vigneron et al. (2007) found that red colour is exposed via dehydration of the porous layers, thereby collapsing the broadband cuticular reflector into a ‘translucent stack’. In this configuration, the change in refractive index between adjacent layers is low enough to permit most incident light to reach the pigment layer, where all but red wavelengths are absorbed.
Crowson (1981) noted that this phenomenon is widespread among cassidine chrysomelids. The function of such a colour change (particularly in leaf-perching insects) seems likely to be aposematism or warning; however, as the transition takes place rather slowly, over at least 2 min (Hinton 1976a), it may be a generalized response to disturbance rather than a warning signal directed at a particular predator.
Non-reversible structural colour change co-occurs with ageing in some donaciine chrysomelids, in which adults undergo a permanent shift from metallic blue to metallic green over the course of several months (M. Barclay 2008, personal communication). The mechanism by which this occurs has not been investigated, but is probably related to hydration or dehydration of a cuticular multilayer reflector.
6. Discussion
Iridescence in Coleoptera clearly arises from a wide variety of mechanisms. Although types of beetle iridescence can be classified into three major groups of analogous structures (multilayer reflectors, three-dimensional photonic crystals and diffraction gratings), iridescence across taxa is neither identical in function nor evolutionarily homologous. We now discuss the demonstrated and theoretical functions of iridescence in Coleoptera, and discuss the hypothesized evolutionary patterns and pathways by which it has most likely arisen.
6.1 Visual functions
6.1.1 Crypsis in foliage
The most common multilayer colour in beetles is green, which is widely speculated to function in crypsis (Crowson 1981; Parker 1998); this is also the most common hue in coloured weevil scales, which may derive their colours either from multilayer structures or crystalline arrays. Parker (1998) suggested that multilayer reflectors coupled with a diffusing (light-scattering) surface texture could be a particularly effective means of substrate matching in beetles, particularly those on substrates for which no insect pigment exists (e.g. green leaves; some flower surfaces). In ruteline scarabs (see circular polarization section below), vivid, saturated green hues are produced by helically arranged multilayer reflectors. Although these colours are highly conspicuous in a museum context, they may well be cryptic in a rainforest or cloud forest environment (Crowson 1981; Thomas et al. 2007). Thomas et al. (2007) noted that Chrysina gloriosa, while vividly coloured in isolation, is one of a suite of green-and-white-striped insects that are relatively cryptic in juniper foliage, their frequent natural perch.
6.1.2 Substrate matching through additive colour
Most tiger beetle (Cicindelinae) species show an inconspicuous dorsal coloration due to the additive mixing of interference colours as described earlier (figure 3a,d). These colours provide crypsis either by matching the general colour of the substrate or by mimicking the colour of small stones (Schultz 1986; Schultz & Bernard 1990; Knisley & Schultz 1997). Indirect evidence of this anti-predator defence is provided by the observation that cicindeline species often exhibit geographical variation in structural coloration that is correlated with the colour of soils on which they occur (Schultz 1986; Hadley et al. 1988; Pearson & Vogler 2001).
6.1.3 Aposematic (warning) colours
Although tiger beetles are, as all carabids, chemically defended to some extent, they have also evolved a remarkable diversity of defensive coloration mechanisms from the same presumably ancestral interference colours. Multilayer reflectors are used in aposematic colour signalling in some cicindelines (Shelly & Pearson 1978; Pearson 1985). Acorn (1988) reported that multilayer colours in some tiger beetles are used in the mimicry of sympatric, chemically defended blister beetles (Meloidae) and velvet wasps (Mutillidae). Vogler & Kelley (1998) analysed cicindeline colour pattern evolution in a phylogenetic context, concluding that bright colours in Cicindela appear to have two functions: either as an aposematic cue in strongly chemically defended beetles (subgenus Cicindelidia) or as a cryptics or disorientation colour in brightly iridescent but weakly defended species (e.g. C. sexguttata). Iridescence may serve to deceive visual predators when tiger beetles that appear bright in the Sun fly into shade and the colours are extinguished (Knisley & Schultz 1997; Schultz 2001).
6.1.4 Sexual signals
Although behavioural and ecological observations are relatively scarce for Coleoptera, several non-crypsis functions of multilayer colours have been posited. The following studies provide evidence for metallic colours as a visual signalling component in male–male competition and mate location.
The pronotal shield of phanaeine scarabs (figure 1e) produces both bright red and bright ultraviolet reflectance when viewed head-on. Vulinec (1997) argued that this surface acts as a ‘backdrop’ emphasizing the size of the horn in males, allowing competitors to assess each other visually; it may also allow females to estimate the parasite load of males. Thery et al. (2008) have documented a similar system in Coprophanaeus lancifer, which has similar male horn morphology but subtler pronotal coloration. Gwynne & Rentz (1983) documented strongly vision-based mate location behaviour in metallic gold buprestids; however, colour cues appear to be less important than chemical cues in other buprestid taxa for which mating habits are known (Alcock 1976). Kurachi et al. (2002) also noted that metallic colours are sexually dimorphic in some leaf beetles (Chrysomelidae), and suggested that coloration plays a role in sexual signalling.
6.1.5 Polarized signalling
Polarized reflectance may act as a receiver-dependent signal system, detectable by polarization-sensitive conspecifics but invisible to vertebrate predators. Experimental evidence indicates that scarabs, as well as desert tenebrionids and many non-beetle insects, can detect polarized light in various wavelength ranges (Dacke et al. 2002; Barta & Horvath 2004). Hegedüs et al. (2006a) discovered that melolonthine scarabs (Melolontha melolontha) are sensitive to green polarized light, and Frantsevich et al. (1977) reported UV-polarized vision in the scarab genus Lethrus (Geotrupidae). Dacke et al. (2002) found that the flightless desert scarab Pachysoma striatum has particularly UV and green polarization-sensitive ommatidia in the dorsal region of the eye, and argue that a UV-polarized skylight is used as an ‘optical compass’. Under laboratory conditions, C. gloriosa with a choice of two polarized light sources (circular and linear) preferentially moved towards the former (P. Brady & M. Cummings 2008, unpublished data). Thus, while various scarabs do appear to use polarization cues to navigate, the possible mate-recognition function of green polarized reflectance has yet to be tested.
6.1.6 Function of three-dimensional photonic crystals
Parker et al. (2003), Welch & Vigneron (2007) and Galusha et al. (2008) all emphasize the angle-independent nature of the iridescent reflectance in weevils (a similar effect is produced in the hopliine scarab Hoplia, members of which have bright green and blue structural colours arising from multilayer reflectors in thousands of minute, body-coating scales; Vigneron et al. 2005). In the case of weevils, while the ‘multifaceted’ arrangement of crystal domains is important, it should be recognized that the distribution of a reflectance mechanism across thousands of scales also contributes to direction independence and could thus function effectively in crypsis among foliage or in producing a dispersive visual signal. It is also of note that the multilayer reflectors that produce bright greens in other beetles (e.g. ruteline scarabs, many buprestids) are almost entirely absent from weevils, occurring only in a few baridines and rarely in pachyrrhynchines. Weevils may therefore have replaced elytral multilayer reflectors with scale-based reflectors; the diversity of colours produced by the latter is certainly comparable to that of the former, and many scale patterns of ‘opal’ weevils seem closer to warning coloration than to crypsis (e.g. Pachyrrhynchus gemmatus, figure 7d).
6.2 Non-visual functions
6.2.1 Thermoregulation
Any form of dorsal coloration has consequences for thermoregulation in diurnal beetles; these effects have been most thoroughly investigated in tiger beetles. In a study of the polymorphic Cicindela horni, Schultz & Hadley (1987) demonstrated that metallic green morphs attained the same body temperatures as black, pigmented morphs under controlled conditions. Expanded white maculations significantly lowered the body temperatures of Cicindela formosa when compared with morphs with reduced maculations. In populations of Neocicindela perhispida, the elytra of beetles that occur on white beaches are entirely white while those on black beaches have greatly reduced maculations and appear black through the additive mixing of interference colours (Hadley et al. 1988). When transplanted to black beaches, the white morphs were able to forage longer without overheating than the resident black morphs, which escaped the heat by burrowing in the sand (Hadley et al. 1992).
6.2.2 Anti-adhesive/friction reduction
Hinton (1969, 1970, 1973a,b, 1976a,b) suggested that the spectral iridescence arising from diffraction gratings serves a visual function, either as a warning colour or as a facultatively conspicuous signal that helps to confuse the depth-perception ability of predators. He also suggested that the usefulness of spectral iridescence as a visual signal is contingent on availability of bright direct illumination, and noted that ‘the highest percentage of beetles with diffraction gratings is found in the sunniest parts of the world’ (Hinton 1973b); however, beetle diversity (and insect diversity, in general) is markedly higher near the equator, so the apparently skewed distribution of diffraction gratings may be an artefact of overall diversity.
Some spectral iridescence is clearly a secondary result of other functions. The abdominal plastron of many torridincollids is brightly iridescent when illuminated; similarly, stridulatory files (documented by Hinton (1969) in the hymenopteran family Mutillidae) are often spectrally iridescent when exposed. The majority of external diffraction gratings in Coleoptera occur on exposed regions of the dorsum and, more rarely, the entire body (Hinton 1973a); their function is not known. However, it is notable that the beetle groups in which diffraction gratings are most common share similar microhabitats and thus similar mechanical challenges.
Nearly all adephagan and polyphagan beetle species with diffraction gratings are associated with a moist, sticky or colloidal substrate, e.g. mud-burrowing carabids (Oodinae (Spence 1982), swamp- or wet-forest-dwelling carabids (Loxandrinae (Ball & Shpeley 1983; Ball 1985)), aquatic beetles (Dytiscidae, Gyrinidae and Noteridae) and small slime-mould and rotten-fungus-feeding beetles, often from wet tropics or cloud forests (Mordellidae, Nitidulidae; Phalacridae, Leiodidae, Staphylinidae: Scaphidiinae (Steiner 1984; Franciscolo 1985; Leschen 1999; Seago & Wheeler 2004))). Interestingly, diffraction gratings are relatively common in adephagan water beetles, but less so in polyphagan water beetles: in Hydrophilidae, for example, diffraction gratings have only been observed in the largely terrestrial and semiaquatic subfamily Sphaeridiinae. Members of this subfamily are found in dung, rich humusy soil and moist decaying leaves (Van Tassel 2001).
Conspicuous iridescence arising from diffraction gratings is also common among burrowing snakes and lizards (Monroe & Monroe 1968; Arnold 2002). Gans & Baic (1977) asserted that diffraction gratings in burrowing snakes function in shedding mud or damp soil, but did not test this across many species nor in any experimental fashion. In carabid beetles, Erwin (1979) and Ball (1985) noted that diffraction gratings are prevalent among swamp-, marsh- and mud-inhabiting species, and rare in species from dry environments. The strong correlation between presence of diffraction gratings and habitat type (moist, concealed, largely nocturnal and crepuscular) suggests that the primary function of this iridescence mechanism is of a friction-reducing or water-repelling nature, and may aid locomotion in many species.
A second possible function of ‘blazed’ gratings (those in which the grating ridges are ratchet-like in cross section) is to aid forward movement in compressed microhabitats. Carabids are known to engage in ‘wedge-pushing’ behaviour with enough force to move through the fibres of wood; Schmalfuss (1978) documented similar ‘terraces’ (essentially large-scale blazed gratings) in burrowing crustaceans, and suggested that these structures function in aiding movement through a dense substrate. Crowson (1981) noted that blazed gratings are relatively common in beetles that move through tight under-bark, rotting wood or packed soil conditions, and uncommon in species found in loosely packed bark or leaf litter.
6.3 Evolution of structural colours
6.3.1 Phylogenetic distribution of iridescence in Coleoptera
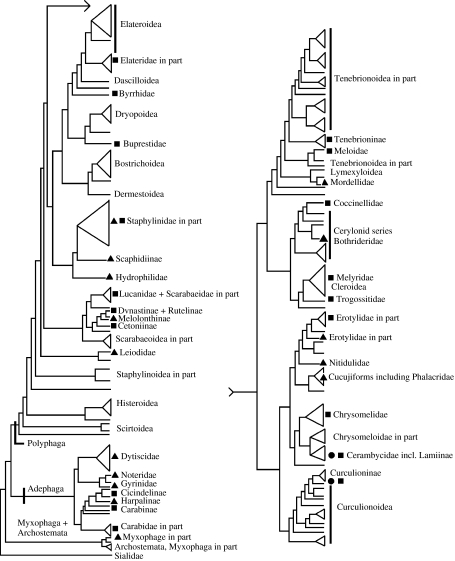
A conservative estimate of phylogenetic distribution of the colour mechanisms described here is given in figure 11. Again, we do not aim to list every species or genus known to possess a particular colour mechanism, but wish to indicate the breadth and frequency with which different types of iridescence appear throughout the order Coleoptera. It is clear that iridescence mechanisms have independently evolved many times, with so great a frequency as to suggest that they are favoured by selection in many circumstances. A number of hypotheses have been advanced as to how specific mechanisms have evolved.
Figure 11.
Approximate phylogenetic distributions of iridescence mechanisms in Coleoptera. Triangles represent diffraction gratings; squares represent multilayer reflectors; circles represent three-dimensional photonic crystals. Tree simplified from that presented by Hunt et al. (2007).
6.3.2 Evolution of multilayer reflectors
Even by the most conservative estimate, there are many repeated evolutionary origins of multilayer reflectors in beetles. The ‘armoured’ body plan of beetles is probably a key innovation that subsequently allowed the group to radiate into the enormous diversity we see today. This feature requires a many-layered cuticle thicker than that of most other insects. Thus, it is possible that beetles are preadapted for multilayer optics. As noted by Parker (1998), selection can act on colour by changing relative thicknesses of chitin or melanin layers in the cuticle, rather than changing materials (i.e. pigments or tagma) outright. The amount of selective pressure needed to drive evolutionary colour change may, therefore, be lower for multilayer structures. Once a laminar structure exists in the cuticle, modification to a broadband reflector is relatively straightforward, requiring enough compression or attenuation during development to effect an increase or decrease in the thickness of chitin layers. Similarly, circularly polarizing reflectance requires a fairly simple modification of the deposition of cuticle, one which already exists in beetle larvae: Crowson (1981, citing Neville 1970) reported that chitin layers in the cuticle of larvae are normally deposited in a helicoidal fashion, while the cuticle layers in adult beetles are not. Retention of larval cuticle-formation patterns could be the mechanism by which helicoidal multilayer reflectors in adult beetles have evolved.
6.3.3 Evolution of three-dimensional photonic crystals
Scales are a common feature in higher weevils, and often form conspicuous colour patterns that are likely to function as crypsis or visual signals. Although scales seem to be a prerequisite for the evolution of photonic crystals, lattices such as those discussed here have not been observed in other scale-bearing beetle groups outside the clade Phytophaga (cf. blue and white scales in the scarabs Hoplia and Cyphochilus). This suggests that there may be some self-assembly mechanism acting in weevils and cerambycid that is not present in other beetles. Self-assembling opal-like crystal lattices occur elsewhere in nature and have been manufactured from a variety of compounds (Parker et al. 2003; Parker & Martini 2006; Pursiainen et al. 2007); those in weevils most likely arise from molecular self-assembly during scale formation.
The crystal lattices described by Galusha et al. (2008) have laminar domains in certain directions and predominantly reflect green light; most non-green structurally coloured weevil scales are white, forming an important component of crypsis and shape disruption and creating false texture used to mimic rough bark, bird droppings, etc. White reflectance in insects can only be produced by the scattering of light rays by an unordered particulate array (see ‘Other mechanisms’ section). Thus, a plausible evolutionary pathway for three-dimensional photonic crystals could proceed along lines of increasing organization, from unordered particles (white reflectance) to two-dimensional ordered laminar structures (green reflectance) to three-dimensional ordered crystal lattices (opal-like reflectance). In this scenario, existing structural colours used in crypsis are exapted to form the conspicuous reflectors of Pachyrrhynchus and other entimines; this hypothesis can be tested using electron microscopy of scale interiors and a tribe- or genus-level phylogeny for higher weevils.
6.3.4 Evolution of diffraction gratings
Diffraction gratings have evolved many times within Coleoptera (‘a hundred times or more’ by Hinton's (1973b) estimate), and from many different morphological precursors. Diffracting structures have arisen from plastron microtrichiae (Torridincolidae), stretched sculpticells (Carabidae, Staphylinidae), what may be modified setae or vestigial sculpticells (Leiodidae: Scotocryptini; Scarabaeidae: Sericini), and unknown microsculptural elements (Nitidulidae). The selective pressures that favour the evolution of diffraction gratings are unlikely to be related to visual function. Although ‘macrogratings’ such as strigae and dense recumbent pubescence may provide a mild crypsis by reducing specular reflectance, gratings that are fine enough to produce iridescence have little effect on specular reflectance (figure 1a,b).
Interestingly, many groups that have diffraction gratings do not have multilayer metallic colours. Rather, in these groups pigment colours predominate, chiefly the brown or black of melanin. Beetle lineages with diffraction gratings have markedly similar ecological proclivities, with the majority of spectrally iridescent taxa living and feeding in concealed, moist, epigean habitats. Although their iridescent reflectance may not serve a visual function as that of leaf feeders, this optical mechanism is no less widespread and has by any estimate evolved many times within Polyphaga and Adephaga alike.
7. Conclusions and recommendations for future studies
There is no single evolutionary pathway by which structural colour evolves in beetles, and no single function for iridescence. Similar iridescence and colour mechanisms are likely to serve different functions in different taxa, not only crypsis but also sexual signalling, aposematism, mimicry, locomotory enhancement and thermoregulation; some may simply be an artefact of other structural constraints, such as the iridescence of the torridincollid plastron or the thin-film colour of an exposed flight wing.
Multilayer reflectors are the most widespread iridescence mechanism, and have evolved many times from similar precursors: stacked chitin layers (optically active or not) are present in all beetles, more so than other, less well-armoured insects. In this sense, the order Coleoptera can be considered preadapted for this particular form of iridescence.
It is clear that multilayer coloration is asymmetrically distributed across the beetle phylogeny: it occurs throughout the order but is most common in the large clades Phytophaga, Scarabaeidae and Buprestidae. In adults of all three groups, surface feeding and daytime activity on plant surfaces is common (Farrell 1998; Bellamy & Nelson 2002). Although there are some dazzling exceptions, the most common colour in these groups is green, with apparent disruptive coloration, aposematism and mimicry less widespread.
It is conceivable that, as various lineages of polyphagan beetles evolved to take full advantage of land plants (and in the process departing from their likely ancestral habits of concealed saprophagy (Farrell 1998; Marvaldi et al. 2002)) they were also subject to increased predation pressure from other invertebrates and vertebrates. If crypsis is the first (and arguably metabolically cheapest) line of defence against predation, and green pigments are rare or unavailable in animals, it can then be argued that multilayer reflectors and photonic crystals are inextricably linked to major evolutionary shifts towards plant feeding in polyphagan beetles. As reflectors that produce green coloration in any exposed condition (i.e. when sunlight is present), structural colour mechanisms grant their bearers an efficient and lasting camouflage among the green foliage of plants.
7.1 Recommendations for future research
When describing the coloration of an organism (in ecological, behavioural and taxonomic works alike), we encourage the biologist to determine the underlying colour mechanism(s); this is particularly important in iridescent taxa, where similar chromatic effects can be caused by very different and non-homologous structures. In this review, we have aimed to provide accurate descriptions, recognitory diagnoses and known distribution of the major mechanisms of iridescence and structural colours in Coleoptera. We also provide a glossary of optical terms of potential use to entomologists (appendix A) and a proposed terminology of iridescence mechanisms (table 1).
Table 1.
Relationships between structural colours, optical mechanisms and visible reflectance in Coleoptera.
| structural colour | mechanism | wavelengths reflected |
|---|---|---|
| simple metallic hues | multilayer reflector | discrete band of visible spectrum |
| silver or gold colour | variable thickness broadband multilayer reflector | all visible |
| circularly polarized colour | helically arranged multilayer reflector | yellow–greena |
| spectral iridescence | diffraction grating | all visible, as ordered spectra |
| opal or diamond effects | three-dimensional photonic crystal | all visible |
| UV and white reflectance | Tyndall scattering | all visible, plus ultraviolet |
| most non-metallic blues | quasi-ordered array | typically blues and purplesa |
Only these colours have been observed in nature; any colour is possible.
Because colour perception is subjective, varying between organisms, and even between human observers, and since we currently lack a fixed vocabulary for structural colours, it is particularly important to characterize chromatic features in the most objective fashion possible. The quickest and most objective method is to measure reflectance peaks with a spectroradiometer (e.g. Endler 1990); this method is effective with colours produced by multilayer reflectors and three-dimensional photonic crystals. Measured reflectance spectra can then be used for precise characterization of multilayer colour effects with or without polarization (e.g. De Silva et al. 2005; Deparis et al. 2008). A less expensive alternative is that proposed by Aguiar (2005), who proposed sampling a colour from within a digital photograph of the specimen in question; graphics programs such as Adobe Photoshop and CorelDraw or Corel PhotoPaint automatically display the RGB coordinates of the sampled colour. This approach is more affordable than the former, but has two notable limitations: unlike spectroradiometry, it cannot detect UV and IR (nor quantify the amount of reflectance of a colour); it will also be affected by the light environment used to take the photograph.
When evaluating the proposed function of a coloration mechanism, it is also important to take into account not only the visual system of study organisms and their potential predators but also the organism's natural context, including optical properties of the substrate and the ambient light conditions (Endler 1990, 1993). This approach has been applied broadly to the study colorations in vertebrates and butterflies, but rarely with beetles. Schultz (2001) quantified the conspicuousness of iridescent anti-predator colorations of two tropical tiger beetles against natural backgrounds and under forest light. Using photoreceptor sensitivities obtained from the related species Onitis alexis, Thery et al. (2008) have determined that the iridescent colour of the crepuscular scarab C. lancifer would be most effective as an intraspecific signal at dusk when the beetles were most active. The visual system of O. alexis is dichromatic with receptor peaks in the UV and green (Warrant & McIntyre 1990); the ancestral condition for beetles is trichromatic vision with peaks in UV, blue and green (Briscoe & Chittka 2001, based on measurements for lampyrid beetles and owlflies). However, more research is needed on the spectral sensitivities of beetles for which iridescent colours may serve as signals.
There is a long entomological tradition of considering beetles and other insects within the context of the museum and laboratory, prizing large and seemingly conspicuous specimens for their jewel-like reflectance or unusual morphology. A similar habit persists in the fields of optics and biomechanics; individual structures are treated in isolation and evaluated on the basis of their material properties or as potential source material for the expanding technological field of biomimicry. Although a mechanistic, experimental approach is critical to elucidate how insect reflectors function, it is important to connect these structures to their role in the organism, which in turn necessitates a phylogenetic and ecological context.
The order Coleoptera is by any standard a prodigious showcase for the extraordinary creativity and flexibility of the evolutionary process. Many optical mechanisms in beetles remain to be fully explored; with more diligent integration of the experimental and evolutionary perspectives in research, we can achieve a fuller understanding of insect chroma.
Acknowledgments
We thank the ASU Frontiers in Life Sciences programme and the organizers of the conference ‘Iridescence: More than Meets the Eye’, as well as A. Slipinksi (CSIRO Entomology, Canberra) and M. Brandley (University of California, Berkeley) for their comments on an early draft of the manuscript.
Appendix A. General definitions of optical terms used in this review and/or commonly encountered in iridescence literature
Anisotropic: having direction-dependent optical properties, i.e. transmitted light travels differently depending on its angle of incidence.
Band gaps: wavelengths (band or range of wavelengths) that are not allowed to propagate through a medium.
Birefringence: ‘double refraction’; splitting of a single light ray into two rays when travelling through anisotropic medium (e.g. crystal or dielectric material).
Bragg grating: can be a surface structure or a transmission structure. Diffracts light but with selective reflectance; differs from transmission or reflection (diffraction) grating in that it does not disperse an ordered spectrum; unlike multilayer reflectors, Bragg gratings can propagate wavelengths for long distances.
Bragg wavelength: wavelength of light that is reflected by a multilayer structure.
Broadband reflectance: bright silver or gold appearance caused by chirped or chaotic multilayer reflector (sensu Parker 1998).
Crystal: any solid where atoms/molecules are regularly ordered in three dimensions.
Dichroism: selective absorption of one polarization state, e.g. a film polarizer. This property converts unpolarized light into polarized light through absorption.
Dielectric: any insulating substance (but not strictly an insulator) that can dissipate electric or magnetic energy, e.g. insect chitin.
Dielectric constant: essentially, the square of the refractive index; also an electrical property of any insulating material.
Diffraction grating: a series of parallel nanoscale ridges that disperses light into ordered spectra.
Iridescence: colour that changes in hue and intensity with change in viewing angle.
Liquid crystal: substances with a phase of matter in between liquid and crystal; different LC phases are distinguished by their optical properties.
Metallic iridescence or metallic colours: structural colours arising from a cuticular multilayer reflector.
Modes: wavelengths of EM that are allowed to propagate through a photonic crystal.
Nanoscale: at the same scale as wavelengths of visible light, usually measured in nanometres.
Photonic crystal: any dielectric material with nanoscale refractive index periodicity affecting the propagation of light waves; can be one-, two- or three-dimensional. In entomological literature, most commonly used to describe three-dimensionally ordered lattice structures.
Refractive index: measure of a material's ability to slow down (bend) a beam of transmitted light.
Spectral iridescence: reflectance of one or more ordered spectra, the apparent position of which changes with angle of observation; caused by diffraction grating.
Appendix B. Anatomical terms important in locating and describing beetle iridescence mechanisms
The most important distinguishing character of beetles is the presence of elytra, the hardened, armour-like forewings that protect the insect's body. Most of the structural studies discussed here have focused on these appendages, which are easily removed from dried specimens for examination. The cross-sectional diagrams provided in figure 2d–f illustrate a section of the integument, the material of which the exoskeleton is composed. The integument comprises a soft, inner epidermis (nucleate cells shown at the bottom of figure 2d–f) and a hard, outer cuticle, which is typically divided into three layers, the inner endocuticle, the exocuticle and the waxy outer epicuticle. Beetle cuticle derives its strength and flexibility from cross-linked fibres of the polysaccharide molecule chitin, which are deposited in a layered matrix exterior to the epidermis.
Footnotes
One contribution of 13 to a Theme Supplement ‘Iridescence: more than meets the eye’.
References
- Acorn J. Mimetic tiger beetles and the puzzle of Cicindelid coloration (Coleoptera: Cicindelidae) Coleopterists Bull. 1988;42:28–33. [Google Scholar]
- Acorn J. The historical development of geographic color variation among dune Cicindela in Western Canada (Coleoptera: Cicindelidae) In: Noonan G.R., Ball G.E., Stork N.E., editors. The biogeography of ground beetles of mountains and islands. Intercept; Andover, UK: 1992. pp. 217–233. [Google Scholar]
- Adachi E. Unexpected variability of millennium green: structural color of Japanese jewel beetle resulted from thermosensitive porous organic multilayer. J. Morphol. 2007;268:826–829. doi: 10.1002/jmor.10557. [DOI] [PubMed] [Google Scholar]
- Aguiar A.P. An accurate procedure to describe colors in taxonomic works, with an example from Ichneumonidae (Hymenoptera) Zootaxa. 2005;1008:31–38. [Google Scholar]
- Alcock J. Courtship and mating in Hippomelas planicosta (Coleoptera: Buprestidae) Coleopterists Bull. 1976;30:343–348. [Google Scholar]
- Allen R., Ball G. Synopsis of Mexican taxa of the Loxandrus series (Coleoptera: Carabidae: Pterostichini) Trans. Am. Entomol. Soc. 1980;105:481–576. [Google Scholar]
- Anderson R., Lanteri A. New genera and species of weevils from the Galapagos Islands, Ecuador, and Cocos Island, Costa Rica (Coleoptera, Curculionidae, Entiminae, Entimini) Am. Museum Novitates. 2000;3299:1–15. doi: 10.1206/0003-0082(2000)299%3C0001:NGASOW%3E2.0.CO;2. [DOI] [Google Scholar]
- Anderson T., Richards A., Jr An electron microscope study of some structural colors of insects. J. Appl. Phys. 1942;13:748–758. doi: 10.1063/1.1714827. [DOI] [Google Scholar]
- Arnold E. History and function of scale microornamentation in lacertid lizards. J. Morphol. 2002;252:145–169. doi: 10.1002/jmor.1096. [DOI] [PubMed] [Google Scholar]
- Ball G. Reconstructed phylogeny and geographical history of genera of the tribe Galeritini (Coleoptera: Carabidae) In: Ball G.E., editor. Taxonomy, phylogeny, and zoogeography of beetles and ants: a volume dedicated to the memory of Philip Jackson Darlington Jr. Dr W. Junk; Dordrecht, The Netherlands: 1985. pp. 1904–1983. [Google Scholar]
- Ball G.E., Shpeley D. The species of eucheiloid Pericalina: classification and evolutionary considerations (Coleoptera: Carabidae: Lebiini) Can. Entomol. 1983;115:743–806. [Google Scholar]
- Barrows E. Life cycles, mating, and color change in tortoise beetles (Coleoptera: Chrysomelidae: Cassidinae) Coleopterists Bull. 1979;33:9–16. [Google Scholar]
- Barta A., Horvath G. Why is it advantageous for animals to detect celestial polarization in the ultraviolet? Skylight polarization under clouds and canopies is strongest in the UV. J. Theor. Biol. 2004;226:429–437. doi: 10.1016/j.jtbi.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Beebe W. Notes on the Hercules beetle, Dynastes hercules (Linn.) at Rancho Grande, Venezuela, with special reference to combat behavior. Zoologica. 1947;32:109–116. [Google Scholar]
- Bellamy, C. L. & Nelson, G. H. 2002 Buprestidae leach 1815. In American beetles, vol. 2 (eds R. H. Arnett & M. C. Thomas), pp. 98–112. New York, NY: CRC Press.
- Berthier S. Springer; Berlin, Germany: 2007. Iridescences: the physical colors of insects. [Google Scholar]
- Briscoe A.D., Chittka L. The evolution of color vision in insects. Annu. Rev. Entomol. 2001;46:471–510. doi: 10.1146/annurev.ento.46.1.471. [DOI] [PubMed] [Google Scholar]
- Caveney S. Cuticle reflectivity and optical activity in scarab beetles: the rôle of uric acid. Proc. R. Soc. B. 1971;178:205–225. doi: 10.1098/rspb.1971.0062. [DOI] [PubMed] [Google Scholar]
- Crowson R. Academic Press; London, UK: 1981. The biology of the Coleoptera. [Google Scholar]
- Dacke M., Nordstrцm P., Scholtz C., Warrant E. A specialized dorsal rim area for polarized light detection in the compound eye of the scarab beetle Pachysoma striatum. J. Comp. Physiol. A Sensory Neural Behav. Physiol. 2002;188:211–216. doi: 10.1007/s00359-002-0295-9. [DOI] [PubMed] [Google Scholar]
- Daly H., Doyen J., Purcell A. 2nd edn. Oxford University Press; New York, NY: 1998. Introduction to insect biology and diversity. [Google Scholar]
- Deparis O., Rassart M., Vandenbem C., Welch V., Vigneron J., Lucas S. Structurally tuned iridescent surfaces inspired by nature. New J. Phys. 2008;10:013 032. doi: 10.1088/1367-2630/10/1/013032. [DOI] [Google Scholar]
- De Silva L., Hodgkinson I., Murray P., Wu Q., Arnold M., Leader J., Mcnaughton A. Natural and nanoengineered chiral reflectors: structural color of manuka beetles and titania coatings. Electromagnetics. 2005;25:391–408. doi: 10.1080/02726340590957399. [DOI] [Google Scholar]
- Doberski J., Walmesley G. Microsculpture in UK ground beetles: are there patterns? Entomol. Sci. 2007;10:425–428. doi: 10.1111/j.1479-8298.2007.00229.x. [DOI] [Google Scholar]
- Durrer H., Villiger W. Schillerfarben von Euchroma gigantea (L.): (Coleoptera: Buprestidae): elektronenmikroskopische Untersuchung der Elytra. Int. J. Insect Morphol. Embryol. 1972;1:233–240. doi: 10.1016/0020-7322(72)90031-1. [DOI] [Google Scholar]
- Endler J. On the measurement and classification of colour in studies of animal colour patterns. Biol. J. Linn. Soc. 1990;41:315–352. doi: 10.1111/j.1095-8312.1990.tb00839.x. [DOI] [Google Scholar]
- Endler J. The color of light in forests and its implications. Ecol. Monogr. 1993;63:1–27. doi: 10.2307/2937121. [DOI] [Google Scholar]
- Erwin T. Thoughts on the evolutionary history of ground beetles: hypotheses generated from comparative faunal analyses of lowland forest sites in temperate and tropical regions. In: Erwin T.L., Ball G.E., Whitehead D.R., Halpern A.L., editors. Carabid beetles, their evolution, natural history, and classification. Dr. W. Junk; The Hague, The Netherlands: 1979. pp. 539–592. [Google Scholar]
- Erwin T. Tropical forests: their richness in Coleoptera and other arthropod species. Coleopterists Bull. 1982;36:74–75. [Google Scholar]
- Farrell B.D. “Inordinate fondness” explained: why are there so many beetles? Science. 1998;281:555–559. doi: 10.1126/science.281.5376.555. [DOI] [PubMed] [Google Scholar]
- Franciscolo M. About a new fungus-eating mordellid beetle from Ecuador (Coleoptera: Mordellidae) Annali del Museo Civico de Storia Naturale, Genova. 1985;85:79–94. [Google Scholar]
- Frantsevich L., Govardovski V., Gribakin F., Nikolajev G., Pichka V., Polanovsky A., Shevchenko V., Zolotov V. Astroorientation in Lethrus (Coleoptera, Scarabaeidae) J. Comp. Physiol. A Sensory Neural Behav. Physiol. 1977;121:253–271. doi: 10.1007/BF00609615. [DOI] [Google Scholar]
- Galusha J.W., Richey L.R., Gardner J.S., Cha J.N., Bartl M.H. Discovery of a diamond-based photonic crystal structure in beetle scales. Phys. Rev. E. 2008;77(Pt 1):050 904. doi: 10.1103/PhysRevE.77.050904. [DOI] [PubMed] [Google Scholar]
- Gans C., Baic D. Regional specialization of reptilian scale surfaces: relation of texture and biologic role. Science. 1977;195:1348–1350. doi: 10.1126/science.195.4284.1348. [DOI] [PubMed] [Google Scholar]
- Goldstein D. Reflection properties of Scarabaeidae. Proc. SPIE. 2005;5888:58880T. doi: 10.1117/12.618546. [DOI] [Google Scholar]
- Greenewalt C., Brandt W., Friel D. The iridescent color of hummingbird feathers. Proc. Am. Philos. Soc. 1960;104:249–253. [Google Scholar]
- Gwynne D., Rentz D. Beetles on the bottle: male buprestids mistake stubbies for females (Coleoptera) Aust. J. Entomol. 1983;22:79–80. doi: 10.1111/j.1440-6055.1983.tb01846.x. [DOI] [Google Scholar]
- Hadley N.F. Wax secretion and color phases of the desert tenebrionid beetle Cryptoglossus verrucosa (LeConte) Science. 1979;203:367–369. doi: 10.1126/science.203.4378.367. [DOI] [PubMed] [Google Scholar]
- Hadley N.F., Schultz T.D., Savill A. Spectral reflectances of three subspecies of the tiger beetle Neocicindela perhispida: correlations with their respective habitat substrates. NZ J. Zool. 1988;15:343–346. [Google Scholar]
- Hadley N.F., Savill A., Schultz T.D. Coloration and its thermal consequences in the New Zealand tiger beetle Neocicindela perhispida. J. Therm. Biol. 1992;17:55–61. doi: 10.1016/0306-4565(92)90020-G. [DOI] [Google Scholar]
- Hariyama T., Takaku Y., Hironaka M., Horiguchi H., Komiya Y., Kurachi M. The origin of the iridescent colors in coleopteran elytron. FORMA-TOKYO. 2002;17:123–132. [Google Scholar]
- Harris, R. 1979 A glossary of surface sculpturing. California Department of Food and Agriculture, Bureau of Entomology. Occasional Papers, no. 28, pp. 1–31.
- Hawks, D. 2001 Taxonomic and nomenclatural changes in Chrysina and a synonymic checklist of species (Scarabaeidae: Rutelinae). Occasional Papers of the Consortium Coleopterorum, no. 4, pp. 1–8.
- Hegedüs R., Horváth Á., Horváth G. Why do dusk-active cockchafers detect polarization in the green? The polarization vision in Melolontha melolontha is tuned to the high polarized intensity of downwelling light under canopies during sunset. J. Theor. Biol. 2006a;238:230–244. doi: 10.1016/j.jtbi.2005.05.033. [DOI] [PubMed] [Google Scholar]
- Hegedüs R., Szél G., Horváth G. Imaging polarimetry of the circularly polarizing cuticle of scarab beetles (Coleoptera: Rutelidae, Cetoniidae) Vision Res. 2006b;46:2786–2797. doi: 10.1016/j.visres.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Hinton H.E. Diffraction gratings in burying beetles (Nicrophorus) Entomologist. 1969;102:185–189. [Google Scholar]
- Hinton H.E. Some little known surface structures. In: Neville A.C., editor. Insect ultrastructure. Royal Entomological Society; London, UK: 1970. pp. 41–58. [Google Scholar]
- Hinton H. Natural deception. In: Gregory R.L., Gombrich E.H., editors. Illusion in nature and art. Duckworth; London, UK: 1973a. pp. 97–159. [Google Scholar]
- Hinton H.E. Some recent work on the colours of insects and their likely significance. Proc. Br. Ent. Nat. Hist. Soc. 1973b;6:43–54. [Google Scholar]
- Hinton H.E. Recent work on physical colors of insect cuticle. In: Hepburn H.R., editor. The insect integument. Elsevier; Amsterdam, The Netherlands: 1976a. pp. 475–496. [Google Scholar]
- Hinton H.E. Colour changes. In: Bligh J., Cloudsley-Thompson J.L., MacDonald A.G., editors. Environmental physiology of animals. Blackwell Scientific; London, UK: 1976b. pp. 389–412. [Google Scholar]
- Hinton H.E., Gibbs D. Diffraction gratings in Phalacrid beetles. Nature. 1969a;221:953–954. doi: 10.1038/221953a0. [DOI] [Google Scholar]
- Hinton H.E., Gibbs D. An electron microscope study of the diffraction gratings of some carabid beetles. J. Insect Physiol. 1969b;15:959–962. doi: 10.1016/0022-1910(69)90136-X. [DOI] [Google Scholar]
- Hinton H.E., Gibbs D. Diffraction gratings in gyrinid beetles. J. Insect Physiol. 1971;17:1023–1025. doi: 10.1016/0022-1910(71)90006-0. [DOI] [Google Scholar]
- Hinton H., Jarman G. Physiological colour change in the Hercules beetle. Nature. 1972;238:160–161. doi: 10.1038/238160a0. [DOI] [Google Scholar]
- Hunt T., et al. A comprehensive phylogeny of beetles reveals the evolutionary origins of a superradiation. Science. 2007;318:1913–1916. doi: 10.1126/science.1146954. [DOI] [PubMed] [Google Scholar]
- Jewell S., Vukusic P., Roberts N. Circularly polarized colour reflection from helicoidal structures in the beetle Plusiotis boucardi. New J. Phys. 2007a;9:99. doi: 10.1088/1367-2630/9/4/099. [DOI] [Google Scholar]
- Jewell S., Vukusic P., Roberts N. Circularly polarized color reflection in the beetle Plusiotis boucardi. Opt. Photon. News. 2007b;18:33. doi: 10.1364/OPN.18.12.000033. [DOI] [Google Scholar]
- Kattawar G. A search for circular polarization in nature. Opt. Photon. News. 1994;5:42–43. [Google Scholar]
- Kinoshita S., Yoshioka S., Miyazaki J. Physics of structural colors. Rep. Prog. Phys. 2008;71:1–30. doi: 10.1088/0034-4885/71/7/076401. [DOI] [Google Scholar]
- Knisley C.B., Schultz T.D. Virginia Museum of Natural History; Martinsville, VA: 1997. The biology of tiger beetles and a guide to the species of the South Atlantic States. [Google Scholar]
- Kurachi M., Takaku Y., Komiya Y., Hariyama T. The origin of extensive colour polymorphism in Plateumaris sericea (Chrysomelidae, Coleoptera) Naturwissenschaften. 2002;89:295–298. doi: 10.1007/s00114-002-0332-0. [DOI] [PubMed] [Google Scholar]
- Land M.F. The physics and biology of animal reflectors. Progr. Biophys. Mol. Biol. 1972;24:75–106. doi: 10.1016/0079-6107(72)90004-1. [DOI] [PubMed] [Google Scholar]
- Lawrence J., Britton E. Melbourne University Press; Carlton, Australia: 1994. Australian beetles. [Google Scholar]
- Leschen R. Systematics of Nitidulinae (Coleoptera: Nitidulidae): phylogenetic relationships, convexity and the origin of phallalophagy. Invertebr. Taxon. 1999;13:845–882. doi: 10.1071/IT98016. [DOI] [Google Scholar]
- Lindroth C. On elytral microsculpture of carabid beetles (Coleoptera: Carabidae) Entomol. Scand. 1974;5:251–264. [Google Scholar]
- Liu F., Yin H., Dong B., Qing Y., Zhao L., Meyer S., Liu X., Zi J., Chen B. Inconspicuous structural coloration in the elytra of beetles Chlorophila obscuripennis (Coleoptera) Phys. Rev. E. 2008;77:12 901. doi: 10.1103/PhysRevE.77.012901. [DOI] [PubMed] [Google Scholar]
- Maldovan M., Thomas E.L. Diamond-structured photonic crystals. Nat. Mater. 2004;3:593–600. doi: 10.1038/nmat1201. [DOI] [PubMed] [Google Scholar]
- Marvaldi A., Sequeira A., O Brien C., Farrell B. Molecular and morphological phylogenetics of weevils (Coleoptera, Curculionoidea): do niche shifts accompany diversification? Syst. Biol. 2002;51:761–785. doi: 10.1080/10635150290102465. [DOI] [PubMed] [Google Scholar]
- Mason C.W. Structural colors in insects, 3. S. Phys. Chem. 1927;31:1856–1872. doi: 10.1021/j150282a008. [DOI] [Google Scholar]
- Mason C. Transient colour changes in the tortoise beetles (Coleoptera: Chrysomelidae) Entomol. News. 1929;40:52–56. [Google Scholar]
- McClain E., Seely M.K., Hadley N.F., Gray V. Wax blooms in tenebrionid beetles of the Namib Desert: correlations with environment. Ecology. 1985;66:112–118. doi: 10.2307/1941311. [DOI] [Google Scholar]
- Michelson A.A. On metallic colouring in birds and insects. Phil. Mag. 1911;21:554–567. [Google Scholar]
- Miyamoto K., Kosaku A. Cuticular microstructures and their relationship to structural color in the shieldbug Poecilocoris lewisi Distant. FORMA-TOKYO. 2002;17:155–167. [Google Scholar]
- Monroe E., Monroe S. Origin of iridescent colors on the indigo snake. Science. 1968;159:97–98. doi: 10.1126/science.159.3810.97-a. [DOI] [PubMed] [Google Scholar]
- Mossakowski D. Reflection measurements used in the analysis of structural colours of beetles. J. Microsc. 1979;116:350–364. [Google Scholar]
- Mossakowski D. Pigmentation as a character for the reconstruction of evolution in cave beetles. In: Mossakowski D., Roth G., editors. Environmental adaptation and evolution. Gustav Fischer; Stuttgart, Germany: 1982. pp. 196–207. [Google Scholar]
- Neville, A. C. 1970 Cuticle ultrastructure in relation to the whole insect. In Insect infrastructure, vol. 5 (ed. A. C. Neville). Symp. R. Entomol. Soc. Lond. pp. 17–39.
- Neville A., Caveney S. Scarabaeid beetle exocuticle as an optical analogue of cholesteric liquid crystals. Biol. Rev. Camb. Philos. Soc. 1969;44:531–562. doi: 10.1111/j.1469-185X.1969.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Noyes J.A., Vukusic P., Hooper I.R. Experimental method for reliably establishing the refractive index of buprestid beetle exocuticle. Opt. Express. 2007;15:4351–4358. doi: 10.1364/OE.15.004351. [DOI] [PubMed] [Google Scholar]
- Oberprieler R.G., Marvaldi A., Anderson R. Weevils, weevils, weevils everywhere. Zootaxa. 2007;1668:491–520. [Google Scholar]
- Paclt J. A chronology of color charts and color terminology for naturalists. Taxon. 1983;32:393–405. doi: 10.2307/1221496. [DOI] [Google Scholar]
- Parker A. The diversity and implications of animal structural colours. J. Exp. Biol. 1998;201:2343–2347. doi: 10.1242/jeb.201.16.2343. [DOI] [PubMed] [Google Scholar]
- Parker A. 515 million years of structural colour. J. Opt. A Pure Appl. Opt. 2000;2:26–31. doi: 10.1088/1464-4258/2/6/201. [DOI] [Google Scholar]
- Parker A. Natural photonic engineers. Mater. Today. 2002;5:26–31. doi: 10.1016/S1369-7021(02)00929-X. [DOI] [Google Scholar]
- Parker A., Martini N. Structural colour in animals—simple to complex optics. Opt. Laser Technol. 2006;38:315–322. doi: 10.1016/j.optlastec.2005.06.037. [DOI] [Google Scholar]
- Parker A.R., McKenzie D. The cause of 50 million-year-old colour. Proc. R. Soc. B. 2003;270:S151–S153. doi: 10.1098/rsbl.2003.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker A., Townley H. Biomimetics of photonic nanostructures. Nat. Nanotechnol. 2007;2:347–353. doi: 10.1038/nnano.2007.152. [DOI] [PubMed] [Google Scholar]
- Parker A., Mckenzie D., Large M. Multilayer reflectors in animals using green and gold beetles as contrasting examples. J. Exp. Biol. 1998;201:1307–1313. doi: 10.1242/jeb.201.9.1307. [DOI] [PubMed] [Google Scholar]
- Parker A., Welch V., Driver D., Martini N. Structural colour: opal analogue discovered in a weevil. Nature. 2003;426:786–787. doi: 10.1038/426786a. [DOI] [PubMed] [Google Scholar]
- Pearson D. Biology of tiger beetles. Annu. Rev. Entomol. 1985;33:123–147. doi: 10.1146/annurev.en.33.010188.001011. [DOI] [Google Scholar]
- Pearson D., Vogler A. Cornell University Press; Ithaca, NY: 2001. Tiger beetles: the evolution, ecology, and diversity of the cicindelids. [Google Scholar]
- Pope R., Hinton H. A preliminary survey of ultraviolet reflectance in beetles. Biol. J. Linn. Soc. 1977;9:331–348. doi: 10.1111/j.1095-8312.1977.tb00275.x. [DOI] [Google Scholar]
- Prum R., Torres R. Structural colouration of avian skin: convergent evolution of coherently scattering dermal collagen arrays. J. Exp. Biol. 2003;206:2409–2429. doi: 10.1242/jeb.00431. [DOI] [PubMed] [Google Scholar]
- Prum R., Cole J., Torres R. Blue integumentary structural colours in dragonflies (Odonata) are not produced by incoherent Tyndall scattering. J. Exp. Biol. 2004;207:3999–4009. doi: 10.1242/jeb.01240. [DOI] [PubMed] [Google Scholar]
- Prum R., Quinn T., Torres R. Anatomically diverse butterfly scales all produce structural colours by coherent scattering. J. Exp. Biol. 2006;209:748–765. doi: 10.1242/jeb.02051. [DOI] [PubMed] [Google Scholar]
- Pursiainen O., Baumberg J., Winkler H., Viel B., Spahn P., Ruhl T. Nanoparticle-tuned structural color from polymer opals. Opt. Express. 2007;15:9553–9561. doi: 10.1364/OE.15.009553. [DOI] [PubMed] [Google Scholar]
- Rassart M., Colomer J.-F., Tabarrant T., Vigneron J.-P. Diffractive hygrochromic effect in the cuticle of the hercules beetle Dynastes hercules. New J. Phys. 2008;10:033 014. doi: 10.1088/1367-2630/10/3/033014. [DOI] [Google Scholar]
- Rayleigh L. The iridescent colours of birds and insects. Proc. R. Soc. A. 1930;128:624–641. doi: 10.1098/rspa.1930.0136. [DOI] [Google Scholar]
- Robertson J., McHugh J., Whiting M. A molecular phylogenetic analysis of the pleasing fungus beetles (Coleoptera: Erotylidae): evolution of colour patterns, gregariousness, and mycophagy. Syst. Entomol. 2004;29:173–187. doi: 10.1111/j.0307-6970.2004.00242.x. [DOI] [Google Scholar]
- Schmalfuss H. Morphology and function of cuticular micro-scales and corresponding structures in terrestrial isopods (Crust., Isop., Oniscoidea) Zoomorphology. 1978;91:263–274. doi: 10.1007/BF00999815. [DOI] [Google Scholar]
- Schultz T.D. The role of structural colors in predator avoidance by tiger beetles of the genus Cicindela. Bull. Entomol. Soc. Am. 1986;32:142–146. [Google Scholar]
- Schultz T.D. Tiger beetle defenses revisited: alternative defense strategies and colorations in two neotropical tiger beetles, Odontocheila nicaraguensis and Pseudoxycheila tarsalis (Carabidae: Cicindelinae) Coleop. Bull. 2001;55:153–163. doi: 10.1649/0010-065X(2001)055%5B0153:TBDRAD%5D2.0.CO;2. [DOI] [Google Scholar]
- Schultz T.D., Bernard G. Pointillistic mixing of interference colors in cryptic tiger beetles. Nature. 1990;337:72–73. doi: 10.1038/337072a0. [DOI] [Google Scholar]
- Schultz T.D., Hadley N.F. Structural colors of tiger beetles and their role in heat transfer through the integument. Physiol. Zool. 1987;60:737–745. [Google Scholar]
- Schultz T.D., Rankin M.A. The ultrastructure of epicuticular interference reflectors of tiger beetles (Cicindela) J. Exp. Biol. 1985a;117:88–110. [Google Scholar]
- Schultz T.D., Rankin M.A. Developmental changes in the interference reflectors and colorations of tiger beetles (Cicindela) J. Exp. Biol. 1985b;117:111–118. [Google Scholar]
- Seago A., Wheeler Q.D. Two new species of Aglyptinus Cockerell with unusual sexually dimorphic antennae and diffraction gratings (Coleoptera: Leiodidae) Coleopterists Bull. 2004;58:235–244. doi: 10.1649/613. [DOI] [Google Scholar]
- Shelly T., Pearson D. Size and color discrimination of the robber fly Efferia tricella (Diptera: Asilidae) as a predator on tiger beetles (Coleoptera: Cicindelidae) Environ. Entomol. 1978;7:790–793. [Google Scholar]
- Silberglied R. Communication in the ultraviolet. Annu. Rev. Ecol. Syst. 1979;10:373–398. doi: 10.1146/annurev.es.10.110179.002105. [DOI] [Google Scholar]
- Smith J.B. Brooklyn Entomological Society; Brooklyn, NY: 1909. An explanation of terms used in entomology. [Google Scholar]
- Spence J. Taxonomic status, relationships, and biogeography of Anatrichis LeConte and Oodinus Motschulsky (Carabidae: Oodini) Coleopterists Bull. 1982;36:567–580. [Google Scholar]
- Steiner W.E. A review of the biology of Phalacrid beetles (Coleoptera) In: Wheeler Q., Blackwell M., editors. Fungus-insect relationships. Perspectives in ecology and evolution. Columbia University Press; New York, NY: 1984. pp. 424–445. [Google Scholar]
- Thery M., Pincebourde S., Feer F. Dusk light environment optimizes visual perception of conspecifics in a crepuscular horned beetle. Behav. Ecol. 2008;19:627–634. doi: 10.1093/beheco/arn024. [DOI] [Google Scholar]
- Thomas D., Seago A., Robacker D. Reflections on golden scarabs. Am. Entomol. 2007;53:224. [Google Scholar]
- Torre-Bueno, J. R. 1989 The Torre-Bueno glossary of entomology (ed. R. T. Schuh). New York, NY: The New York Entomological Society.
- Van Tassel, E. R. 2001 Hydrophilidae latreille 1802. In American beetles, vol. 2 (eds R. H. Arnett & M. C. Thomas), pp. 187–208. New York, NY: CRC Press.
- Vigneron J., Colomer J., Vigneron N., Lousse V. Natural layer-by-layer photonic structure in the squamae of Hoplia coerulea (Coleoptera) Phys. Rev. E. 2005;72:61 904. doi: 10.1103/PhysRevE.72.061904. [DOI] [PubMed] [Google Scholar]
- Vigneron J., Rassart M., Vandenbem C., Lousse V., Deparis O., Biró L., Dedouaire D., Cornet A., Defrance P. Spectral filtering of visible light by the cuticle of metallic woodboring beetles and microfabrication of a matching bioinspired material. Phys. Rev. E. 2006;73:041 905. doi: 10.1103/PhysRevE.73.041905. [DOI] [PubMed] [Google Scholar]
- Vigneron J., et al. Switchable reflector in the Panamanian tortoise beetle Charidotella egregia (Chrysomelidae: Cassidinae) Phys. Rev. E. 2007;76:31 907. doi: 10.1103/PhysRevE.76.031907. [DOI] [PubMed] [Google Scholar]
- Vogler A., Kelley C. Covariation of defensive traits in Cicindela tiger beetles: a phylogenetic approach using mtDNA. Evolution. 1998;52:357–366. doi: 10.2307/2411088. [DOI] [PubMed] [Google Scholar]
- Vukusic P., Hallam B., Noyes J. Brilliant whiteness in ultrathin beetle scales. Science. 2007;315:348. doi: 10.1126/science.1134666. [DOI] [PubMed] [Google Scholar]
- Vulinec K. Iridescent dung beetles: a different angle. Florida Entomol. 1997;80:132–141. doi: 10.2307/3495550. [DOI] [Google Scholar]
- Warrant E.J., McIntyre P.D. Limitations to the resolution of superposition eyes. J. Comp. Physiol. A. 1990;167:785–803. doi: 10.1007/BF00189768. [DOI] [Google Scholar]
- Welch V., Vigneron J. Beyond butterflies—the diversity of biological photonic crystals. Opt. Quantum Electron. 2007;39:295–303. doi: 10.1007/s11082-007-9094-4. [DOI] [Google Scholar]
- Welch V., Lousse V., Deparis O., Parker A., Vigneron J. Orange reflection from a three-dimensional photonic crystal in the scales of the weevil Pachyrrhynchus congestus pavonius (Curculionidae) Phys. Rev. E. 2007;75:41919-1–41919-9. doi: 10.1103/PhysRevE.75.041919. [DOI] [PubMed] [Google Scholar]
- Yablonovitch E. Photonic band-gap structures. Opt. Soc. Am. B. 1993;10:283–295. [Google Scholar]