Abstract
Betel quid (BQ)-chewing oral cancer is a prevalent disease in many countries of Southeast Asia. Yet, the precise disease mechanism remains largely unknown. Here, we show that BQ extract-induced cell motility in three oral cancer cells (Ca9-22, SAS, and SCC9) presumably involves the Src family kinases (SFKs). Besides, BQ extract can markedly induce cell migration of wild type mouse embryonic fibroblasts (MEFs) but not MEFs lacking three SFK members, namely, Src, Yes, and Fyn, indicating the requirement of SFKs for BQ-induced cell motility. Betel quid extract can also elevate cellular SFK activities because phosphorylation of tyrosine 416 at the catalytic domain is increased, which in turn promotes phosphorylation of an in vitro substrate, enolase. Furthermore, we identified that areca nut, a major component of BQ, is the key factor accounting for BQ-induced cell migration and invasion through SFKs-mediated signaling pathways. Immunohistochemistry revealed that, particularly in BQ-chewing cases, the activity of SFKs was significantly higher in tumor-adjacent mucosa than that in solid tumor areas (P < .01). These results suggest a possible role of SFKs in tumor-host interface and thus in early tumor invasion in vivo. Consistent with this is the observation that activation of SFKs is colocalized with invasive tumor fronts in oral squamous cell carcinoma. Together, we conclude that SFKs may represent a potential biomarker of invasion and therapeutic target in BQ-induced oral cancer.
Introduction
Betel quid (BQ) generally consists of areca nut (AN), inflorescence of Piper betle (IPB) and slaked lime (SL). Betel quid chewing has been widely practiced in many parts of Southeast Asian countries and Asian-migrant communities elsewhere, adding up to a total of 600 million BQ chewers worldwide [1]. In Taiwan, the incidence of oral cancer death has been steadily increasing during the past decade. According to recent statistics by the Department of Health, oral cancer deaths have become the fourth common cause of overall cancer death in Taiwanese male population. It has been well documented that the high incidence of oral cancer death correlates intimately with BQ chewing [2,3]. In fact, mounting evidence indicates a causative relationship between BQ chewing and carcinogenesis of oral squamous cell carcinoma (OSCC) [1,4,5]. Despite mainly epidemiologic evidence, a recent report by the International Agency for Research on Cancer further confirmed that BQ without tobacco and AN alone are sufficient to cause carcinogenic effects in human [6]. However, the molecular mechanisms of BQ-induced oral carcinogenesis remain virtually unknown.
Substantial research efforts have been made in an attempt to illuminate how BQ chewing could lead to oral carcinogenesis. For example, it has been shown that AN extract and arecoline, one of the AN alkaloids, can induce mutagenic and genotoxic effects in vitro [7–9]. Also, BQ ingredients have been demonstrated to induce reactive oxygen species and inflammatory mediators such as prostaglandins [10,11], two critical factors thought to promote chemical carcinogenesis. Noticeably, two recent reports went further to verify that AN ingredients can activate mitogen-activated protein kinases in oral keratinocytes, leading to the induction of inflammatory mediators and other stress responses such as activation of NF-κB signaling pathway [12,13]. There are, however, rather limited studies that directly link BQ ingredients-induced cellular responses to cancer-specific phenotypes during the course of multistep chemical carcinogenesis.
Squamous cell carcinoma is the most common malignant neoplasm of the oral cavity, in that metastasis to cervical lymph nodes of the neck is the most important prognostic indicator [14]. Invasion or metastasis of OSCC requires active cell migration through the extracellular matrix with simultaneous remodeling of intercellular adhesions. This process is primarily assisted by up-regulation of the extracellular matrix-degrading enzymes matrix metalloproteinases (MMPs), in particular MMP-2 and MMP-9, and disruption of cell-cell adhesion such as methylation of E-cadherin and degradation of β-catenin [15–17]. Interestingly, previous studies by others suggest that galectins, especially galectin-7, could promote cancer cell migration of head and neck squamous cell carcinomas through upregulating the expression of MMP-9 [18,19]. In addition, there is increasing evidence that overexpression of epidermal growth factor receptor (EGFR) is correlated with oral cancer metastasis [20]. However, whether BQ ingredients might affect oral cancer cell behavior such as invasion and/or metastasis is still unclear. Here, we report that BQ and AN extracts can both significantly induce oral cancer cell migration and invasion in vitro presumably through activation of the Src family kinases (SFKs). Notably, AN seems to be the key factor of the three major BQ components responsible for these biologic effects. Moreover, our immunohistochemical analyses suggest that the activity of SFKs might be involved in early invasion of BQ-induced oral carcinogenesis.
Materials and Methods
BQ Extract and BQ Ingredients' Extracts Preparations
Betel quid and respective BQ components including AN, IPB, and SL were obtained from local venders. Water-soluble extracts of BQ or each component (AN, IPB, or SL) were prepared and optimized working concentrations were determined as described previously [21]. Briefly, cells were treated with BQ ingredients for 24 hours, and cytotoxicity was evaluated by measuring lactate dehydrogenase activity in culture medium. Working concentration at 0.2 mg/ml for AN, IPB, or SL has no detectable cytotoxicity.
Cell Culture and Transient Transfection
Oral cancer cell lines Ca9-22, SAS, and SCC9 were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with nutrient mixture F-12 (DMEM/F12; Invitrogen Technologies, Carlsbad, CA) and 10% FBS. SYF cells, obtained from American Type Culture Collection (ATCC, Manassas, VA), and mouse embryonic fibroblasts (MEFs) were cultured in DMEM supplemented with 10% FBS. For transient transfection, 8 x 105 Ca9-22 cells were seeded on 60-mm dishes 24 hours before transfection. Afterward, cells were transfected with pcDNA3.0-HA-CSK or the control vector by using FuGENE 6 transfection reagent (Roche Applied Science, Indianapolis, IN). At 36 to 48 hours after transfection, cells were harvested for subsequent migration assay or Western blot analysis.
Plasmid Construction
HA-CSK expression construct was generated by amplifying the full-length C-terminal Src kinase (CSK) cDNA from astrocytoma cDNA libraries by polymerase chain reaction using forward (5′-GAATTCATGTCAGCAATACAGGCCGCC-3′) and reverse (5′-CTCGAGTCACAGGTGCAGCTCGTG-3′) primers. The cDNA fragment was cloned into pGEM-T Easy vector (Promega, Madison, WI), sequenced, and then subcloned into BamHI/XhoI site in the pcDNA3.0-HA vector.
Migration and Invasion Assays
Toxin A (Clostridium difficile) and PP2 (4-amino-5-(4-chlorophenyl-7-(t-butyl) pyrazolo [3, 4-d] pyrimidine) were purchased from Calbiochem (San Diego, CA). A total of 3 x 104 oral cancer cells in serum-free culture medium containing toxin A (1 ng/ml), PP2 (2.5–10 µM), and/or BQ ingredients' extracts (1.6 or 0.2 mg/ml) were seeded onto the upper chamber of cell migration inserts with 8.0-µm pore size polycarbonate membrane (BD Biosciences, San Diego, CA), and the inserts were then placed into a culturing well containing 10% FBS medium and treatments as in the upper chamber. Afterward, cells were allowed to migrate for 24 hours, and nonmigrating cells on the upper chamber were removed with a cotton swab. Cells that migrated to the lower side of the inserts were fixed with 100% methanol and stained with Giemsa (Merck, Germany). Cell migration assays for SYF and MEF cells were performed as described above except that the seeding cell number was 1 x 104 and migrating period was 3 hours for MEF and 4 hours for SYF cells. For invasion assays, 1 x 105 cells were seeded onto the BD BioCoat Matrigel Chambers (BD Biosciences) with the remaining treatments or procedures similar to migration assays. Total migrated or invaded cell number was counted under brightfield optics at an original magnification of x100.
Western Blot Analysis
For the detection of ectopic expression of HA-CSK or endogenous expression of Src family proteins, the transiently transfected cells were lysed in RIPA buffer [100 mM Tris (pH 8.2), 150 mM NaCl, 1% deoxycholic acid, 1% Triton X-100, 0.1% SDS, and 5 mM EDTA] supplemented with protease inhibitor cocktail tablets (Roche Molecular Biochemicals, Mannheim, Germany). Protein concentrations of cell extracts were determined by bicinchoninic acid protein assay reagent (Pierce, Rockford, IL) using BSA as a standard. Total cell extracts (12 µg per lane) were resolved on 10% SDS PAGE, followed by transfer to Immobilon-P PVDF membrane (Millipore, Billerica, MA). The membranes were probed with anti-HA (Covance, Berkeley, CA) or anti-Src (Src2; Santa Cruz Biotechnology, Santa Cruz, CA) antibody, visualized with enhanced chemiluminescence reagents, SuperSignal, (Pierce) and underwent autoradiography. For the detection of endogenous phospho-Src (pY 416), cells were treated with BQ (1.6 mg/ml) or AN (0.2 mg/ml) extract at various time points and then lysed directly in 2 x SDS sample buffer. Immunoblot analysis was carried out as above except that the primary antibody was anti-Src (pY416; Cell Signaling Technology, Inc., Danvers, MA), and the incubation was done at 4°C overnight.
In Vitro Kinase Assay
The cellular activity of SFKs treated or untreated with BQ ingredients was detected by in vitro kinase assay as described by Chan et al. [22] with minor modifications. Src family kinases were immunoprecipitated by 5 µg of anti-Src (Src2; Santa Cruz Biotechnology) and protein A/G-Sepharose beads (Calbiochem) overnight at 4°C. The immunoprecipitates were then washed thrice with 1% Nonidet P-40 lysis buffer and twice with 25 mM Tris-HCl, pH 7.4, with 10 mM MnCl2. Later, the beads were incubated with 20 µl of kinase buffer (50 mM Tris-HCl, pH 7.4, 10 mM MnCl2, 1 mM DTT) containing 2 µg of acid-denatured enolase (Sigma, St. Louis, MO) and 10 mCi of [γ-32P]ATP (PerkinElmer Life Sciences, Waltham, MA) per reaction for 10 minutes at 37°C. Reactions were stopped by adding Laemmli sample buffer and resolved by SDS-PAGE followed by autoradiography.
Tissue Preparation and Immunohistochemistry
Surgical resection specimens including normal mucosa, tumor-adjacent mucosa, leukoplakia, and squamous cell carcinoma were obtained from patients undergoing maxillofacial surgery. The Institutional Review Board of National Taiwan University Hospital approved the use of these tissues. Subsequently, tissues were embedded in an optimal cutting temperature compound and frozen at -70°C. Frozen tissue sections of 5 µm in thickness were fixed in TBS containing 4% paraformaldehyde for 15 minutes. Endogenous peroxidase was depleted by incubating with 0.3% H2O2 and 10% methanol for 30 minutes. Blocking reaction was carried out in TBST containing 10% goat serum for 60 minutes. Later, tissue sections were first incubated with anti-Src (pY416; Biosource, Camarillo, CA) at 1:200 dilution in TBST for 60 minutes at room temperature, followed by washing with TBST and then incubating with SuperPicture HRP Polymer Conjugate secondary antibody (Zymed Laboratories, San Francisco, CA) for 30 minutes at room temperature. To detect immunoreactivity, DAB chromagen (Zymed Laboratories) was applied to the tissue sections until the desired staining intensity was achieved. Sections were then counterstained with hematoxylin (DAKO, Carpinteria, CA) and mounted with Entellan (Merck). Interpretations of staining results were performed by a pathologist from the Department of Pathology at the National Taiwan University Hospital.
Semiquantitation of Src (pY416) Immunoreactivities and Statistics
Immunoreactivities were quantified with a 12-point weighted score [23]. First, the percentage of positive staining in each section was scored with a 5-point scale: 0 for <5%, 1 for 5% to 25%, 2 for 25% to 50%, 3 for 50% to 75%, and 4 for >75%. Second, the intensity of positive staining was scored with a 3-point scale: 1 for weak, 2 for medium, and 3 for strong. The weighted score for each section was obtained by adding the percentage score and the intensity score. The staining intensity was compared to that in colon cancer sections, which served as the positive control, and was scored as 3. Correlations between immunoreactivities for tumor-adjacent mucosa sections and those for the remaining tissue categories were calculated by using an independent two-population t test. Significance was defined at P < .05. Graphing and statistics were plotted by Microcal Origin 6.0 software.
Results
Betel Quid Extract Induces Oral Cancer Cell Migration through SFKs-Mediated Signaling Pathways
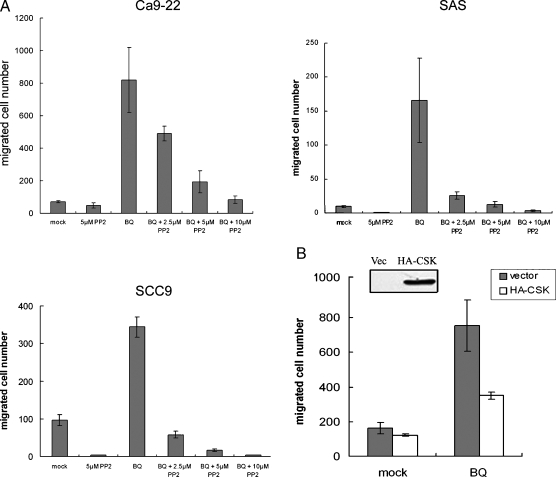
To test the hypothesis that BQ extract might increase oral cancer cell motility through certain cellular signaling pathway(s), oral cancer cells Ca9-22 were treated with BQ extract while being allowed to migrate for 24 hours in a Boyden's chamber-based apparatus. Treating Ca9-22 cells with BQ extract at 1.6 mg/ml, the optimal working concentration determined by cytotoxicity [21], resulted in a dramatic six-fold increase in cell migration. Because the SFKs and the Rho family GTPase are the two central cellular signalings implicated in cell migration, we asked whether PP2 and toxin A (C. difficile), the two respective selective inhibitors of the aforementioned signaling pathways, could act against the observed BQ-induced cell migration. Interestingly, our findings revealed that PP2, but not toxin A, could considerably counteract BQ-induced cell migration, raising the possibility that BQ extract might enhance cancer cell motility through selective activation of SFKs (data not shown). To exclude serendipity, we examined whether BQ-induced cell migration could be observed in additional oral cancer cells and how BQ-treated cancer cells responded to increasing concentrations of PP2 (2.5–10 µM). We found that BQ extract could evidently increase cell migration in all three oral cancer cell lines, whereas this was counteracted by PP2 in a dose-dependent manner (Figure 1A). To confirm this result, an expression vector carrying CSK, the cellular-negative regulator of SFKs instead of a synthetic inhibitor such as PP2, was introduced into Ca9-22 cells followed by allowing cell migration in the presence or absence of BQ extract. Ectopic overexpression of Csk reduced BQ-induced cell migration by about two-fold (Figure 1B). These results suggest that BQ extract can significantly increase oral cancer cell motility presumably through SFKs-mediated signaling pathways.
Figure 1.
Betel quid extract induces oral cancer cell migration through activation of SFKs. (A) Cell migration of three oral cancer cell lines Ca9-22, SAS, and SCC9 was all significantly induced when treated with BQ extract (1.6 mg/ml), as indicated by the increased total migrated cell numbers. The elevated cell motility could be progressively counteracted by addition of an increasing concentration of PP2 (2.5–10 µM). Each treatment contains DMSO, the solvent of PP2, for control purpose. (B) Ectopic overexpression of the cellular-negative regulator of SFKs, CSK, resulted in reduced cell migration in the presence of BQ extract. Ca9-22 cells were transfected with HA-CSK construct or control vector, and protein expression was confirmed by Western blot analysis as shown. All migration assays were done in triplicates, and results were expressed as mean ± SD.
Requirement of SFKs for BQ-Induced Cell Migration
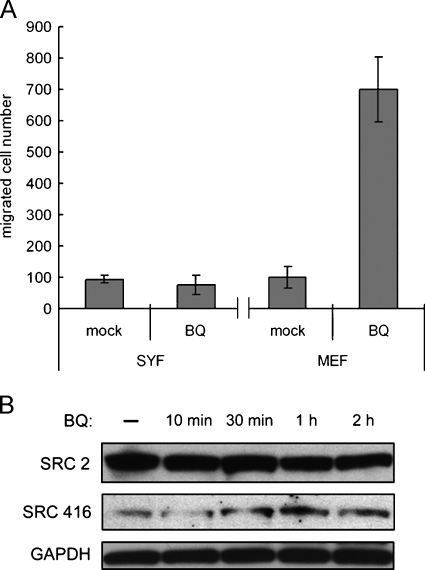
The fact that PP2 effectively antagonizes the increased cell migration brought about by BQ stimulation prompted us to address whether SFKs are required for BQ-induced cell migration. To this end, we sought to demonstrate the necessity of SFKs for BQ-induced cell migration using SYF cells, an MEF lacking all three ubiquitously expressed Src family members, namely, Src, Fyn, and Yes. Our results showed that, in the presence of BQ, cell migration was notably increased in MEFs, the legitimate wild type control cells. In contrast, treating SYF cells with BQ extract exhibited negligible effects on cell migration (Figure 2A). To confirm whether BQ-induced MEF cell migration could be attributable to the activation of SFKs, we investigated the phosphorylation status of tyrosine 416 (pY416), because autophosphorylation at this residue of the catalytic domain is a putative indicator of cellular activity of SFKs [24]. Immunoblot analysis results indicated an increase in pY416 after treating MEF cells with BQ extract for 30 minutes or longer, thereby suggesting enhanced kinase activities. More importantly, protein expression of SFKs, as revealed by a “pan-Src” antibody (Src2), remained virtually unchanged on BQ stimulation (Figure 2B). As expected, both protein expressions of SFKs and kinase activities were not detectable in SYF cells (data not shown). These findings indicate that BQ extract can induce cell migration through elevating the kinase activity but not regulating the protein expression of endogenous SFKs.
Figure 2.
Betel quid-induced cell migration was abolished in SFK-deficient cells. (A) Wild type MEFs and SYF, MEF cells lacking Src, Yes, and Fyn, were treated with BQ extract while being allowed to migrate for 3 hours and 4 hours, respectively. Betel quid extract could induce cell migration of MEF cells by about seven-fold, whereas no induction was observed in SYF cells. (B) Mouse embryonic fibroblast cells were treated with BQ extract for various periods as indicated, and phosphorylation of Src (pY416) was determined using Western blot analysis. Phosphorylation of SFKs at tyrosine 416 (Src 416) was increased in MEF cells 30 minutes after BQ extract treatment. Anti-SFKs antibody (Src2) detected total proteins of SFKs. GAPDH was the loading control.
Betel Quid Extract Stimulates the Cellular Activity of SFKs in Oral Cancer Cells
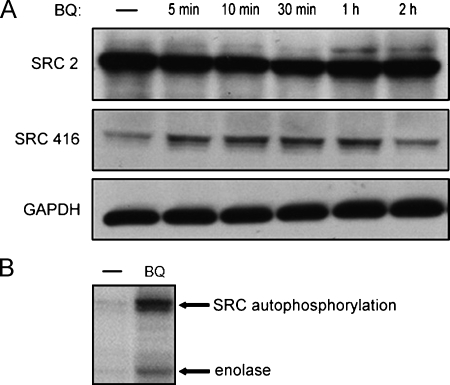
To provide direct evidence that BQ extract can activate SFKs in oral cancer cells, leading to an overt increase in cell motility, we likewise checked Src (pY416) in BQ-treated Ca9-22 cells. We found that SFK activities were persistently activated for at least 1 hour during a time-course BQ treatment, whereas, resembling the results in MEF cells, the protein expression level was constant throughout the treatment period (Figure 3A). We then confirmed whether the elevated kinase activity was caused by the stimulation of BQ extract by in vitro kinase assay. Immunoprecipitates of SFKs from BQ-treated cell lysates were used for measuring the capacity of tyrosine phosphorylation of an in vitro substrate, enolase. Treatment of Ca9-22 cells with BQ extract resulted in a pronounced increase in both SFK autophosphorylation and kinase activities (Figure 3B), indicating that BQ could truly stimulate the cellular activity of SFKs, which was further validated by their elevated catalytic activities in vitro. These, in conjunction with the previous results in which PP2 profoundly counteracts BQ-induced cell migration, have clearly proven that BQ extract can stimulate oral cancer cell motility through the activation of SFKs.
Figure 3.
Enhancement of SFK activities by BQ extract in oral cancer cell Ca9-22. (A) Ca9-22 cells were treated with BQ extract for different periods as indicated and subjected to detection of phospho-Src (pY416) as described in Figure 2B. Phosphorylation at tyrosine 416 was quickly induced within 5 minutes on BQ stimulation. (B) The cellular activity of SFKs treated or untreated with BQ ingredients was detected by in vitro kinase assay. Ca9-22 cells were treated with BQ extract for 30 minutes and then the cell lysates were immunoprecipitated with anti-SFKs antibody (Src2). Cellular Src kinase activities were directly measured on protein A/G agarose beads carrying Src immunocomplex. In the presence of BQ, Src kinases autophosphorylation was significantly increased, leading to increased phosphorylation of the in vitro substrate, enolase (arrows).
Areca Nut Is the Major Component Responsible for BQ-Induced Biologic Effects
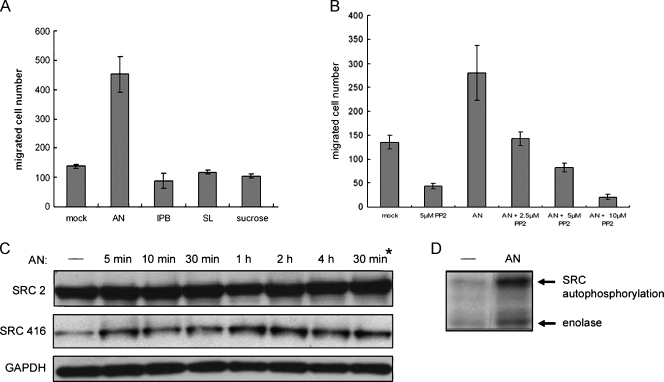
Having demonstrated that BQ extract, the whole mixture of the three major components AN, IPB, and SL, could activate SFKs and thereby cancer cell motility, it was tantalizing to determine which component is likely to be the key factor for these biologic effects. We directly tested the ability of each component to promote cell migration of Ca9-22 cells. We found, remarkably, that under the same treatment concentration (0.2 mg/ml), AN extract enhanced cell migration by about three-fold, whereas IPB and SL extracts had negligible effects (Figure 4A). In addition, the control experiment represented by an irrelevant substance sucrose excluded possible serendipity associated with AN-induced cell migration. To verify whether AN-induced cell migration was also mediated through SFK signaling pathways, we examined AN-induced cell migration in the presence of increasing concentrations of PP2. As anticipated, PP2 seemed to act against AN-induced cell migration in a dose-dependent manner, consistent with its effects on BQ-induced cell motility (Figure 4B). Moreover, in a time-course treatment with AN extract, cellular SFK activities were elevated as indicated by an increase in Src (pY416), whereas endogenous protein expression remained unchanged throughout the treatment period (Figure 4C). Likewise, AN-induced SFK activities were further confirmed by in vitro kinase assay as described (Figure 4D). Collectively, these findings have identified AN as the major component contributing to BQ-induced oral cancer cell migration through SFKs-mediated signaling pathways.
Figure 4.
Areca nut is the key component of BQ that induces oral cancer cell migration through activation of the SFKs. Water-soluble extracts of BQ ingredients including AN, IPB, and SL were prepared, and optimal working concentrations were determined as described. (A) Ca9-22 cells were treated with the respective BQ components at an identical concentration (0.2 mg/ml) and migration assays were performed as above. Sucrosewas the irrelevant substance control. (B) Areca nut-induced oral cancer cellmigrationwas further examined as was done in Figure 1A, in that the concentration of AN extract was 0.2 mg/ml and PP2 was 2.5 to 10 µM. (C) Induction of phosphorylation at tyrosine 416 (Src416) at different time points by AN extract (0.2 mg/ml) was detected as previously described. The asterisk indicates a 30-minute period of BQ extract treatment on Ca9-22 cells. (D) Ca9-22 cells were treated with AN extract (0.2 mg/ml) for 30 minutes and then cellular Src kinase activities were directly measured on protein A/G-Sepharose beads carrying Src immunocomplex by in vitro kinase assay.
SFKs Mediate BQ- and AN-Enhanced Oral Cancer Cell Invasion
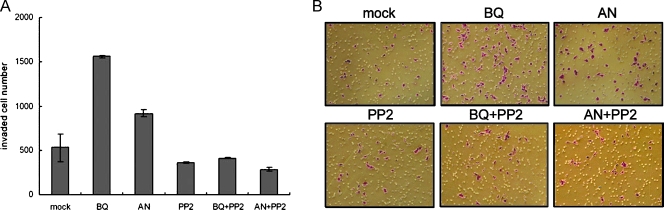
We next tested whether BQ or AN extract can induce oral cancer cell invasion in vitro. Betel quid- or AN-treated Ca9-22 cells were seeded onto invasion chambers to assess their ability to pass through a thin layer of Matrigel membrane. In good agreement with previous migration assays, we found that both BQ and AN extracts can induce cell invasion by about three- and two-fold, respectively. Conversely, because PP2 seemed to significantly inhibit cell invasion in the presence of BQ or AN extract, we thus confirmed that BQ and AN extracts conferred this enhanced invasive property by activating the SFK signaling pathway (Figure 5, A and B).
Figure 5.
The BQ and AN extracts induce oral cancer cell invasion through SFKs-mediated signaling pathways. (A) Cell invasion of Ca9-22 cells was assayed using Matrigel-coated invasion chambers with different treatments as indicated. Cells were allowed to invade through the Matrigel membrane for 22 hours and then total invaded cell numbers were counted. Invasion assays were done in triplicates, and results were expressed as mean ± SD. (B) Representative fields of invaded cells corresponded to the invasion assay in (A). Original magnification, x100.
Activity of SFKs May Be Important for Oral Cancer Cell Invasion In Vivo
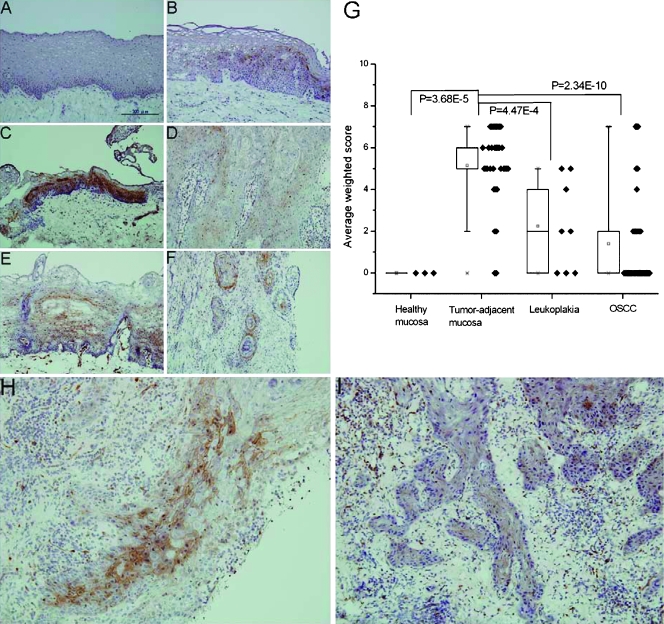
To explore the possibility that SFKs could be a biomarker for tumor invasion and/or metastasis in clinicopathologic conditions, oral tissue sections containing healthy mucosa without BQ exposure, corresponding tumor-adjacent mucosa, leukoplakia (or commonly deemed “precancer”), and tumor (OSCC) [21] were subjected to immunohistochemical staining using anti-Src (pY416). Consistent with the in vitro results, BQ-chewing oral cavity lesions including 2/4 of leukoplakia, 14/14 of tumor-adjacent mucosa, and 7/16 of OSCC had positive staining, whereas all of BQ-free healthy oral mucosa had negative staining (Figure 6, A–D). These results have confirmed the induction of SFK activities by BQ in clinical tissues. Conversely, the activity of SFKs also increased in both BQ-free tumor-adjacent mucosa (17/19) and OSCC (6/19; Figure 6, E and F). Because it seemed that SFKs might not be activated exclusively by BQ, we then compared the staining of Src (pY416) among the four categories of oral tissue specimens regardless of BQ-chewing. Our results indicate that the average weighted scores for Src (pY416) immunoreactivities in tumor-adjacent mucosa, leukoplakia, and OSCC were generally higher than in healthy mucosa. Interestingly, we found that the average weighted score of tumor-adjacent mucosa was significantly higher than that of healthy mucosa (P = 3.68e-5), leukoplakia (P = 4.47e-4), and OSCC (P = 2.34e-10), respectively (Figure 6G). These data indicate that the activity of SFKs may be involved mostly in early invasion when neoplastic cells begin to invade adjacent normal tissues. In addition, despite its diminished activity at OSCC compared to tumor-adjacent mucosa, the staining of Src (pY416) is localized at the invasion front of both BQ-chewing and BQ-free OSCC, suggesting that the activity of SFKs is necessary for tumor cells to invade the neighboring stromal tissues (Figure 6, H and I).
Figure 6.
Immunohistochemical and statistical analyses of SFKs in oral tissue sections. Representative results of immunohistochemical staining of Src (pY416) in (A) BQ-free healthy mucosa, (B) BQ-chewing leukoplakia, (C) BQ-chewing tumor-adjacent mucosa, (D) BQ-chewing OSCC, (E) BQ-free tumor-adjacent mucosa, and (F) BQ-free OSCC. (G) Average weighted scores ± SD of Src (pY416) immunoreactivities for the four categories of oral tissue specimens. Score distribution of samples in each category was denoted by solid diamonds (◆), whereas statistical difference by P values. Immunohistochemical staining of Src (pY416) at the invasion front of (H) BQ-chewing and (I) BQ-free OSCC. Microscopic magnification was x200 in all cases.
Discussion
The SFKs play a central role in a variety of signal transduction pathways, regulating fundamental cellular processes including cell growth, differentiation, adhesion, motility, angiogenesis, and survival [25,26]. In addition, SFKs have been implicated in the development of many different types of human cancers [27,28]. Of particularly importance is the intimate correlation between the three widely expressed members Src, Fyn, and Yes and malignant phenotypes such as tumor invasion and metastasis [29]. For example, elevated Src protein expression and/or activity have been linked to high metastatic potential in colon and in many other epithelial cancers [30,31]. More importantly, compounds targeting SFKs have been evaluated in preclinical models for treatment of solid tumors including head and neck squamous cell carcinoma [32]. To date, BQ-chewing oral cancer has had a serious impact on especially male health of Southeast countries despite an increasing research effort. Indeed, it is conceivable that a lack of understanding on the precise mechanism behind the multistep chemical carcinogenesis including invasion or metastasis has prevented a breakthrough in clinical therapeutics.
In the present study, to better simulate tumor cell movement in a three-dimensional microenvironment in vivo, we used Boyden's chamber-based apparatus to analyze the effects of BQ and its components on in vitro oral cancer cell migration and invasion. Although the Rho family GTPase has been well implicated in cancer cell migration and invasion [33,34], BQ-induced cancer cell migration is selectively and effectively inhibited by PP2 but not toxin A, the specific inhibitor of Rho GTPases. Moreover, we showed that BQ-induced cell migration in MEFs necessitates SFKs. These results suggest that BQ-induced cell migration is primarily triggered by SFKs-mediated signaling pathways. However, activation of SFKs seems to be delayed in MEFs compared to that in oral cancer cells, suggesting that BQ can induce distinct signaling pathways in different cell types. It is noteworthy that SFKs can promptly respond to BQ stimulation while their protein expressions remain nearly constant during the treatment period, raising the possibility that this BQ-induced biologic effect is unlikely to involve gene regulation normally taking place in the nucleus. Furthermore, we identified that AN is the key ingredient contributing to BQ-induced cell migration and invasion through the activation of SFKs. However, the fact that BQ, but not AN, can significantly promote cell motility may thus suggest a synergistic effect of the heterogeneous BQ compositions on cell motility. It is possible that this synergistic effect can activate additional signaling pathways different from those induced by AN. In addition, whether arecoline and arecaidine, the two major alkaloids of AN, can induce similar biologic effects is currently unknown. Verification of this should further potentiate clinical and therapeutic applications.
The activity of SFKs induced by BQ extract in cultured cells was recapitulated in BQ-chewing oral cavity lesions including leukoplakia, corresponding tumor-adjacent mucosa, and OSCC. However, despite relatively weak Src (pY416) immunoreactivities, we found that SFK activities were also elevated in BQ-free tumor-adjacent mucosa and OSCC specimens. These results indicate that etiologic factors except BQ chewing may induce SFK activities in clinicopathologic cases. Indeed, a recent study showed that nicotine, a component of cigarette smoke, could increase phospho-Src (pY416) level and thus the activity of SFKs [35]. Whereas clinicopathologic parameters other than BQ chewing for these BQ-free tissue specimens are uncertain, we nonetheless speculate that persistent smoking and alcohol drinking, two popular habits frequently associated with BQ chewing in local populations, are likely to stimulate SFK activities in tissues of oral cavity. Moreover, regardless of BQ chewing, the three categories of oral cavity lesions seemed to have elevated average weighted scores for Src (pY416) immunoreactivities, in that, surprisingly, the activity of SFKs (or average weighted score) in tumor-adjacent mucosa was significant higher than that in leukoplakia and OSCC. These results have raised an intriguing possibility that SFK activities could play an important role in early invasion, during which invasive cancer cells begin to migrate from the primary tumor site into the surrounding normal tissues. This is consistent with a most recent study, in that the tumor-host interface has the highest activity of SFKs, which triggers invasion of glioblastoma in vivo. In contrast, a reduced activity of SFKs was found in the inner tumor mass [36]. Interestingly, we also found that the activity of SFKs was increased in vitro by BQ extract in normal human oral keratinocytes (data not shown), which may support the observation that SFK activities are significantly higher in BQ-chewing than in BQ-free tumor-adjacent mucosa (Figure 6, C and E). Besides, we demonstrated that SFKs activities were located at the invasion front of OSCC, suggesting a possible function of SFKs in basement membrane penetration and stromal invasion inside the tumor mass.
The molecular mechanism underlying BQ-induced SFKs activation is still unclear. It has been shown that AN extract does not activate EGFR in oral keratinocytes [13]. Consistent with this result are our preliminary data suggesting an unlikely role of EGFR in BQ-induced cell migration because of a lack of inhibition on this biologic effect when EGFR inhibitor is present. Whether BQ ingredients can activate motility-promoting signaling pathways including SFKs in an EGFR-independent manner is under investigation. Likewise, it remains elusive what additional downstream signaling molecules or effectors might bridge between the relatively upstream BQ-induced SFK activities and the finally displayed biologic effects. A recent report by others indicates that AN extract can activate mitogen-activated protein kinases such as c-Jun N-terminal kinase and the stress-regulated transcription factor NF-κB [13]. However, whether these AN-activated signaling pathways are directly linked to biologic functions of oral cancer cells is still unknown. Thus, our current studies have been aimed to globally identify cellular signaling pathways mediating BQ-induced cancer cell motility. Another important question to address is whether the SFKs including Src, Yes, and Fyn could be differently stimulated by BQ or AN, thereby to various extents modulating BQ- or AN-induced oral cancer motility. Our preliminary results suggest that Src kinase activity per se plays a role in BQ-induced cell motility, yet the detailed mechanism requires further studies.
In conclusion, we have shown that BQ extract can significantly induce in vitro oral cancer cell motility, including migration and invasion, through the activation of SFKs. Given that BQ generally comprises AN, IPB, and SL, we further dissected the effects of these major components and found that AN seemed to be the sole component that dramatically enhanced cell motility. More importantly, our results also reveal that such AN-induced cell motility can be largely mediated by SFKs, too. Finally, taking together our in vitro and immunohistochemical studies, we propose that SFKs could be a potential biomarker for cancer cell motility and tumor invasion of oral carcinogenesis. More importantly, considering the therapeutic perspectives, we have validated SFKs a potential target for the development of antimigratory compounds against oral cancer. Verifications by other in vitro cell migration assays [37] should further warrant its clinical applications.
Acknowledgments
The authors thank Hong-Chen Chen for providing enolase and technical assistance and A-Mei Huang for MEF cells.
Abbreviations
- SFK
Src family kinase
- BQ
betel quid
- AN
areca nut
- IPB
inflorescence of Piper betle
- SL
slaked lime
- OSCC
oral squamous cell carcinoma
- MEF
mouse embryonic fibroblast
- CSK
C-terminal Src kinase
- PP2
4-amino-5-(4-chlorophenyl-7-(t-butyl) pyrazolo [3, 4-d] pyrimidine
Footnotes
This work was supported mainly by National Science Council (grant NSC 95-2311-B-037-004-MY2) and by Kaohsiung Medical University (grant KMU-EM-97-1.1ab).
References
- 1.Sharan RN. Association of betel nut with carcinogenesis. Cancer J. 1996;9:13–19. [Google Scholar]
- 2.Kwan HW. A statistical study on oral carcinoma in Taiwan with emphasis on the relationship with betel nut chewing: a preliminary report. Taiwan Yi Xue Hui Za Zhi. 1976;75:497–505. [PubMed] [Google Scholar]
- 3.Ko YC, Huang YL, Lee CH, Chen MJ, Lin LM, Tsai CC. Betel quid chewing, cigarette smoking and alcohol consumption related to oral cancer in Taiwan. J Oral Pathol Med. 1995;24:450–453. doi: 10.1111/j.1600-0714.1995.tb01132.x. [DOI] [PubMed] [Google Scholar]
- 4.Bhide SV, Shivapurkar NM, Gothoskar SV, Ranadive KJ. Carcinogenicity of betel quid ingredients: feeding mice with aqueous extract and the polyphenol fraction of betel nut. Br J Cancer. 1979;40:922–926. doi: 10.1038/bjc.1979.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeng JH, Chang MC, Hahn LJ. Role of areca nut in betel quid- associated chemical carcinogenesis: current awareness and future perspectives. Oral Oncol. 2001;37:477–492. doi: 10.1016/s1368-8375(01)00003-3. [DOI] [PubMed] [Google Scholar]
- 6.IARC, author. Betel-quid and areca nut chewing and some areca nut-related nitrosamines. IARC Monogr Eval Carcinog Risk Chem Hum. 2003;85:11–18. [Google Scholar]
- 7.Sundqvist K, Grafstrom RC. Effects of areca nut on growth, differentiation and formation of DNA damage in cultured human buccal epithelial cells. Int J Cancer. 1992;52:305–310. doi: 10.1002/ijc.2910520225. [DOI] [PubMed] [Google Scholar]
- 8.Jeng JH, Hahn LJ, Lin BR, Hsieh CC, Chan CP, Chang MC. Effects of areca nut, inflorescence Piper betle extracts and arecoline on cytotoxicity, total and unscheduled DNA synthesis in cultured gingival keratinocytes. J Oral Pathol Med. 1999;28:64–71. doi: 10.1111/j.1600-0714.1999.tb01998.x. [DOI] [PubMed] [Google Scholar]
- 9.Lai KC, Lee TC. Genetic damage in cultured human keratinocytes stressed by long-term exposure to areca nut extracts. Mutat Res. 2006;599:66–75. doi: 10.1016/j.mrfmmm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Chang MC, Ho YS, Lee PH, Chan CP, Lee JJ, Hahn LJ, Wang YJ, Jeng JH. Areca nut extract and arecoline induced the cell cycle arrest but not apoptosis of cultured oral KB epithelial cells: association of glutathione, reactive oxygen species and mitochondrial membrane potential. Carcinogenesis. 2001;22:1527–1535. doi: 10.1093/carcin/22.9.1527. [DOI] [PubMed] [Google Scholar]
- 11.Jeng JH, Wang YJ, Chiang BL, Lee PH, Chan CP, Ho YS, Wang TM, Lee JJ, Hahn LJ, Chang MC. Roles of keratinocyte inflammation in oral cancer: regulating the prostaglandin E2, interleukin-6 and TNF-alpha production of oral epithelial cells by areca nut extract and arecoline. Carcinogenesis. 2003;24:1301–1315. doi: 10.1093/carcin/bgg083. [DOI] [PubMed] [Google Scholar]
- 12.Chang MC, Wu HL, Lee JJ, Lee PH, Chang HH, Hahn LJ, Lin BR, Chen YJ, Jeng JH. The induction of prostaglandin E2 production, interleukin-6 production, cell cycle arrest, and cytotoxicity in primary oral keratinocytes and KB cancer cells by areca nut ingredients is differentially regulated by MEK/ERK activation. J Biol Chem. 2004;279:50676–50683. doi: 10.1074/jbc.M404465200. [DOI] [PubMed] [Google Scholar]
- 13.Lin SC, Lu SY, Lee SY, Lin CY, Chen CH, Chang KW. Areca (betel) nut extract activates mitogen-activated protein kinases and NF-kappaB in oral keratinocytes. Int J Cancer. 2005;116:526–535. doi: 10.1002/ijc.21104. [DOI] [PubMed] [Google Scholar]
- 14.Regezi JA, Sciubba JJ, Jordan RCK, editors. Oral Pathology: Clinical-Pathologic Correlations. Philadelphia, PA: WB Saunders Company; 1989. pp. 70–83. [Google Scholar]
- 15.Coussens LM, Werb Z. Matrix metalloproteinases and the development of cancer. Chem Biol. 1996;3:895–904. doi: 10.1016/s1074-5521(96)90178-7. [DOI] [PubMed] [Google Scholar]
- 16.Shinohara M, Hiraki A, Ikebe T. Immunohistochemical study of desmosomes in oral squamous cell carcinoma: correlation with cytokeratin and E-cadherin staining, and with tumor behavior. J Pathol. 1998;184:369–381. doi: 10.1002/(SICI)1096-9896(199804)184:4<369::AID-PATH1236>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 17.Kudo Y, Kitajima S, Ogawa I, Hiraoka M, Sargolzaei S, Keikhaee MZ, Sato S, Miyauchi M, Takata T. Invasion and metastasis of oral cancer cells require methylation of E-cadherin and/or degradation of membranous β-catenin. Clin Cancer Res. 2004;10:5455–5463. doi: 10.1158/1078-0432.CCR-04-0372. [DOI] [PubMed] [Google Scholar]
- 18.Saussez S, Camby I, Toubeau G, Kiss R. Galectins as modulators of tumor progression in head and neck squamous cell carcinomas. Head Neck. 2007;29:874–884. doi: 10.1002/hed.20559. [DOI] [PubMed] [Google Scholar]
- 19.Demers M, Magnaldo T, St-Pierre Y. A novel function for galectin-7: promoting tumorigenesis by up-regulating MMP-9 gene expression. Cancer Res. 2005;65:5205–5210. doi: 10.1158/0008-5472.CAN-05-0134. [DOI] [PubMed] [Google Scholar]
- 20.Shintani S, Li C, Mihara M, Nakashiro K, Hamakawa H. Gefitinib (“Iressa”), an epidermal growth factor receptor tyrosine kinase inhibitor, mediates the inhibition of lymph node metastasis in oral cancer cells. Cancer Lett. 2003;201:149–155. doi: 10.1016/s0304-3835(03)00464-6. [DOI] [PubMed] [Google Scholar]
- 21.Chen JYF, Chang YL, Yu YJ, Chao CC, Kao HW, Wu CT, Lin WC, Ko JY, Jou YS. Specific induction of the high-molecular-weight microtubuleassociated protein 2 (hmw-MAP2) by betel quid extract in cultured oral keratinocytes: clinical implications in betel quid-associated oral squamous cell carcinoma (OSCC) Carcinogenesis. 2004;25:269–276. doi: 10.1093/carcin/bgh006. [DOI] [PubMed] [Google Scholar]
- 22.Chan PC, Chen YL, Cheng CH, Yu KC, Cary LA, Shu KH, Ho WL, Chen HC. Src phosphorylates Grb2-associated binder 1 upon hepatocyte growth factor stimulation. J Biol Chem. 2003;278:44075–44082. doi: 10.1074/jbc.M305745200. [DOI] [PubMed] [Google Scholar]
- 23.Chan WY, Cheung KK, Schorge JO, Huang LW, Welch WR, Bell DA, Berkowitz RS, Mok SC. Bcl-2 and p53 protein expression, apoptosis, and p53 mutation in human epithelial ovarian cancers. Am J Pathol. 2000;156:409–417. doi: 10.1016/S0002-9440(10)64744-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calalb MB, Polte TR, Hanks SK. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol. 1995;15:954–963. doi: 10.1128/mcb.15.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parsons SJ, Parsons JT. Src family kinases, key regulator of signal transduction. Oncogene. 2004;23:7906–7909. doi: 10.1038/sj.onc.1208160. [DOI] [PubMed] [Google Scholar]
- 26.Playford MP, Schaller MD. The interplay between Src and integrins in normal and tumor biology. Oncogene. 2004;23:7928–7946. doi: 10.1038/sj.onc.1208080. [DOI] [PubMed] [Google Scholar]
- 27.Frame MC. Src in cancer: deregulation and consequences for cell behavior. Biochim Biophys Acta. 2002;1602:114–130. doi: 10.1016/s0304-419x(02)00040-9. [DOI] [PubMed] [Google Scholar]
- 28.Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell. 2004;6:209–214. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22:337–358. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- 30.Cartwright CA, Coad CA, Egbert J. Elevated c-Src tyrosine kinase activity in premalignant epithelia of ulcerative colitis. J Clin Invest. 1994;93:509–515. doi: 10.1172/JCI117000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biscardi JS, Tice DA, Parsons SJ. c-Src, receptor tyrosine kinases, and human cancer. Adv Cancer Res. 1999;76:61–119. doi: 10.1016/s0065-230x(08)60774-5. [DOI] [PubMed] [Google Scholar]
- 32.Egloff AM, Grandis JR. Targeting epidermal growth factor receptor and Src pathways in head and neck cancer. Semin Oncol. 2008;35:286–297. doi: 10.1053/j.seminoncol.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cruz-Monserrate Z, O'Connor KL. Integrin α6β4 promotes migration, invasion through Tiam1 upregulation, and subsequent Rac activation. Neoplasia. 2008;10:408–417. doi: 10.1593/neo.07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hall CL, Dubyk CW, Riesenberger TA, Shein D, Keller ET, van Golen KL. Type I collagen receptor (α2β1) signaling promotes prostate cancer invasion through RhoC GTPase. Neoplasia. 2008;10:797–803. doi: 10.1593/neo.08380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dasgupta P, Rastogi S, Pillai S, Ordonez-Ercan D, Morris M, Haura E, Chellappans S. Nicotine induces cell proliferation by β-arrestin-mediated activation of Src and Rb-Raf-1 pathways. J Clin Invest. 2006;116:2208–2217. doi: 10.1172/JCI28164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klber S, Sancho-Martinez I, Wiestler B, Beisel A, Gieffers C, Hill O, Thiemann M, Mueller W, Sykora J, Kuhn A, et al. Yes and PI3K bind CD95 to signal invasion of glioblastoma. Cancer Cell. 2008;13:235–248. doi: 10.1016/j.ccr.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Decaestecker C, Debeir O, van Ham P, Kiss R. Can anti-migratory drugs be screened in vitro? A review of 2D and 3D assays for the quantitative analysis of cell migration. Med Res Rev. 2007;27:149–176. doi: 10.1002/med.20078. [DOI] [PubMed] [Google Scholar]