Abstract
A marked heterogeneity exists among stressors in their ability to reinstate alcohol seeking in rats. We have reported that the pharmacological stressor yohimbine, an alpha-2 adrenoceptor antagonist, potently reinstated alcohol seeking, but FG-7142, a benzodiazepine inverse agonist was ineffective. In rats, we determined that yohimbine elicits patterns of brain expression of the mRNAs for c-fos, a marker of neuronal activation, and corticotropin-releasing factor (CRF) a stress-related peptide, distinct from that produced by FG-7142. The purpose of the present experiment is to determine if these differential effects of yohimbine and FG-7142 on regional c-fos and CRF mRNA expression generalize to another animal commonly used in alcohol research, the C57 BL/6J mouse. In comparing the results of the present study to those of our previous one, we found a number of commonalities in the patterns of activation elicited by yohimbine and FG-7142 between the two species, and some notable differences. As we found in the rat, yohimbine selectively increased c-fos mRNA in the mouse NACs, BLA and CeA. Yohimbine increased CRF mRNA only in the mouse PVN, but was without effect on CRF mRNA in extrahypothalamic sites, the BNST and CeA. This differs from what we saw in the rat, where yohimbine increased CRF mRNA in these extrahypothalamic regions, but not the PVN. The selective induction of c-fos in the NACs, BLA and CeA of mice and rats by yohimbine offers further support for the idea that activation of these structures participates in reinstatement induced by such stressors.
Keywords: Stress, alcohol, reinstatement, in situ hybridization, corticotropin-releasing factor, c-fos
Introduction
The effects of stressors on alcohol seeking in laboratory rodents have been investigated for a number of years using the reinstatement procedure. We and others have shown that exposure to the environmental stressor intermittent footshock induces reinstatement of extinguished responding for alcohol, while social defeat or restraint has no effect [13, 23, 27, 30, 49]. Different pharmacological stressors also vary in their effects on reinstatement. Yohimbine, a prototypical alpha-2 adrenoceptor antagonist, induces a marked reinstatement of responding for alcohol [25], while FG-7142, an inverse benzodiazepine receptor agonist is ineffective [49]. In studies following up on these initial observations, we determined that the stress-related neuropeptide, corticotropin-releasing factor (CRF) plays a key role in these effects of yohimbine and footshock on alcohol seeking [24, 26, 29].
In a study designed to further define the biological basis for such differences, we reported that c-fos and CRF mRNA were selectively increased by stressors effective in inducing reinstatement, footshock and yohimbine, in three brain regions previously implicated in the rewarding effects of alcohol and other drugs of abuse [14]. We observed that only these two stressors induced c-fos mRNA in the shell of the nucleus accumbens, and in the basolateral and central amygdalar nuclei; footshock and yohimbine also selectively induced CRF mRNA in the dorsal bed nucleus of the stria terminalis. These results imply that neuronal activity in these regions may determine the effectiveness of stressor in the reinstatement of alcohol seeking.
In order to help establish the generality of these effects, we will determine in the present study whether another species, the mouse, shows such stressor-specific patterns of neuronal activation. Mice consume alcohol [9, 34, 50], and show increased consumption in response to footshock stress in a strain-dependent manner [31, 32]. Interestingly, there is evidence that rats and mice differ in behavioral and neural responses to stressors [1, 2, 5, 21, 48] and in the neuroanatomical organization of brain areas implicated in stress [1, 2, 5, 21, 48]. We will therefore contrast the effects of the pharmacological stressors yohimbine and FG-7142 on the expression of c-fos and CRF mRNA in the brains of C57 BL/6J mice. The results will be discussed in light of the previous findings in our study on rats [14].
Materials and methods
Animals
Twenty-four male C57 BL/6J mice (Charles River, Montreal, Canada) weighing 20–30 g were maintained at 22 °C (light phase 07.00 h–19.00 h) with free access to food and water. Experimental procedures were done in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication No. 80-23, revision 1996) and were approved by the local animal care committee. All efforts were made to minimize the animals pain and discomfort during the experiments. C57 BL/6J mice were used as they have been shown to consume more alcohol than other strains [4, 33].
Drug injections
Mice received vehicle injections (distilled water) prior to being placed into test chambers for 1 h on 3 separate occasions to habituate them to experimental procedures. On the test day, they were administered distilled water vehicle (n=8), the alpha-2 adrenoceptor antagonist yohimbine (Sigma, St. Louis, MO, 2.5 mg/kg, i.p., n=8) or the benzodiazepine inverse agonist FG-7142 (Sigma, 10 mg/kg, i.p, n=8) prior to placement in the test chambers. Mice were decapitated 60 min after vehicle or drug injections and their brains processed for in situ hybridization of c-fos and CRF mRNA. Increased c-fos mRNA induced by various stimuli are readily detectible in rats at this time point [8, 14]. Likewise, we and others have shown CRF mRNA to be significantly up regulated in the rat brain at this time after stress exposure [14, 18, 20, 28]. The doses of yohimbine and FG-7142 chosen were based on our findings in rats [14, 25, 49], and produce comparable levels of HPA activation and behavioral signs of anxiety in rats and mice [3, 7, 19, 35, 41, 42, 44].
In Situ Hybridization
The brains were rapidly removed, frozen in isopentane (−40 °C), and stored at −70 °C. Subsequently, coronal sections (10 µm) through the brain areas of interest were sliced on a cryostat and thaw-mounted onto glass slides coated with poly-l-lysine. Slicing was guided by the use of a brain atlas [39]. Brain areas sampled included those previously implicated in alcohol and drug seeking as well as others involved in responses to stress in laboratory rodents. These areas were the medial, orbital and anterior cingulate regions of the frontal cortex (MFC, OFC, ACg), the core and shell of the nucleus accumbens (NACc, NACs), the medial and lateral septum (MS, LS), dorsal and ventral bed nucleus of the stria terminalis (dBNST, vBNST), basolateral and central amygdala (BLA, CeA), paraventricular nucleus of the hypothalamus (PVN), dorsal and ventral hippocampus (dHIPP, vHIPP), the dorsal and median raphe nuclei (DRN, MRN) and the locus coeruleus (LC). All of these regions were processed for c-fos mRNA. The main CRF-containing nuclei in the brain namely, the BNST, PVN and CeA were processed for CRF mRNA [46]. In Table 1, the anterior-posterior distances from bregma for each of the areas sampled is shown.
Table 1.
Effects of pharmacological stressors on c-fos (A) and CRF (B) mRNA in mouse brain. The anterior-posterior location of each site is given in mm from bregma [39] and the field sizes used to measure each site are given in µm2. The results of the one way ANOVAs done on the integrated optical densities are also presented for each brain region. n=6–8 animals/group. Data are presented as group means ± S.E.M. of the percentage of the vehicle condition.
| A. c-fos mRNA | |||||||
|---|---|---|---|---|---|---|---|
| Site | A-P (mm) |
Area (µm2) |
df | F | p | Yohimbine (% Vehicle) |
FG-7142 (% Vehicle) |
| MFC | +2.1 | 1050 | 2,23 | 0.52 | .601 | 123.18±11.29 | 103.41±16.47 |
| ACg | +2.1 | 900 | 2,23 | 3.55 | .047 | 135.67±10.79 | 96.79±14.06 |
| OFC | +1.7 | 660 | 2,23 | 1.92 | .172 | 148.37±13.38 | 101.93±13.88 |
| NACc | +1.7 | 900 | 2,23 | 6.28 | .007 | 139.13±12.35 | 77.99±10.07 |
| NACs | +1.7 | 230 | 2,23 | 5.12 | .016 | 163.99±15.00* | 104.33±13.96 |
| CPu | +1.1 | 560 | 2,21 | 0.59 | .562 | 74.10±10.82 | 72.48±15.07 |
| MS | +1.1 | 370 | 2,23 | 2.32 | .123 | 116.02±8.44 | 89.98±7.57 |
| LS | +1.1 | 580 | 2,23 | 8.54 | .002 | 151.00±10.91* | 98.13±8.65 |
| dBNST | +0.2 | 450 | 2,23 | 5.36 | .013 | 185.81±31.33* | 84.49±14.90 |
| vBNST | +0.2 | 320 | 2,23 | 8.43 | .002 | 260.53±45.54* | 96.99±19.27 |
| PVN | −0.8 | 210 | 2,22 | 16.18 | .001 | 284.85±42.86* | 76.10±17.19 |
| BLA | −1.3 | 210 | 2,23 | 8.16 | .002 | 158.13±10.96* | 92.71±6.71 |
| CeA | −1.3 | 210 | 2,23 | 13.6 | .001 | 182.69±110.30* | 99.36±7.44 |
| dHIPP | −1.3 | 210 | 2,19 | 0.83 | .454 | 130.50±19.28 | 75.73±17.29 |
| vHIPP | −3.0 | 280 | 2,23 | 0.66 | .525 | 113.48±8.03 | 106.24±8.27 |
| VTA | −3.0 | 280 | 2,23 | 0.10 | .903 | 98.73±9.12 | 107.58±15.51 |
| DR | −4.0 | 210 | 2,23 | 1.65 | .216 | 117.62±10.33 | 90.02±13.26 |
| MR | −4.0 | 210 | 2,23 | 0.57 | .577 | 97.05±7.34 | 86.72±8.41 |
| LC | −5.4 | 140 | 2,23 | 17.6 | .001 | 177.29±11.19* | 107.78±9.48 |
| B. CRF mRNA | |||||||
| dBNST | −0.3 | 450 | 2,23 | 1.06 | .366 | 109.91±11.61 | 114.08±9.97 |
| vBNST | −0.3 | 320 | 2,23 | 0.02 | .985 | 101.06±12.73 | 98.03±8.93 |
| PVN | −1.8 | 210 | 2,23 | 4.95 | .017 | 136.49±8.62* | 108.21±11.01 |
| CeA | −2.6 | 210 | 2,22 | 2.79 | .085 | 114.06±14.89 | 148.99±19.89 |
Significantly different from the vehicle condition, p<0.05 (Bonferroni t-test).
In situ hybridization for c-fos and CRF mRNA was conducted according to a published protocol [16]. Oligonucleotide probes complementary to mouse c-fos (5′-CGG GCA GTG GCA CGT CTG GAT GCC GGC TGC CTT GCC TTC TCT GAC TGC-3′, [10]) and mouse CRF (5′-CAG TTT CCT GTT GCT GTG AGC TTG CTG AGC TAA CTG CTC TGC CCG GGC-3′, [38]) were 3′ end-labeled with 35S d-ATP using terminal deoxynucleotidyl transferase. Brain sections were hybridized overnight at 37° with the probe. After washing to a final stringency of 0.5X SSC, rinsing and dehydrating, the slides were placed on x-ray film for 7–18 days before developing and autoradiographic analysis. All brain sections from a particular site were processed simultaneously. Control experiments with probes made from the sense sequences of c-fos and CRF mRNA verified the specificity of hybridization of the antisense probes to their respective targets.
Semiquantitative Analysis of Autoradiograms and Statistics
Autoradiograms were digitized using a CCD camera (DXC-390, Sony, Toronto, ON) and a fluorescent transilluminator (Fotodyne, Hartland, WI). Semiquantitative analysis of the digitized images was done as described previously [15]. Guided by a brain atlas [39], 8 brain sections for each region were sampled bilaterally for each mouse, and a mean integrated optical density value was determined for each site using a sampling area of standard size by a technician blind to the treatment group. Results were calculated as integrated optical density values (mean grey value of pixels over threshold × number of pixels over threshold). The threshold was calculated as the mean background density + 3.5 × S.D. Table 1 shows the sizes of the sampling areas used to measure each brain region.
Separate ANOVAs were run on the integrated optical density values of c-fos or CRF mRNA in each brain area measured, with the between groups factor of Drug (Vehicle, 2.5 mg/kg yohimbine, 10 mg/kg FG-7142). Bonferroni-corrected t-tests were used to assess group differences when a significant main effect of Drug was found. For presentation, integrated optical density values (±S.E.M.) for each treatment in each brain region were calculated as percentage of the mean of the relevant vehicle treated group.
Results
c-fos mRNA
The effects of administration of the pharmacological stressors yohimbine or FG-7142 on c-fos mRNA in discrete regions of the mouse brain are shown in Table 1, panel A. ANOVAs revealed a significant effect of Drug in the ACg, NACc and NACs, the LS, BNST (dorsal and ventral), PVN, amygdalar nuclei (BLA and CeA) and LC (p's<0.05). Post hoc analysis revealed significant differences between the vehicle- and yohimbine-treated groups in these sites with the exception of the ACg and NACc. FG-7142 did not significantly affect c-fos mRNA in any brain region analyzed.
CRF mRNA
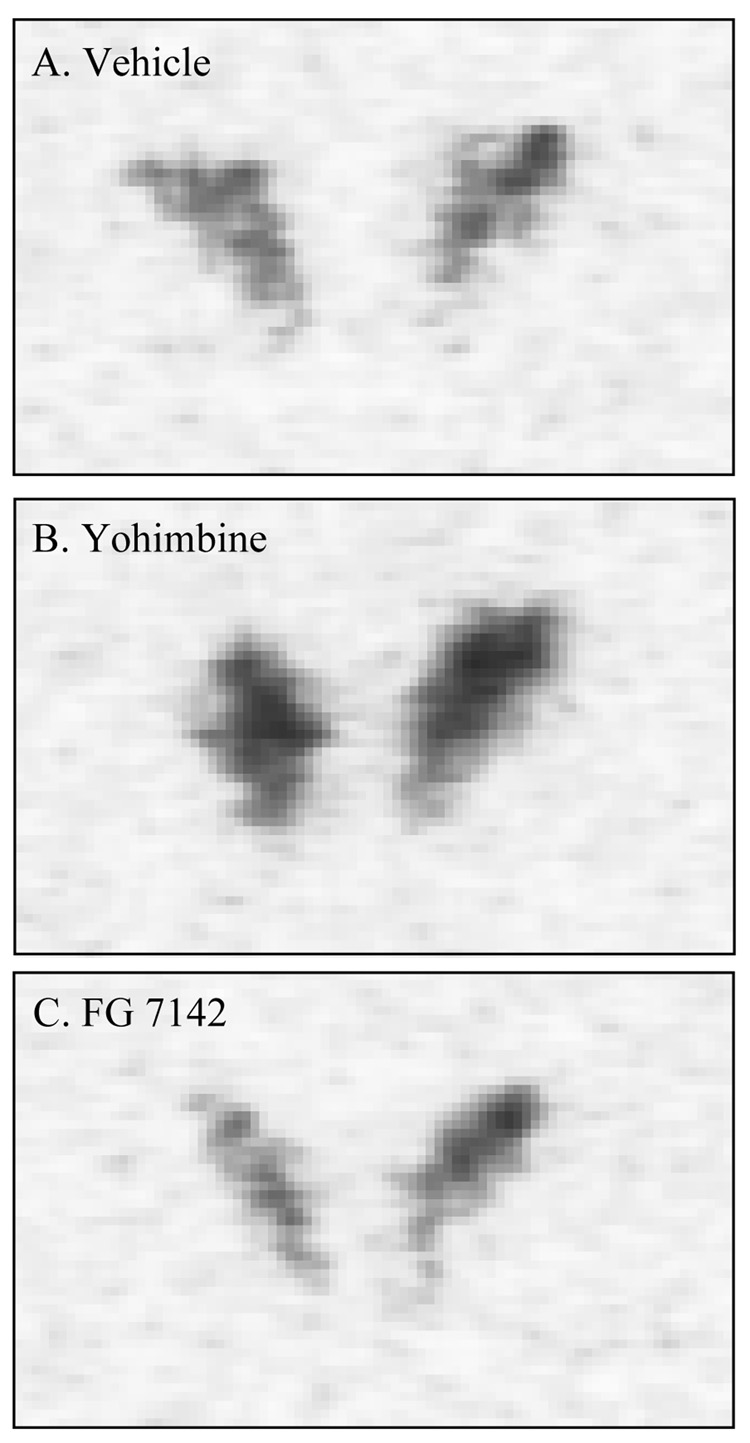
The results of the ANOVAs done examining the effects of drug treatment on the CRF mRNA in the BNST, CEA and PVN are shown in Table 1, panel B. Administration of yohimbine significantly increased CRF mRNA expression in the mouse PVN (p<0.05), but not in any other region. FG-7142 did not affect CRF mRNA in any region. Fig. 1 shows the effects of vehicle, yohimbine and FG-7142 on CRF mRNA in the PVN in photomicrographs from representative animals.
Figure 1.
Effects of pharmacological stressors on CRF mRNA in the PVN of mice. A, vehicle, B, yohimbine (2.5 mg/kg), C, FG-7142 (10 mg/kg). The digitized autoradiograms are from representative animals. Image contrast has been adjusted for clarity.
Discussion
In this report, we extended the results of our previous study describing the effects of pharmacological stressors on the regional brain activation of rats to C57 BL/6J mice. We found yohimbine-induced activation in regions of the mouse brain known to be involved in the rewarding effects of drugs of abuse. Although the patterns of drug-induced activation observed in mice share commonalities with those we observed in rats in our previous study [14], there were also a number of differences between the two species.
Yohimbine increased the expression of mRNA for the immediate early gene c-fos in the NAC shell, dorsal and ventral BNST, PVN, BLA, CeA and LC in the mouse brain. These observations are in agreement with our previous report and those of others on the effects of this drug in rat brain [36]. In our previous study, which employed multiple types of stressors, we noted that only the two stressors shown to produce reinstatement of alcohol seeking, footshock and yohimbine, induced c-fos in the NACs, BLA and CeA. A similar pattern was observed in mice; we found that yohimbine, but not FG-7142, selectively activated these brain regions. It appears, therefore, that yohimbine activates a common set of neural elements in rats and mice. Taken together with the behavioral data, these results further suggest that the NACs, BLA and CeA may comprise common circuitry underlying relapse to alcohol and other drugs.
We found differences between mice and rats in yohimbine-induced induction of c-fos in three brain regions, the OFC, NACc, DR and LS. The OFC, NACc and DR showed yohimbine-induced increases in c-fos in rats, but not in mice, while the LS was only activated in mice. These regions have been shown to be activated in both species by a variety of stimuli, including psychostimulants and stressors [6, 45, 47], but a systematic study of species differences in their neuroanatomy of function has not been done.
Only one other study describes the acute effects of yohimbine on c-fos in the mouse, at a dose twice that employed in the present study [43]. In agreement with our findings, they reported that yohimbine increased c-fos expression in the BLA, CeA and PVN. They did not find a significant effect in the LC, a region where we did see increased c-fos mRNA in response to yohimbine. One possible explanation of this is they measured c-fos immunoreactivity while we measured c-fos mRNA, that is known to be more rapidly induced [8, 22].
The other pharmacological stressor tested, FG-7142, was without effect on c-fos expression in mice in any brain region. This contrasts with the activation in the frontal cortices, MS and hippocampus we observed when yohimbine was administered to rats [14]. Although there are no other reports on the effects of FG-7142 administration on c-fos in mice, there are a number of studies on the behavioral effects of FG-7142 in this species. Since FG-7142 has been reported to reliably induce anxiety-like behavior in both rats [12, 40, 41] and mice [7, 19, 42], this failure to observe the induction of c-fos was unexpected. The most straightforward interpretation of this is that mice may be less sensitive to the neuronal effects of FG-7142. Had we tested higher doses of the drug effects on mRNAs for c-fos and CRF may have been noted.
In studies on rats, we have shown that CRF plays a key role in reinstatement induced by yohimbine [29]. The importance of CRF was underscored by our finding that CRF mRNA was significantly induced by yohimbine, but not by FG-7142, in the dBNST of rats [14]. In the present study in mice, CRF mRNA was unaffected by yohimbine or FG-7142 in the dBNST. The reasons for this apparent discrepancy are not known. There is, however, evidence of differences in the neuroanatomical organization of CRF-related elements in the BNST. For example, mice have fewer CRF1, and more CRF2 receptor mRNA-containing neurons in the dBNST [48].
Likewise, we did not observe a significant effect of yohimbine in the CeA of the mouse, but did in the rat. One possible explanation for this is that there is relatively low basal expression of CRF mRNA in the mouse CeA [1]. One observation that may support this idea is CRF mRNA in the CeA is activated in the mouse by systemic injection of cyclophosphamide 6 h after injection, but not at 1 h [37]. It is possible that the CRF mRNA response is slower in the mouse, and had we measured it at a later timepoint, we may have observed drug-induced differences.
Limitations
The effects of yohimbine on reinstatement of alcohol seeking have not been examined in mice. The dose of yohimbine chosen is based on our studies in rats on its effects on alcohol seeking [25] and produces similar effects on anxiety-like behavior in rats and mice [11, 17, 43]. Nevertheless, in the absence of a dose-response analysis on reinstatement in mice, the possibility that species differences in sensitivity to yohimbine underlie the differential patterns of induction of c-fos and CRF mRNA that we observed cannot be completely ruled out. This potential limitation also applies to FG-7142, as the effects of this compound on drug seeking are unknown in mice.
Related to this point, another factor that may limit the interpretation of the present results is that the mice in this study were alcohol naive. It could be argued that patterns of induction of c-fos and CRF mRNA would be different in animals that have self-administered alcohol and undergone extinction. An important extension of this study, therefore, would be to test the effects of yohimbine, FG-7142 or other stressors on c-fos and CRF mRNA in alcohol-experienced mice.
In this study, we investigated only one post-injection sacrifice interval (1 h). If mice differ from rats in the rate of c-fos and/or CRF mRNA induction and degradation, we may have missed drug-induced changes occurring at other times. Another extension of this study would therefore be to determine drug effects on mRNA expression at different times after injection in rats and mice.
Summary and conclusions
We found regionally specific effects of yohimbine on c-fos and CRF mRNA in the mouse brain. Yohimbine, a drug we have shown to potently induce the reinstatement of alcohol seeking in rats [25, 29], selectively increased c-fos mRNA in the mouse NACs, BLA and CeA, which corresponds to our findings in the rat. Yohimbine increased CRF mRNA only in the mouse PVN, but was without effect on CRF mRNA in extrahypothalamic sites, the BNST and CeA. In contrast, rats did not show a CRF response in the PVN, but did in the BNST and CeA. The selective induction of c-fos the NACs, BLA and CeA of mice and rats by yohimbine offers further support for the idea that their activation may be involved in the behavioral effects of yohimbine.
Acknowledgments
This work was support by grants from the Integrative Neuroscience Initiative on Alcoholism (East) to D. Funk and NIAAA (AA13108) to A.D. Lê.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Asan E, Yilmazer-Hanke DM, Eliava M, Hantsch M, Lesch KP, Schmitt A. The corticotropin-releasing factor (CRF)-system and monoaminergic afferents in the central amygdala: investigations in different mouse strains and comparison with the rat. Neuroscience. 2005;131:953–967. doi: 10.1016/j.neuroscience.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 2.Bain MJ, Dwyer SM, Rusak B. Restraint stress affects hippocampal cell proliferation differently in rats and mice. Neurosci Lett. 2004;368:7–10. doi: 10.1016/j.neulet.2004.04.096. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin HA, Johnston AL, File SE. Antagonistic effects of caffeine and yohimbine in animal tests of anxiety. Eur J Pharmacol. 1989;159:211–215. doi: 10.1016/0014-2999(89)90709-7. [DOI] [PubMed] [Google Scholar]
- 4.Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl) 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- 5.Brodkin ES, Carlezon WA, Jr, Haile CN, Kosten TA, Heninger GR, Nestler EJ. Genetic analysis of behavioral, neuroendocrine, and biochemical parameters in inbred rodents: initial studies in Lewis and Fischer 344 rats and in A/J and C57BL/6J mice. Brain Research. 1998;805:55. doi: 10.1016/s0006-8993(98)00663-5. [DOI] [PubMed] [Google Scholar]
- 6.Calandreau L, Jaffard R, Desmedt A. Dissociated roles for the lateral and medial septum in elemental and contextual fear conditioning. Learn Mem. 2007;14:422–429. doi: 10.1101/lm.531407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costall B, Jones BJ, Kelly ME, Naylor RJ, Tomkins DM. Exploration of mice in a black and white test box: validation as a model of anxiety. Pharmacol Biochem Behav. 1989;32:777–785. doi: 10.1016/0091-3057(89)90033-6. [DOI] [PubMed] [Google Scholar]
- 8.Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- 9.Dole VP, Ho A, Gentry RT, Chin A. Toward an analogue of alcoholism in mice: analysis of nongenetic variance in consumption of alcohol. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:827. doi: 10.1073/pnas.85.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drago J, Gerfen CR, Westphal H, Steiner H. D1 dopamine receptor-deficient mouse: cocaine-induced regulation of immediate-early gene and substance P expression in the striatum. Neuroscience. 1996;74:813–823. doi: 10.1016/0306-4522(96)00145-5. [DOI] [PubMed] [Google Scholar]
- 11.File SE. Aversive and appetitive properties of anxiogenic and anxiolytic agents. Behav. Brain Res. 1986;21:189–194. doi: 10.1016/0166-4328(86)90236-6. [DOI] [PubMed] [Google Scholar]
- 12.File SE, Pellow S, Braestrup C. Effects of the beta-carboline, FG 7142, in the social interaction test of anxiety and the holeboard: correlations between behaviour and plasma concentrations. Pharmacol. Biochem. Behav. 1985;22:941–944. doi: 10.1016/0091-3057(85)90299-0. [DOI] [PubMed] [Google Scholar]
- 13.Funk D, Harding S, Juzytsch W, Le AD. Effects of unconditioned and conditioned social defeat on alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2005;183:341–349. doi: 10.1007/s00213-005-0194-1. [DOI] [PubMed] [Google Scholar]
- 14.Funk D, Li Z, Le AD. Effects of environmental and pharmacological stressorson c-fos and corticotropin-releasing factor mRNA in rat brain: Relationship to the reinstatement of alcohol seeking. Neuroscience. 2006;138:235–243. doi: 10.1016/j.neuroscience.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 15.Funk D, Li Z, Shaham Y, Le AD. Effect of blockade of corticotropin-releasing factor receptors in the median raphe nucleus on stress-induced c-fos mRNA in the rat brain. Neuroscience. 2003;122:1–4. doi: 10.1016/j.neuroscience.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 16.GeneDetect. In-situ hybridization using GeneDetect oligonucleotide probes. Auckland: GeneDetect; 2001. [Google Scholar]
- 17.Haapalinna A, Viitamaa T, MacDonald E, Savola JM, Tuomisto L, Virtanen R, Heinonen E. Evaluation of the effects of a specific alpha 2-adrenoceptor antagonist, atipamezole, on alpha 1- and alpha 2-adrenoceptor subtype binding, brain neurochemistry and behaviour in comparison with yohimbine. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:570–582. doi: 10.1007/pl00005092. [DOI] [PubMed] [Google Scholar]
- 18.Herman JP, Schafer KH, Sladek CD, Day R, Young EA, Akil H, Watson SJ. Chronic electroconvulsive shock treatment elicits up-regulation of CRF and AVP mRNA in select populations of neuroendocrine neurons. Brain Res. 1989;501:235–246. doi: 10.1016/0006-8993(89)90641-0. [DOI] [PubMed] [Google Scholar]
- 19.Holmes A, Rodgers RJ. Prior exposure to the elevated plus-maze sensitizes mice to the acute behavioral effects of fluoxetine and phenelzine. Eur J Pharmacol. 2003;459:221–230. doi: 10.1016/s0014-2999(02)02874-1. [DOI] [PubMed] [Google Scholar]
- 20.Hsu DT, Chen FL, Takahashi LK, Kalin NH. Rapid stress-induced elevations in corticotropin-releasing hormone mRNA in rat central amygdala nucleus and hypothalamic paraventricular nucleus: an in situ hybridization analysis. Brain Res. 1998;788:305–310. doi: 10.1016/s0006-8993(98)00032-8. [DOI] [PubMed] [Google Scholar]
- 21.Imaki T, Katsumata H, Konishi SI, Kasagi Y, Minami S. Corticotropin-releasing factor type-1 receptor mRNA is not induced in mouse hypothalamus by either stress or osmotic stimulation. J Neuroendocrinol. 2003;15:916–924. doi: 10.1046/j.1365-2826.2003.01071.x. [DOI] [PubMed] [Google Scholar]
- 22.Kovacs KJ. c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem Int. 1998;33:287–297. doi: 10.1016/s0197-0186(98)00023-0. [DOI] [PubMed] [Google Scholar]
- 23.Le A, Shaham Y. Neurobiology of relapse to alcohol in rats. Pharmacol. Ther. 2002;94:137–156. doi: 10.1016/s0163-7258(02)00200-0. [DOI] [PubMed] [Google Scholar]
- 24.Le AD, Harding S, Juzytsch W, Fletcher PJ, Shaham Y. The role of corticotropin-releasing factor in the median raphe nucleus in relapse to alcohol. J. Neurosci. 2002;22:7844–7849. doi: 10.1523/JNEUROSCI.22-18-07844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology (Berl) 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- 26.Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- 27.Le AD, Quan B, Juzytsch W, Fletcher PJ, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology. 1998;135:169. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- 28.Makino S, Shibasaki T, Yamauchi N, Nishioka T, Mimoto T, Wakabayashi I, Gold PW, Hashimoto K. Psychological stress increased corticotropin-releasing hormone mRNA and content in the central nucleus of the amygdala but not in the hypothalamic paraventricular nucleus in the rat. Brain Res. 1999;850:136–143. doi: 10.1016/s0006-8993(99)02114-9. [DOI] [PubMed] [Google Scholar]
- 29.Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, Lê AD. The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2007;195:345–355. doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- 30.Martin-Fardon R, Ciccocioppo R, Massi M, Weiss F. Nociceptin prevents stress-induced ethanol- but not cocaine-seeking behavior in rats. Neuroreport. 2000;11:1939–1943. doi: 10.1097/00001756-200006260-00026. [DOI] [PubMed] [Google Scholar]
- 31.Matthews DB, Bhave SV, Belknap JK, Brittingham C, Chesler EJ, Hitzemann RJ, Hoffmann PL, Lu L, McWeeney S, Miles MF, Tabakoff B, Williams RW. Complex genetics of interactions of alcohol and CNS function and behavior. Alcohol Clin Exp Res. 2005;29:1706–1719. doi: 10.1097/01.alc.0000179209.44407.df. [DOI] [PubMed] [Google Scholar]
- 32.Matthews DB, Morrow LA, O'Buckley T, Flanigan TJ, Berry RB, Cook MN, Mittleman G, Goldowitz D, Tokunage S, Silvers JM. Acute mild footshock alters ethanol drinking and plasma corticosterone levels in C57BL/6J male mice, but not DAB2/J or A/J male mice. Alcohol. doi: 10.1016/j.alcohol.2008.05.001. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McClearn GE, Rodgers DA. Differences in alcohol preference among inbred strains of mice. Quarterly Journal of Studies of Alcohol. 1959;20:691. [Google Scholar]
- 34.Miczek KA, de Almeida RM. Oral drug self-administration in the home cage of mice: alcohol-heightened aggression and inhibition by the 5-HT1B agonist anpirtoline. Psychopharmacology (Berl) 2001;157:421–429. doi: 10.1007/s002130100831. [DOI] [PubMed] [Google Scholar]
- 35.Mikkelsen JD, Soderman A, Kiss A, Mirza N. Effects of benzodiazepines receptor agonists on the hypothalamic-pituitary-adrenocortical axis. Eur J Pharmacol. 2005;519:223–230. doi: 10.1016/j.ejphar.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 36.Myers EA, Banihashemi L, Rinaman L. The anxiogenic drug yohimbine activates central viscerosensory circuits in rats. J. Comp. Neurol. 2005;492:426–441. doi: 10.1002/cne.20727. [DOI] [PubMed] [Google Scholar]
- 37.Nishii H, Nomura M, Aono H, Fujimoto N, Matsumoto T. Up-regulation of galanin and corticotropin-releasing hormone mRNAs in the key hypothalamic and amygdaloid nuclei in a mouse model of visceral pain. Regul Pept. 2007;141:105–112. doi: 10.1016/j.regpep.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 38.Nomura M, Serino R, Yamamoto Y, Ozaki Y, Saito J, Matsumoto T, Yamashita H, Ueta Y. Upregulation of CRH gene expression and downregulation of arginine vasopressin gene expression in the hypothalamus of bilateral nephrectomized rats. Life Sci. 2002;72:501–509. doi: 10.1016/s0024-3205(02)02287-7. [DOI] [PubMed] [Google Scholar]
- 39.Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates. Amsterdam: Elsevier Academic Press; 2004. [Google Scholar]
- 40.Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986;24:525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- 41.Pellow S, File SE. The effects of putative anxiogenic compounds (FG 7142, CGS 8216 and Ro 15-1788) on the rat corticosterone response. Physiol Behav. 1985;35:587–590. doi: 10.1016/0031-9384(85)90145-3. [DOI] [PubMed] [Google Scholar]
- 42.Rodgers RJ, Cole JC, Aboualfa K, Stephenson LH. Ethopharmacological analysis of the effects of putative 'anxiogenic' agents in the mouse elevated plus-maze. Pharmacol Biochem Behav. 1995;52:805–813. doi: 10.1016/0091-3057(95)00190-8. [DOI] [PubMed] [Google Scholar]
- 43.Sahun I, Gallego X, Gratacos M, Murtra P, Trullas R, Maldonado R, Estivill X, Dierssen M. Differential responses to anxiogenic drugs in a mouse model of panic disorder as revealed by Fos immunocytochemistry in specific areas of the fear circuitry. Amino Acids. 2007;33:677–688. doi: 10.1007/s00726-006-0464-1. [DOI] [PubMed] [Google Scholar]
- 44.Stephens DN, Schneider HH, Kehr W, Jensen LH, Petersen E, Honore T. Modulation of anxiety by beta-carbolines and other benzodiazepine receptor ligands: relationship of pharmacological to biochemical measures of efficacy. Brain Res Bull. 1987;19:309–318. doi: 10.1016/0361-9230(87)90099-2. [DOI] [PubMed] [Google Scholar]
- 45.Stone EA, Quartermain D, Lin Y, Lehmann ML. Central alpha1-adrenergic system in behavioral activity and depression. Biochem Pharmacol. 2007;73:1063–1075. doi: 10.1016/j.bcp.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Swanson LW, Sawchenko P, Rivier J, Vale W. Organization of bovine corticotropin releasing factor immunoreactive cells and fibers in the rat brain. Neuroendocrinology. 1983;36:165. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- 47.Trinh JV, Nehrenberg DL, Jacobsen JP, Caron MG, Wetsel WC. Differential psychostimulant-induced activation of neural circuits in dopamine transporter knockout and wild type mice. Neuroscience. 2003;118:297–310. doi: 10.1016/s0306-4522(03)00165-9. [DOI] [PubMed] [Google Scholar]
- 48.Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J. Comp. Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 49.Wang L. The Role of Stress in Relapse to Alcohol. University of Toronto; 2002. [Google Scholar]
- 50.Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]