Abstract
Crop rotation is a common means of reducing pathogen populations in soil. Several rotation crops have been shown to reduce soybean cyst nematode (Heterodera glycines) populations, but a comprehensive study of the optimal crops is needed. A greenhouse study was conducted to determine the effect of growth and decomposition of 46 crops on population density of H. glycines. Crops were sown in soil infested with H. glycines. Plants were maintained until 75 days after planting, when the soil was mixed, a sample of the soil removed to determine egg density, and shoots and roots chopped and mixed into the soil. After 56 days, soil samples were again taken for egg counts, and a susceptible soybean (‘Sturdy’) was planted in the soil as a bioassay to determine egg viability. Sunn hemp (Crotalaria juncea), forage pea (Pisum sativum), lab-lab bean (Lablab purpureus), Illinois bundleflower (Desman-thus illinoensis), and alfalfa (Medicago sativa) generally resulted in smaller egg population density in soil or number of cysts formed on soybean in the bioassay than the fallow control. Sunn hemp most consistently showed the lowest numbers of eggs and cysts. As a group, legumes resulted in lower egg population densities than monocots, Brassica species, and other dicots.
Keywords: Brassica, Crotalaria juncea, crop rotation, Desmanthus illinoensis, Glycine max, Heterodera glycines, Lablab purpureus, management, Medicago sativum, Pisum sativum, population, soybean cyst nematode
The soybean cyst nematode, Heterodera glycines Ichinohe, is the most damaging pest of soybeans (Glycine max (L.) Merr.) in the US (Wrather et al., 2003; Monson and Schmitt, 2004). Rotation with resistant soybean cultivars and nonhost plants are the principle tactics for management of H. glycines (Niblack and Chen, 2004; Niblack, 2005). However, effectiveness of management using resistant cultivars and crop rotation depends on numerous factors: mainly the availability of cultivars resistant to the various H. glycines HG Types or races; variability of H. glycines populations in pathogenicity on different soybean genotypes; species of rotation crops; number of years rotation crops used; and nematode survival ability in different geographical locations.
A number of crops have been evaluated in greenhouse and field studies for their effectiveness in lowering H. glycines population densities. Some of the crops effectively reduced H. glycines population densities when grown in rotation or as cover crops with soybean. A cover crop is any crop grown to provide soil cover, either inter-seeded or in rotation with other crops. Studies in the southern US demonstrated that American jointvetch (Aeschynomene americana), bahiagrass (Paspalum notatum), cotton (Gossypium hirsutum), sorghum (Sorghum bicolor), hairy indigo (Indigofera hirsuta), velvetbean (Mucuna pruiens), and wheat (Triticum aestivum) used as rotation crops or as winter/summer cover crops effectively lowered H. glycines population densities and in most cases increased soybean yield (Dabney et al., 1988; Rodriguez-Kabana et al., 1988, 1989, 1990, 1991a, 1991b; Weaver et al., 1993; Dillon et al., 1997; Weaver et al., 1998; Vargas-Ayala and Rodriguez-Kabana, 2001; Hague and Overstreet, 2002). In Japan, Nishizawa (1978) reported that millet (Pennisetum glaucum), rape (Brassica napus), and potato (Solanum tuberosum) were also effective in lowering H. glycines population density.
In the North Central region of the US, soybean is commonly rotated annually with corn (Zea mays), and this cropping system is conducive to H. glycines population development when soybean is grown. A diversified cropping system including additional crops in rotation or using cover or trap crops is needed for long-term, effective management of the nematode in the region. Several studies have been carried out to evaluate other crops for their potential as rotation crops in managing H. glycines populations and crop yields. A Kansas study showed that crop rotation with nonhosts grain sorghum and wheat was effective in reducing pre-plant populations of H. glycines, but high levels of nematode reproduction when soybean was planted resulted in serious damage to the susceptible cultivar during the first year back into soybean production (Long and Todd, 2001). Double cropping of soybean and winter wheat in rotation with grain sorghum did not help in cyst management, as yield suppression in the susceptible cultivar was comparable to that seen in full-season soybean (Long and Todd, 2001). Jackson et al. (2005) found that nonhost crops oat (Avena sativa), canola (Brassica napus), sesame (Sesamum indicum), corn, sorghum, and red clover (Trifolium pratense) did not appear to decrease the ability of H. glycines to infect and develop on subsequent soybean crops in Missouri, so the benefits of rotation with these nonhost crops are limited to reducing H. glycines populations and the frequent increase in yield in subsequent soybean crops. Under Minnesota conditions, a 5-yr rotation of nonhost crops or rotation of resistant soybean with a nonhost crop was needed to lower H. glycines population densities to below damaging level (e.g., 200–500 eggs/100 cm3 soil) (Chen et al., 2001b).
A few studies have been conducted in Minnesota to determine the effectiveness of diverse crops in the greenhouse and in fields as rotation, trap, or cover crops in lowering H. glycines population densities. Sortland and MacDonald (1987) found in a greenhouse study that crop rotation to decrease population of H. glycines race 5 must extend through two growing periods and preferably through three. They found that adzuki bean (Phaseolus angularis cv. Manoka) and pea (Pisum sativum) allowed some development of H. glycines females on roots, but could work as rotation crops; corn led to the lowest H. glycines population levels and was the most effective rotation crop of the study, and lambsquarters (Chenopodium album) and pigweed (Amaranthus retroflexus) are not hosts but may detrimentally affect the growth of the subsequent soybean crop (Sort-land and MacDonald, 1987). In another Minnesota field study, Miller et al. (2006) evaluated 16 crops commonly produced in Minnesota or having potential for use in the state as rotation crops: barley (Hordeum vulgare), flax (Linum usitatissimum), oats, sorghum, wheat, buckwheat (Fagopyrum sagittatum), canola, corn, rye (Secale cereale), sugar beet (Beta vulgaris), potato, sunflower (Helianthus annuus), alfalfa, hairy vetch (Vicia villosa), red clover, and pea. They found that legumes that are not or are poor hosts appeared to be the best crops for reducing the H. glycines population density, while monocots including corn appeared to be the least effective. Hairy vetch, a leguminous crop, supported the development of H. glycines females on its roots in the field and was probably a moderate host of H. glycines (Miller et al., 2006). Chen et al. (2006) studied the effects of alfalfa, red clover, and perennial ryegrass as cover crops inter-seeded with soybean on H. glycines and soybean and corn yields. Their results were inconsistent among three sites, and any reductions in H. glycines populations were minimal. Pea, when inter-seeded with corn as a trap crop, reduced H. glycines population density as compared with the corn-only control, but it was not cost-effective to manage H. glycines in this way (Chen et al., 2001a).
The objective of this screening study was to measure the changes in H. glycines egg population density in soils planted and subsequently incorporated with 46 crops and the changes in number of cysts formed on subsequent susceptible soybeans planted in these soils. The research was designed to better evaluate rotation crops that could be used to manage H. glycines in Minnesota, such as additional untested crops and the mechanisms involved in control of H. glycines. The results of this study, along with those of field experiments and previous greenhouse studies (Sortland and MacDonald, 1987; Miller et. al., 2006), may help determine which crops could be chosen for use as alternative rotation or cover crops to manage soybean cyst nematode in the region.
Materials and Methods
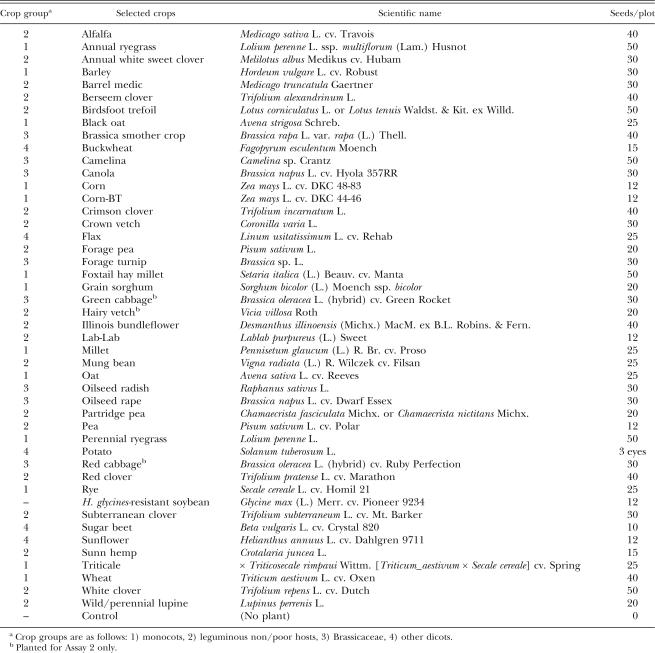
Forty-six crops were evaluated in the greenhouse for their potential as rotation or cover crops in managing for H. glycines by lowering soil egg population densities. The plant species chosen for this study are either common crops or have potential as alternative crops in Minnesota. Some of them, such as sunn hemp, have been shown to have potential in managing plant-parasitic nematodes including H. glycines (Wang et al., 2002; Kushida et al., 2003). Several of the crops, namely black oat (Avena strigosa), brassica smother crop (Brassica campestris), camelina (Camelina sp.), crown vetch (Coronilla varia), foxtail hay millet (Setaria italica cv. Manta), and triticale (Triticosecale rimpaui cv. Spring), included in this study had apparently not been included in any H. glycines host range, hatch, or rotation tests previously. Some of the other crops needed to be re-tested due to mixed results in other research.
Soil infested with H. glycines race 3 (HG Type 0-) was collected from field plots of a crop rotation experiment (Chen et. al., 2001b) on a commercial farm in Waseca County, MN, on November 6, 2003 (Assay 1), and in spring 2004 (Assay 2). The soil was a Webster clay loam (fine-loamy, mixed, mesic Typic Endoaquoll) (38.7% sand; 29.8% silt; 31.5% clay; pH 7.9). The soil was mixed thoroughly and supplemented with egg-free field soil to obtain an even distribution of cysts at a density of approximately 20,000 (Assay 1) or 23,000 (Assay 2) eggs/100 cm3 soil. Approximately 1,250 g of this soil was placed in 16-cm-diam. clay pots and planted with the selected crops (Table 1). Planting was done by pouring most of each 1,250 g soil portion into its pot, except for a ∼2-cm layer, which was added after the seeds were scattered on the soil surface. Seeding rate was determined by estimating how many plants of each species would appropriately fit in a 16-cm-diam. pot as they developed over the course of the experiment. There was also a fallow (no plant) control. Six replicates of each crop were used.
Table 1.
Crops selected and seeding rates for screening study.
Pots were arranged in randomized blocks by replicate and were maintained in a greenhouse (with temperatures estimated at 20–33°C). They were watered every day to keep the soil moist. Plants were given N, P, K fertilizer in the irrigation water (1.2 g N, 1.2 g P, and 1.2 g K/liter) after about 30 d of growth. After 75 d, the plants were cut at the soil surface, and the fresh weight of aerial material was recorded. Soil in each pot was mixed thoroughly, and a sub-sample of 50 cm3 soil in Assay 1 and 100 cm3 soil in Assay 2 was taken to determine H. glycines egg densities. Cysts were extracted using hand decanting, sieving (850-μm aperture for the top sieve, 250-μm aperture for the bottom sieve), and 63% sucrose solution flotation, and eggs were released using a cyst crusher (Faghihi and Ferris, 2000). Eggs were then collected on nested sieves (75-μm aperture for the top sieve, 25-μm aperture for the bottom sieve), cleaned using centrifugation in a 38% sucrose solution, and counted.
The aerial material of each crop was cut into 3-cm sections and evenly distributed among the six pots in which it was grown. Plant materials were mixed with the remaining soil and roots in a container and then returned to the same pot. These pots were maintained (watered daily and hand-weeded when necessary) in the greenhouse for 56 d, and then the soil was again mixed thoroughly and 100 cm3 (190 g) samples were taken from each pot to determine egg population density following the procedures described above.
Approximately 150 cm3 of the remaining soil of each pot was placed in a 7-cm-diam. clay pot. ‘Sturdy’ soybean seeds were soaked in water for 3.25 hr and planted two per pot. After 7 d, the seedlings were thinned to one plant per pot. The pots were maintained in the greenhouse and watered every day. After 32 d, the soybeans with the soil were removed from their pots, each placed in a 1-liter beaker, soaked in tap water for at least 30 min, then gently washed to remove soil. Cysts (females) were dislodged from the roots with a high-pressure water jet over a set of nested sieves (850-μm aperture for the top sieve, 250-μm aperture for the bottom sieve). Cysts caught on the bottom sieve were collected and counted.
Data analysis: To determine nematode population change during the rotation crop period (first 75 d) and the following fallow period (the following 56 d), egg population change factors (PCF) during these two periods were computed: PCF1 = nematode egg population density at the end of the rotation crop growing period/initial egg population density; PCF2 = egg population density at the end of fallow period/egg population density at the end of crop-growing period; and PCF1+2 = egg population density at the end of fallow period/initial egg population density at planting the rotation crops. Nematode reproduction factor (Rf) on the susceptible soybean following the fallow period was computed: Rf = cyst counts × 1,000/initial eggs per pot, which represents the number of females produced per 1,000 eggs. Initially, data were tested for normality using the Shapiro-Wilk test (Shapiro and Wilk, 1965) using the Statistix 8.0 program (Analytical Software, Tallahassee, FL). PCF1 and PCF1+2 were not transformed for statistical analysis. PCF2, cysts formed per plant, and Rf in Assay 1 were transformed with Ln(x), x 0.65, and x 0.63, respectively, and PCF2, cysts formed per plant, and Rf in Assay 2 were transformed with x 0.2, and x 0.1, and Ln(x), respectively, to improve homogeneity of variance before being subjected to analysis of variance. Means of individual crops were compared using Tukey's Studentized Range (HSD) test at α = 0.05. To determine differences among groups of crops, the data were averaged by four groups: 1) monocots; 2) leguminous, non/poor hosts; 3) Brassicaceae; and 4) “other dicots” besides the leguminous, non/poor hosts and Brassicaceae (Table 1). Orthogonal contrasts among the groups were performed.
Results
Assay 1
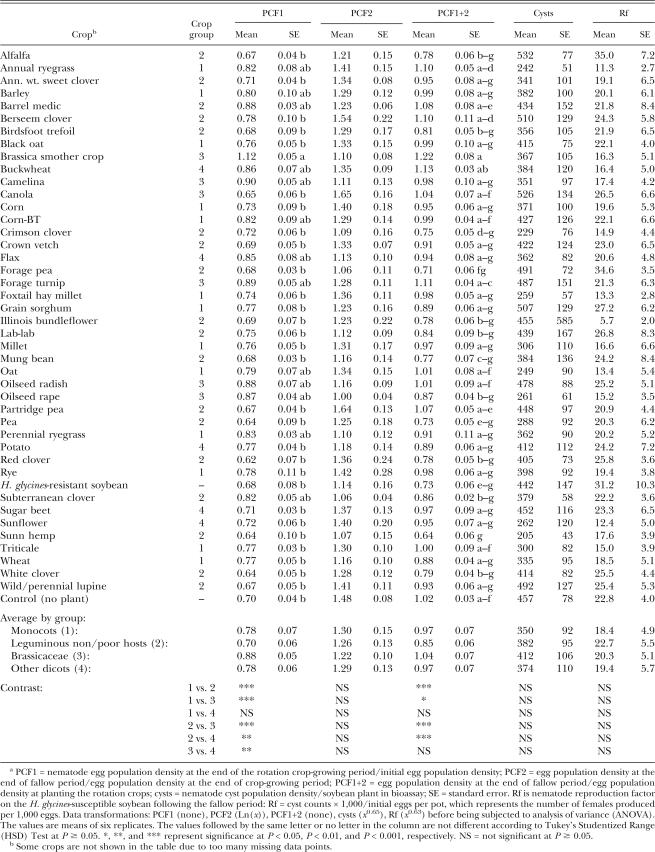
Egg population 75 d after planting (PCF1): After the crops had been growing in the infested soil for 75 d, soil samples were taken to determine soil egg population density. Of all the crops evaluated, only the brassica smother crop soil had a mean egg count which was higher (61%) than that of the fallow control. The egg count in the brassica smother crop was also higher than many other crops (Table 2). As a group, leguminous non/poor hosts led to the lowest egg counts. The Brassicaceae family group resulted in greater egg population density than monocots, “other dicots,” and leguminous non/poor hosts (Table 2).
Table 2.
Population density of Heterodera glycines in response to rotation crops in Assay 1 (fall soil) of a greenhouse study.a
Egg population 56 d after harvesting crops (PCF2 and PCF1+2): The egg population change factor during the 56 d of fallow period after harvesting the crop (PCF2) did not differ among the individual crops and the crop groups (Table 2). However, the population change between the time of planting the crops and the end of fallow period (PCF1+2) differed among the individual crops (Table 2). Brassica smother crop did not show greater egg populations than the control at this point of time. Sunn hemp lowered egg population densities (38% lower) compared with the fallow control. When compared with sunn hemp, PCF1+2 values were greater for the following crops: annual ryegrass, barrel medic, berseem clover, brassica smother crop, buckwheat, canola, corn-BT, forage turnip, oat, oilseed radish, partridge pea, and triticale. As a group, the leguminous non/poor hosts again led to the lowest PCF1+2 values compared with all other groups. The Brassicaceae group also resulted in slightly greater PCF1+2 than monocots (Table 2).
Female number on soybean roots (Cysts and Rf): When the susceptible soybean cultivar ‘Sturdy’ was planted in the soils as a bioassay, there was no difference in cyst number and Rf among individual crops or the crop groups. However, sunn hemp resulted in the numerically lowest mean cyst number, 55% lower than the mean of the fallow control.
Assay 2
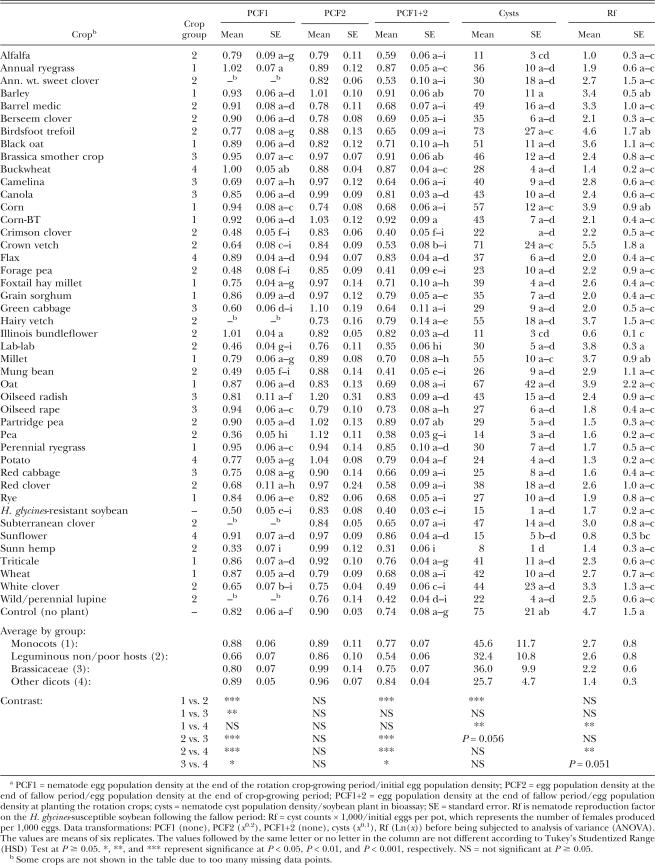
Egg population 75 d after planting (PCF1): The results were slightly different for Assay 2 compared with Assay 1. Seventy-five days after planting the crops in the infested soil, the following crops showed decreases in egg population density as compared with the fallow control: lab-lab (44% lower), pea (56% lower), and sunn hemp (60% lower) (Table 3). Compared with sunn hemp, all crops except crimson clover, crown vetch, forage pea, green cabbage, lab-lab, mung bean, pea, resistant soybean, and white clover had higher PCF1 values (Table 3). As a group, the leguminous non/poor hosts led to the lowest egg counts. Unlike the result in Assay 1 (Table 2), plants in the Brassicaceae family in Assay 2 resulted in smaller egg population densities than the monocots and the “other dicots” (Table 3).
Table 3.
Population density of Heterodera glycines in response to rotation crops in Assay 2 (spring soil) of a greenhouse study.a
Egg population 56 d after harvesting (PCF2 and PCF1+2): After plant materials were mixed into the soil and were maintained with no plant growth for 56 d in the greenhouse, lab-lab (53% lower) and sunn hemp (58% lower) showed differences in PCF1+2 as compared with the fallow control (Table 3). Compared with sunn hemp, the following crops led to greater PCF1+2: annual ryegrass, barley, black oat, brassica smother crop, buckwheat, canola, corn-BT, flax, foxtail hay millet, grain sorghum, hairy vetch, Illinois bundleflower, millet, oilseed radish, oilseed rape, partridge pea, perennial ryegrass, potato, sunflower, and triticale. As a group, leguminous non/poor hosts had a smaller PCF1+2 than any of other three groups (Table 3). Plants in the Brassicaceae family also resulted in smaller PCF1+2 than the group of “other dicots.” There were no significant differences in PCF2 among the crops.
Number of females on soybean roots (Cysts and Rf): The results from Assay 2 showed some differences in H. glycines cyst counts among the crops (Table 3). The lowest cyst count was again from the sunn hemp soil, which was 89% lower than the mean cyst count in the fallow control soil. Illinois bundleflower (86% lower) and alfalfa (85% lower) soils also led to cyst numbers that were lower than the fallow control. Compared with sunn hemp, the number of cysts was higher for the following crops: barley, birdsfoot trefoil, corn, crown vetch, and millet. As groups, the leguminous non/poor hosts and other dicots not belonging to the Brassicaceae family resulted in lower cyst counts than the monocots (Table 3). The Rf values for Assay 2 also showed some differences among crops. Illinois bundle-flower and sunflower led to lower Rf values than the fallow control. As a group, the “other dicots” led to the lowest average Rf value (Table 3).
Discussion
The results of this study are generally consistent with results from recent field experiments by Miller et al. (2006), which also found that, compared with monocots and nonleguminous dicots, rotation with leguminous crops as a group led to lower H. glycines populations in field soil. Perhaps because this study was carried out under controlled greenhouse conditions, there were greater differences among the individual crops and groups of crops as compared with the previous field studies. This study not only confirmed the results from the previous field study, but also identified additional effective plant species as rotation or cover crops for managing H. glycines. Some of the crops such as sunn hemp and lab-lab are highly effective in reducing H. glycines population density and may have great potential in managing H. glycines and increasing soybean productivity in the North Central region.
As noted above, several crops produced significantly lower H. glycines numbers than the fallow control in various parts of the experiment. However, the crop in which H. glycines population density was most consistently the lowest was sunn hemp. As a group, the legumes, to which sunn hemp belongs, supported the lowest egg or cyst numbers. However, heterogeneity existed among species within a group, and selection of crops for H. glycines management should be based on the data from individual species rather than the groups.
There was some variation between Assay 1 and Assay 2. Fewer of the measurements in Assay 1 were statistically significant. In general, the trends in both assays were similar, with the crops falling in roughly the same order when ranked by egg or cyst counts. The variation may be due to the fact that the soil in Assay 1 was collected in fall, and soil for Assay 2 was collected in spring. Therefore, the eggs in the two Assays would be at different stages of diapause and might respond differently (i.e., they may or may not hatch) to the crops grown in the soil.
In Assay 1, an unexpected result was that PCF1 values were generally greater than PCF1+2 values. This may have been due to lower cyst extraction efficiency of egg population at the end of the rotation crop growing period. Theoretically, PCF should be less than 1 if there is no nematode reproduction. However, many PCF values, especially in Assay 1, were greater than 1 even for the crops that are nonhosts. This doesn't mean that the nematode population increased in these crops, but rather, the higher PCF values were perhaps due to experimental error in soil sampling and sample processing.
The mechanism involved in reducing H. glycines populations during the crop-growing period and the fallow period is not fully understood. Because PCF1 and PCF1+2 varied among crops more than PCF2 did, this suggests that the effect of the crops on H. glycines egg population density was mainly from the crop-growing period. Because PCF2 values did not differ significantly, H. glycines death by plant residue (egg death or hatch stimulation followed by J2 death in the absence of a living host) may not be the main mechanism in lowering H. glycines population density. Inducing egg hatch was probably the main mechanism in lowering the egg population density during the crop-growing period. The Rf (number of cysts formed on susceptible soybean per 1,000 eggs) may indicate the viability and infectivity of the eggs. While there was no difference in Rf in Assay 1, differences in Rf were observed in Assay 2. Whether the lower Rf in the “other dicots” group was due to lower viability or infectivity as compared with the other groups of crops could not be determined without further study and additional data.
Crop growth and the nematode population response may differ in different environments. We used the natural field soil rather than sterilized soil to provide soil microbial communities as similar as possible to those under field conditions. The clay loam soil used in this study is common in southern Minnesota and the North Central region. This screening study provided a basis for selecting crops to manage H. glycines in the region. However, further studies are needed under different field conditions to ascertain the effectiveness.
In conclusion, leguminous non/poor hosts as a group resulted in the greatest reduction in H. glycines egg population densities and cyst numbers, and sunn hemp was the crop which consistently led to the lowest H. glycines populations overall. The mechanisms involved in reduction of the H. glycines populations are unclear, but the results suggest that the effect occurs during the crop-growing period and is perhaps due to induced egg hatch.
Footnotes
The authors thank D. Miller, C. Johnson, W. Gottschalk, and K. Betts for technical assistance. This research was supported by Cooperative State Research, Education, and Extension Service, USDA, under Agreement Number 2002-34103-11990.
This paper was edited by Steve Koenning.
Literature Cited
- Chen SY, Porter PM, Reese CD, Klossner LD, Stienstra WC. Evaluation of soybean and pea as trap crops for managing Heterodera glycines . Journal of Nematology. 2001a;33:214–218. [PMC free article] [PubMed] [Google Scholar]
- Chen SY, Porter PM, Reese CD, Stienstra WC. Crop sequence effects on soybean cyst nematode and soybean and corn yields. Crop Science. 2001b;41:1843–1849. [Google Scholar]
- Chen SY, Wyse DL, Johnson GA, Porter PM, Stetina SR, Miller DR, Betts KJ, Klossner LD, Haar MJ. Effect of cover crops alfalfa, red clover, and perennial ryegrass on soybean cyst nematode population and soybean and corn yields in Minnesota. Crop Science. 2006;46:1890–1897. [Google Scholar]
- Dabney SM, McGawley EC, Boethel DJ, Berger DA. Short-term crop rotation systems for soybean production. Agronomy Journal. 1988;80:197–204. [Google Scholar]
- Dillon CR, Keisling TC, Riggs RD, Oliver LR. The profit potential of soybean production rotation systems in Arkansas. Communications in Soil Science and Plant Analysis. 1997;28:1693–1709. [Google Scholar]
- Faghihi J, Ferris JM. An efficient new device to release eggs from Heterodera glycines . Journal of Nematology. 2000;32:411–413. [PMC free article] [PubMed] [Google Scholar]
- Hague SS, Overstreet C. Crop rotation effects on nematode populations. In: van Santen E, editor. Making conservation tillage conventional: Building a future on 25 years of research, Proceedings of 25th annual southern conservation tillage conference for sustainable agriculture, Auburn, AL, USA, June 24–26, 2002. Auburn, AL: Alabama Agricultural Experiment Station, Auburn University; 2002. pp. 156–160. [Google Scholar]
- Jackson TA, Smith GS, Niblack TL. Heterodera glycines infectivity and egg viability following nonhost crops and during overwintering. Journal of Nematology. 2005;37:259–264. [PMC free article] [PubMed] [Google Scholar]
- Kushida A, Suwa N, Ueda Y, Momota Y. Effects of Crotalaria juncea and C. spectabilis on hatching and population density of the soybean cyst nematode, Heterodera glycines (Tylenchida: Heteroderidae) Applied Entomology and Zoology. 2003;38:393–399. [Google Scholar]
- Long JH, Todd TC. Effect of crop rotation and cultivar resistance on seed yield and the soybean cyst nematode in full-season and double-cropped soybean. Crop Science. 2001;41:1137–1143. [Google Scholar]
- Miller DR, Chen SY, Porter PM, Johnson GA, Wyse DL, Stetina SR, Klossner LD, Nelson GA. Evaluation of rotation crops for management of the soybean cyst nematode in Minnesota. Agronomy Journal. 2006;98:569–578. [Google Scholar]
- Monson M, Schmitt DP. Economics. In: Schmitt DP, Wrather JA, Riggs RD, editors. Biology and management of the soybean cyst nematode. Marceline, MO: Schmitt & Associates of Marceline; 2004. pp. 41–53. [Google Scholar]
- Niblack TL. Soybean cyst nematode management reconsidered. Plant Disease. 2005;89:1020–1026. doi: 10.1094/PD-89-1020. [DOI] [PubMed] [Google Scholar]
- Niblack TL, Chen SY. Cropping systems. In: Schmitt DP, Wrather JA, Riggs RD, editors. Biology and management of the soybean cyst nematode. Marceline, MO: Schmitt & Associates of Marceline; 2004. pp. 181–206. [Google Scholar]
- Nishizawa T. Annual population changes of soil nematodes in the field of continuous cropping or rotation. Kasetsart Journal. 1978;12:3–13. [Google Scholar]
- Rodriguez-Kabana R, King PS, Robertson DG, Weaver CF, Carden EL. New crops with potential for management of soybean nematodes. Nematropica. 1988;18:45–52. [Google Scholar]
- Rodriguez-Kabana R, Weaver DB, Garcia R, Robertson DG, Carden EL. Bahiagrass for the management of root-knot and cyst nematodes in soybean. Nematropica. 1989;19:185–193. [Google Scholar]
- Rodriguez-Kabana R, Weaver DB, Robertson DG, Carden EL, Pegues ML. Additional studies on the use of bahiagrass for the management of root-knot and cyst nematodes in soybean. Nematropica. 1991a;21:203–210. [Google Scholar]
- Rodriguez-Kabana R, Weaver DB, Robertson DG, Weaver F, Carden EL. Rotations of soybean with tropical corn and sorghum for the management of nematodes. Journal of Nematology. 1991b;23:662–667. [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Kabana R, Weaver DB, Robertson DG, Young RW, Carden EL. Rotations of soybean with two tropical legumes for the management of nematode problems. Nematropica. 1990;20:101–110. [Google Scholar]
- Shapiro SS, Wilk MB. An analysis of variance for normality (complete samples) Biometrika. 1965;52:591–611. [Google Scholar]
- Sortland ME, MacDonald DH. Effect of crop and weed species on development of a Minnesota population of Heterodera glycines race 5 after one to three growing periods. Plant Disease. 1987;71:23–27. [Google Scholar]
- Vargas-Ayala R, Rodriguez-Kabana R. Bioremediative management of soybean nematode population densities in crop rotations with velvetbean, cowpea, and winter crops. Nematropica. 2001;31:37–46. [Google Scholar]
- Wang KH, Sipes BS, Schmitt DP. Crotalaria as a cover crop for nematode management: A review. Nematropica. 2002;32:35–57. [Google Scholar]
- Weaver DB, Rodriguez-Kabana R, Carden EL. Velvetbean in rotation with soybean for management of Heterodera glycines and Meloidogyne arenaria . Journal of Nematology. 1993;25:809–813. [PMC free article] [PubMed] [Google Scholar]
- Weaver DB, Rodriguez-Kabana R, Carden EL. Velvetbean and bahiagrass as rotation crops for management of Meloidogyne spp. and Heterodera glycines in soybean. Journal of Nematology. 1998;30:563–568. [PMC free article] [PubMed] [Google Scholar]
- Wrather JA, Koenning SR, Anderson TR. Effect of diseases on soybean yields in the United States and Ontario (1999 to 2002). Plant Health Progress. 2003 http://www.plantmanagementnetwork.org/sub/php/review/2003/soybean/