Abstract
Down-regulation of the KAI1 (CD82) metastasis suppressor is common in advanced human cancer, but underlying mechanism(s) regulating KAI1 expression are only now being elucidated. Recent data provide evidence that low levels of KAI1 mRNA in LNCaP cells are caused by binding of β-catenin/Reptin complexes to a specific motif in the proximal promoter, which prevents binding of Tip60/Pontin activator complexes to the same motif, thus inhibiting transcription. Here, we explored a pathway by which phorbol 12-myristate 13-acetate (PMA) up-regulates KAI1 transcription in LNCaP prostate cancer cells. Pretreatment with specific inhibitors showed that induction of KAI1 by PMA uses classic isoforms of protein kinase C (cPKC), is independent of Ras and Raf, and requires activation of MEK1/2 and ERK1/2, but does not involve p38MAPK. Induction of KAI1 transcription by PMA was associated with enhanced overall acetylation of histones H3 and H4, but only acetylation of H3 was blocked by a PKC inhibitor. Chromatin immunoprecipitation showed that PMA induces recruitment of Tip60/Pontin activator complexes to NFκB-p50 motifs in the proximal promoter, and this was blocked by a PKC inhibitor. These changes were not associated with differences in overall levels of Tip60, Pontin, β-catenin, or Reptin protein expression but with PMA-induced nuclear translocation of Tip60.
Introduction
Down-regulated expression of the KAI1 metastasis suppressor is common in the advanced stages of many human cancer types [1,2]. Experimental studies using a combination of in vitro and in vivo approaches have demonstrated that loss of KAI1 expression is associated with reduced homotypic cell adhesion, increased cell migration, and altered ability of tumor cells to bind specific extracellular proteins, such as fibronectin [3–5]. The consequences of these changes are increased in vitro invasive [4,6,7] and in vivo metastatic [5,7] ability of tumor cells. Given this importance to tumor cell behavior, our knowledge of factors regulating KAI1 expression is limited. Studies of mechanisms underlying down-regulation in advanced cancers and cancer cell lines have shown that loss of heterozygosity [8], mutations in the KAI1 gene [8] and promoter hypermethylation [9,10] are unlikely to be involved. Transient transfection approaches have identified several promoter regions important for basal transcription [11] and have also provided evidence for the importance of a 76-bp enhancer-like sequence upstream of the transcription start site in a wide range of cancer cell types [12]. Other studies have linked transcriptional regulation of KAI1 to changes in the composition of specific chromatin-remodeling protein complexes binding to a specific motif in the proximal KAI1 promoter [13,14]. Thus, in nonmetastatic cancer cells, activation of KAI1 transcription is mediated by the binding of a Tip60/Pontin complex with associated histone acetylase activity to a specific p50 motif in the proximal promoter. In metastatic cancer cells, such as LNCaP prostate cancer cells, Tip60/Pontin-mediated activation of KAI1 transcription is blocked by an inhibitory complex consisting of β-catenin and Reptin recruiting the histone deacetylase HDAC1 [14]. Currently, the relationship between the p53, AP1, and AP2 proteins, which bind the enhancer, and the role of the chromatin remodeling complexes to overall KAI1 transcription remain to be elucidated.
Biochemical pathways that determine transcriptional responses of KAI1 to extracellular signals remain to be studied. Phorbol 12-myristate 13-acetate (PMA) [15], nerve growth factor [16], tumor necrosis factor alpha [17], and sodium butyrate (NaB) [18] all upregulate KAI1 mRNA levels in prostate cancer cells, which express little or no KAI1 mRNA, but detailed signaling pathways used by these factors have not been characterized. Because phorbol ester is an established model for studying pathways used by growth factors and hormones to regulate cell behavior, PMA was chosen as a starting point to elucidate specific signaling pathways, which induce transcription of KAI1. In addition, because previous studies have used LNCaP prostate cancer cells to explore transcriptional regulation of the KAI1 gene, we focused our studies on the effects of PMA in this cell line. Results presented in this report show that PMA induced KAI1 in LNCaP prostate cancer cells by activation of classic protein kinase C (cPKC) isoforms. This up-regulation was Ras- and Raf-independent and required activation of MEK/ERK signaling factors. The data also provide support for the idea that PMA induces KAI1 transcription by recruiting a histone acetyl-transferase activator complex of Pontin and Tip60 to specific motifs within the KAI1 promoter region.
Materials and Methods
Chemicals and Reagents
Phorbol 12-myristate 13-acetate, AG126, BAPTA/AM, bis-indolylmaleimide III (Bim III), bryostatin 1 (Bryo 1), FPT inhibitor III, H89 dihydrochloride, PD98059, PP2, PP3, SB203580, staurosporine, thymeleatoxin, trichostatin A, and ZM336372 were from Calbiochem (San Diego, CA). Actinomycin D, apigenin, 6-dichlorobenzimidazole 1-β-d-ribofuranoside, and TriReagent were purchased from Sigma-Aldrich (Castle Hill, NSW, Australia). U0126 was from Cell Signaling Technology (Beverly, MA).
Tissue Culture
LNCaP were from Leland Chung (Department of Urology, Emory University School of Medicine, Atlanta, GA) and cultured in T-medium [19]. Media and supplements were all from Invitrogen (Mount Waverley, Victoria, Australia). Cells were grown in a humidified incubator at 37°C with 5% CO2. For experiments, LNCaP cells (1 x 106) were seeded into 10-cm-diameter Petri dishes containing 10 ml of T-medium. After 24 hours, cells were pretreated with inhibitors for 1 hour before exposure to 20 nM PMA for 6 hours.
Reverse Transcription-Polymerase Chain Reaction
Total RNA was isolated from cell cultures using TriReagent as per manufacturer's instructions. After phenol/chloroform extraction to remove residual DNA, 2 µg RNA was used to prepare cDNA, as described [6]. Forward and reverse primers for amplification of specific targets, together with sizes of amplified products, are shown in Table 1. Reactions contained 2.5 µl of 10x reaction buffer, 0.25 µl of 25 mM dNTP mix, 0.65 µl each of forward and reverse primers (20 pmol/µl), 1.0 µl of cDNA, 0.5 µl of Taq DNA polymerase (5 U/ml), and 1.0 µl (KAI1) or 1.5 µl (GAPDH, Pontin, Reptin, Tip60, and β-catenin) of 25 mM MgCl2, in a total volume of 25 µl, and were performed in a Hybaid Touchdown Thermocycler (Hybaid Limited, Basingstoke, United Kingdom). KAI1, Pontin, Reptin, Tip60, and β-catenin were amplified at 94°C for 4 minutes, followed by 94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 30 seconds, with a final step at 72°C for 10 minutes. Conditions were similar for amplification of GAPDH, except that the elongation step was extended to 45 seconds. In preliminary experiments, numbers of cycles required to reach linear amplification for each product were determined. Subsequently, the levels of expression for each gene in control- or PMA-treated cells were examined in the middle of the linear amplification phase (24 cycles, GAPDH; 25 cycles, Pontin, Tip60 and β-catenin; 28 cycles, KAI1; 29 cycles, Reptin) to ensure detection of increases or decreases in mRNA levels after drug treatment. Reaction products were separated in 2.0% (w/v) agarose, visualized with ethidium bromide and analyzed by Kodak Digital Science 1D Image Analysis Software (Eastman Kodak, Rochester, NY). Graphically presented data show relative levels of expression (compared with GAPDH) before and after treatment, expressed as mean ± SE of at least three independent experiments. Where calculated, statistical significance was determined by Student's t test, with confidence levels indicated in the figure legends. Illustrated results are representative of these experiments.
Table 1.
Primer sequences used for RT-PCR.
| Gene | Amplicon | Sequences (5′ to 3′) |
| KAI1 Forward | 344bp | CCCGGCAACAGGACCCAGAGT |
| KAI1 Reverse | TCAGTCAGGGTGGGCAAGAGG | |
| Tip60 Forward | 184bp | CAGGACAGCTCTGATGGAATA |
| Tip60 Reverse | GAGGACAGGCAATGTGGTGAG | |
| β-catenin Forward | 298bp | ACAACTGTTTTGAAAATCCA |
| β-catenin Reverse | CGAGTCATTGCATACTGTCC | |
| Pontin Forward | 218bp | GCATGTCGAAGAGATCAGTGA |
| Pontin Reverse | CTGAACTGACAGCGCTGCA | |
| Reptin Forward | 239bp | GAAATTGTCGAGGAACAAGAGAGT |
| Reptin Reverse | TTTATTCAGGTAATTGAGCAGGTG | |
| GAPDH Forward | 600bp | CCACCCATGGCAAATTCCATGGCA |
| GAPDH Reverse | TCTAGACGGCAGGTCAGGTCCA |
Western Blot Analysis
Total cell extracts were prepared by lysing cells in 250 µl M-Per buffer (Pierce Endogen, Rockford, IL) containing 1x Protease Inhibitor Cocktail 1 (5 µM AEBSF hydrochloride, 1.5 nM aprotinin, 10 nM E-64 protease inhibitor, 5 µM EDTA, and 10 nM leupeptin; EMD Biosciences, San Diego, CA) with added sodium orthovanadate (0.4 mM). Protein concentration of supernatants was determined by BCA assay (Pierce Endogen). Extracts, containing 20 µg (p38MAPK, MEK, ERK, β-catenin, and histones) or 50 µg (Tip60 and Reptin) of protein, were separated in 7.5% (β-catenin), 10% (p38MAPK, MEK, ERK, Tip60, Reptin), or 16% (acetylated histones) SDS-polyacrylamide gels and transferred to nitrocellulose membranes. After blocking with PBSTM [0.1% (v/v) Tween 20 in PBS (PBST) containing 5% (w/v) skimmed milk] for 1 hour at room temperature, filters were probed overnight at 4°C with the following specific antibodies. Antibodies to total or phosphorylated forms of ERK1/2 (p44/p42) and p38MAPK, as well as HRP-conjugated secondary antibodies, were from Cell Signaling Technology. Monoclonal antibodies to acetylated histone H3 or H4 and Tip60 were from Upstate (Charlottesville, CA) and to β-catenin (clone 14) from BD Biosciences (Heidelberg, Germany). Rabbit polyclonal antibodies to β-catenin (sc-7199) and tubulin (sc-1094) were from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal and rabbit polyclonal antibodies to human Reptin and Pontin have been described previously [20–22]. After rinsing in PBST (3 x 5 minutes), filters were incubated with an appropriate HRP-linked secondary antibody for 1 hour at room temperature. After a final rinsing with PBST (3 x 5 minutes) to remove unbound antibody, specific proteins were visualized by the Supersignal WestPico (Pierce Endogen) or Lumilight (Roche, Germany) chemiluminescence method and were exposed to Fuji X-ray film or Hyperfilm (GE Healthcare, Freiburg, Germany). Cytoplasmic and nuclear fractionation was performed as previously reported [23] using the Nuclear Isolation Kit: Nuclei EZ Prep (Sigma, Deisenhofen, Germany) according to the manufacturer's recommendations except that nuclei were resuspended in RIPA buffer [150 mM NaCl, 25 mM Tris pH 6.8, 5 mM EDTA, 1% (w/v) sodiumdeoxcholate, 1% (v/v)NP-40, 0.1% (w/v) SDS]. Total lysates were generated in ice-cold lysis buffer [10 mM imidazole pH 6.8, 0.1 M KCl, 0.3 M sucrose, 2 mM MgCl2, 10 mM EGTA, 1 mM NaF, 1 mM Na2MoO4, 1 mM NaVO3, 0.2% (v/v) Triton X-100, and complete EDTA protease inhibitor cocktail].
Chromatin Immunoprecipitation
Chromatin immunoprecipitation (ChIP) was performed as described previously [24] with minor modifications. LNCaP cells were grown on 100-mm dishes to a confluence of 80% to 90%. At the indicated times after the addition of 20 nM PMA, cells were washed twice with PBS, fixed with 2 mM disuccinimidyl-glutarate for 45 minutes at room temperature, and then cross-linked for 10 minutes at room temperature using 1% (v/v) formaldehyde. For inhibitor studies, cells were preincubated for 60 minutes with 10 µM Bim III before addition of PMA. In other control studies, cells were stimulated with 5 ng/ml interleukin-1β (IL-1β) for the indicated times. For immunoprecipitation, 2 µg of anti-NCoR (sc-1069; Santa Cruz Biotechnology), 2 µg of anti-β-catenin (BD Biosciences), 2 µg of anti-Tip60 (Upstate Biotechnology), 2 µg of anti-Reptin (L12E) [20], and 5 µg of anti-Pontin (5G3-11) [22] antibodies were used. For re-ChIP, the immunocomplexes were eluted with 100 µl of 10 mM DTT at 37°C for 30 minutes and diluted at 1:40 in ChIP dilution buffer followed by incubating with antibodies for the second immunoprecipitation. Second ChIP was performed as described for the first immunoprecipitation. For subsequent polymerase chain reaction (PCR) analysis, 2 µl of extracted DNA (50 µl) was used as template for the amplification. Primers to detect the p50 binding motif in the KAI1 proximal promoter have been previously described [14]. Polymerase chain reaction was performed using the following amplification parameters: an initial incubation of 2 minutes at 94°C was followed by 32 cycles at 94°C for 15 seconds, annealing for 30 seconds at 59°C, and elongation for 45 seconds at 72°C, with a final extension for 3 minutes at 72°C. Polymerase chain reaction products were separated on an 8% polyacrylamide gel, stained with ethidium bromide, and subsequently analyzed under UV light.
Results
PMA Up-regulates KAI1 mRNA Levels in LNCaP Prostate Cancer Cells
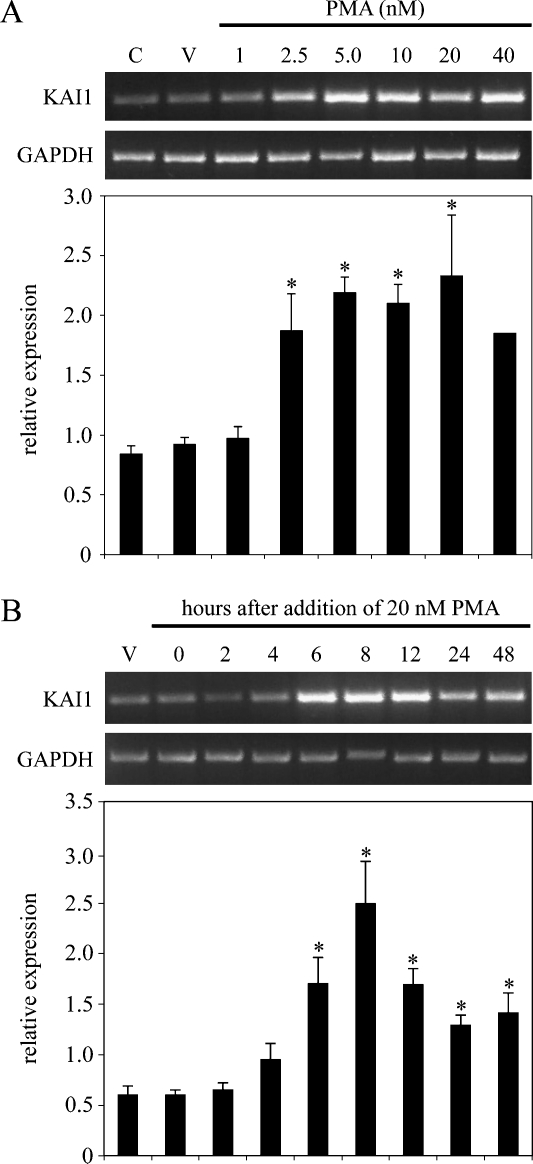
Initial experiments where mRNA levels were examined 24 hours after treatment confirmed that PMA induced KAI1 in LNCaP cells (Figure 1A). A significant induction was first observed with 2.5 nM PMA, maximal (approximately three-fold) with 5.0 nM PMA, and this level of induction was maintained with up to 20 nM PMA. At 40 nM PMA, the effect was decreased (Figure 1A). A time-course experiment demonstrated that induction of KAI1 mRNA was first clearly detectable from 4 hours after adding 20 nM PMA and that this increase was statistically significant from 6 hours after addition (Figure 1B). A maximal induction of approximately four-fold was observed 8 hours after addition; thereafter, levels of KAI1 mRNA declined. On the basis of these data, in subsequent experiments with inhibitors in LNCaP cells, PMA was used at a concentration of 20 nM for 6 hours.
Figure 1.
Phorbol 12-myristate 13-acetate up-regulates KAI1 mRNA levels in LNCaP prostate cancer cells. (A) LNCaP cells were untreated (C), treated with DMSO vehicle only for 24 hours (V), or treated with the indicated concentrations of PMA for 24 hours. KAI1 and GAPDH mRNA levels were determined from total RNA by reverse transcription-polymerase chain reaction (RT-PCR). (B) LNCaP cells were treated withDMSOvehicle only (V) or treated with 20 nM PMA for the indicated times. KAI1 and GAPDH mRNA levels were determined from total RNA by RT-PCR. Upper panels in (A) and (B) show ethidium bromide-stained gels of representative data. Lower panels showthe relative levels of KAI1 mRNA (KAI1/GAPDH) in treated cells. Data represent the means ± SD of at least three independent experiments. *P < .05, when compared with vehicle only-treated cells.
To determine whether this effect of PMA was generally applicable to metastatic prostate cancer cells, we repeated these experiments using DU145 and PC3 cells, both of which also express very low levels of KAI1 mRNA. Data summarized in Figure W1 confirmed that addition of PMA resulted in a dose-dependent induction of KAI1 mRNA levels in both DU145 and PC3 cells, with a maximum 1.5- to 3-fold induction at 8 (DU145) or 24 hours (PC3) after the addition of 20 nM PMA. Although induction of KAI1 mRNA by PMA was seen in all three prostate cancer cell lines tested, the clearest induction was obtained using LNCaP cells. Subsequent studies into the effects of PMA on KAI1 expression focused on the LNCaP cell line, but similar results were obtained for both DU145 and PC3 cell lines (data not shown).
Classic Isoforms of Protein Kinase C Directly Mediate Up-regulation of KAI1 mRNA by PMA
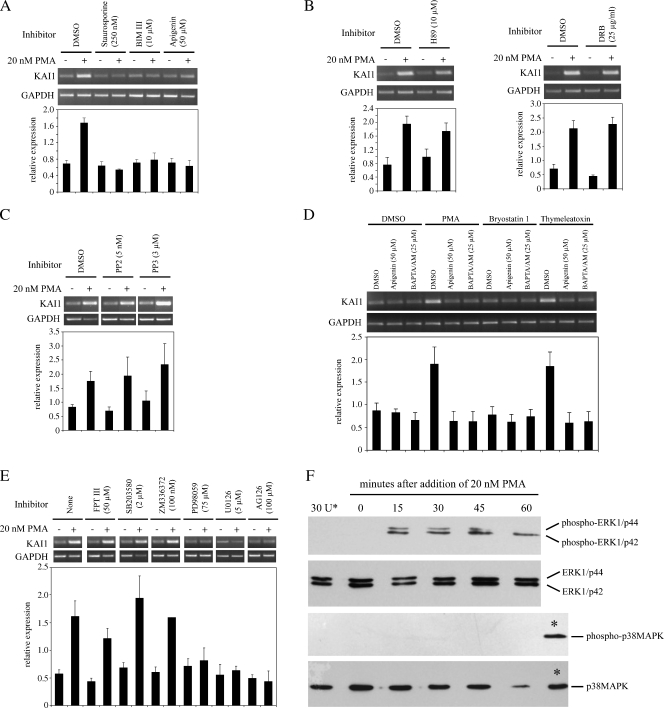
Phorbol 12-myristate 13-acetate mediates its effects by mimicking diacylglycerol (DAG), the endogenous activator of protein kinase C (PKC). Pretreatment of LNCaP cells for 1 hour with 10 µM Bim III, a highly selective cell-permeable PKC inhibitor, had no effect on low basal levels of KAI1 mRNA but completely blocked induction of KAI1 by PMA (Figure 2A). Pretreatment of LNCaP cells with two less-specific inhibitors of PKC, staurosporine (250 nM) and apigenin (50 µM), also completely blocked induction of KAI1 by PMA (Figure 2A). In contrast, pretreatment of cells with H-89 dihydrochloride (10 µM), a potent and specific inhibitor of protein kinase A or with the casein kinase II inhibitor 6-dichloro-benzimidazole 1-β-d-ribofuranoside (25 µg/ml) did not markedly affect basal levels of KAI1 or prevent up-regulation of KAI1 by PMA (Figure 2B). Similarly, the Src-dependent tyrosine kinase inhibitor PP2 (5 nM) had no effect on the induction of KAI1 by PMA (Figure 2C). PP3, a negative control for PP2 and a potent inhibitor of epidermal growth factor receptor kinase, failed to prevent induction of KAI1 by PMA. These data suggested that PKC was directly mediating up-regulation of KAI1 mRNA by PMA in prostate cancer cells.
Figure 2.
Phorbol 12-myristate 13-acetate uses a classic PKC isoform-dependent, Raf-independent pathway involving MEK1/2 and ERK1/2 for induction of KAI1/CD82 mRNA. Reverse transcription-polymerase chain reaction analysis of RNA from LNCaP cells pre-treated for 1 hour with the indicated concentrations of PKC inhibitors (A), PKA and CKII inhibitors (B), Src tyrosine kinase inhibitors (C), specific PKC activators (D), and specific Ras downstream pathway inhibitors (E) before the addition of 20 nM PMA for 6 hours. Cells were harvested, then RNA-isolated, converted to cDNA and used in PCR analyses for KAI1 and GAPDH, as described in the Materials and Methods section. Graphs show relative levels of KAI1 (KAI1/GAPDH). Data are means ± SE of at least three independent experiments. (F) LNCaP cells were treated with 20 nM PMA for the indicated periods; 20 µg per lane of total protein cell lysate was subjected to electrophoresis, blotted to nitrocellulose membrane, and then probed for nonphosphorylated and phosphorylated forms of ERK1/2 and p38MAPK, as indicated. In lane 30U*, as a control, cells were pretreated for 1 hour with U0126 before addition of PMA. Lanes labeled * for p38MAPK and phospho-p38MAPK, show signal generated by positive control lysates to verify antibodies were effective.
The PKC family consists of 12 serine-threonine kinases divided into three major groups: classic (α, β, and γ), novel (δ, ε, η, and θ), and atypical (μ. ξ, and ι). Activation of classic enzymes requires calcium and DAG, novel enzymes are activated by DAG, and atypical forms are activated independently of calcium or DAG [25]. Involvement of specific PKC enzymes in the induction of KAI1 by PMA was tested using an endogenous calcium-chelator (BAPTA/AM; 25 µM) or apigenin (50 µM) and then adding PMA (20 nM). Pretreatment of cells for 1 hour with BAPTA/AM blocked induction of KAI1 by PMA as efficiently as apigenin (Figure 2D), suggesting that cPKC mediated the effect of PMA. To test this possibility, cells were pretreated for 1 hour with BAPTA/AM or apigenin, and then for 6 hours with Bryo 1 (50 nM) or thymeleatoxin (50 nM), which specifically activate cPKC enzymes. Thymeleatoxin up-regulated KAI1 as effectively as PMA and this was blocked by apigenin and BAPTA/AM (Figure 2D). In contrast, Bryo 1 had little effect on KAI1 mRNA levels (Figure 2D). A possible explanation for this discrepancy can be proposed from results of previous studies that have reported that although Bryo 1 activates cPKC enzymes [26], long-term exposure (>4 hours, as in the present study) can dramatically and specifically down-regulate cPKCα [27,28]. These data suggested that induction of KAI1 by PMA in prostate cancer cells is mediated by a calcium-dependent cPKC enzyme and that the specific isoform responsible is possibly cPKCα.
PKC Directly Activates MEK and ERK1/2 in Up-regulation of KAI1 mRNA by PMA
Downstream targets of PKC activation by PMA can use either Ras-dependent [29–31] or Ras-independent [32–34] signaling pathways. To assess the role of Ras in the induction of KAI1 by PMA, LNCaP cells were pretreated with a potent inhibitor of the Ras farnesyl protein transferase that blocks activation of Ras (FPT III; 50 µM). FPT III had little effect on basal levels of KAI1 or on the induction of KAI1 by PMA (Figure 2E). The involvement of downstream effector molecules in the signaling pathway was first tested with ZM 336372 (100 nM), a selective and potent inhibitor of c-Raf. Again, pretreatment of cells with this inhibitor failed to block induction of KAI1 by PMA (Figure 2E). To investigate the importance of alternative signaling pathways downstream of c-Ras, cells were first pretreated with either PD98059 (75 µM) or U0126 (5 µM) to selectively block MEK1/2. As a control, we used SB203580 (2 µM), a highly specific inhibitor of p38MAP kinase. PD98059 and U0126 inhibitors each had little effect on basal levels of KAI1 mRNA but completely blocked induction of KAI1 mRNA by PMA (Figure 2E). Conversely, SB203580 did not block induction of KAI1 mRNA by PMA (Figure 2E). Effects of the MAP kinase inhibitors were confirmed by Western blot analysis with antibodies to either total ERK1/2 or p38MAPK or with antibodies specific for phosphorylated activated forms of these proteins. Results shown in Figure 2F verified that ERK1 (p44), ERK2 (p42) and p38MAPK were present in untreated LNCaP but that no detectable phosphorylated protein was present. Phorbol 12-myristate 13-acetate had no effect on total levels of p44 or p42 but caused some reduction in p38MAPK by 60 minutes of treatment. ERK proteins (particularly p42) were phosphorylated by 15 minutes after treatment with 20 nM PMA. Phosphorylated p42 was still present 60 minutes after the addition of PMA, but the levels of phosphorylated p44 were decreased by 45 minutes after adding PMA and not detectable by 60 minutes. Importantly, phosphorylation of p44 and p42 was completely blocked by pretreatment of cells with 5 µM U0126. In contrast, there was no phosphorylation of p38MAPK by PMA (Figure 2F), lending further support to the notion that a signaling pathway involving this protein is not involved in the induction of KAI1 by PMA and providing an explanation for the failure of SB203580 to block induction (Figure 2E). The importance of ERK1/2 to the induction of KAI1 by PMA was then tested using AG126 (100 µM), an inhibitor of the ERK2 tyrosine kinase (Figure 2F). This inhibitor completely abrogated the effect of PMA on KAI1. In summary, these data suggested that the pathway of KAI1 induction by PMA involved direct signaling from PKC through MEK1/2 and ERK1/2.
Up-regulation of KAI1 mRNA by PMA Involves Histone Acetylation
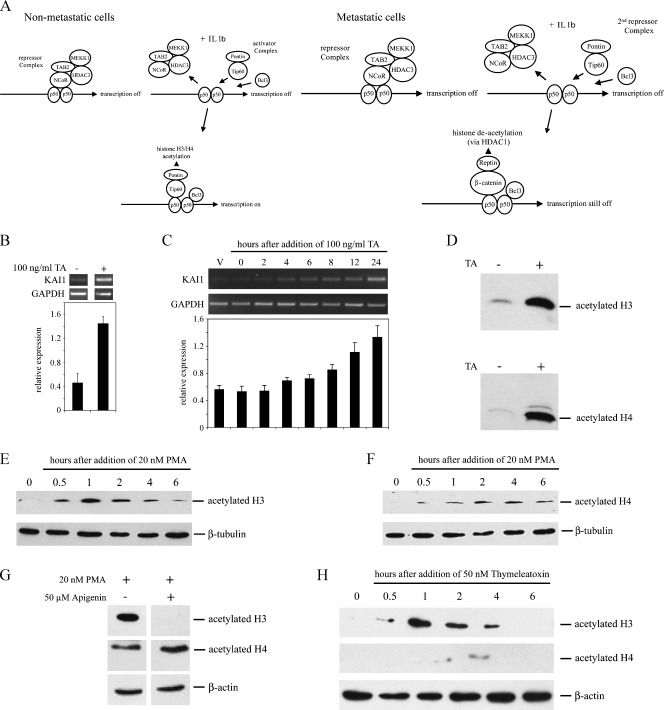
Depending on the stimulus, downstream effectors of ERK1/2 activation target either protein translation or transcription (reviewed in Hazzalin and Mahadevan [35]). Consistent with a previous study [15], pretreatment of cells with actinomycin D or cycloheximide before the addition of PMA clearly blocked the induction of KAI1, implying a role for de novo transcription and translation in the effects of PMA (not shown). Furthermore, ERK1/2-dependent up-regulation of transcription can involve altered chromatin structure (for example, [36,37]; reviewed in Clayton and Mahadevan [38]). Chromatin immunoprecipitation has provided compelling evidence for the importance of chromatin remodeling complexes binding a specific NF-κB (p50) motif in the proximal promoter [14] to KAI1 transcription (summarized in Figure 3A). In nonmetastatic cells, IL-1β-dependent activation of the KAI1 promoter involves displacement of an NCoR-repressor complex by an activator complex of Tip60 and Pontin (which acetylates histones H3 and H4). Although the NCoR complex is displaced in metastatic LNCaP cancer cells treated with IL-1β, subsequent binding of the Tip60/Pontin activator complex is blocked by a repressor complex containing β-catenin and Reptin, which inhibits transcription by recruiting HDAC1 [14].
Figure 3.
Trichostatin A and PMA induce acetylation of H3 and H4 histones in LNCaP cells. (A) Schematic of the proposed arrangement of Tip60 or β-catenin containing complexes on the KAI1 promoter, in nonmetastatic and metastatic cancer cells, before and after stimulation with IL-1β. Diagram modified from Kim et al. [14]. (B) Polymerase chain reaction analysis of KAI1 and GAPDH levels in LNCaP treated for 24 hours with DMSO vehicle or with 100 ng/ml trichostatin A in DMSO (TA). Graph shows relative levels of KAI1 (KAI1/GAPDH). Data are means ± SE of at least three independent experiments. (C) Polymerase chain reaction analysis of KAI1 and GAPDH in LNCaP treated for up to 24 hours with 100 ng/ml trichostatin A in DMSO. Vehicle-treated cells (V) were exposed to DMSO only for 24 hours. Graph summarizes mean ± SE from four independent experiments. *P < .05 compared with vehicle-only control. (D) Western blot analysis of acetylated histones H3 and H4, in lysates from LNCaP cells treated for 8 hours with DMSO vehicle (-) or 100 ng/ml trichostatin A (TA; +). (E, F) Western blot analysis of acetylated H3 (E) and H4 (F) histones in lysates from LNCaP cells treated with 20 nM PMA for the indicated times. (G) Western blot analysis for acetylated histones H3 and H4 in LNCaP cells after a 1-hour exposure to 20 nM PMA either without (-) or with (+) 1 hour of pretreatment with 50 µM apigenin. (H) Cells were treated with 50 nM thymeleatoxin for the indicated times, and lysates were used for Western blot analysis of acetylated histones H3 and H4 as described in the Materials and Methods section.
A role for HDAC1 in suppressing KAI1 transcription is supported by previous studies showing that NaB [18] and trichostatin A [10] can elevate KAI1 mRNA levels. Induction of KAI1 mRNA by NaB (data not shown) and trichostatin A was confirmed in LNCaP cells (Figure 3B). Induction occurred within a time frame (first detected 4–6 hours after drug addition) similar to that of PMA (Figure 3C) and was accompanied by overall acetylation of histones H3 and H4 (Figure 3D). H3 and H4 were also strongly acetylated in LNCaP cells from 30 minutes after the addition of 20 nM PMA (Figure 3, E and F). Acetylation of H3 was maximal at 1 hour, remained elevated 2 to 4 hours after addition, but declined to near basal levels by 6 hours (Figure 3E). Acetylation of H4 was maximal 2 to 4 hours after the addition of PMA but still elevated 6 hours after addition (Figure 3F). Acetylation of H3 by PMA was blocked by pretreatment of the cells with the PKC inhibitor, apigenin (Figure 3G), but acetylation of H4 by PMA was not blocked by either apigenin (Figure 3G) or U0126 (data not shown), suggesting that the effect of PMA on histone H4 acetylation might be not specific to the PKC activation pathway described previously. Confirming the importance of histone H3 acetylation to KAI1 transcription, thymeleatoxin, another inducer of KAI1, which acts through cPKC (Figure 2D), also clearly stimulated overall acetylation of histone H3 over a time course similar to that of PMA but only weakly affected acetylation of H4 (Figure 3H).
Together, these data were consistent with a model whereby PMA activates KAI1 transcription in LNCaP cells through a signaling pathway, which directly results in increased acetylation of cellular H3 and is associated with acetylation of H4. However, this does not imply that histone acetylation directly contributes to the upregulation of KAI1 transcription by phorbol ester.
PMA Recruits Specific Activator Protein Complexes to the KAI1 Promoter
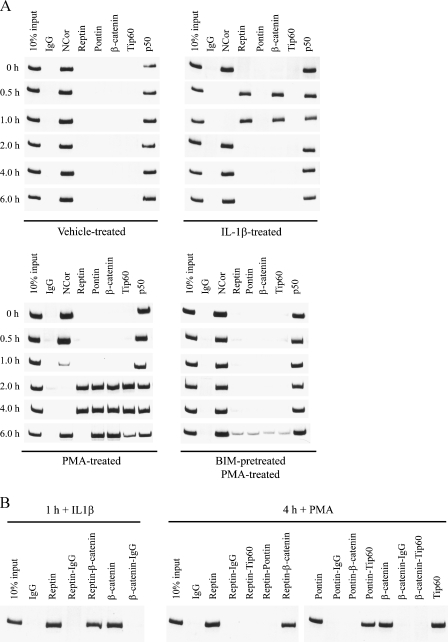
Given these results and data from preliminary experiments using the inhibitor anacardic acid, which suggested that p300 and PCAF were not the histone acetyltransferases targeted by PMA to induce KAI1 (data not shown), ChIP was used to assess binding of p50, repressor (NCoR, β-catenin, Reptin), and activator (Pontin and Tip60) complexes to the p50 binding motif identified by Kim et al. [14] and Baek et al. [39] in vehicle-treated LNCaP cells and cells treated with 20 nM PMA. Association of these proteins in response to PMA was compared with their binding to the p50 motif in response to IL-1β treatment (5 ng/ml) for the indicated times). Results illustrated in Figure 4A (top left panel) show that in untreated LNCaP cells, NCoR and p50 bind this motif. Consistent with Kim et al. [14], treatment with IL-1β resulted in displacement of NCoR but not p50 within 0.5 hours of IL-1β addition and in recruitment of β-catenin and Reptin to the promoter (Figure 4A, top right panel). By 2 hours after the addition of IL-1β, both β-catenin and Reptin had been displaced again and NCoR reappeared on the promoter, thereby keeping the promoter in the inactive state. There was no evidence for binding of the activator proteins Tip60 or Pontin to the promoter at any time after the addition of IL-1β. NCoR, but not p50, was also lost from the promoter after the addition of 20 nM PMA (Figure 5A, lower left panel), but the kinetics of this loss was slower than that observed for IL-1β, with NCoR taking 2 hours to be completely lost from the promoter. In the absence of NCoR, β-catenin and Reptin appeared on the promoter 2 hours after the addition of PMA. In contrast to the response to IL-1β, Tip60 and Pontin also appeared on the promoter at this time after PMA addition. All four proteins were still present 4 hours after the addition of PMA, but after 6 hours, Reptin had been lost from the promoter, Tip60 levels were decreasing, and NCoR had reappeared, suggesting that the promoter was returning to an inactive state. Importantly, these effects caused by PMA were significantly delayed by pretreating cells with the PKC inhibitor Bim III (Figure 4A, lower right panel), providing evidence that the PKC signaling pathway described was responsible for mediating PMA-dependent changes in proteins binding the KAI1 promoter.
Figure 4.
Phorbol 12-myristate 13-acetate induces changes in the composition of chromatin-remodeling protein complexes at specific NFκB-p50 sites in the promoter of the KAI1 gene. (A) LNCaP cells were either treated with DMSO vehicle, 5 ng/ml IL-1β, and 20 nM PMA for 1 hour or treated with 10 µM Bim III before 20 nM PMA for the indicated times and then processed for ChIP analysis of proteins binding to the KAI1 promoter, as described in the Materials and Methods section. Data are representative of two independent experiments. (B) Samples from (A) were subsequently analyzed by two-step ChIP to determine interactions between specific proteins binding the KAI1 promoter in cells treated with PMA for 1 and 4 hours.
Figure 5.
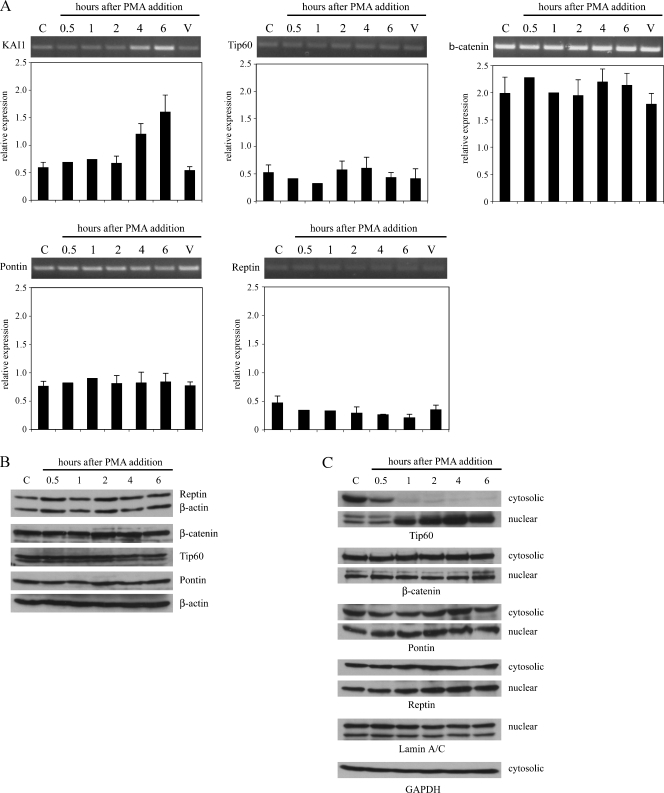
Expression of chromatin remodeling proteins in the 6 hours after the addition of PMA. (A) Reverse transcription-polymerase chain reaction analysis of KAI1, Tip60, Pontin, β-catenin, Reptin, and GAPDH mRNA levels in control LNCaP cells (C), vehicle only-treated LNCaP cells (V), and LNCaP cells treated for 0 to 6 hours with 20 nM PMA. Graph shows relative expression of each gene compared with GAPDH in control and treated cells. Data are means ± SE of at least three independent experiments. (B) Western blot analysis of Tip60, β-catenin, Pontin, Reptin, and β-actin protein levels in LNCaP cells treated for 0 to 6 hours with DMSO or with 20 nM PMA in DMSO. Cells were lysed in RIPA buffer and 25 µg of protein was loaded per lane. (C) Cells were treated with PMA as in (B), and cytoplasmic and nuclear fractionations were analyzed for Tip60, β-catenin, Pontin, and Reptin by Western blot analysis. Protein loading was controlled with anti-lamin A/C and anti-GAPDH antibodies. Data are representatives of three independent experiments.
A two-step ChIP approach was then used to assess the composition of complexes binding the KAI1 promoter at 1 hour after the addition of IL-1β and 4 hours after the addition of PMA. These studies confirmed that complexes binding the promoter after the addition of IL-1β were composed of β-catenin and Reptin (Figure 4B, lanes 1–7) as described previously [14]. At 4 hours after the addition of PMA, β-catenin, Reptin, Tip60, and Pontin are all detected on the KAI1 promoter (Figure 4A). However, these data cannot determine whether all four proteins are bound to the same promoter or whether proteins forming a repressor complex (β-catenin and Reptin) are on different promoters to those proteins that form an activator complex (Tip60 and Pontin). Data in Figure 4B (lanes 8–22) show that, also in response to PMA, Reptin forms complexes only with β-catenin and not with Pontin or Tip60 (lanes 11–14). Furthermore, no interaction between β-catenin and Tip60 was detected when β-catenin was on the promoter (lane 21). In contrast, if Pontin was present on the promoter, only complexes with Tip60 were detected (lanes 15–18). These data were in agreement with those of Kim et al. [14] who showed that binding of β-catenin and Reptin complexes, or Pontin and Tip60 complexes to the KAI1 promoter is mutually exclusive. Our studies suggested that 4 hours after the addition of PMA, a proportion of KAI1 promoters in the treated cell population had recruited Tip60/Pontin activator complexes to the KAI1 promoter.
PMA Induces Nuclear Translocation of Tip60
Changing relative levels of Tip60, β-catenin, or Reptin by overexpression of Tip60 or down-regulation of β-catenin or Reptin is sufficient to restore IL-1β-dependent acetylation of histones H3 and H4 and, consequently, activation of the KAI1 promoter in LNCaP cells by favoring formation of Tip60/Pontin activator complexes on the KAI1 promoter [14]. To determine whether PMA-induced changes in relative levels of these proteins might underlie the observed changes in the composition of protein complexes binding to the p50 motif in the KAI1 promoter, mRNA and protein levels for Tip60, β-catenin, Reptin, and Pontin were examined in cells treated with 20 nM PMA for up to 6 hours. Data presented in Figure 5 show that mRNA (Figure 5A) and protein levels (Figure 5B) for Tip60, β-catenin, Pontin, and Reptin were unchanged by PMA, whereas KAI1 mRNA levels were clearly elevated by 4 hours after the addition of PMA. Not only Pontin and Reptin but also Tip60 and β-catenin are proteins that have been localized both in the cytoplasm and nucleus (see, for example, control samples in Figure 5C), but the fraction of these proteins within the nucleus is responsible for their effects on transcription. In the absence of any PMA-dependent changes in the overall expression of these proteins, the observed effects might be explained by an altered intracellular distribution of one or more of these proteins in response to PMA; specifically, an increase in the levels of Tip60 and Pontin within the nucleus. To address this possibility, LNCaP cells were stimulated with PMA, and at different time points after the addition of PMA, cytosolic and nuclear fractions were analyzed for the expression of Tip60, β-catenin, Pontin, and Reptin by Western blot analysis. Interestingly, there was a rapid loss of Tip60 from the cytosolic fraction (detectable by 30 minutes after the addition of PMA) and a concomitant accumulation of Tip60 within the nuclear fraction (Figure 5C). This distribution was maintained for up to 6 hours after the addition of PMA (Figure 5C). In contrast, the distribution of β-catenin, Pontin, and Reptin between the cytoplasm and nucleus was not altered after the addition of PMA. These data overall suggested that increasing nuclear levels of Tip60 may be responsible for changes in the composition of protein complexes binding the KAI1 promoter associated with induction of transcription by PMA, by favoring the formation of Tip60/Pontin activator complexes.
Discussion
We have characterized a signaling pathway and downstream molecular events used by PMA to induce the expression of the KAI1 metastasis-suppressor gene in LNCaP prostate cancer cells. Consistent with the current understanding of signaling pathways involving PMA, induction of KAI1 occurred through a pathway, which used classic isoforms of protein kinase C and directly activated MEK and ERK intermediates. Whereas signaling pathways used by PMA generally involve both Ras- and Raf-dependent steps (see Ming et al. [33]), in this study, the use of inhibitors suggested no requirement for either of these intermediates. Other studies have shown that PKC can bypass Ras and directly activate Raf [34,40,41]. Additionally, in certain cell types and for certain responses, Ras and Raf may both be bypassed. Thus, a combination of specific inhibitors and overexpression of dominant-negative proteins has been used to show that the pathway of PMA-induced growth arrest in HepG2 cells requires PKCα and ERK but not Ras or Raf [42]. Similarly, growth arrest of Bcr/Abl-transformed leukemia cells by perillyl alcohol can occur by directly inhibiting MEK signaling and bypass of both Ras and Raf [43]. Whereas our studies support the idea that PMA activates a cPKC-MEK/ERK-dependent signaling pathway resulting in the up-regulation of KAI1 transcription, we cannot exclude the possibility that more global effects of PMA on cellular metabolism may also contribute to the overall effects of PMA on KAI1 mRNA levels. Consistent with this possibility, pretreatment of cells with translational inhibitors such as cyclohexamide also partially inhibited induction of KAI1 mRNA by PMA.
Recent studies have demonstrated the importance of specific protein complexes containing β-catenin, Reptin, Pontin, and Tip60 binding to a specific NFκB-p50 motif in the proximal promoter regulating KAI1 transcription in response to IL-1β [13,14]. Data from the present study suggest that this p50 motif is also a key element in regulating the transcriptional response to PMA. The chromatin remodeling components mediating the response to PMA are similar to those involved in the response to IL-1β. However, whereas NCoR is completely replaced by β-catenin/Reptin repressor complexes in LNCaP cells exposed to IL-1β, thus preventing the binding of activator complexes containing Pontin and Tip60, the displacement of NCoR in response to PMA results in the binding of Tip60/Pontin complexes at least to a proportion of promoters within the cell population, resulting in transcriptional activation. The kinetics of changes in proteins binding to the KAI1 promoter in response to PMA treatment, however, suggested that transcriptional regulation of KAI1 is complex. At the moment, we cannot explain how the assembly of β-catenin/Reptin complexes and reappearance of NCoR at the NFκB site on the KAI1 promoter, within the first 6 hours after PMA treatment, can be correlated with an overall increase of KAI1 mRNA from 6 to 24 hours after the addition of PMA. Currently, we are considering three possible mechanisms to explain this phenomenon, which may act together to regulate the KAI1 promoter. 1) In addition to the specific site investigated in this paper, other regulatory cis-elements may contribute to the observed changes of mRNA levels induced by PMA; for example, the upstream enhancer-like motif containing p53, AP1, and AP2 motifs identified by Marreiros et al. [44]. 2) Recruitment of additional unidentified factors may be involved in the regulation at the site investigated in the present study and/or additional sites. 3) Recent observations report that transcription is regulated by a highly dynamic assembly/disassembly of protein complexes at regulatory cis-elements at gene promoters with cycles of association and dissociation of regulatory factors [45,46]. Further studies will be required to clarify the importance of these different elements.
The underlying basis for a lack of transcriptional response to IL-1β in metastatic cells, such as LNCaP cells, is proposed to be due, at least in part, to very low levels of Tip60 relative to β-catenin in these cells, thus favoring formation of repressive β-catenin/Reptin complexes [14]. This model suggests that one possible mechanism by which PMA might allow formation of Tip60/Pontin complexes on the promoter is by changing relative levels of Tip60 and β-catenin, such that the chances of a Tip60/Pontin complex binding the promoter are increased. Although examination of the overall expression of these four proteins after the addition of PMA provided no evidence to support this proposal, we did observe that PMA caused a rapid and specific redistribution of Tip60 from the cytoplasm to the nucleus. Increased levels of Tip60 would favor the chances of Tip60 binding the promoter. Further studies will need to define whether PKC and/or MEK/ERK signaling is responsible for the nuclear translocation of Tip60 caused by PMA and how activation of PKC and/or MEK/ERK signaling specifically affects transcription, for example, which proteins are being affected by phosphorylation induced by this pathway, and how signaling induced by IL-1β induces different effects on proteins binding the KAI1 promoter.
How might PMA promote binding of Tip60 and Pontin to the regulatory site in the KAI1 gene in LNCaP cells? Recent reports indicate a role of sumoylation/desumoylation during prostate cancer development and metastasis [47,48]. A recent study has shown that metastatic cells express higher levels of the small ubiquitin-like modifier (SUMO)-conjugating enzyme Ubc9, which attaches SUMO to Reptin, and lower levels of SUMO-processing enzymes, such as SENP1 and SUSP1 [13]. SUMOylation stimulates the repressive effects of Reptin by promoting nuclear localization and increasing interaction with HDAC1 [13]. Levels of SUMOylated repressive Reptin are higher in metastatic than in nonmetastatic cells, and this form of Reptin prevents binding of Tip60 to the KAI1 promoter [13]. Conceivably, PMA might favor formation of Tip60/Pontin complexes on the KAI1 cis-regulatory element by de-SUMOylating Reptin or promoting SUMOylation of Pontin [49]. Despite exhaustive attempts, we were unable to convincingly show the presence of any SUMOylated Reptin or Pontin in this study (data not shown). In ChIP experiments, with anti-SUMO antibodies, it was possible to detect binding of a SUMOylated component/s to the KAI1 promoter (not shown), but cross-linking during ChIP experiments does not allow specific identification of the SUMOylated component. In addition, it was not possible to completely correlate changes in SUMOylation with the time-dependent changes in the complex composition at the cis-regulatory sites observed in the ChIP experiments, even when assuming that Reptin and Pontin are the only candidates to be SUMOylated (data not shown). Interestingly, only a small fraction (5%) of the overall Reptin population is SUMOylated, yet more than a third of the total pool is nuclear [50]. This has raised questions as to why the non-SUMOylated Reptin in the nucleus does not normally compete with SUMOylated Reptin for binding to the KAI1 promoter to activate transcription [50]. It will be important to determine whether PMA has any effect on the proportion of SUMOylated Reptin or Pontin associated with the KAI1 promoter.
A third mechanism by which the transcriptional activity of Pontin and Reptin can be modulated has been recently described [22]. Hint 1 (histidine triad nucleotide-binding protein 1) binds both Pontin and Reptin, and it was shown to disrupt Pontin/Reptin complexes and thus may contribute to the formation of Pontin/Tip60 or β-catenin/Reptin transcriptional complexes. Because Hint1 was previously identified as PKC interacting protein-1 (PKCI-1), it can be speculated that PKC through Hint1 may affect the transcriptional output at target gene promoters [51]. It is intriguing to speculate that PKC-dependent phosphorylation of Tip60 might be involved in its accumulation in the nucleus or in enhancing of the binding of Tip60 to Pontin.
Taken together, our data and the increased expression and enhanced nuclear localization of Pontin and Reptin observed in cancer tissues [52–54] suggest that an exact regulation of nuclear Tip60/Pontin and β-catenin/Reptin levels and function is important for the transcriptional output at KAI1 and perhaps other promoters. However, the molecular mechanisms have to be further investigated in vitro and in vivo [55], and their general role in the metastasis of tumor cells of other origins such as breast or colon remains to be examined. In this context, here, we have identified a signaling pathway by which PMA up-regulates KAI1 transcription by stimulating the formation of specific activator complexes on the KAI1 promoter. These data provide further support for the central importance of the Reptin/β-catenin: Tip60/Pontin axis in regulating gene expression.
Supplementary Material
Abbreviations
- (Bim III)
bis-indolylmaleimide III
- ChIP
chromatin immunoprecipitation
- cPKC
classic isoforms of protein kinase C
- H3
histone H3
- H4
histone H4
- HDAC
histone deacetylase
- NaB
sodium butyrate
- IL-1β
interleukin-1 β
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
Footnotes
This study was supported by a Faculty of Medicine Research Grant from the University of New SouthWales (P.J.), and by the Charité - Universitätsmedizin Berlin and the Sonnenfeld Stiftung (O.H.).
This article refers to supplementary material, which is designated by Figure W1 and is available online at www.neoplasia.com.
References
- 1.Liu WM, Zhang XA. KAI1/CD82, a tumor metastasis suppressor. Cancer Lett. 2006;240:183–194. doi: 10.1016/j.canlet.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 2.Tonoli H, Barrett JC. CD82 metastasis suppressor gene: a potential target for new therapeutics? Trends Mol Med. 2005;11:563–570. doi: 10.1016/j.molmed.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Jee B, Jin K, Song HG, Lee H. Metastasis-suppressor KAI1/CD82 induces homotypic aggregation of human prostate cancer cells through a Src-dependent pathway. Exp Mol Med. 2003;35:30–37. doi: 10.1038/emm.2003.5. [DOI] [PubMed] [Google Scholar]
- 4.Takaoka A, Hinoda Y, Satoh S, Adachi Y, Itoh F, Adachi M. Suppression of invasive properties of colon cancer cells by a metastasis suppressor KAI1 gene. Oncogene. 1998;16:1443–1453. doi: 10.1038/sj.onc.1201648. [DOI] [PubMed] [Google Scholar]
- 5.Yang JL, Jackson P, Yu Y, Yoshie O, Ham JL, Russell PJ, Crowe PJ. Invasion and metastasis correlate with down-regulation of KAI1 expression in human colon cancer cell lines. GI Cancer. 2001;3:313–322. [Google Scholar]
- 6.Jackson P, Kingsley EA, Russell PJ. Inverse correlation between KAI1 mRNA levels and cell hehaviour in bladder cancer cell lines. Cancer Lett. 2000;156:9–17. doi: 10.1016/s0304-3835(00)00427-4. [DOI] [PubMed] [Google Scholar]
- 7.Yang X, Wei LL, Tang C, Slack R, Mueller S, Lippman ME. Overexpression of KAI1 suppresses in vitro invasiveness and in vivo metastasis in breast cancer cells. Cancer Res. 2001;61:5284–5288. [PubMed] [Google Scholar]
- 8.Dong JT, Suzuki H, Pin SS, Bova GS, Schalken JA, Isaacs WB, Barrett JC, Isaacs JT. Down-regulation of the KAI1 metastasis suppressor gene during the progression of human prostatic cancer infrequently involves gene mutation or allelic loss. Cancer Res. 1996;56:4387–4390. [PubMed] [Google Scholar]
- 9.Jackson P, Millar D, Kingsley E, Yardley G, Ow K, Clark S, Russell PJ. Methylation of a CpG island within the promoter region of the KAI1 metastasis suppressor gene is not responsible for down-regulation of KAI1 expression in invasive cancer cell lines. Cancer Lett. 2000;157:169–176. doi: 10.1016/s0304-3835(00)00483-3. [DOI] [PubMed] [Google Scholar]
- 10.Sekita N, Suzuki H, Ichikawa T, Kito H, Akakura K, Igarashi T, Nakayama T, Watanabe M, Shiraishi T, Toyota M, et al. Epigenetic regulation of the KAI1 metastasis suppressor gene in human prostate cancer cell lines. Jpn J Cancer Res. 2001;92:947–951. doi: 10.1111/j.1349-7006.2001.tb01185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao AC, Lou W, Dong JT, Barrett JC, Danielpour D, Isaacs JT. Defining regulatory elements in the human KAI1 (CD82) metastasis suppressor gene. Prostate. 2003;57:256–260. doi: 10.1002/pros.10309. [DOI] [PubMed] [Google Scholar]
- 12.Marreiros A, Czolij R, Yardley G, Crossley M, Jackson P. Identification of regulatory regions within the KAI1 promoter: a role for binding of AP1, AP2 and p53. Gene. 2003;302:155–164. doi: 10.1016/s0378-1119(02)01101-0. [DOI] [PubMed] [Google Scholar]
- 13.Kim JH, Choi HJ, Kim B, Kim MH, Lee JM, Kim IS, Lee MH, Choi SJ, Kim KI, Chung CH, et al. Roles of sumoylation of a reptin chromatinremodelling complex in cancer metastasis. Nat Cell Biol. 2006;8:631–639. doi: 10.1038/ncb1415. [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, Kim B, Cai L, Choi HJ, Ohgi KA, Tran C, Chen C, Chung CH, Huber O, Rose DW, et al. Transcriptional regulation of a metastasis suppressor gene by Tip60 and β-catenin complexes. Nature. 2005;434:921–926. doi: 10.1038/nature03452. [DOI] [PubMed] [Google Scholar]
- 15.Akita H, Iizuka A, Hasimoto Y, Kohri K, Ikeda K, Nakanishi M. Induction of KAI-1 expression in metastatic cancer cells by phorbol esters. Cancer Lett. 2000;153:79–83. doi: 10.1016/s0304-3835(00)00352-9. [DOI] [PubMed] [Google Scholar]
- 16.Sigala S, Faraoni I, Botticini D, Paez-Pereda M, Missale C, Bonmassar E, Spano P. Suppression of telomerase, re-expression of KAI1, and abrogation of tumorigenicity by nerve growth factor in prostate cancer cell lines. Clin Cancer Res. 1999;5:1211–1218. [PubMed] [Google Scholar]
- 17.Shinohara T, Miki T, Nishimura N, Nokihara H, Hamada H, Mukaida N. Nuclear factor κB-dependent expression of metastasis suppressor KAI1/CD82 gene in lung cancer cell lines expressing mutant p53. Cancer Res. 2001;61:673–678. [PubMed] [Google Scholar]
- 18.Joseph J, Mudduluru G, Antony S, Vashistha S, Ajitkumar P, Somasundaram K. Expression profiling of sodium butyrate (NaB)-treated cells: identification of regulation of genes related to cytokine signaling and cancer metastasis by NaB. Oncogene. 2004;23:6304–6315. doi: 10.1038/sj.onc.1207852. [DOI] [PubMed] [Google Scholar]
- 19.Thalmann GN, Sikes RA, Chang SM, Johnston DA, von Eschenbach AC, Chung LWK. Suramin-induced decrease in prostate-specific antigen expression with no effect on tumor growth in the LNCaP model of human prostate cancer. J Natl Cancer Inst. 1996;88:794–801. doi: 10.1093/jnci/88.12.794. [DOI] [PubMed] [Google Scholar]
- 20.Bauer A, Chauvet S, Huber O, Usseglio F, Rothbächer U, Aragnol D, Kemler R, Pradel J. Pontin52 and Reptin52 function as antagonistic regulators of β-catenin signalling activity. EMBO J. 2000;19:6121–6130. doi: 10.1093/emboj/19.22.6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauer A, Huber O, Kemler R. Pontin52, an interaction partner of β-catenin, binds to the TATA box binding protein. Proc Natl Acad Sci USA. 1998;95:14787–14792. doi: 10.1073/pnas.95.25.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiske J, Huber O. The histidine triad protein Hint1 interacts with Pontin and Reptin and inhibits TCF-β-catenin-mediated transcription. J Cell Sci. 2005;118:3117–3129. doi: 10.1242/jcs.02437. [DOI] [PubMed] [Google Scholar]
- 23.Weiske J, Albring KF, Huber O. The tumor suppressor Fhit acts as a repressor of β-catenin transcriptional activity. Proc Natl Acad Sci USA. 2007;104:20344–20349. doi: 10.1073/pnas.0703664105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiske J, Huber O. The histidine triad protein Hint1 triggers apoptosis independent of its enzymatic activity. J Biol Chem. 2006;281:27356–27366. doi: 10.1074/jbc.M513452200. [DOI] [PubMed] [Google Scholar]
- 25.Koivunen J, Aaltonen V, Peltonen J. Protein kinase C (PKC) family in cancer progression. Cancer Lett. 2006;235:1–10. doi: 10.1016/j.canlet.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 26.Song JC, Hanson CM, Tsai V, Farokhzad OC, Lotz M, Matthews JB. Regulation of epithelial transport and barrier function by distinct protein kinase C isoforms. Am J Physiol Cell Physiol. 2001;281:C649–C661. doi: 10.1152/ajpcell.2001.281.2.C649. [DOI] [PubMed] [Google Scholar]
- 27.Clarke H, Ginanni N, Laughlin KV, Smith JB, Pettit GR, Mullin JM. The transient increase of tight junction permeability induced by bryostatin 1 correlates with rapid down-regulation of protein kinase Cα. Exp Cell Res. 2000;261:239–249. doi: 10.1006/excr.2000.5035. [DOI] [PubMed] [Google Scholar]
- 28.Stewart JR, O'Brian CA. Protein kinase C-α mediates epidermal growth factor receptor transactivation in human prostate cancer cells. Mol Cancer Ther. 2005;4:726–732. doi: 10.1158/1535-7163.MCT-05-0013. [DOI] [PubMed] [Google Scholar]
- 29.Hess A, Wijayanti N, Neuschäfer-Rube AP, Katz N, Kietzmann T, Immenschuh S. Phorbol ester-dependent activation of peroxiredoxin I gene expression via a protein kinase C, Ras, p38 mitogen-activated protein kinase signaling pathway. J Biol Chem. 2003;278:45419–45434. doi: 10.1074/jbc.M307871200. [DOI] [PubMed] [Google Scholar]
- 30.Hewson CA, Edbrooke MR, Johnston SL. PMA induces the MUC5AC respiratory mucin in human bronchial epithelial cells, via PKC, EGF/TGFα, Ras/Raf, MEK, ERK and Sp1-dependent mechanisms. J Mol Biol. 2004;344:683–695. doi: 10.1016/j.jmb.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 31.Lee HW, Ahn DH, Crawley SC, Li JD, Gum JR, Jr, Basbaum CB, Fan NQ, Szymkowski DE, Han SY, Lee BH, et al. Phorbol 12-myristate 13-acetate up-regulates the transcription of MUC2 intestinal mucin via Ras, ERK, and NF-κB. J Biol Chem. 2002;277:32624–32631. doi: 10.1074/jbc.M200353200. [DOI] [PubMed] [Google Scholar]
- 32.Lee MH, Kim JY, Anderson WB. Src tyrosine kinase inhibitor PP2 markedly enhances Ras-independent activation of Raf-1 kinase by phorbol myristate acetate and H2O2. J Biol Chem. 2004;279:48692–48701. doi: 10.1074/jbc.M403132200. [DOI] [PubMed] [Google Scholar]
- 33.Ming XF, Burgering BMT, Wennstrom S, Claesson-Welsh L, Heldin CH, Bos JL, Kozma SC, Thomas G. Activation of p70/p85 S6 kinase by a pathway independent of rp21ras. Nature. 1994;371:426–429. doi: 10.1038/371426a0. [DOI] [PubMed] [Google Scholar]
- 34.Tobin D, Nilsson M, Toftgard R. Ras-independent activation of Rel-family transcription factors by UVB and TPA in cultured keratinocytes. Oncogene. 1996;12:785–793. [PubMed] [Google Scholar]
- 35.Hazzalin CA, Mahadevan LC. MAPK-regulated transcription: a continuously variable gene switch? Nat Rev Mol Cell Biol. 2002;3:30–40. doi: 10.1038/nrm715. [DOI] [PubMed] [Google Scholar]
- 36.Lucas M, Zhang X, Prasanna V, Mosser DM. ERK activation following macrophage FcγR ligation leads to chromatin modifications at the IL-10 locus. J Immunol. 2005;175:469–477. doi: 10.4049/jimmunol.175.1.469. [DOI] [PubMed] [Google Scholar]
- 37.Pennini ME, Pai RK, Schultz DC, Boom WH, Harding CV. Mycobacterium tuberculosis 19-kDa lipoprotein inhibits IFN-γ-induced chromatin remodeling of MHC2TA by TLR2 and MAPK signalling. J Immunol. 2006;176:4323–4330. doi: 10.4049/jimmunol.176.7.4323. [DOI] [PubMed] [Google Scholar]
- 38.Clayton AL, Mahadevan LC. MAP kinase-mediated phosphoacetylation of histone H3 and inducible gene regulation. FEBS Lett. 2003;546:51–58. doi: 10.1016/s0014-5793(03)00451-4. [DOI] [PubMed] [Google Scholar]
- 39.Baek SH, Ohgi KA, Rose DW, Koo EH, Glass CK, Rosenfeld MG. Exchange of N-CoR corepressor and Tip60 coactivator complexes links gene expression by NFγB and β-amyloid precursor protein. Cell. 2002;110:55–67. doi: 10.1016/s0092-8674(02)00809-7. [DOI] [PubMed] [Google Scholar]
- 40.Ho PD, Zechner DK, He H, Dillmann WH, Glembotski CC, McDonough PM. The Raf-MEK-ERK cascade represents a common pathway for alteration of intracellular calcium by Ras and protein kinase C in cardiac myocytes. J Biol Chem. 1998;273:21730–21735. doi: 10.1074/jbc.273.34.21730. [DOI] [PubMed] [Google Scholar]
- 41.Ueda Y, Hirai S, Osada S, Suzuki A, Mizuno K, Ohno KS. Protein kinase C activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J Biol Chem. 1996;271:23512–23519. doi: 10.1074/jbc.271.38.23512. [DOI] [PubMed] [Google Scholar]
- 42.Wen-Sheng W. Protein kinase C α trigger Ras- and Raf-independent MEK/ERK activation for TPA-induced growth inhibition of human hepatoma cell HepG2. Cancer Lett. 2006;239:27–35. doi: 10.1016/j.canlet.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 43.Clark SS, Zhong L, Filiault D, Perman S, Ren Z, Gould M, Yang X. Anti-leukemia effect of perillyl alcohol in Bcr/Abl-transformed cells indirectly inhibits signaling through Mek in a Ras- and Raf-independent fashion. Clin Cancer Res. 2003;9:4494–4504. [PubMed] [Google Scholar]
- 44.Marreiros A, Dudgeon K, Dao V, Grimm MO, Czolij R, Crossley M, Jackson P. KAI1 promoter activity is dependent on p53, junB and AP2: evidence for a possible mechanism underlying loss of KAI1 expression in cancer cells. Oncogene. 2005;24:637–649. doi: 10.1038/sj.onc.1208216. [DOI] [PubMed] [Google Scholar]
- 45.Perissi V, Rosenfeld MG. Controlling nucelar receptors: the circular logic of cofactor cycles. Nat Rev Mol Cell Biol. 2005;6:542–554. doi: 10.1038/nrm1680. [DOI] [PubMed] [Google Scholar]
- 46.Sierra J, Yoshida T, Joazeiro CA, Jones KA. The APC tumor suppressor counteracts β-catenin activation and H3K4 methylation at Wnt target genes. Genes Dev. 2006;20:586–600. doi: 10.1101/gad.1385806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baek SH. A novel link between SUMO modification and cancer metastasis. Cell Cycle. 2006;5:1492–1495. doi: 10.4161/cc.5.14.3008. [DOI] [PubMed] [Google Scholar]
- 48.Cheng J, Bawa T, Lee P, Gong L, Yeh ETH. Role of desumoylation in the development of prostate cancer. Neoplasia. 2006;8:667–676. doi: 10.1593/neo.06445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim JH, Lee JM, Nam HJ, Choi HJ, Yang JW, Lee JS, Kim MH, Kim SI, Chung CH, Kim KI, et al. SUMOylation of pontin chromatin-remodeling complex reveals a signal integration code in prostate cancer. Proc Natl Acad Sci USA. 2007;104:20793–20798. doi: 10.1073/pnas.0710343105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gallant P. Control of transcription by Pontin and Reptin. Trends Cell Biol. 2007;17:187–192. doi: 10.1016/j.tcb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Huber O, Weiske J. β-Catenin takes a HIT. Cell Cycle. 2008;7:1326–1331. doi: 10.4161/cc.7.10.5926. [DOI] [PubMed] [Google Scholar]
- 52.Huber O, Ménard L, Haurie V, Nicou A, Taras D, Rosenbaum J. Pontin and Reptin, two related ATPases with multiple roles in cancer. Cancer Res. 2008;68:6873–6876. doi: 10.1158/0008-5472.CAN-08-0547. [DOI] [PubMed] [Google Scholar]
- 53.Lauscher JC, Loddenkemper C, Kosel L, Gröne J, Buhr HJ, Huber O. Increased pontin expression in human colorectal cancer tissue. Hum Pathol. 2007;38:978–985. doi: 10.1016/j.humpath.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 54.Rousseau B, Ménard L, Haurie V, Taras D, Blanc JF, Moreau-Gaudry F, Metzler P, Hugues M, Boyault S, Lemière S, et al. Overexpression and role of the ATPase and putative DNA helicase RuvB-like 2 in human hepatocellular carcinoma. Hepatology. 2007;46:1108–1118. doi: 10.1002/hep.21770. [DOI] [PubMed] [Google Scholar]
- 55.Havens AM, Pedersen EA, Shiozawa Y, Ying C, Jung Y, Sun Y, Neeley C, Wang J, Mehra R, Keller ET, et al. An in vivo mouse model for human prostate cancer metastasis. Neoplasia. 2008;10:371–379. doi: 10.1593/neo.08154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.