Abstract
Knowledge of the virulence phenotypes of soybean cyst nematode, Heterodera glycines populations is important in choosing appropriate sources for breeding resistant cultivars and managing the nematode. We investigated races of 59 H. glycines populations collected from 1997 to 1998 and races and HG Types of 94 populations collected in 2002 from soybean fields across southern and central Minnesota. In the 1997 to 1998 samples, race 3 was predominant and represented 78% of the populations. The remaining populations were 11.9% race 1, 1.7% race 4, 6.8% race 6, and 1.7% race 14. In the 2002 samples, the populations were classified as 15.3% race 1, 77.6% race 3, 2.4% race 5, 3.5% race 6 and 1.2% race 9. Percentage of 1997 to 1998 populations with female indices (FI) higher than 10 were 10.2% on Pickett 71, 3.4% on Peking, 13.6% on PI 88788, 3.4% on PI 90763, 1.7% on PI 209332, and 1.7% on PI 437654. Percentage of 2002 populations with FI >10 was 1.1% on Peking, 17.0% on PI88788, 14.9% on PI 209332, 33.0% on PI 548316, 11.7% on Pickett 71, and 0% on the other three indicators, PI 90763, PI 437654, and PI 89772. The line PI 548316 was relatively susceptible to the Minnesota H. glycines populations and may not be recommended for breeding resistant cultivars in the state. There was no noticeable change of frequencies of virulence phenotypes in response to the use of resistant cultivars during 1997 to 2002 in Minnesota except that FI increased on the PI 209332.
Keywords: Heterodera glycines, HG Type, host-parasitic relationship, race, soybean cyst nematode, virulence phenotype
Soybean cyst nematode, Hetorodera glycines Ichinohe, is a major yield-limiting pathogen of soybean (Glycine max (L.) Merr.) in the world. A conservative estimate based on the assumption of a mean of 3% direct loss from the nematode is over US$1.1 billion annually worldwide (Schmitt, 2004). The major tactic for managing H. glycines is the use of host resistance. Breeding and using resistant soybean cultivars are the most economical and practical methods for control of this nematode in most soybean-growing regions throughout the world. The knowledge about virulence of H. glycines populations is essential for breeding appropriate cultivars for a region and for using the cultivars effectively in fields.
Soon after the first confirmation of the H. glycines infestation in the US in 1954 (Winstead et al., 1955), resistance of soybean to H. glycines was discovered (Ross and Brim, 1957) and the variability in virulence of H. glycines among populations was recognized (Ross, 1962). The variability of H. glycines virulence was first classified to four races based on number of females formed on four differential soybean lines, Pickett, Peking, PI 88788, and PI 90763, relative to the female number on the susceptible cultivar Lee (Golden et al., 1970). If the female number is less than 10% of the number on Lee, the response of the soybean line is “–”, and if ≥10%, the response is “+”. In this scheme, there are 16 possible combinations of responses of the four lines, and thus 16 potential races were proposed later (Riggs and Schmitt, 1988; Riggs, 1988). In 2002, the virulence classification scheme was modified in an effort to have a more accurate test name and to more adequately define the diversity in virulence phenotype, and the term “HG Type” was used instead of “race” for the virulence phenotype (Niblack et al., 2002). In the HG Type classification, another four indicator lines, PI 437654, PI 209332, PI 89772, and PI 548316 (Cloud), were added besides the three indicator lines Peking, PI 88788, and PI 90763 from the race tests, and the description of HG Type indicates the positive response of a population on individual lines.
The first confirmation of H. glycines infestation in Minnesota was from Faribault County in 1978 (MacDonald et al., 1980). In that report, MacDonald et al. (1980) documented that the race of H. glycines populations found in Faribault, MN, was probably race 5. In a later regional survey, two populations from Minnesota were classified to race 3 (Kim et al., 1997). The nematode infestation has been confirmed in 57 counties in southern and central regions, and it continues to spread towards northern counties in the Red River Valley in the state (Chen, unpublished data, 2006). Over the past two decades, especially recent years, a number of resistant soybean cultivars have been developed and used in managing H. glycines in the state (Chen et al., 2001b). For the effective use of the resistant cultivars, knowledge about the virulence phenotypes and the frequencies of H. glycines populations is necessary. During 1997 to 1998, a survey was conducted to determine the H. glycines races in the state. The H. glycines populations collected in 2002 were analyzed again for their virulence phenotypes. This paper reports the results of these two survey analyses. The objective of this research was to determine the types and frequencies of virulence of the H. glycines populations in Minnesota and monitor changes that may occur over the 5 years between the two sampling times.
Materials and Methods
A total of 59 soil samples randomly selected from the pool of H. glycines-infested soil samples (2,543 infested out of 3,449 total samples) submitted by soybean growers in 1997 to 1998 and 94 soil samples from the pool of samples (1,142 infested out of 1,271 total samples) submitted in 2002 were analyzed. In general, each of the soil samples was collected from 5 to 20 acres of an area in a corn or soybean field. The selected soil samples generally represented all H. glycines-infested areas in Minnesota, although in 1997 to 1998, most soil samples were from Waseca and surrounding counties (south central). Before used, the soils had been stored at room temperature (22–24°C) for a period of time depending on when the soil samples were received. The soils were placed in pots and planted with the H. glycines-susceptible soybean ‘Sturdy’ to increase population densities. Newly formed females on the roots were collected, and eggs were released from the cysts. The eggs were used as inoculum for H. glycines race and HG-Type tests following the procedures described previously (Riggs and Schmitt, 1988; Niblack et al., 2002) with some modification.
For samples collected in 1997 to 1998, the virulence was tested on the four race differential lines Pickett 71, Peking, PI 88788, and PI 90763, along with PI 209332 and PI 437654. For the 2002 samples, the virulence was analyzed based on the HG-Type test procedures including the seven indicator lines PI 548402 (Peking), PI 88788, PI 90763, PI 437654, PI 209332, PI 89772, and PI 548316 (Cloud) (Niblack et al., 2002), and the line Pickett 71 was also included for the comparison of races between the two batches of samples. All soybean lines and cultivar Lee (Lee 68 in 1997/1998 and Lee 74 in 2002) were obtained from the USDA Soybean Germ-plasm Collection in Urbana, Illinois. The soybean seeds were germinated on a moist, sterilized germination paper in petri dishes in an incubator at 29°C. After 48 hr, the seedlings with root radicals 2 to 3 cm long were selected for the tests.
A cone-tainer (4-cm-diam. and 13.5-cm high) was filled with autoclaved sandy loam soil to half and 2,000 eggs in 2.5 ml of water were added. Additional soil was placed in the cone-tainer to approximately 1 cm from the top. A hole was made at the center to a depth of 3 cm with a glass stick (0.5-cm-diam.). A soybean seedling was placed in the hole with radical toward the ground. Another inoculum of 2,000 eggs in 2.5 ml of water was added near the seedling, and the seedling was covered with additional soil to about 1-cm depth. Each soybean line was replicated five times (five cone-tainers). All of the cone-tainers for each nematode population were inserted into autoclaved sand in a container (35 × 31 × 15 cm) that had five holes at bottom for draining excess water. The cone-tainers were maintained in the greenhouse at an average of 25°C (20–30°C) and watered daily.
After 30 d, the soybean plants with soil were removed from the cone-tainers and soaked in water in 1-liter beakers for at least 30 min. The soybean plants were gently removed from the beakers, and the soil on the roots was gently washed off. The females on roots were counted directly with or without the aid of a magnifier. Female index was determined for each soybean line by dividing the female count on the indicator line by the female count on the control ‘Lee’ and expressed as percentage. If the number of females on Lee was fewer than 50, the data was discarded and the population was retested.
Results
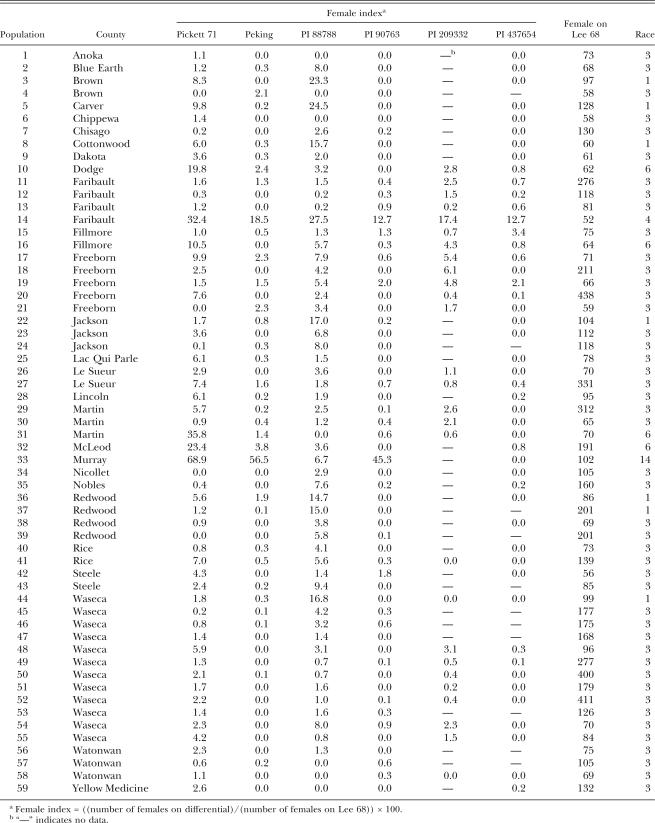
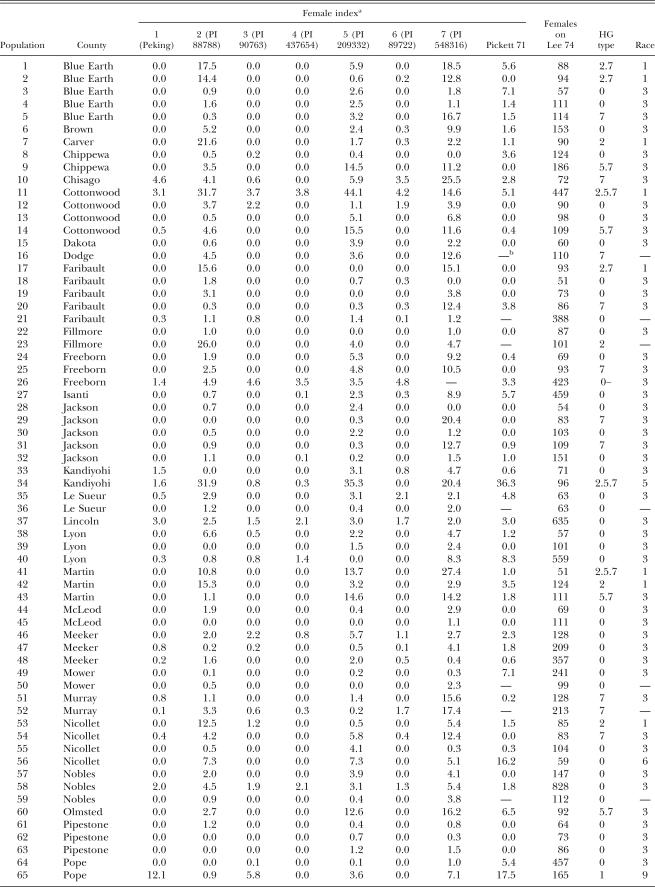
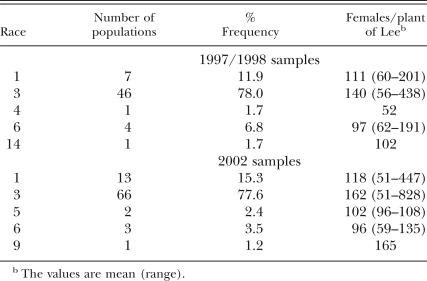
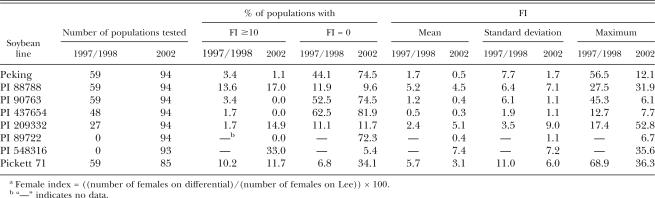
A total of 59 populations collected from 27 counties in 1997 to 1998 and 94 populations collected from 38 counties in 2002 were analyzed (Tables 1 and 2). There was a wide range of female numbers formed on Lee (Tables 1, 2, 3, and 4). Among the 1997 to 1998 samples, race 3 was predominant and represented 78% of the populations. The remaining populations were 11.9% race 1, 1.7% race 4, 6.8% race 6 and 1.7% race 14 (Table 3). In the 2002 samples, 85 populations were classified to races. A similar high frequency of race 3(77.6%) was found, and the remaining races were 15.3% race 1, 2.4% race 5, 3.5% race 6, and 1.2% race 9 in the 85 samples (Table 3).
Table 1.
Races of Heterodera glycines in Minnesota in 1997–1998.
Table 2.
HG Types and races of Heterodera glycines in Minnesota fields in 2002.
Table 3.
Race frequencies of Heterodera glycines in soil samples collected in 1997, 1998, and 2002 in Minnesota.
Table 4.
Frequencies of Heterodera glycines HG Types in soil samples collected in 2002 in Minnesota.
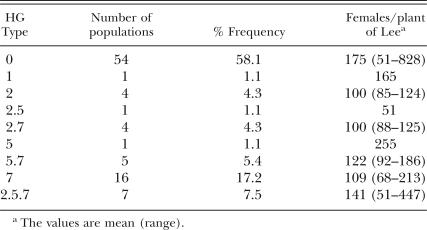
In the 2002 samples, nine HG Types were found (Table 4): HG Types 0, 1, 2, 5, 7, 2.5, 2.7, 5.7 and 2.5.7. The HG Type 0 was predominant and represented 58.1% of the populations, most (87%) of which belonged to race 3. HG Type 7 represented 17.2%, and all other HG Types had low (<8%) frequencies (Table 4).
No soybean line was immune to all populations tested in this study; despite that, reproduction of Minnesota H. glycines populations varied among soybean lines (Table 5). PI 88788, the major resistance source for breeding soybean cultivars in the region, had less resistance than most lines and yielded FI >10 in 13.6% of 1997 to 1998 populations and 17% of 2002 populations. Peking, also the resistance source for some cultivars in the region, was relatively resistant to Minnesota populations, although high reproduction (FI 56.5) was observed in one population (Tables 1 and 5). Pickett 71, which was derived from Peking, had much less resistance to the H. glycines populations than Peking and produced FI >10 in 10 to 12% of populations (highest FI 68.9). PI 90763 had a similarly high level of resistance as Peking. PI 437654 and PI 89722 had the highest resistance to H. glycines and supported no reproduction in 82% and 72.3% populations, respectively, in the 2002 samples. PI 209332 supported a relatively high percentage (14.9%) of H. glycines populations with FI >10 in 2002, but a low percentage of populations in 1997 to 1998. PI 548316 was probably the least resistant to Minnesota populations of H. glycines, and it yielded FI >10 in 33% of the 2002 populations.
Table 5.
Female indices (FI) of Heterodera glycines on individual soybean lines.a
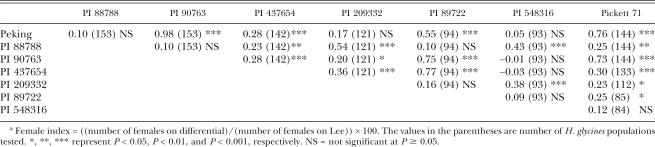
Pearson correlation analysis showed that the three lines, PI 88788, PI 209332, and PI 548316, which had relative high FI, were highly correlated in terms of resistance to the Minnesota populations based on FI (Table 6). Correlation of these three lines was not significant with the other four indicator lines, Peking, PI 90763, PI 437654, and PI 89722, except for PI 88788 vs. PI 437654, and PI 209332 vs. PI 90763 or PI 437654 (Table 6). Peking and PI 90763 had the highest correlation coefficient (0.98). The soybean cultivar Picket 71 was significantly correlated with all lines except PI 548316.
Table 6.
Correlation coefficients among soybean lines with resistance to Heterodera glycines based on female indices (FI) of Minnesota populations collected in 1997, 1998, and 2002.a
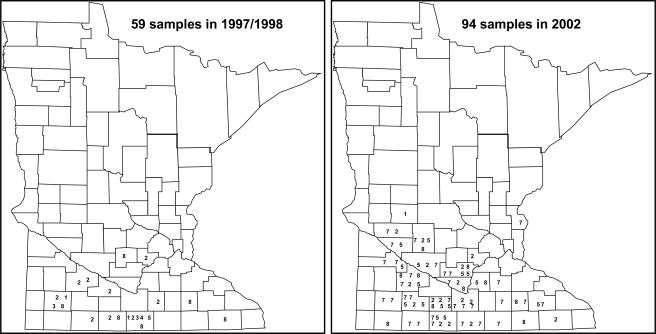
The virulent populations distributed in all areas across the southern and central Minnesota where the H. glycines infestation has been confirmed (Fig. 1). Although H. glycines has also been observed in the counties along the southwest and southeast borders, fewer virulent populations were found in these border counties, probably due to limited soybean acreage and few samples from these counties.
Fig. 1.
Distribution of virulent populations of Heterodera glycines in Minnesota. The numbers represent soybean lines on which the H. glycines populations produced female index greater than 10.1 = Peking, 2 = PI 88788, 3 = PI 90763, 4 = PI 437654, 5 = PI 209332, 6 = PI 89722, 7 = 548316, and 8 = Pickett 71.
Discussion
In this study, the races of 59 H. glycines populations in 1997 to 1998 samples, races of 85 populations and HG Types of 94 populations in 2002 samples collected from soybean fields across H. glycines-infested counties in southern and central Minnesota were characterized. Among these populations, a total of seven races and nine HG Types were detected (Tables 3, 4). In addition, race 2 was found in Minnesota, but the sample was not included in this survey. Race 3, which is avirulent on the four race-differential lines, and HG Type 0, which is avirulent on all seven indicator lines, were predominant. The results suggest that majority of populations in the state were avirulent on these breeding lines, and there was small risk to use the resistant soybean cultivars derived from Peking and PI 88788 in most fields in Minnesota. However, a few populations that can parasitize PI 88788, Peking and other soybean lines have been found. Growers are advised to monitor performance of resistant cultivars in fields or have virulence phenotype tested in order to assure appropriate use of resistant cultivars.
The frequencies of races or virulent phenotypes varied among different geographical regions. There was a trend that virulence decreased from south to north in the US (Kim et al., 1997). Most populations collected north of 37°N latitude in 1992 to 1993 were race 3, while only one out of 41 populations south of 37°N was classified to race 3 (Kim et al., 1997). In soil samples collected from North Carolina during 1994 to 1997, no race 3 was found, and FI was high on most soybean lines except for Hartwig (Koenning and Barker, 1998). Relatively high percentages of virulent populations were also reported from surveys conducted in other southern states (Lehman and Dunn, 1987; Young, 1990; Lewis et al., 1993). Although the avirulent (on the four race differential lines) race 3 was predominant in all states in the North Central region (Sikora and Noel, 1991; Niblack et al., 1993; Willson et al., 1996; Kim et al., 1997), differences in frequency of virulent populations and level of virulence may exist among the states in the region. For example, the mean FI on PI 88788 was only 4.5 (SD 7.1 and highest 31.9) in Minnesota in 2002 (Table 5), 10.4 (SD 14.9 and highest 67.1) in Ohio in 1992 to 1995 (Willson et al., 1996), 12.3 (SD 13.7 and highest 53.1) in Illinois in 1989 to 1990, and 12.3 (highest 70) in Missouri in 1998 to 1992 (Niblack et al., 1993). The populations in Minnesota appeared to be less virulent to PI 88788 (main resistant source of the commercial cultivars) than the populations in the other three states.
The greater diversity and higher level of virulence of the populations in the southern region compared with the northern region may be mainly due to longer history of planting resistant cultivars (Kim et al., 1997). However, other possibilities cannot be ruled out. A similar pattern of H. glycines race distribution was observed in China where race 3 was predominant in the northeast and northern regions, and more diverse races and higher virulent populations were detected in the central regions (Liu et al., 1997; Chen et al., 2001a). There was no pattern or history of using H. glycines-resistant cultivars in China. Therefore, it is possible that the difference in virulence in different geographical regions may be partially due to different fitness of the virulence genotypes under climatic conditions in the different regions.
Based on the data of 1997 to 1998 samples and 2002 samples, there was no noticeable change of frequency of H. glycines races and virulent populations over the 5 years on the indicator lines, except for PI 209332 on which frequency of populations with FI >10 increased from 1.7% in 1997 to 1998 to 14.9% in 2002. It is unclear whether there was a true increase of virulence on PI 209332 during the 5 years or if the difference was experimental error. Nevertheless, the change of virulent phenotypes has been documented in field plot experiments (Young et al., 1986; Young and Hartwig, 1992; Young, 1994) and state-wide surveys. In North Carolina, higher frequencies of virulent H. glycines populations were detected in 1994 to 1996 (Koenning and Barker, 1998) as compared with the samples collected during 1985 to 1986 (Schmitt and Barker, 1987). Similarly, smaller percentages of populations were classified to race 3, and higher frequencies and levels of virulence of populations were found in 1998 to 1999 samples (Niblack et al., 2003) than in samples collected in 1988 to 1992 (Niblack et al., 1993) in Missouri. Increase of frequencies of virulent populations has also been reported from Illinois (Niblack et al., 2006). Although the frequency and level of virulence of H. glycines populations did not change on PI 88788 during the 5 years from 1997 to 2002 in Minnesota, it is expected that the level of virulence will increase and the diverse types of virulent populations will emerge with the use of the resistant cultivars in the state. In fact, increase of virulence on PI 88788 (change from race 3 to race 1) has occurred in field experimental plots after 8 years of continuously planting the same resistant cultivar Freeborn (PI 88788 resistance source) (unpublished data).
Currently, most commercial cultivars have PI 88788 as a resistance source, although a small proportion of cultivars is derived from Peking. A few cultivars derived from CystX (PI 437654 resistance source) have been released in the past few years. Because many populations can reproduce at least one female on PI 437654, the cultivars from CystX could not be immune to Minnesota H. glycines populations and will place a selection pressure on the Minnesota H. glycines populations, as do cultivars from PI 88788 and Peking. PI 90763 and PI 89722 are highly resistant to Minnesota populations and may be used in breeding cultivars for the state. PI 548316 is the least resistant to Minnesota populations and may not be a preferred resistance source for the state.
Footnotes
This research was supported by Minnesota Soybean Producers check-off funding through the Minnesota Soybean Research and Promotion Council and the Minnesota Agricultural Experiment Station. The authors thank C. Reese, D. Miller, C. Johnson, W. Gottschalk and J. Ballman for technical assistance.
This paper was edited by Steve Koenning.
Literature Cited
- Chen P.S., Qi J.S., Wang S.H., Hu Q.Y. Studies on identification and monitoring of physiologic variation of Heterodera glycines in China. Acta Phytopathologia Sinica. 2001a;31:336–341. [Google Scholar]
- Chen S.Y., Porter P.M., Orf J.H., Reese C.D., Stienstra W.C., Young N.D., Walgenbach D.D., Schaus P.J., Arlt T.J., Breiten-bach F.R. Soybean cyst nematode population development and associated soybean yields of resistant and susceptible cultivars in Minnesota. Plant Disease. 2001b;85:760–766. doi: 10.1094/PDIS.2001.85.7.760. [DOI] [PubMed] [Google Scholar]
- Golden A.M., Epps J.M., Riggs R.D., Duclos L.A., Fox J.A., Bernard R.L. Terminology and identity of infraspecific forms of the soybean cyst nematode (Heterodera glycines) Plant Disease Reporter. 1970;54:544–546. [Google Scholar]
- Kim D.G., Riggs R.D., Robbins R.T., Rakes L. Distribution of races of Heterodera glycines in the Central United States. Journal of Nematology. 1997;29:173–179. [PMC free article] [PubMed] [Google Scholar]
- Koenning S.R., Barker K.R. Survey of Heterodera glycines races and other plant-parasitic nematodes on soybean in North Carolina. Journal of Nematology. 1998;30:569–576. [PMC free article] [PubMed] [Google Scholar]
- Lehman P.S., Dunn R.A. Distribution of Florida populations of the soybean cyst nematode with previously undescribed genetic variation. Plant Disease. 1987;71:68–70. [Google Scholar]
- Lewis S.A., Drye C.E., Saunders J.A., Shipe E.R., Halbrendt J.M. Plant-parasitic nematodes on soybean in South Carolina. Journal of Nematology. 1993;25:890–894. [PMC free article] [PubMed] [Google Scholar]
- Liu X.Z., Li J.Q., Zhang D.S. History and status of soybean cyst nematode in China. International Journal of Nematology. 1997;7:18–25. [Google Scholar]
- MacDonald D.H., Noel G.R., Lueschen W.E. Soybean cyst nematode, Heterodera glycines, in Minnesota. Plant Disease. 1980;64:319–321. [Google Scholar]
- Niblack T.L., Arelli P.R., Noel G.R., Opperman C.H., Orf J.H., Schmitt D.P., Shannon J.G., Tylka G.L. A revised classification scheme for genetically diverse populations of Heterodera glycines. Journal of Nematology. 2002;34:279–288. [PMC free article] [PubMed] [Google Scholar]
- Niblack T.L., Colgrove K.B., Colgrove A.C. Soybean cyst nematode in Illinois from 1990 to 2006: Shift in virulence phenotype of field populations. Journal of Nematology. 2006;38:285. (Abstr.). [Google Scholar]
- Niblack T.L., Heinz R.D., Smith G.S., Donald P.A. Distribution, density, and diversity of Heterodera glycines in Missouri. Journal of Nematology. 1993;25:880–886. [PMC free article] [PubMed] [Google Scholar]
- Niblack T.L., Wrather J.A., Heinz R.D., Donald P.A. Distribution and virulence phenotypes of Heterodera glycines in Missouri. Plant Disease. 2003;87:929–932. doi: 10.1094/PDIS.2003.87.8.929. [DOI] [PubMed] [Google Scholar]
- Riggs R.D. Races of Heterodera glycines . Nematropica. 1988;18:163–170. [Google Scholar]
- Riggs R.D., Schmitt D.P. Complete characterization of the race scheme for Heterodera glycines . Journal of Nematology. 1988;20:392–395. [PMC free article] [PubMed] [Google Scholar]
- Ross J.P. Physiological strains of Heterodera glycines . Plant Disease Reporter. 1962;46:766–769. [Google Scholar]
- Ross J.P., Brim C.A. Resistance of soybean to the soybean cyst nematode as determined by a double-row method. Plant Disease Reporter. 1957;41:923–924. [Google Scholar]
- Schmitt D.P. Introduction. In: Schmitt D.P., Wrather J.A., Riggs R.D., editors. Biology and management of the soybean cyst nematode. Marceline, MO: Schmitt & Associates of Marceline; 2004. pp. 1–7. [Google Scholar]
- Schmitt D.P., Barker K.R. Incidence of plant-parasitic nematodes in the coastal plain of North Carolina. Plant Disease. 1987;72:107–110. [Google Scholar]
- Sikora E.J., Noel G.R. Distribution of Heterodera glycines races in Illinois. Journal of Nematology. 1991;23:624–628. [PMC free article] [PubMed] [Google Scholar]
- Willson H.R., Riedel R.M., Eisley J.B., Young C.E., Jasinski J.R., Wheeler T.A., Kauffman P.H., Pierson P.E., Stuart M.C. Distribution of Heterodera glycines in Ohio. Journal of Nematology. 1996;28:599–603. [PMC free article] [PubMed] [Google Scholar]
- Winstead N.N., Skotland C.B., Sasser J.N. Soybean-cyst nematode in North Carolina. Plant Disease Reporter. 1955;39:9–11. [Google Scholar]
- Young L.D. Survey of soybean cyst nematode races in Tennessee. Journal of Nematology. 1990;22:672–675. [PMC free article] [PubMed] [Google Scholar]
- Young L.D. Changes in the Heterodera glycines female index as affected by ten-year cropping sequences. Journal of Nematology. 1994;26:505–510. [PMC free article] [PubMed] [Google Scholar]
- Young L.D., Hartwig E.E. Cropping sequence effects on soybean and Heterodera glycines . Plant Disease. 1992;76:78–81. [Google Scholar]
- Young L.D., Hartwig E.E., Anand S.C., Widick D. Responses of soybeans and soybean cyst nematodes to cropping sequences. Plant Disease. 1986;70:787–791. [Google Scholar]