Abstract
Ovarian carcinoma arises from the ovarian surface epithelium, which undergoes phenotypic changes characteristic of müllerian epithelium during the first stages of tumorigenesis. The variant isoform of the hepatocyte nuclear factor 1 (vHNF1) is a transcription factor involved in the development of tissues derived from the müllerian duct. Here, we show that vHNF1 knockdown in two ovarian carcinoma cell lines, SKOV3 and IGROV1, leads to reduced E-cadherin (E-cadh) expression and decreased proliferation rate. Accordingly, SKOV3 cells ectopically expressing a dominant-negative (DN) vHNF1 mutant undergo an epithelial-mesenchymal-like transition, acquiring a spindle-like morphology, loss of E-cadh, and disrupted cell-cell contacts. Gene expression profiling of DNvHNF1 cells on the basis of a newly compiled list of epithelial-mesenchymal transition-related genes revealed a correlation between vHNF1 loss-of-function and acquisition of the mesenchymal phenotype. Indeed, phenotypic changes were associated with increased Slug transcription and functionality. Accordingly, vHNF1-transfected immortalized ovarian surface epithelial cells showed down-regulation of Snail and Slug transcripts. In DNvHNF1-transfected SKOV3 cells, growth rate decreased, and in vHNF1-transfected immortalized ovarian surface epithelial cells, growth rate increased. By immunohistochemistry, we found a strong association of vHNF1 with E-cadh in clear cell and in a subset of serous carcinomas, data that could potentially contribute in distinguishing different types of ovarian tumors. Our results may help in understanding the biology of ovarian carcinoma, identifying early detection markers, and opening potential avenues for therapeutic intervention.
Introduction
The pathophysiology of epithelial ovarian cancers (EOCs) remains poorly defined. One widely supported hypothesis is that they are derived from inclusion cysts. These cysts originate from the ovarian surface epithelium (OSE), which is the monolayer of cells covering the ovaries [1,2]. Ovarian surface epithelium cells appear as a simple epithelium with some characteristics typical of mesenchymal cells. Ovarian surface epithelium cells remain plastic in short-term culture, expressing vimentin together with cytokeratins 8 and 18. Conversely, invaginations and inclusion cysts have properties characteristic of müllerian epithelium, including expression of the specific epithelial marker E-cadherin (cadh) at the cell-cell junctions. After transformation, EOC cells can coexpress E-cadh and the mesenchymal marker vimentin as well as epithelial cytokeratins [3]. Unlike the tumor suppressor function of E-cadh in breast, prostate, and colon carcinomas [4,5], expression of E-cadh in ovarian epithelium seems to be associated with the development of EOCs [6].Nonetheless, themechanismof E-cadh-associated malignant OSE transformation is controversial [7,8]. In some advanced-stage EOCs, the so-called mesenchymal-epithelial transition (MET), which occurs during the first stages of transformation, is followed by an epithelial-mesenchymal transition (EMT) with loss of E-cadh expression [9].
Epithelial-mesenchymal transition is required for morphogenesis during embryonic development but has also been implicated in the acquisition of invasiveness by end-stage tumors [10–12]. This conversion results in loss of expression of adhesion molecules, such as E-cadh, ZO-1, and occludin, with consequent loss of cell-cell contacts and extensive remodeling of the cytoskeleton. Loss of E-cadh during development and cancer progression in tumors, other than EOCs, is mainly caused by transcriptional repression resulting from interaction of regulators with specific E-boxes in the proximal promoter of Cdh1, the gene encoding E-cadh [13]. Most prominent in this respect are the Snail-related zinc-finger transcription factors Snail and Slug.
The variant isoform of the transcription factor HNF-1 (vHNF1) activates transcription on homodimerization or heterodimerization with its companion protein HNF1α [14]. A role for HNF1 proteins in tumors has not yet been defined. For HNF1α, a biallelic inactivation of the relevant gene has been found in 50% of human liver adenomas [15], and somatic mutations were observed in 11% of endometrial carcinomas but not in breast and ovarian carcinomas [16]. Regarding vHNF1, the complete inactivation by germ line mutation of TCF2, the gene encoding for vHNF1, seemed to be associated to renal cell carcinoma [17] hypothesizing a tumor suppressor function. More recently, two variants within TCF2 have been found to be associated to prostate cancer risk [18]. vHNF1 is involved in the development of tissues organized in tubules, such as the pancreatic exocrine ducts and the kidney tubules [19,20], and in müllerian duct-derived tissues [21]. The transcription of the FR gene, which encodes the folate receptor (FR) α, is strongly activated in EOCs. We recently showed that the FR gene is regulated by vHNF1 [22], which is expressed in ovarian tumor specimens but not in OSE cells or in specimens obtained from tumors of other oncotypes.
Here, we addressed the potential role of vHNF1 in the MET-like taking place during ovarian cell transformation. We used in vitro approaches to negatively or positively affect vHNF1 expression and/or functionality in ovarian normal and transformed cells. We found that vHNF1 expression and functionality are directly correlated with epithelial differentiation, positively associated with growth potential, and inversely correlated with expression and functionality of E-box-binding transcriptional repressors. Immunohistochemical analysis of normal and transformed ovarian tissues showed that vHNF1 is not expressed in OSE cells but is expressed in 33% of E-cadh-expressing EOCs independently of tumor grading. The overall results demonstrate that vHNF1 is a new player in the epithelial differentiation of a subset of normal and transformed ovary cells.
Materials and Methods
Cell Culture
The ovarian carcinoma cell lines IGROV1 and SKOV3 (American Type Culture Collection, Manassas,VA) were maintained in RPMI 1640 medium (Sigma, St. Louis, MO) supplemented with 10% FCS (Sigma) and 2 mM l-glutamine. hTERT-IOSE (hereafter designated IOSE), obtained as described [23], were maintained in 199-MCDB105 medium (Sigma) supplemented with 15% FCS, 2 mmol l-glutamine, 200 µg/ml G418, and 50 µg/ml hygromycin.
Reagents and Antibodies
Triton X-100 (TX-100) and MES were from Sigma-Aldrich Fine Chemicals (St. Louis, MO); geneticin sulfate (G418) was from Gibco BRL (Paisley, Scotland). The following primary antibodies (Abs) were used at the dilution recommended by the manufacturer: anti-vHNF1 (goat), anti-HNF1 (rabbit), anti-ZO-1, and anti.occludin 1 (Santa Cruz Biotechnology, Santa Cruz, CA); anti-E-cadh mAb (mouse; Transduction Laboratories, BD Biosciences Pharmingen, Palo Alto, CA); anti-S100A4 (rabbit; DakoCytomation, Glostrup, Denmark). Horseradish peroxidase-labeled secondary Abs were from Amersham Bioscience-GE Healthcare (Piscataway, NJ). Secondary fluorochrome-conjugated Alexa Fluor 488 (green) was from Molecular Probes (Eugene, OR).
Small interfering RNA Treatment
IGROV1 and SKOV3 cells (5 x 105) were seeded in 24-well plates and transfected 24 hours later with 80 pmol/ml of small interfering RNA (siRNA) duplex against vHNF1 mRNA (SmartPool; Dharmacon, Lafayette, CO) or Luciferase siRNA as control (Quiagen-Xeragon, Germantown, MD). siRNA transfection was performed by using Lipofectamine 2000 (Invitrogen, Paisley, UK) according to the manufacturer's protocol. Cells were harvested 48 hours later and analyzed for RNA and protein expression by quantitative reverse transcription-polymerase chain reaction (RT-PCR) and Western blot analysis, respectively.
Construction of DNvHNF1 and vHNF1 Expression Vectors
vHNF1 cDNA was obtained from the vector RSV-LFB3 (kindly provided by C. Toniatti, IRBM,Merck Research Laboratories, Pomezia, Italy). Dominant-negative vHNF1 (DNvHNF1; nt 1-729 of the open reading frame) was obtained by standard PCR with sense and antisense primers containing HindIII and XbaI restriction sites, respectively (sense, 5′-AGGAGGTCTAGAATGGTGTCCAAGCTCACG-3′; antisense, 5′-AAGGGAAGCTTTCACCAGGCTTGTAGAGG-3′). The purified fragment was inserted into the HindIII and XbaI sites of the expression vector pcDNAIneo (Invitrogen). For the expression vector encoding vHNF1, the vHNF1 open reading frame was inserted into the HindIII and XbaI restriction sites of the pcDNA3.1/Hygro vector (Invitrogen). Before transfection, both vHNF1-pcDNA3.1/Hygro and DNvHNF1-pcDNAIneo were verified by sequencing.
Quantitative Real-time RT-PCR
Total RNA was isolated with the RNeasy Total RNA kit (Quiagen, Hilden, Germany) according to the manufacturer's instructions. One microgram of total RNA was reverse-transcribed using the ABI High Capacity cDNA Archiving Kit (Applied Biosystem, Foster City, CA). Three replicates were run for each gene in each sample in a 96-well format plate. The probes and primer sets were the following Assays on Demand: Ref Hs00170423_m1 for Cdh1, Hs00195591_m1 for Snail, Hs00161904_m1 for Slug, HS00170182_mi for PLAU, and Hs00277509_m1 for FN (Applied Biosystems). GADPH mRNA levels were used as a control for the RNA extraction and RT experiments. Data were analyzed with the Sequence Detector v1.9 software. Relative gene expression for each sample was determined using the formula 2(-ΔCt) reflecting target gene expression normalized to GAPDH levels.
Cell Solubilization, Fractionation, and Western Blot Analysis
For preparation of total cell lysates, cells were washed with ice-cold PBS and lysed in SDS sample buffer (62.5 mM Tris-HCl pH 6.8, 2.3% SDS, 10% glycerol, 5% β-mercaptoethanol, 0.005% bromophenol blue). Proteins were separated on precast 4% to 12% SDS-PAGE (Invitrogen) and transferred onto nitrocellulose membranes (Amersham Bioscience-GE Healthcare) as described [24]. Visualization was by the enhanced chemiluminescence method (Amersham Biosciences) using a Chemidocxrs and the Quantity One software (Bio-Rad, Hercules, CA). For cellular fractionation, confluent cells were treated as described [24]. Protein concentration of the fractions was determined by the BCA protein reagent assay (Pierce, Rockford, IL).
Cell Transfection
IGROV1 and SKOV3 cells were transfected with the DNvHNF1 construct essentially as described [25] using Lipofectamine 2000 according to the manufacturer's suggestions (Invitrogen). Forty-eight hours after transfection, fresh medium containing 400 µg/ml G418 (Gibco BRL) was added to the cell culture. DNvHNF1-positive clones were identified by RT-PCR using oligonucleotides that amplify only DNvHNF1 but not wild type (wt) vHNF1 (data not shown). Stable clones were tested by Western blot analysis on total cell lysates using rabbit anti-HNF1 Ab, which recognized both wt and DNvHNF1 proteins (Santa Cruz Biotechnology).
Immunofluorescence
Immunofluorescence was performed essentially as described [24], 2 x 104 cells seeded on glass coverslips were grown for 48 hours, washed with cold PBS, and fixed with cold methanol for 10 minutes before immunoreaction. Samples were mounted with Mowiol solution and examined with an Eclipse TE2000-S microscope with a 40x PanFluor objective (Nikon, Melville, NY). Images were acquired with ACT-1 software (Nikon) at a resolution of 2250 x 1800 pixels. All procedures were carried out at room temperature.
Electrophoretic Mobility Shift Assay
Preparation of nuclear extracts (NEs) and electrophoretic mobility shift assay were carried out essentially as described [22].
Microarray Analysis
Gene expression in DNvHNF1 and mock transfectants was compared in three different RNA preparations pooled for each cell line. Total RNA from transfected SKOV3 cultures was extracted, further purified on RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's protocols, and treated with DNase (RNase-free DNase Set; Qiagen). Targets were obtained by synthesizing cDNA from 15 µg of total RNA. To verify the reproducibility of the observations, we performed two separate retrotranscriptions from both cell types to obtain four separate targets for hybridization. Genome set Human U133 Plus 2.0 chips (Affymetrix, Santa Clara, CA) were used in duplicate. Data from the two biologic replicates for each target (DNvHNF1 and mock cells) were tested in duplicate chips (a total of four samples for target) after normalization. The statistical analyses of the microarray data were performed with GenePicker software designed by the IFOM Institute (Milan, Italy). This software allowed to set up analysis schemes and to search the data for regulated genes using t test and Change-Fold Change analysis.We performed the reported analysis selecting the probe sets with significant statistical analysis (P < .05 for t test) and a fold change >1.5 or <-1.5, obtaining a list of 621 probe sets.
Compilation of Gene Lists Associated with the Epithelial or Mesenchymal Phenotype
A list of EMT-related genes was compiled after a literature search for genes modulated during processes activated by EMT [10,12] and taking into consideration two studies of gene expression profiling: one on Ha-ras-transformed polarized mammary epithelial cell line EpH4 induced to EMT by TGFβ treatment [26] and another on Madison-Darby canine kidney epithelial cells expressing the E-box-binding repressors Snail, Slug, and E47 [27]. Table W1 shows a list of genes associated with epithelial (140) and mesenchymal (186) phenotypes and passing criteria as that reported above. The categorization reported in both Tables 1 and W1 was done according to the Gene Ontology categories.
Table 1.
Genes Associated with Epithelial and Mesenchymal Phenotypes* and Found to Be Differentially Expressed in DNvHNF1 Versus Mock Cells.
| Gene Symbol | GenBank ID | Gene Name | Fold Change | P |
| Epithelial genes (n = 134)† | ||||
| Actin cytoskeleton organization (n = 6) | ||||
| PODXL | NM_005397 | podocalyxin-like | 1.94 | .0002 |
| Cell adhesion/ECM-related (n = 22) | ||||
| ANXA4 | BC000182 | annexin A4 | -1.84 | .0000 |
| CD99 | U82164 | CD99 antigen | 1.66 | .0000 |
| CDH1 | NM_004360 | cadherin 1, type 1, E-cadherin | -3.33 | .0404 |
| CLDN1 | AF101051 | claudin 1 | -2.07 | .0002 |
| CLDN7 | NM_020412 | claudin 7 | -7.26 | .0000 |
| EVA1 | AF275945 | epithelial V-like antigen 1 | 3.00 | .0002 |
| FBLP-1 | AL133035 | filamin-binding LIM protein-1 | -3.01 | .0131 |
| ITGA3 | NM_002204 | integrin, alpha 3 | 1.62 | .0002 |
| ITGB6 | AK026736 | integrin, beta 6 | 2.50 | .0002 |
| PDZKIIPI | NM_005764 | PDZK1-interacting protein 1 | -2.03 | .0000 |
| NID2 | NM_007361 | nidogen 2 | -1.91 | .0005 |
| OCLN | AI829721 | occludin | -1.70 | .0512 |
| Cell cycle (n = 8) | ||||
| DUSP1 | NM_004417 | dual-specificity phosphatase 1 | -1.94 | .0053 |
| Cell growth/maintenance (n = 42) | ||||
| BPAG1 | AI798790 | bullous pemphigoid antigen 1, 230/240 kDa | 1.64 | .0000 |
| DEFB1 | U73945 | defensin, beta 1 | -7.78 | .0000 |
| GDI2 | D13988 | GDP dissociation inhibitor 2 | -1.86 | .0000 |
| SEMA3C | NM_006379 | sema domain, immunoglobulin domain (Ig), secreted, (semaphorin) 3C | 6.22 | .0000 |
| TACSTD2 | J04152 | tumor-associated calcium signal transducer 2 | -4.03 | .0325 |
| Cell motility (n = 8) | ||||
| F11R | AF154005 | F11 receptor | -2.09 | .0325 |
| JAG1 | U77914 | jagged 1 | 2.19 | .0000 |
| Metabolism (n = 29) | ||||
| CA2 | M36532 | carbonic anhydrase II | -17.04 | .0000 |
| CITED2 | AF109161 | Cbp/p300-interacting factor, with Glu/Asp-rich carboxy-terminal domain, 2 | -2.02 | .0340 |
| EXT1 | NM_000127 | exostoses (multiple) 1 | -1.63 | .0005 |
| Mesenchymal genes (n = 173) | ||||
| Actin cytoskeleton organization (n = 9) | ||||
| ACTN1 | AI082078 | actinin, alpha 1 | 1.9 | .0005 |
| PLEK2 | NM_016445 | pleckstrin 2 | 2.02 | .0008 |
| Cell adhesion/ECM-related (n = 21) | ||||
| BGN | BC002416 | biglycan | 10.5 | .0000 |
| CD44 | AF098641 | CD44 antigen | 2.10 | .0000 |
| COL5A1 | NM_000393 | collagen, type V, alpha 1 | 2.05 | .0000 |
| COL5A2 | AL564683 | collagen, type V, alpha 2 | 39.29 | .0000 |
| FN1 | AK026737 | fibronectin 1 | -10.39 | .0425 |
| Lamb1 | M20206 | laminin, beta 1 | 2.91 | .0000 |
| Cell cycle (n = 10) | ||||
| CDC2 | NM_001786 | cell division cycle 2, G1 to S and G2 to M | -1.69 | .0523 |
| Cell growth and/or maintenance (n = 59) | ||||
| CXCL1 | NM_001511 | chemokine (C-X-C motif) ligand 1 | -1.78 | .0015 |
| EPS8 | NM_004447 | epidermal growth factor receptor pathway substrate 8 | 2.07 | .0000 |
| FZD1 | NM_003505 | frizzled homolog 1 | 1.86 | .0006 |
| FZD2 | L37882 | frizzled homolog 2 | 1.66 | .0001 |
| HMGA2 | NM_003483 | high mobility group AT-hook 2 | 2.76 | .0001 |
| IGFBP1 | NM_000596 | nsulin-like growth factor binding protein 1 | -9.94 | .0067 |
| IGFBP3 | M31159 | insulin-like growth factor binding protein 3 | -2.90 | .0006 |
| KIFAP3 | NM_014970 | kinesin-associated protein 3 | 2.71 | .0067 |
| Met | BG170541 | met proto-oncogene (hepatocyte growth factor receptor) | 2.77 | .0015 |
| PMP22 | L03203 | peripheral myelin protein 22 | 2.05 | .0013 |
| PTPRM | NM_002845 | protein tyrosine phosphatase, receptor type, M | -2.74 | .0013 |
| SLC29A1 | AF079117 | solute carrier family 29 (nucleoside transporters), member 1 | 1.90 | .0003 |
| Cell motility (n= 25) | ||||
| MMP10 | NM_002425 | matrix metalloproteinase 10 (stromelysin 2) | 3.59 | .0000 |
| MMP2 | NM_004530 | matrix metalloproteinase 2 | 4.10 | .0000 |
| MMP7 | NM_002423 | matrix metalloproteinase 7 | 7.51 | .0000 |
| PLAU | NM_002658 | plasminogen activator, urokinase | 2.90 | .0000 |
| PLAUR | X74039 | plasminogen activator, urokinase receptor | 4.02 | .0000 |
| S100A2 | NM_005978 | S100 calcium binding protein A2 | 2.20 | .0000 |
| S100A3 | NM_002960 | S100 calcium binding protein A3 | 2.04 | .0004 |
| S100A4 | NM_002961 | S100 calcium binding protein A4 | 2.05 | .0000 |
| S100A6 | NM_014624 | S100 calcium binding protein A6 | 1.64 | .0001 |
| S100P | NM_005980 | S100 calcium binding protein P | -29.48 | .0002 |
| SERPINH1 | BF316352 | serine (or cysteine) proteinase inhibitor | -2.44 | .0000 |
| Development/differentiation (n = 23) | ||||
| ID1 | D13889 | inhibitor of DNA binding 1, dominant negative helix-loop-helix protein | -1.94 | .0000 |
| ID3 | NM_002167 | inhibitor of DNA binding 3, dominant negative helix-loop-helix protein | -23.23 | .0001 |
| ID4 | U16153 | inhibitor of DNA binding 4, dominant negative helix-loop-helix protein | -3.89 | .0001 |
| LUM | NM_002345 | lumican | 18.65 | .0001 |
| SNAI2 | AI572079 | snail homolog 2 (Drosophila) | 4.91 | .0000 |
| SPARC | NM_003118 | secreted protein, acidic, cysteine-rich (osteonectin) | 8.94 | .0000 |
| Metabolism (n = 22) | ||||
| ACVR1 | NM_001105 | activin A receptor, type I | 2.74 | .0045 |
| BHLHB2 | NM_003670 | basic helix-loop-helix domain containing, class B, 2 | 2.36 | .0000 |
| PTGIS | NM_000961 | prostaglandin I2 (prostacyclin) synthase | -2.17 | .0012 |
| Biological process unknown (n = 3) | ||||
| PSTPIP2 | NM_024430 | proline-serine-threonine phosphatase interacting protein 2 | -2.67 | .0003 |
| UPP1 | NM_003364 | uridine phosphorylase 1 | -2.80 | .0000 |
The genes listed in the EMT-related gene database (Table W1) were extracted from the DNvHNF1/Mock data set generated using the GeneChip Human Genome U133 Plus 2.0 Array. Note that 307 (94%) of 326 EMT-related genes were present in the chip.
The total number of genes belonging to each category is shown in parentheses.
Luciferase Assay
Cells were transfected with TOP- and FOP-promoter-reporter gene constructs (Upstate Biotechnology, Lake Placid, NY) using Lipofectamine 2000 according to the manufacturer's suggestions (Invitrogen). Cotransfection with thymidine kinase-Renilla was performed to evaluate transfection efficiency. After 48 hours, cells were lysed and analyzed for promoter activity. The dual-luciferase assay was performed essentially as suggested by the manufacturer (Promega, Madison, WI).
Growth Potential Measurements
In vitro proliferation of stable transfected cells was measured with the CellTiter-Glo luminescent cell viability assay kit (Promega) according to the manufacturer's suggestions. Cells (4 x 104 cells per well) were cultured in 96-well plates for up to 5 days. In vitro proliferation of siRNA-treated cells was evaluated as radiolabeled thymidine incorporation. Briefly, cells were plated in 96-well plates at a density of 1 x 104 cells per well and transiently transfected with siRNA duplex against vHNF1 mRNA or control siRNA. Cells were pulsed for 4 hours with [methyl-3H]thymidine (Amersham; 1 µCi per well) and 24 (for IGROV1 cells) or 48 hours (for SKOV3cells) later washed twice with ice-cold PBS. After fixation with 100 µl of 10% trichloroacetic acid for 30 minutes at 4°C, cells were lysed with 100 µl per well 0.2 N NaOH and radiolabeled thymidine incorporation was measured by scintillation counting.
Immunohistochemistry
All clinical specimens were obtained with approval from the institutional review board and informed consent from all participating patients to use excess biologic material for investigative purposes. Immunohistochemistry (IHC) was performed by using routine tissue blocks and a commercially available tissue arrays (Ovary cancer, AccuMax Array, Petagen and CJ1 Human, Ovary cancer, and Super Bio Chips) essentially as described [28]. For antigen retrieval and primary Ab dilutions, see Supplementary data. Two observers (M.L.C. and A.T.) independently assessed positivity or negativity of staining on the basis of intensity and the percentage of positive cells.
Statistical Analyses
GraphPad Prism software (GraphPad Software, San Diego, CA) was used to analyze all data. Differences between mean values were determined by Student's t test, and Fisher's test was used to determine whether the percentage of EMT-related genes is different by chance. The correlation of vHNF1 and E-cadh expression levels in IHC was evaluated by χ2 test. P values <.05 (2-sided) were considered significant.
Results
vHNF1 Silencing Impairs Epithelial Differentiation of Ovarian Tumor Cells
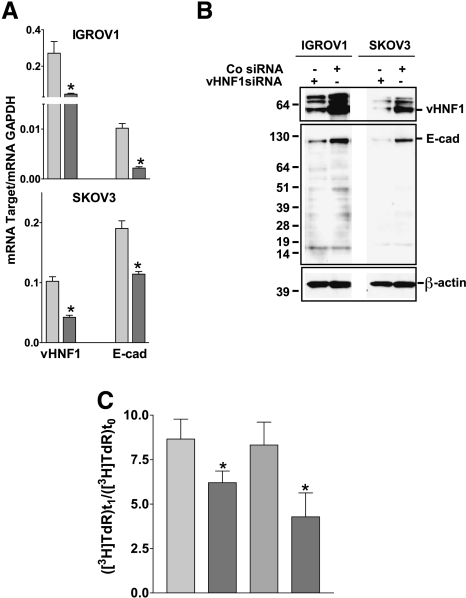
To identify the role of vHNF1 in ovarian carcinoma cells, IGROV1 and SKOV3 cells were transfected with vHNF1-specific siRNA or control siRNA (Figure 1A). The reduction of endogenous vHNF1 transcription and protein expression correlated directly with a decrease in the levels of E-cadh transcription and protein expression in both cell lines. The decreased intensity of the 120-kDa band corresponding to the full-size E-cadh protein in lysates of vHNF1 siRNA-treated cells (Figure 1B) was not caused by degradation [29] because proteins of lower molecular weight were equally abundant in all lysates.
Figure 1.
vHNF1 silencing impairs epithelial differentiation of ovarian tumor cells. IGROV1 and SKOV3 cells were treated with a vHNF1-specific siRNA. (A) Quantitative RT-PCR on total RNA extracts from cells treated with a control (light gray bar) or vHNF1-specific (dark gray bar) siRNA. Data represent mean (SD) for the vHNF1 and E-cadh genes normalized to the housekeeping gene GAPDH in at least six determinations. Asterisks indicate significant differences (P < .05). (B) In a parallel experiment, cells were lysed and analyzed by Western blot analysis with Abs against vHNF1 and E-cadh, respectively. Co siRNA indicates control siRNA. β-Actin was used for normalization of gel loading. One of three experiments is shown. (C) IGROV1 and SKOV3 cells were treated with a control (light gray bar) or a vHNF1-specific (dark gray bar) siRNA as in Figure 2, and proliferation was evaluated by incorporation of radiolabeled thymidine. Data are mean (SD) of six replicates; one of two experiments is shown. Asterisks indicate significant differences (P < .05).
Further, we measured the growth capability of IGROV1 and SKOV3 cells treated with vHNF1-specific siRNA (Figure 1C). By radio-labeled thymidine incorporation, we observed that reduction of endogenous vHNF1 expression led to a 25% (P = .026) and 45% (P = .018) decrease in proliferation of IGROV1 and SKOV3 cells, respectively.
vHNF1 Loss-of-Function Impairs Epithelial Differentiation of Ovarian Tumor Cells
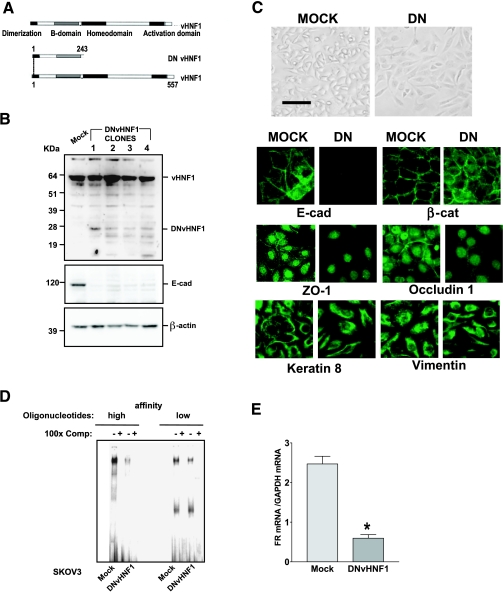
o scrutinize the role of vHNF1 expression in ovarian carcinomas, we stably transfected IGROV1 and SKOV3 cells with an expression plasmid containing a DNvHNF1 cDNA encoding the truncated vHNF1 form that occurs naturally in pancreatic β-cells of patients bearing maturity-onset diabetes of the young type 5 (Figure 2A) [30].We were unable to obtain any stable clone despite repeated DNvHNF1 transfections in IGROV1 cells (data not shown), but we obtained several stable clones by transfecting SKOV3 cells. Western blot analysis of total cell lysates from DNvHNF1-transfected SKOV3 clones (hereafter designated DNvHNF1) using an Ab that detects both wt vHNF1 and DNvHNF1 revealed the truncated 27-kDa band only in DNvHNF1 cells (Figure 2B). Probing the same blots with an Ab against E-cadh showed a large decrease in the expression of this protein in all DNvHNF1 clones analyzed. Furthermore, we obtained the same results on transfection of MDCK cells with the same construct (data not shown). Clone 1 was further characterized.
Figure 2.
vHNF1 loss-of-function impairs epithelial differentiation of ovarian tumor cells. (A) Schematic representation of wt vHNF1, with its functional domains, and the truncated DNvHNF1. Note that DNvHNF1 only maintains the N-terminal dimerization and B domains. (B) Western blot analysis of total cell lysates from Mock cells and DNvHNF1 clones was performed using a rabbit anti-HNF1 Ab. The 15- to 24-kDa bands in DNvHNF1 lysates might represent shorter DNvHNF1 products; β-actin was used for normalization of gel loading. (C) Upper panel: Morphology of Mock and DNvHNF1 cells. Cells were grown to confluence in six-well plates, and images were obtained by phase-contrast microscopy with a 10x objective. Bar, 100 µm. Lower panel: IF was performed on methanol-fixed Mock and DNvHNF1 cells with Abs against the molecules indicated. Images were obtained with a 40x objective. (D) Electrophoretic mobility shift assay of NEs prepared from Mock and DNvHNF1 cells was performed using two oligonucleotides containing the HNF1 consensus DNA-binding site and corresponding to the proximal elements of the sequences of the albumin (high affinity) and FR (low affinity) promoters, respectively. Specific DNA-protein complexes were competed with a 100-fold molar excess of unlabeled probes (100x Comp), as indicated. (E) Quantitative RT-PCR of the FR transcript using total RNA extracted from Mock and DNvHNF1 transfectants. Data represent mean (SD) for FR expression normalized to the housekeeping gene GAPDH in at least six determinations. Asterisk indicates a significant difference (P < .01).
Consistent with the loss of E-cadh expression, in phase-contrast microscopy DNvHNF1 clones revealed a spindle-like shape and loss of defined cell-cell borders compared with the more epithelial morphology of mock-transfected cells (hereafter referred to as Mock cells; Figure 2C, upper panel). Immunofluorescence analysis of adherens junctions showed that E-cadh and β-catenin (ctn) expressions were confined to cell-cell contacts in Mock cells, whereas in DNvHNF1 cells, there was loss of E-cadh staining and discontinuous β-ctn staining at cell-cell contacts (Figure 2C, lower panel). Moreover, both ZO-1 and occluding 1, markers of tight junctions, were clearly present at cell-cell contacts in Mock cells, but they were mainly concentrated in the nuclei of DNvHNF1 cells, as previously shown in other cell systems [31,32]. Normal and transformed ovarian cells may display both epithelial and mesenchymal features [3], and accordingly, SKOV3 cells coexpress both cytokeratins and vimentin. Interestingly, DNvHNF1 cells maintained coexpression of cytokeratins and vimentin but failed to display the typical pattern of cell-cell junctional cytokeratin filaments observed in Mock cells. Similar expression patterns were seen in the other DNvHNF1 clones (data not shown).
Electrophoretic mobility shift assay was performed with NEs from DNvHNF1 cells using two different vHNF1-specific oligonucleotides corresponding to the proximal elements of the sequences of the albumin (high affinity) and FR (low affinity) promoters [22,33]. This analysis indicated that the ability of NEs from DNvHNF1 cells to form DNA complexes with the oligonucleotides was substantially reduced compared to those from Mock cells (Figure 2D). On the basis of our previous demonstration that vHNF1 binds to and activates the FR promoter in ovarian carcinoma cells, we evaluated the FR transcript levels in DNvHNF1 cells to confirm the effective down-regulation of endogenous vHNF1 transcriptional activity by expression of the DNvHNF1 protein. Indeed, real-time RT-PCR analysis revealed a fourfold lower level of the FR transcript in DNvHNF1 cells (Figure 2E).
vHNF1 Loss-of-Function Induces a Gene Expression Profile Resembling That of EMT
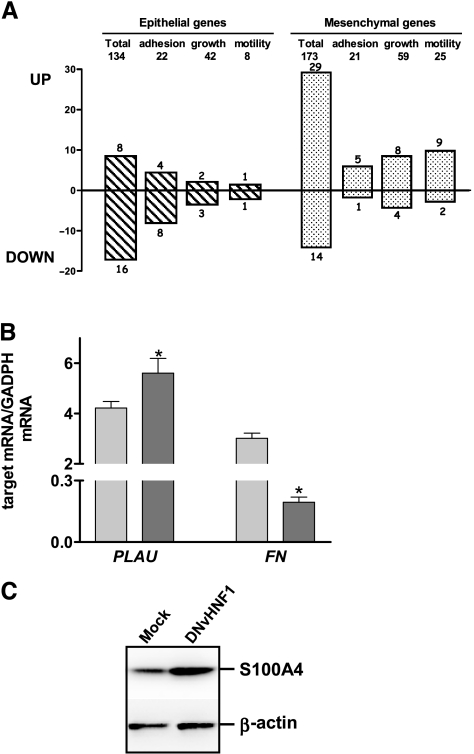
We compared the gene expression profiles of DNvHNF1 cells and Mock cells by the GeneChip Human Genome U133 Plus 2.0 Array. Using a cutoff of 1.5-fold, we identified 459 upregulated and 473 downregulated genes in DNvHNF1 cells. A preliminary analysis of the differentially expressed genes indicated a pattern suggestive of EMT (data not shown). Thus, we focused on EMT and compiled a list of specific epithelial (140) and mesenchymal (186) genes that are reported in Table W1 (for list compilation, refer to the Materials and Methods section). In the epithelial gene list, genes associated with cell growth/maintenance, metabolism, cell adhesion/extracellular cell matrix (ECM)-related, and development/differentiation were the largest classes; in the mesenchymal gene list, cell growth/maintenance and cell motility-associated genes formed the largest classes. We extracted the expression data for each gene of this EMT-related list from the DNvHNF1 versus Mock data sets. A search for variation of expression of these genes in DNvHNF1 cells was consistent with our initial observation: 22% (67/307) of EMT-related genes were expressed differently in the two cell lines (Table 1), compared to the 13% expected by chance (P < .0001). More specifically, 24 of 134 epithelial genes in DNvHNF1 cells were differentially expressed: 16 of them were down-regulated and 8 were upregulated, which is consistent with published EMT data [26,27] (Figure 3A). In addition, 43 of 173 mesenchymal genes were differentially expressed and 29 of them (67%) were upregulated in DNvHNF1 cells. Most modulated genes in DNvHNF1 cells were associated with cell adhesion/ECM (18 genes), cell growth/maintenance (16 genes), or motility (13 genes). Among the mesenchymal genes, we observed up-regulation of CD44, Met, PLAU, PLAUR, MMP2, MMP7, S100A4, HMG2A, SNAI2, and SPARC, all of which are expressed during EMT. Real-time RT-PCR for PLAU showed upregulation of these genes in DNvHNF1 cells (Figure 3B).Western blot analysis with anti-S100A4 Ab indicated increased expression of this protein in DNvHNF1 cells compared to Mock cells (Figure 3C). Among epithelial genes, we found down-regulation of CDH1, which encodes E-cadh, and OCLN, which encodes for occluding, that had been shown before to be downregulated in DNvHNF1 cells (Figure 2), and TACSTD2, encoding Ep-CAM protein, which is highly expressed in ovarian carcinoma cells [34].
Figure 3.
vHNF1 loss-of-function induces a gene expression profile resembling that of EMT. (A) Upper panel: we compiled a list of specific epithelial (134) and mesenchymal (173) genes that are reported in Table W1. The number of genes in the largest functional classes is reported. Lower panel: we extracted the expression data for each gene of this EMT-related list from the DNvHNF1 versus Mock data sets. Epithelial (dashed bars) and mesenchymal (dotted bars) genes differentially expressed in DNvHNF1 versus Mock data sets. (B) Quantitative RT-PCR for target mRNA was performed with total RNA extracted from Mock (light gray bars) and DNvHNF1 (dark gray bars) cells. Data represent mean (SD) for the relevant genes normalized to the housekeeping gene GAPDH in at least six determinations. Asterisks indicate significant difference (P < .02). (C) Western blot analysis of total cell lysates from Mock and DNvHNF1 cells was performed using a rabbit anti-S100A4 Ab. β-Actin was used for normalization of gel loading. One of three gels is shown.
Some apparent discrepancies with published data were found, such as the up-modulation of ITGB6 and SEMA3C, described before as down-modulated in the epithelial phenotype [26], and the down-regulation of typical mesenchymal genes, such as FN and IDs [9,35]. Nevertheless, real-time RT-PCR confirmed the down-regulation of FN in DNvHNF1 cells (Figure 3B).
These results strongly suggest that vHNF1 loss-of-function impairs cell-cell adhesion and leads to a more mesenchymal phenotype in the SKOV3 ovarian carcinoma cell line.
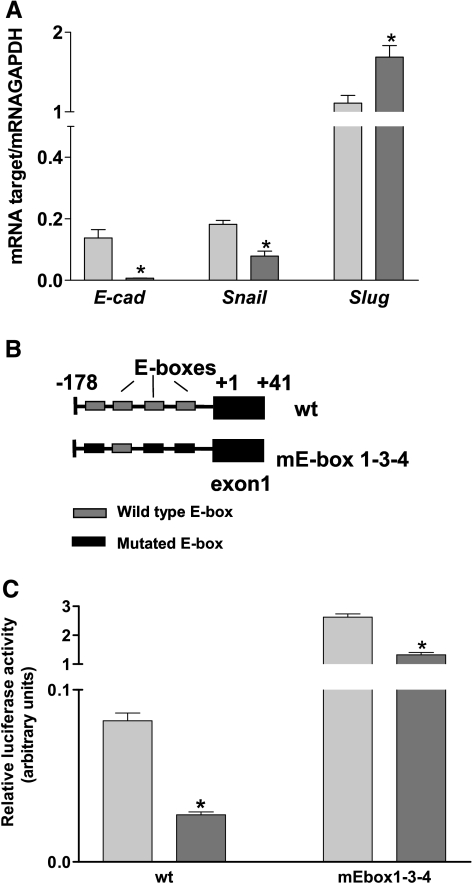
vHNF1 Loss-of-Function Leads to Slug Expression and Functionality
E-cadh down-regulation could have been caused by transcriptional repression by E-box transcription factors, so we performed quantitative real-time RT-PCR to evaluate the expression of E-cadh and its transcriptional regulators Snail and Slug. Compared to Mock cells, DNvHNF1 cells showed absence of E-cadh mRNA, 2-fold decreased Snail mRNA levels, and 1.8-fold increased Slug mRNA levels (Figure 4A). We then analyzed in these cell lines the effect of vHNF1 loss-of-function on Cdh1 promoter activity (Figure 4C) by transiently transfecting the promoter constructs depicted in Figure 4B. Luciferase reporter assay showed that the activity of the E-box-containing Cdh1 promoter significantly decreased about threefold in DNvHNF1 cells compared to Mock cells. The activities of the mutated E-box-containing construct were less derepressed in DNvHNF1 cells than in Mock cells, indicating that in DNvHNF1 cells, other mechanisms may contribute to the repression of the Cdh1 promoter in addition to the activity of E-box-binding proteins.
Figure 4.
vHNF1 loss-of-function leads to Slug expression and functionality. (A) Quantitative RT-PCR for E-cadh, Snail, and Slug transcripts was performed on total RNA extracted from Mock (light gray bars) and DNvHNF1 (dark gray bars). Data represent mean (SD) for the genes indicated, after normalization to the housekeeping gene GAPDH in at least six determinations. Asterisks indicate significant differences (P ≤ .02). (B) Schematic representation of Chd1 proximal promoter containing four putative E-box sequences cloned upstream of the luciferase gene and transiently transfected in Mock and DNvHNF1 cells. Mutations within the E-boxes are as indicated. (C) Luciferase-promoter gene assay of Mock (light gray bars) and DNvHNF1 (dark gray bars) cells transiently transfected with reporter plasmids containing the wt Cdh1 proximal promoter or the same promoter with mutated E-box sequences (mEbox) as reported in panel B. Data are mean (SD) normalized for transfection efficiency in three independent experiments performed in triplicate. Asterisk indicates a significant difference (P ≤ .01).
These results indicate that loss of E-cadh expression in DNvHNF1 cells might be caused by transcriptional repression partly mediated by Slug binding to specific E-boxes of the Cdh1 promoter.
Ectopic Expression of vHNF1 in IOSE Cells Is Sufficient to Induce Snail and Slug
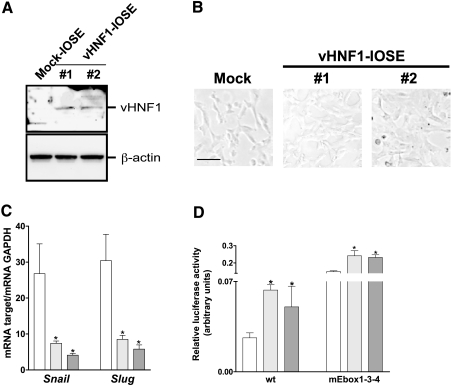
The data presented above indicate that vHNF1 participates in determining the epithelial phenotype of ovarian cancer cells. To evaluate whether vHNF1 is sufficient to activate a differentiation program toward MET in normal ovary cells, we stably transfected hTERT-IOSE cells [23] with vHNF1 cDNA. Western blot analysis with anti-vHNF1 Ab revealed that two selected clones of vHNF1-transfected hTERT-IOSE (hereafter designated vHNF1-IOSE #1 and #2) express a 62-kDa vHNF1 protein not expressed by mock-transfected hTERT-IOSE (hereafter designated Mock-IOSE; Figure 5A). By phase-contrast microscopy, vHNF1-IOSE #2, which represents a clone grown in vitro for longer time than vHNF1-IOSE #1 cell line, appeared to lose the typicalmesenchymalmorphology,whereas both Mock- and vHNF1-IOSE #1 maintained a more spindle-like morphology (Figure 5B).
Figure 5.
Ectopic expression of vHNF1 in IOSE cells is sufficient to induce Snail and Slug. (A) Western blot analysis of total cell lysates from Mock and DNvHNF1-IOSE clones #1 and #2 was performed with a rabbit anti-vHNF1 Ab. (B) Morphology of Mock and DNvHNF1-IOSE cells. While Mock- and vHNF1-IOSE #1 showed a more spindle-like morphology, vHNF1-IOSE #2, which represents a clone grown in vitro for longer time than vHNF1-IOSE #1 cell line, appeared larger in size acquiring a more compacted morphology. Images were obtained by phase-contrast microscopy using a 10x objective. Bar, 100 µm. (C) Quantitative RT-PCR for Snail and Slug transcripts was performed on total RNA extracted from Mock (white bar) and vHNF1-IOSE #1 (light gray bars) and #2 (dark gray bars) cells. Data represent mean (SD) for the relevant genes normalized to the housekeeping gene GAPDH in at least six determinations. Asterisks indicate significant differences (P ≤ .05). (D) Luciferase-promoter gene assay using Mock (white bar) and DNvHNF1-IOSE #1 (light gray bars) and #2 (dark gray bars) cells transiently transfected with promoter reporter plasmids containing the wt Cdh1 proximal promoter or the same promoter with mutations in the E-box sequences (mEbox) as reported in Figure 4B.
By quantitative RT-PCR on total RNA, the E-cadh transcript was slightly detectable in all transfected IOSE (data not shown). Expression of Snail transcript in vHNF1-expressing clones was found to be downregulated 3- and 4-fold, and Slug transcript 2.5- and 3.5-fold, respectively (Figure 5C).
We then analyzed the effect of vHNF1 loss-of-function on the Cdh1 promoter activity in Mock and vHNF1-IOSE cells (Figure 5D) by transiently transfecting the promoter constructs (shown in Figure 4B). Luciferase reporter assay showed that the activity of the E-box-containing wt Cdh1 promoter increased approximately 2.5-fold in vHNF1-IOSE cells in comparison to Mock-IOSE cells. The activities of the mutated E-box1-3-4 construct was approximately twofold de-repressed in vHNF1-IOSE cells compared toMock-IOSE, suggesting that E-boxes 1, 3, and 4 are relevant for the CDH1 gene transcription in this type of cells.
These results together demonstrate that vHNF1 negatively regulates specific E-box-binding repressors in normal ovarian cells, which is in line with the data on ovarian carcinoma cells reported above.
vHNF1 Modulates the Proliferative Potential of Ovarian Cancer and Normal Cells
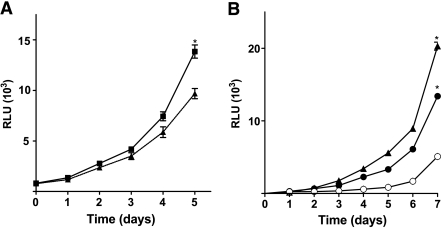
We aimed also to evaluate vHNF1-dependent growth potential in both DNvHNF1 and vHNF1-transfected-IOSE. In culture, DNvHNF1 cells grew slower than Mock cells, so that the DNvHNF1 cell density was 30% lower on day 5 after seeding (P = .0001; Figure 6A), consistent with the decrease in proliferation after transient vHNF1 silencing (Figure 1C). The slower growth rate was paralleled by reduced colony-forming capability (data not shown).
Figure 6.
vHNF1 modulates the proliferative potential of ovarian cancer and normal cells. Cells were seeded in 96-well plates, and growth was measured for up to 5 to 7 days with a CellTiter-Glo luminescent cell viability assay kit (Promega). (A) Mock (▪) and DNvHNF1 (▴): data represent mean (SD) of six determinations from three independent experiments. (B) Mock (○) and vHNF1-IOSE #1 (●) and #2 (▴): data represent mean (SD) of five determinations from one of three experiments. Asterisks indicate significant differences (P ≤ .05).
Growth potential was also evaluated in the IOSE transfected cells up to 7 days. It is noteworthy that ectopic vHNF1 expression caused a two- and three-fold increase of the growth rate of vHNF1-IOSE #1 and #2, respectively, in comparison to Mock-IOSE cells (Figure 6B).
These results are in line with those shown above (Figure 1A) and suggest a positive involvement of vHNF1 in controlling the proliferation of normal and transformed ovarian cells.
vHNF1 Is Expressed in a Subset of Normal and Transformed E-cadh-Expressing Ovarian Cells
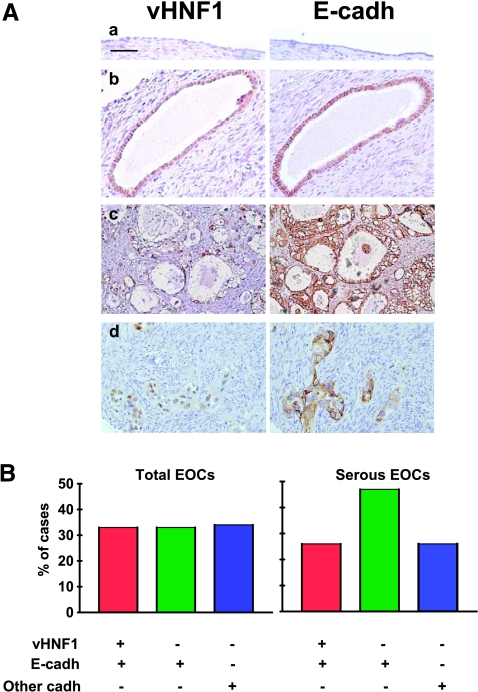
We previously showed that vHNF1 is expressed only in ovarian carcinoma cell lines and not in short-term cultures of OSE cells [22]. Here, we used IHC to evaluate vHNF1 expression together with E-cadh in sections from four normal human ovaries, selected for having a normal monolayer epithelium or for presenting invaginations and inclusion cysts lined by a single layer of cells, and in samples from benign, low malignant potential (LMP) and malignant ovarian tumors of different histotypes (Figure 7). When detected, anti-vHNF1 staining was observed only in the nucleus or in the nucleus and the cytoplasm, whereas anti-E-cadh mainly stained the cell membrane. No vHNF1 expression was detected in OSE from different individuals. In 20% of cysts, the single, normal cell layer reacted with anti-vHNF1 together with anti-E-cadh (representative example in Figure 7). Cells from four benign tumors and four of six LMP tumors stained for vHNF1 and E-cadh (Table 2). One LMP tumor that did not stain with anti-vHNF1 was endometrioid. Among the 38 carcinomas tested, 18 reacted with anti-vHNF1: 7 of these were clear cell carcinomas, 5 were serous (representative examples in Figure 7A), and 1 was mucinous. Four of 7 endometrioid EOCs were vHNF1-positive.
Figure 7.
vHNF1 is expressed in a subset of normal and transformed E-cadh-expressing ovarian cells. Immunohistochemical analyses with anti-vHNF1 and -E-cadh Abs on paraffin-embedded normal and tumor-derived ovarian tissues. The immunohistochemical analysis is also reported in Table 2. (A) Representative examples of normal ovarian epithelium (a), an inclusion cyst (b), and two serous EOCs (c and d). Images were obtained with a 20x objective. Bar, 100 µm. (B) Epithelial ovarian carcinomas analyzed for vHNF1 and/or E-cadh expressions. Anti-E-cadh-negative samples comprise other cadh-expressing tumors. Total number of EOCs, n = 38; total number of serous EOCs, n = 19. Vertical bars, percentage of immunoreactive samples.
Table 2.
Expression of vHNF1 and E-cadh Detected by Immunohistochemistry in Ovarian Tumor Samples.
| Ovarian Tumors* | No. of Cases (n = 49) | Presence of vHNF1/E-cadh by IHC | |||
| +/+ | +/- | -/+ | -/- | ||
| Benign | 5 | 4 | — | 1 | — |
| LMP | 6 | 4 | — | 1 | 1† |
| Carcinoma‡: | |||||
| Serous | 19 | 5 | 1 | 9 | 4 |
| Mucinous | 2 | 1 | — | 1 | — |
| Clear cell | 10 | 7 | 1 | — | 2 |
| Endometrioid | 7 | — | 3 | 3 | 1 |
Commercially available tissue arrays.
Endometrioid LMP tumor.
Mucinous and endometrioid carcinomas comprise a grade I tumor; all other carcinomas were grades II and III.
Interestingly, 66% of EOCs tested were reactive with anti-E-cadh monoclonal Ab, and within these EOCs, 33% were positive for both vHNF1 and E-cadh (Figure 7B). Within serous histotype, which represents most EOCs, 73% expressed E-cadh and 26% together with vHNF1. Note that E-cadh-negative tumors could express N-cadh or cadh-11, as previously reported [23].
In conclusion, vHNF1 appears to be expressed in inclusion cysts, and it is clearly expressed in clear cell carcinomas and in some serous carcinomas, but it is not expressed in OSE. Interestingly, vHNF1 expression is significantly associated with E-cadh expression in a subset of samples comprising some cysts as well as in benign and malignant tumors of serous or clear cell histotype, whereas no coexpression was observed in endometrioid tumors (P = .0024).
Discussion
Here, we demonstrate that vHNF1 may act as an initial regulator of OSE plasticity and proliferation, thereby contributing significantly to the changes in differentiation of OSE cells during neoplastic transformation and progression. Indeed, a DN form of the transcription factor vHNF1 induces EMT when expressed in an ovarian carcinoma cell line (SKOV3), as confirmed by a change in mRNA expression profile resembling that of EMT and by a loss at the protein level of E-cadh and components of tight junctions. DNvHNF1 expression in SKOV3 ovarian carcinoma cells downregulated Slug expression and functionality, and conversely, vHNF1 ectopically expressed in hTERT-IOSE cells decreased Snail and Slug expression and functionality. Overall, our results uncover a novel role of vHNF1 in the epithelial differentiation of ovary cells.
The HNF transcription factors have been related mainly to hepatocyte and pancreatic β-cell differentiation, and vHNF1 in particular seems to be required for maintenance of the differentiation state and functional activity of mouse pancreatic β-cells. Mutations and/or deletions that impair vHNF1 functionality cause major alterations in expression of important metabolic genes. Indeed, vHNF1 knock-out mice die within a few days after gastrulation [19]. In adult mice, β-cells show impaired glucose tolerance and reduced insulin secretion when vHNF1 is selectively deleted using Cre recombinase [36]. The present results suggest for the first time that vHNF1 is one of the transcription factors governing the epithelial differentiation of OSE.
To evaluate the molecular signature associated with vHNF1 loss-of-function in SKOV3 cells, we performed an EMT-guided comparative expression profile analysis on the basis of a newly compiled EMT-related gene database (Table W1). The observed profile is in agreement with an EMT shift, with a few remarkable exceptions, such as down-modulation of FN1, ID1, and ID4 and up-regulation of ITGB6 and SEMA3C. This could simply be caused by cell line specificity of these molecules. Note that vHNF1 loss-of-function does not completely revert the malignancy of SKOV3 cells and that further analysis is needed to determine whether particular molecular mechanisms regulate the expression of these genes in a tumor type-specific way. Conversely, several genes characteristic of either EMT or MET but not yet associated with EOCs were identified as differentially expressed. Microarray and immunofluorescence analyses showed that vHNF1 functionality was associated mainly with genes that modulate cell adhesion and are ECM-related, which is consistent with a role for HNF transcription factors in regulating cell adhesion [37]. The potential usefulness of this information in the context of clinical screening markers and possible genetic or pharmacologic targeting awaits further validation of the role of vHNF1 and the newly identified associated genes, together with detailed gene expression comparisons between OSE and EOC samples.
Consistent with a previous report that only EOC with clear cell histotype expresses vHNF1 [38], our immunohistochemical analysis revealed vHNF1 expression in most clear cell EOCs, as well as in approximately 30% of serous EOCs, which are most EOCs. We detected heterogeneous expression of vHNF1 associated to E-cadh expression in 32% of the EOC samples, independently of tumor grading. These results confirm a role for vHNF1 in the epithelial phenotype of EOCs. An analysis to define the molecular signature that characterizes this subset of EOCs, which include mainly the clear cell and serous histotypes, is ongoing.
Previous studies indicated that the proximal vHNF1 promoter was methylated in 26% of the EOCs analyzed, but no such methylation was observed in OSE cells that do not express vHNF1 [39]. In renal cell carcinomas, TCF2 inactivation was caused by germ line mutations [17]. Therefore, in vHNF1-negative ovarian carcinomas, TCF2 gene could have either methylated promoter or inactivating mutations. We further hypothesize that cysts undergo transformation if they contain genetic mutations and possibly express vHNF1 conferring a further growth advantage. Epithelial ovarian carcinomas derived from those cysts continue to express vHNF1, but once tumors progress, epigenetic mechanisms such as methylation of the vHNF1 promoter might be activated, resulting in loss of vHNF1 expression. One key function of vHNF1 seems to be the negative modulation of EMT-inducing transcription factors such as Snail and Slug, leading to the positive modulation of E-cadh and other epithelial proteins. Previously, vHNF1 has been shown to be involved in METoccurring during kidney development, whereas kidney fibrosis has been associated with the binding of the E-box-binding repressors Snail or Slug to the promoters of vHNF1 and E-cadh encoding genes [40]. These results together with ours favor the hypothesis of a delicate reciprocal transcriptional regulation between E-box repressors and vHNF1. Recently another embryonic transcription factor, FOXC2, was identified as a central modulator of the EMT program in metastatic basal-like breast cancer [41]. These observations, together with ours, support the hypothesis that embryonic transcription factors are necessary for execution of transformation or invasion programs in different types of cancers. Of course, this hypothesis does not exclude that other mechanisms, such as those involving specific HOX genes, might contribute to determining the morphologic heterogeneity of EOCs [42].
In addition to its role in OSE cell plasticity, vHNF1 seems to contribute to the increased growth potential of normal and transformed ovarian cells. Indeed, siRNA-mediated silencing of vHNF1 or its inhibition by a DN mutant was associated with decreased growth proliferation, whereas de novo expression of vHNF1 increased proliferation. The increase in proliferation could be attributed to the modulation of cell cycle progression but did not confer unlimited growth potential to OSE cells (unpublished observation). We can also hypothesize that the vHNF1 confers a growth advantage in vitro so that ovarian carcinoma cell lines maintain vHNF1 expression, whereas in tumors the genomic modifications described above lead to the loss of vHNF1 expression.
Recently, a new model for the pathogenesis of EOCs has been proposed in which ovarian tumors are divided in two types [43]. Type I tumors, which include low-grade serous, mucinous, endometrioid, and clear cell carcinomas, are slowly growing and are generally confined to the ovary. Type II tumors are rapidly growing and highly aggressive. Despite considerable efforts, it is not yet possible to distinguish these different types of ovarian tumors at early stage and set up the most successful therapy. In this context, the strong association of vHNF1 with E-cadh in clear cell and in a subset of serous carcinomas could potentially contribute in distinguishing different types of ovarian tumors, on a more extensive molecular analysis. Epithelial-mesenchymal transition has been recognized as a potential mechanism for carcinoma progression. The mechanisms governing EMT in tumor progression recapitulate many of those identified in embryogenesis [10–12]. However, besides EOC with endometrioid histotype, EOCs seem to diverge in other ways from the general EMT scenario. For example, β-ctn does not detectably activate β-ctn/TCF-responsive genes on progression [44], whereas E-cadh expression is maintained in advanced EOCs [6,45]. Therefore, increasing knowledge of the molecular mechanisms of METoccurring at first stages of tumorigenesis and controlled by vHNF1 in EOCs may provide new and fascinating insights into the biology of this important disease and likely to identifying early detection markers and to opening potential avenues for therapeutic intervention.
Supplementary Material
Acknowledgments
The authors thank M.L. Sensi and A. Bredan for critical reading of the article and Gloria Bosco for her secretarial assistance.
Abbreviations
- Ab
antibody
- cadh
cadherin
- ctn
catenin
- DN
dominant-negative
- EMT
epithelial-mesenchymal transition
- EOC
epithelial ovarian carcinoma
- FR
folate receptor
- IHC
immunohistochemistry
- LMP
low malignant potential
- MET
mesenchymal-epithelial transition
- NE
nuclear extract
- OSE
ovarian surface epithelium
- vHNF1
variant hepatocyte nuclear factor
- wt
wild type
Footnotes
Financial support: This work was supported by grants to S.C. from Associazione Italiana Ricerca Cancro and the Cariplo Foundation, grant number 2003-1740.
References
- 1.Shih IeM, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164:1511–1518. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell DA. Origins and molecular pathology of ovarian cancer. Mod Pathol. 2005;18(Suppl 2):S19–S32. doi: 10.1038/modpathol.3800306. [DOI] [PubMed] [Google Scholar]
- 3.Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev. 2001;22:255–288. doi: 10.1210/edrv.22.2.0422. [DOI] [PubMed] [Google Scholar]
- 4.Strumane K, Van Roy F, Berx G. The role of E-cadherin in epithelial differentiation and cancer progression. Recent Res Devel Cell Biochem. 2003;1:33–37. [Google Scholar]
- 5.Richmond PJM, Karayiannakis AJ, Nagafuchi A, Kaisary AV, Pignatelli M. Aberrant E-cadherin and α-catenin expression in prostate cancer: correlation with patient survival. Cancer Res. 1997;57:3189–3193. [PubMed] [Google Scholar]
- 6.Sundfeldt K. Cell-cell adhesion in the normal ovary and ovarian tumors of epithelial origin; an exception to the rule. Mol Cell Endocrinol. 2003;202:89–96. doi: 10.1016/s0303-7207(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 7.Auersperg N, Pan J, Grove BD, Peterson T, Fisher J, Maines-Bandiera S, Somasiri A, Roskelley CD. E-cadherin induces mesenchymal-to-epithelial transition in human ovarian surface epithelium. Proc Natl Acad Sci USA. 1999;96:6249–6254. doi: 10.1073/pnas.96.11.6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maines-Bandiera SL, Huntsman D, Lestou VS, Kuo WL, Leung PC, Horsman RD, Wong AS, Woo MM, Choi KK, Roskelley CD, et al. Epithelio-mesenchymal transition in a neoplastic ovarian epithelial hybrid cell line. Differentiation. 2004;72:150–161. doi: 10.1111/j.1432-0436.2004.07204003.x. [DOI] [PubMed] [Google Scholar]
- 9.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 11.Thompson EW, Newgreen DF, Tarin D. Carcinoma invasion and metastasis: a role for epithelial-mesenchymal transition? Cancer Res. 2005;65:5991–5995. doi: 10.1158/0008-5472.CAN-05-0616. [DOI] [PubMed] [Google Scholar]
- 12.Savagner P. Rise and Fall of Epithelial Phenotype: Concept of Epithelial-Mesenchymal Transition. Landes Bioscience Ed. New York, NY: Kluwer Academic/Plenum Publishers; 2005. [Google Scholar]
- 13.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 14.Pontoglio M. Hepatocyte nuclear factor 1, a transcription factor at the crossroads of glucose homeostasis. J Am Soc Nephrol. 2000;11(Suppl 16):S140–S143. [PubMed] [Google Scholar]
- 15.Bluteau O, Jeannot E, Bioulac-Sage P, Marques JM, Blanc JF, Bui H, Beaudoin JC, Franco D, Balabaud C, Laurent-Puig P, et al. Bi-allelic inactivation of TCF1 in hepatic adenomas. Nat Genet. 2002;32:312–315. doi: 10.1038/ng1001. [DOI] [PubMed] [Google Scholar]
- 16.Rebouissou S, Rosty C, Lecuru F, Boisselier S, Bui H, Frere-Belfa MA, Sastre X, Laurent-Puig P, Zucman-Rossi J. Mutation of TCF1 encoding hepatocyte nuclear factor 1alpha in gynecological cancer. Oncogene. 2004;23:7588–7592. doi: 10.1038/sj.onc.1207989. [DOI] [PubMed] [Google Scholar]
- 17.Rebouissou S, Vasiliu V, Thomas C, Bellanne-Chantelot C, Bui H, Chretien Y, Timsit J, Rosty C, Laurent-Puig P, Chauveau D, et al. Germline hepatocyte nuclear factor 1alpha and 1beta mutations in renal cell carcinomas. Hum Mol Genet. 2005;14:603–614. doi: 10.1093/hmg/ddi057. [DOI] [PubMed] [Google Scholar]
- 18.Gudmundsson J, Sulem P, Steinthorsdottir V, Bergthorsson JT, Thorleifsson G, Manolescu A, Rafnar T, Gudbjartsson D, Agnarsson BA, Baker A, et al. Two variants on chromosome 17 confer prostate cancer risk, and the one in TCF2 protects against type 2 diabetes. Nat Genet. 2007;39:977–983. doi: 10.1038/ng2062. [DOI] [PubMed] [Google Scholar]
- 19.Haumaitre C, Barbacci E, Jenny M, Ott MO, Gradwohl G, Cereghini S. Lack of TCF2/vHNF1 in mice leads to pancreas agenesis. Proc Natl Acad Sci USA. 2005;102:1490–1495. doi: 10.1073/pnas.0405776102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gresh L, Fischer E, Reimann A, Tanguy M, Garbay S, Shao X, Hiesberger T, Fiette L, Igarashi P, Yaniv M, et al. A transcriptional network in polycystic kidney disease. EMBO J. 2004;23:1657–1668. doi: 10.1038/sj.emboj.7600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbacci E, Reber M, Ott MO, Breillat C, Huetz F, Cereghini S. Variant hepatocyte nuclear factor 1 is required for visceral endoderm specification. Development. 1999;126:4795–4805. doi: 10.1242/dev.126.21.4795. [DOI] [PubMed] [Google Scholar]
- 22.David KA, Milowsky MI, Kostakoglu L, Vallabhajosula S, Goldsmith SJ, Nanus DM, Bander NH. Clinical utility of radiolabeled monoclonal antibodies in prostate cancer. Clin Genitourin Cancer. 2006;4:249–256. doi: 10.3816/CGC.2006.n.003. [DOI] [PubMed] [Google Scholar]
- 23.De Cecco L, Marchionni L, Gariboldi M, Reid JF, Lagonigro MS, Caramuta S, Ferrario C, Bussani E, Mezzanzanica D, Turatti F, et al. Gene expression profiling of advanced ovarian cancer: characterization of molecular signature involving the fibroblast growth factor 2. Oncogene. 2004;23:8171–8183. doi: 10.1038/sj.onc.1207979. [DOI] [PubMed] [Google Scholar]
- 24.Sanna E, Miotti S, Mazzi M, De Santis G, Canevari S, Tomassetti A. Binding of nuclear caveolin-1 to promoter elements of growth-associated genes in ovarian carcinoma cells. Exp Cell Res. 2007;313:1307–1317. doi: 10.1016/j.yexcr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Bagnoli M, Tomassetti A, Figini M, Flati S, Dolo V, Canevari S, Miotti S. Downmodulation of caveolin-1 expression in human ovarian carcinoma is directly related to a-folate receptor overexpression. Oncogene. 2000;19:4754–4763. doi: 10.1038/sj.onc.1203839. [DOI] [PubMed] [Google Scholar]
- 26.Jechlinger M, Grunert S, Tamir IH, Janda E, Ludemann S, Waerner T, Seither P, Weith A, Beug H, Kraut N. Expression profiling of epithelial plasticity in tumor progression. Oncogene. 2003;22(46):7155–7169. doi: 10.1038/sj.onc.1206887. [DOI] [PubMed] [Google Scholar]
- 27.Moreno-Bueno G, Cubillo E, Sarrio D, Peinado H, Rodriguez-Pinilla SM, Villa S, Bolos V, Jorda M, Fabra A, Portillo F, et al. Genetic profiling of epithelial cells expressing E-cadherin repressors reveals a distinct role for Snail, Slug, and E47 factors in epithelial-mesenchymal transition. Cancer Res. 2006;66:9543–9556. doi: 10.1158/0008-5472.CAN-06-0479. [DOI] [PubMed] [Google Scholar]
- 28.Castellano G, Reid JF, Alberti P, Carcangiu ML, Tomassetti A, Canevari S. New potential ligand-receptor signaling loops in ovarian cancer identified in multiple gene expression studies. Cancer Res. 2006;66:10709–10719. doi: 10.1158/0008-5472.CAN-06-1327. [DOI] [PubMed] [Google Scholar]
- 29.Janda E, Nevolo M, Lehmann K, Downward J, Beug H, Grieco M. Raf plus TGFbeta-dependent EMT is initiated by endocytosis and lysosomal degradation of E-cadherin. Oncogene. 2006;25:7117–7130. doi: 10.1038/sj.onc.1209701. [DOI] [PubMed] [Google Scholar]
- 30.Barbacci E, Chalkiadaki A, Masdeu C, Haumaitre C, Lokmane L, Loirat C, Cloarec S, Talianidis I, Bellanne-Chantelot C, Cereghini S. HNF1beta/TCF2 mutations impair transactivation potential through altered co-regulator recruitment. Hum Mol Genet. 2004;13:3139–3149. doi: 10.1093/hmg/ddh338. [DOI] [PubMed] [Google Scholar]
- 31.Gottardi CJ, Arpin M, Fanning AS, Louvard D. The junction-associated protein, zonula occludens-1, localizes to the nucleus before the maturation and during the remodeling of cell-cell contacts. Proc Natl Acad Sci USA. 1996;93:10779–10784. doi: 10.1073/pnas.93.20.10779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Traweger A, Fuchs R, Krizbai IA, Weiger TM, Bauer HC, Bauer H. The tight junction protein ZO-2 localizes to the nucleus and interacts with the heterogeneous nuclear ribonucleoprotein scaffold attachment factor-B. J Biol Chem. 2003;278:2692–2700. doi: 10.1074/jbc.M206821200. [DOI] [PubMed] [Google Scholar]
- 33.Tronche F, Ringeisen F, Blumenfeld M, Yaniv M, Pontoglio M. Analysis of the distribution of binding sites for a tissue-specific transcription factor in the vertebrate genome. J Mol Biol. 1997;266:231–245. doi: 10.1006/jmbi.1996.0760. [DOI] [PubMed] [Google Scholar]
- 34.Heinzelmann-Schwarz VA, Gardiner-Garden M, Henshall SM, Scurry J, Scolyer RA, Davies MJ, Heinzelmann M, Kalish LH, Bali A, Kench JG, et al. Overexpression of the cell adhesion molecules DDR1, Claudin 3, and Ep-CAM in metaplastic ovarian epithelium and ovarian cancer. Clin Cancer Res. 2004;10:4427–4436. doi: 10.1158/1078-0432.CCR-04-0073. [DOI] [PubMed] [Google Scholar]
- 35.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelialmesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Coffinier C, Thomas MK, Gresh L, Eddu G, Manor T, Levitsky LL, Yaniv M, Rhoads DB. Selective deletion of the Hnf1beta (MODY5) gene in beta-cells leads to altered gene expression and defective insulin release. Endocrinology. 2004;145:3941–3949. doi: 10.1210/en.2004-0281. [DOI] [PubMed] [Google Scholar]
- 37.Battle MA, Konopka G, Parviz F, Gaggl AL, Yang C, Sladek FM, Duncan SA. Hepatocyte nuclear factor 4alpha orchestrates expression of cell adhesion proteins during the epithelial transformation of the developing liver. Proc Natl Acad Sci USA. 2006;103:8419–8424. doi: 10.1073/pnas.0600246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kato N, Sasou S, Motoyama T. Expression of hepatocyte nuclear factor-1beta (HNF-1beta) in clear cell tumors and endometriosis of the ovary. Mod Pathol. 2006;19:83–89. doi: 10.1038/modpathol.3800492. [DOI] [PubMed] [Google Scholar]
- 39.Terasawa K, Toyota M, Sagae S, Ogi K, Suzuki H, Sonoda T, Akino K, Maruyama R, Nishikawa N, Imai K, et al. Epigenetic inactivation of TCF2 in ovarian cancer and various cancer cell lines. Br J Cancer. 2006;94:914–921. doi: 10.1038/sj.bjc.6602984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boutet A, De Frutos CA, Maxwell PH, Mayol MJ, Romero J, Nieto MA. Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. EMBO J. 2006;25:5603–5613. doi: 10.1038/sj.emboj.7601421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mani SA, Yang J, Brooks M, Schwaninger G, Zhou A, Miura N, Kutok JL, Hartwell K, Richardson AL, Weinberg RA. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc Natl Acad Sci USA. 2007;104:10069–10074. doi: 10.1073/pnas.0703900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng W, Liu J, Yoshida H, Rosen D, Naora H. Lineage infidelity of epithelial ovarian cancers is controlled by HOX genes that specify regional identity in the reproductive tract. Nat Med. 2005;11:531–537. doi: 10.1038/nm1230. [DOI] [PubMed] [Google Scholar]
- 43.Kurman RJ, Shih IeM. Pathogenesis of ovarian cancer: lessons from morphology and molecular biology and their clinical implications. Int J Gynecol Pathol. 2008;27:151–160. doi: 10.1097/PGP.0b013e318161e4f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu R, Zhai Y, Fearon ER, Cho KR. Diverse mechanisms of betacatenin deregulation in ovarian endometrioid adenocarcinomas. Cancer Res. 2001;61:8247–8255. [PubMed] [Google Scholar]
- 45.Ahmed N, Thompson EW, Quinn MA. Epithelial-mesenchymal interconversions in normal ovarian surface epithelium and ovarian carcinomas: an exception to the norm. J Cell Physiol. 2007;213:581–588. doi: 10.1002/jcp.21240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.