Abstract
Extracts from the plants Plantago lanceolata and P. rugelii were evaluated for toxicity to the root-knot nematode Meloidogyne incognita, the beneficial microbes Enterobacter cloacae, Pseudomonas fluorescens and Trichoderma virens, and the plant-pathogenic fungi Fusarium oxysporum f. sp. gladioli, Phytophthora capsici, Pythium ultimum, and Rhizoctonia solani. Wild plants were collected, roots were excised from shoots, and the plant parts were dried and ground to a powder. One set of extracts (10% w/v) was prepared in water and another in methanol. Treatments included extract concentrations of 25%, 50%, 75% and 100%, and water controls. Meloidogyne incognita egg hatch was recorded after 7-day exposure to the treatments, and second-stage juvenile (J2) activity after 48 hours. All extracts were toxic to eggs and J2, with P. lanceolata shoot extract tending to have the most activity against M. incognita. Numbers of active J2 remained the same or decreased in a 24-hour water rinse following the 48-hour extract treatment, indicating that the extracts were lethal. When data from water- and methanol-extracted roots and shoots of both plant species were combined for analysis, J2 tended to be more sensitive than eggs to the toxic compounds at lower concentrations, while the higher concentrations (75% and 100%) were equally toxic to both life stages. The effective concentrations causing 50% reduction (EC50) in egg hatch and in J2 viability were 44.4% and 43.7%, respectively. No extract was toxic to any of the bacteria or fungi in our assays.
Keywords: Enterobacter cloacae, Fusarium oxysporum f. sp. gladioli, Meloidogyne incognita, natural product, Phytophthora capsici, Plantago lanceolata, Plantago rugelii, plantain, Pseudomonas fluorescens, Pythium ultimum, Rhizoctonia solani, root-knot nematode, Trichoderma virens
Species of the genus Plantago (commonly known as plantain) have been used for medicinal purposes (Blumenthal et al., 2000) and have also been tested for inhibitory activity against viruses, microbes, and insects. For example, P. intermedia extracts were inhibitory to Escherichia coli (Uzun et al., 2004), extracts of P. major were active against certain viruses (Chiang et al., 2002), bacteria and fungi (Samuelsen, 2000), and a P. lanceolata line with high levels of iridoid glycosides enhanced resistance to the insect Spodoptera exigua and the fungus Diaporthe adunca (Biere et al., 2004).
Studies have also examined activity of several Plantago spp. against plant-parasitic nematodes. Plantago major reduced damage caused by Xiphinema index on grapes (Aballay et al., 2005), and extracts were nematicidal to X. americanum and X. index (Insunza et al., 2001a, 2001b) and controlled Ditylenchus dipsaci on garlic (Insunza and Valenzuela, 1995). Plantago asiatica extracts had a negative but reversible effect on activity of Meloidogyne javanica and were lethal to Pratylenchus vulnus (Ferris and Zheng, 1999). Plantago lanceolata plants reduced population densities of Mesocriconema xenoplax on peach seedlings, but also suppressed seedling growth (Whittington and Zehr, 1992).
Except for these studies, little is known about effects of naturally produced Plantago compounds on plant-parasitic nematodes. We therefore investigated two species of Plantago, P. lanceolata and Plantago rugelii. Plantago lanceolata was selected because it is a common plant with a wide geographic range, is used or is being studied as a pasture and forage plant (Skinner, 2005), and is cultivated for medicines and has exhibited some antimicrobial and nematicidal activity (Whittington and Zehr, 1992; Blumenthal et al., 2000; Biere et al., 2004). Plantago rugelii is native to North America and has not been tested for effects on nematodes or microbes, so it was selected as a little-studied species to compare with P. lanceolata.
This study was conducted to determine whether extracts from P. lanceolata and P. rugelii were toxic to Meloidogyne incognita (a major nematode pest with a wide geographic and host distribution) and to seven plant-associated microbes. The tested microbes were the beneficial bacteria Enterobacter cloacae and Pseudomonas fluorescens, the beneficial fungus Trichoderma virens, and the plant-pathogenic fungi Fusarium oxysporum f. sp. gladioli, Phytophthora capsici, Pythium ultimum, and Rhizoctonia solani. The specific objectives were to ascertain whether water- or methanol-soluble extracts from roots and shoots of P. lanceolata and P. rugelii contained compounds toxic to M. incognita eggs or J2 or to the selected microbes and to determine the effective concentration values (EC50) of plant extracts resulting in 50% reduction in activity or 50% mortality of the target organisms.
Materials and Methods
Preparation of plant extracts: For the first trial of the experiment, wild P. lanceolata and P. rugelii plants were collected on 13 and 15 September 2005, respectively, from grassy areas at the Henry A. Wallace Beltsville Area Research Center, Beltsville, MD. For both species, the plants were dug from the soil and washed, the flowering and fruiting stalks were removed, and roots were excised from shoots. The roots and shoots were dried at 60°C for 8 d and subsequently refrigerated in plastic bags at 7°C. A second set of plant material was collected and similarly processed for the second trial of the experiment approximately 2 wk after the first collection date for each species.
Dried plant parts were coarsely ground with a mortar and pestle or a Cuisinart Mini-Prep Processor (Model DLC-1BK, Cuisinart, East Windsor, NJ) and subsequently ground to a powder in a Cyclone Sample Mill (Model No. 3010–030, UDY Corporation, Fort Collins, CO). Powdered plant material was stored in a refrigerator at 7°C until use.
Soluble compounds were extracted from the powdered plant material for 24 hr at 4°C in a refrigerated incubator shaker at 100 rpm in deionized (DI) water or in methanol (10% dry weight plant material/volume liquid) in 500-ml Erlenmeyer flasks sealed with Parafilm “M” (Pechiney Plastic Packaging, Chicago, IL) and foil. After the extraction, the suspensions were filtered through eight layers of cheesecloth. Methanol was removed with a rotary evaporator (Büchi Rotary Evaporator, Model RE, Brinkman Instruments, Inc., West-bury, NY) and replaced with a volume of DI water equivalent to the methanol volume recorded after filtering through cheesecloth. All extracts were then centrifuged for 10 min at 3,046g, and the supernatants were filtered sequentially through GD/X series syringe filters (Whatman, Clifton, NJ) using a procedure modified from Meyer et al. (2004). Extracts were filtered through 1.0-μm pore size GF/B filters (and 0.45-μm filters if initial filtering was difficult) and then through sterile 0.2-μm PES filters, dilutions of the plant extracts were made with autoclaved DI water (that had also been filtered through sterile 0.2-μm PES filters), and all concentrations of extracts were filtered again through sterile 0.2-μm filters so that contaminating microbes were removed. Extracts were frozen at −15°C until use. The pH values of the water control and of all extracts were measured on sample portions that were then discarded. The pH values were not at levels toxic to nematodes and were not adjusted.
Meloidogyne incognita assays: Meloidogyne incognita race 1, originally isolated from a Maryland field, was cultured in the greenhouse on pepper (Capsicum annuum) ‘PA-136.’ Egg masses were picked from plant roots, collected in tap water, and rinsed three times with sterile DI water. Egg masses were broken apart and eggs were surface-sterilized by agitation for 3½ min in 0.5% sodium hypochlorite. The sterilized eggs were collected, refrigerated overnight at 7°C in sterile DI water, and used the next day for assays. J2 were obtained by placing sterilized eggs on a Spectra/Mesh Nylon Filter (openings 30 μm in diameter; Spectrum Laboratories Inc., Rancho Dominguez, CA) in an autoclaved storage dish. J2 that passed through the filter within 72 hr were collected and used immediately for assays.
Eight extracts, P. lanceolata and P. rugelii shoots and roots extracted in water and in methanol, were each tested against eggs and J2 at four concentrations: 25%, 50%, 75%, and 100%. The 25%, 50%, and 75% dilutions were prepared from the original undiluted extracts (prior to addition of egg or J2 suspensions), and the control was 0% (sterile DI water). Nematode eggs and J2 were suspended in water and placed into 24-well cell culture plates (Costar, Corning Incorporated, Corning, NY). Each well received 100 μl sterile DI water containing approximately 200 eggs or 50 J2 and 900 μl plant extract or sterile, filtered DI water control. Undiluted extract (900 μl) added to 100 μl egg or J2 suspension was then designated as the 100% concentration. The culture plates were incubated at 28°C, and three determinations were then made: percentage egg hatch, percentage active J2 in extracts, and percentage active J2 after water rinse. Egg hatch was determined 7 d after placement of eggs into treatments by counting the number of hatched J2 in each well. J2 activity was determined 48 hr after placement of J2 into treatments by counting mobile and immobile nematodes. To establish whether the extracts were affecting J2 viability and not just mobility, the treatments were removed after the 48-hr J2 activity counts and immediately replaced with sterile DI water (water rinse), and J2 activity was determined 24 hr later. Two trials were conducted, with five replicate wells per treatment in each trial.
Microbe assays: The seven microbes exposed to Plantago extracts were E. cloacae 501R3, F. oxysporum f. sp. gladioli 99-glad-Y2, P. capsici 599, P. fluorescens PF5, P. ultimum Pucz, R. solani R-23A, and T. virens GL3 (Howell and Stipanovic, 1980; Roberts et al., 1992; Meyer et al., 2001; Roberts et al., 2005). Bacteria were maintained on nutrient agar (NA) and fungi on potato dextrose agar (PDA) (Sigma Chemical Company, St. Louis, MO). All microbial isolates were from the culture collections of the Sustainable Agricultural Systems Laboratory or the Floral and Nursery Plants Research Unit, USDA-ARS, Beltsville, MD.
PDA and NA were both used for assays with bacteria and fungi. Agar plugs (12-mm diam.) from fungal isolates grown on NA and PDA for approximately 8 d were placed in the centers of plates containing the same medium used to produce the plug. Bacteria were grown overnight in nutrient broth and spread over the entire PDA or NA plate surface. Three discs of Whatman Chromatography #3 filter paper (10-mm diam.) were placed around the periphery of each plate. Approximately 25 μl of each plant extract was placed on two filters in each Petri dish. One filter received 25 μl of sterile water or methanol as a control. Plates were incubated at room temperature and rated daily for zones of inhibition of fungal or bacterial growth around the filter disks. There were three replicate plates for NA and for PDA for each treatment (plant extract × fungal or bacterial combination). The experiment was performed twice.
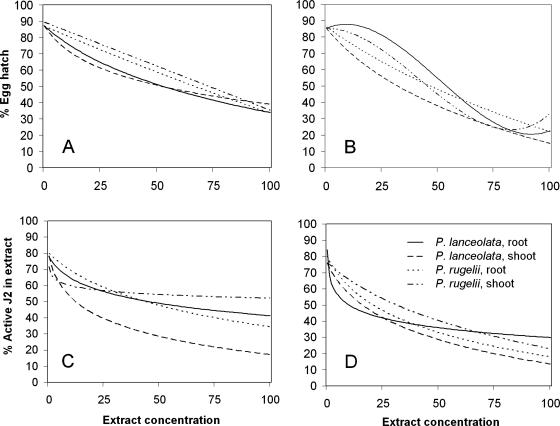
Statistical analysis: 1) Model toxicity trends. For each of the 24 observed combinations of nematode stages (eggs, J2 in extracts, and J2 rinsed in water), plant species (P. rugelii and P. lanceolata), plant parts (roots and shoots), and extraction solvents (water and methanol), the relationship between concentration and percentage hatched eggs or percentage active J2 was modeled by fitting a regression model (SAS, Proc NLIN, Cary, NC) to the 50 observed percentage hatched or percentage active values (i.e., five wells for each of two trials at each of the five observed concentrations). In each case, the appropriate regression model was a log-linear model (percentage hatched eggs or percentage active J2 = a + b*[% concentration-C]), a simple linear, or a cubic polynomial model. A single, parsimonious nonlinear analysis of covariance model was obtained (Fig. 1, models for eggs and J2 in extract treatments) by combining statistically similar parameters from the 24 regression models using SAS Proc NLMIXED (Milliken and Johnson, 2002).
Fig. 1.
Toxicity trends showing relationships among Plantago extract concentrations and egg hatch or second-stage juvenile (J2) viability of Meloidogyne incognita. The single analysis of covariance model described R2 = 84% of the total observed data variability with root mean- square-error, s = 10.28. Observed data points were 0%, 25%, 50%, 75%, and 100% extract concentrations; the concentration designated as 100% was prepared as 10% w/v and diluted 10% with nematode suspension. Percentage egg hatch determined after 7-d exposure to A) methanol extracts and B) water extracts. Percentage active J2 determined after 48-hr exposure to C) methanol extracts and D) water extracts.
2) Estimated percentage egg hatch or percentage active J2 at observed levels of each extract concentration, estimated EC50 values, compared among the 24 treatments. These estimates are influenced by the count of hatched or active juveniles in each well and by the toxicity trend models. To incorporate this influence and obtain accurate estimates, the observed data were resampled by bootstrapping procedures (Efron and Tibshirani, 1993) 500 times for each treatment, extract concentration, and trial.
A single “resampling” was defined as the repeated selection of one well from the five observed wells, replacing the selected well before repeating the selection to obtain a “resample” composed of five wells. For each “resampling” of a treatment, extract concentration, and trial: a) percentage egg hatch or percentage active J2 was calculated, b) a regression model (primarily the log-linear model with some linear, cubic, or Gompertz models) was fitted to the percentages obtained for the five extract concentrations, and c) the regression model provided a predicted percentage egg hatch or percentage active J2 at each observed extract concentration and an estimate of the EC50.
The 500 resamplings and regressions provided 500 individual percentage egg hatch or percentage active J2 estimates and EC50 estimates for each treatment and trial. These 500 estimates yielded an extensive representation of how percentage egg hatch or percentage active J2 and EC50 might vary in reality, had 2,500 wells/extract concentration been available for each trial. The average of these 500 estimates, called the bootstrap estimate (Efron and Tibshirani, 1993), was used as the accurate representation for a trial. Hence, for each treatment there were duplicate percentage egg hatches or percentage active J2 estimates at a specific extract concentration and duplicate EC50 estimates that resulted in one value per trial.
The effects of nematode stage, plant species, plant part, and extraction solvent on percentage egg hatch or percentage active J2 were analyzed at specific observed extract concentrations or on the EC50. Four-way ANOVAs with subsequent Sidak-adjusted mean comparisons were conducted, using SAS 9.1.3 Proc MIXED, on the duplicate bootstrap estimates of percentage egg hatch or percentage active J2 or EC50. The GROUP = option of the REPEATED statement was used to define and model heterogeneous within-treatment variances.
Results
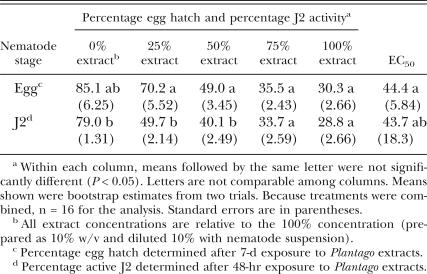
Meloidogyne incognita assays: Extracts from P. lanceolata and P. rugelii roots and shoots decreased M. incognita egg hatch and J2 activity. Mean egg hatch and J2 activity were high in the water controls (0% concentration), with values ≥ 79% (Table 1). In the 24-hr water rinse following the 48-hr water control treatment, there was a slight but significant increase to 82.7% active J2. This increase in activity may have been associated with increased aeration during rinsing and indicated that water rinsing did not negatively affect activity of healthy J2. When results from both plant species, from root and shoot extracts, and from both solvents were combined for analysis, the extracts were significantly more toxic to J2 than to eggs at the 25% concentration, with less than half of the J2 active after the 48-hr exposure to the extracts (Table 1). Percentage egg hatch and J2 activity both decreased as extract concentrations increased (Table 1), and effects on J2 activity were not reversible in water rinse after any extract treatment (data not shown). This response to the water rinse demonstrated that the extracts were lethal to J2 and were not just rendering them immobile. At the 50% extract concentration, J2 were still somewhat more affected by the treatments than eggs. However, when M. incognita eggs and J2 were exposed to 75% and 100% extract concentrations, there were no significant differences in the effects on egg hatch vs. J2 activity, and the EC50 for eggs was similar to the EC50 for J2 (Table 1).
Table 1.
Comparison of Meloidogyne incognita egg hatch and second-stage juvenile (J2) activity following treatment with extracts from Plantago lanceolata and P. rugelii, and the effective concentrations causing 50% reduction in egg hatch or 50% J2 mortality (EC50). Data from water- and methanol-extracted roots and shoots of both plant species were combined for analysis.
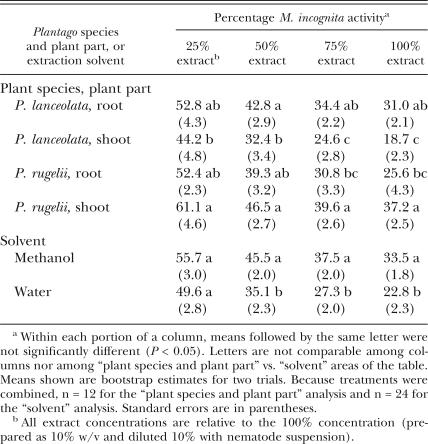
While all extracts were toxic to M. incognita, P. lanceolata shoot extracts were generally the most toxic to the nematode at all concentrations (Table 2). However, the effects were not significantly different from those recorded with extracts from P. rugelii roots. There was an overall difference in effect between the two solvents; extraction with water resulted in greater toxicity to M. incognita than methanol extraction, except at the lowest concentration.
Table 2.
Comparisons of Plantago lanceolata and P. rugelii root and shoot extracts for effects on Meloidogyne incognita egg hatch and second-stage juvenile (J2) activity (data from methanol and water extracts were combined for analysis), and separate comparison of hatch and activity in all treatments extracted in methanol vs. all treatments extracted in water. Percentages of egg hatch, J2 viability in extracts, and J2 viability following water rinse were all combined for analysis and labeled “activity.”
With a few exceptions, toxicity trends for individual extracts demonstrated that there was increasing toxicity to eggs and to J2 as extract concentrations increased (Fig. 1). However, the interactions between the extracts and the nematode did not all follow the same pattern (Fig. 1). When methanol extracts were compared with each other for effects on egg hatch, P. lanceolata shoot extract was the most toxic to egg hatch below the 50% concentration, while P. lanceolata root extract was the most toxic at the higher concentrations (Fig. 1A). Among the water extracts, P. lanceolata shoots were the most active against eggs at all concentrations (Fig. 1B). The water extracts from P. lanceolata shoots were more toxic to eggs than the corresponding concentrations of methanol extracts from P. lanceolata shoots (Fig. 1A,B). Overall, P. lanceolata shoot extract tended to be the most effective treatment for reducing egg hatch at the lowest concentrations, regardless of extraction solvent.
When methanol-extracted treatments were tested for effects on percentage J2 activity, P. lanceolata shoot extract was the most toxic to J2 at all observed concentrations (Fig. 1C). In the extracts prepared with water, P. lanceolata shoots were the most toxic treatment to J2 at concentrations above 25% (Fig. 1D), although a similar trend in activity against J2 resulted from treatment with water-extracted P. rugelii roots (Fig. 1D). Unlike the assays with eggs, P. lanceolata shoot extracts exhibited somewhat similar trends in activity against J2 in both solvents (Fig. 1C,D).
The pH of the water controls was approximately 7.10. The pH of the treatments ranged from 4.62 to 5.68, generally increasing a little as each extract was diluted.
Microbe assays: None of the plant extracts at any dilution were inhibitory to E. cloacae, F. oxysporum f. sp. gladioli, P. capsici, P. fluorescens, P. ultimum, R. solani, or T. virens under the conditions of our filter paper assay, as indicated by the lack of zones of inhibition around any of the filter papers containing the extracts.
Discussion
The eight Plantago extracts (P. lanceolata and P. rugelii roots and shoots extracted in methanol and in water) were toxic to eggs and J2 of M. incognita. A water rinse following extract treatment did not result in increased J2 activity, indicating that the extracts were lethal, rather than nematostatic. When all treatments were combined, eggs were not as strongly affected as J2 at lower extract concentrations, but both life stages were equally affected by the 75% and 100% dilutions. However, none of the Plantago extracts inhibited growth of the tested bacteria or fungi. Compounds such as the iridoid glycosides aucubin and catalpol, the aglycone aucubigenin, and caffeic acid derivatives including plantamajoside and acteoside have been isolated from Plantago spp. and have demonstrated antimicrobial activity (Blumenthal et al., 2000; Samuelsen, 2000). The chemicals extracted in our studies were not identified; they may have been toxic to nematodes and not to the tested microbes, or higher concentrations might be required to visibly affect the plant-associated microbes.
Although the Plantago extracts were toxic to M. incognita, both P. lanceolata and P. rugelii are nematode hosts. Plantago lanceolata is parasitized by Aphelenchoides ritzemabosi, D. dipsaci, and Meloidogyne sp., and P. rugelii by D. dipsaci (Buhrer et al., 1933; Buhrer, 1938; Nooij and Mook, 1992; Knight et al., 2002). The toxic compounds therefore do not result in complete protection of the plant from nematodes, although symptoms from nematodes were described as minor (Nooij and Mook, 1992).
Live P. lanceolata plants reduced population levels of M. xenoplax on peach in microplot and greenhouse studies, but not to levels considered to be economically significant (Whittington and Zehr, 1992). Some of the reduction in nematode numbers may have resulted from competition between P. lanceolata and peach plants that reduced the peach root systems. The research did not investigate effects of plant extracts, so it is not known whether or not nematicidal compounds contributed to the suppressive effects of the plantain.
Ferris and Zheng (1999) investigated effects of Plantago extracts on M. javanica and P. vulnus. Pratylenchus vulnus individuals and M. javanica J2 were placed into water extracts prepared from P. asiatica. Similar to our studies with P. lanceolata and P. rugelii extracts, P. vulnus individuals became inactive and the effect was irreversible. However, M. javanica J2 activity decreased in the extracts but the nematodes recovered. This difference from our results may be due to the Plantago species tested, to variability in root-knot nematode species responses, or both. It is also notable that M. javanica was not affected by pH from 4.0 to 8.5 (Ferris and Zheng, 1999); the lowest pH value recorded for the Plantago extracts in our study was 4.62, which should not be a factor in nematode viability.
Our study demonstrated that extracts from P. lanceolata and P. rugelii were toxic to M. incognita but not to the tested bacteria and fungi. Plantago lanceolata shoot extracts tended to be the most active against M. incognita eggs and J2, making this species/plant part combination the leading candidate for further study of nematicidal compounds. However, the compounds found in both species have potential for use in selectively targeting plant-parasitic nematodes in pest management systems. Further research is needed to isolate and identify Plantago-specific compounds, to determine their toxicity to additional plant-parasitic nematodes, and to understand the fate of these compounds in soil.
Footnotes
The authors thank Paula Crowley, Carol Masler, Laurie McKenna and Sharon Ochs for assistance in the laboratory, Don Kobayashi, Rutgers University, for Pseudomonas fluorescens PF5, and Robert McGovern, University of Florida, and Kathryn Kamo, USDA, ARS, Floral and Nursery Plants Research Unit, for Fusarium oxysporum f. sp. gladioli 99-glad-Y2.
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Literature Cited
- Aballay E, Parraguez A, Insunza V. Nematicidal evaluation of five plant species incorporated into the soil as organic matter on the population of Xiphinema index in Vitis vinifera L. cv. Thompson Seedless. Fitopatología. 2005;40:35–42. [Google Scholar]
- Biere A, Marak HB, van Damme JMM. Plant chemical defense against herbivores and pathogens: Generalized defense or trade-offs? Oecologia. 2004;140:430–441. doi: 10.1007/s00442-004-1603-6. [DOI] [PubMed] [Google Scholar]
- Plantain. In: Blumenthal M, Busse WR, Goldberg A, Gruenwald J, Hall T, Riggins CW, Rister RS, editors; Klein S, Rister RS, translators. Texas: American Botanical Council; Boston: Integrative Medicine Communications; 2000. [Google Scholar]
- Buhrer EM. Additions to the list of plants attacked by the root-knot nematode (Heterodera marioni) Plant Disease Reporter. 1938;22:216–234. [Google Scholar]
- Buhrer EM, Cooper C, Steiner G. A list of plants attacked by the root-knot nematode (Heterodera marioni) Plant Disease Reporter. 1933;17:64–96. [Google Scholar]
- Chiang LC, Chiang W, Chang MY, Ng LT, Lin CC. Antiviral activity of Plantago major extracts and related compounds in vitro. Antiviral Research. 2002;55:53–62. doi: 10.1016/s0166-3542(02)00007-4. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ. An introduction to the bootstrap, Monographs on statistics and applied probability #57. New York: Chapman & Hall; 1993. [Google Scholar]
- Ferris H, Zheng L. Plant sources of Chinese herbal remedies: Effects on Pratylenchus vulnus and Meloidogyne javanica . Journal of Nematology. 1999;31:241–263. [PMC free article] [PubMed] [Google Scholar]
- Howell CR, Stipanovic RD. Suppression of Pythium ultimum-induced damping-off of cotton seedlings by Pseudomonas fluorescens and its antibiotic, pyoluteorin. Phytopathology. 1980;70:712–715. [Google Scholar]
- Insunza V, Aballay E, Macaya J. In vitro nematicidal activity of aqueous plant extracts on Chilean populations of Xiphinema americanum sensu lato. Nematropica. 2001a;31:47–54. [Google Scholar]
- Insunza V, Aballay E, Macaya J. Nematicidal activity of aqueous plant extracts on Xiphinema index. Nematologia Mediterranea. 2001b;29:35–40. [Google Scholar]
- Insunza BV, Valenzuela A. Control of Ditylenchus dipsaci on garlic (Allium sativum) with extracts of medicinal plants from Chile. Nematropica. 1995;25:35–41. [Google Scholar]
- Knight KWL, Hill CF, Sturhan D. Further records of Aphelenchoides fragariae and A. ritzemabosi (Nematoda: Aphelenchida) from New Zealand. Australasian Plant Pathology. 2002;31:93–94. [Google Scholar]
- Meyer SLF, Huettel RN, Liu XZ, Humber RA, Juba J, Nitao JK. Activity of fungal culture filtrates against soybean cyst nematode and root-knot nematode egg hatch and juvenile mobility. Nematology. 2004;6:23–32. [Google Scholar]
- Meyer SLF, Roberts DP, Chitwood DJ, Carta LK, Lumsden RD, Mao W. Application of Burkholderia cepacia and Trichoderma virens, alone and in combinations, against Meloidogyne incognita on bell pepper. Nematropica. 2001;31:75–86. [Google Scholar]
- Milliken GA, Johnson DE. Analysis of Messy Data— Volume 3: Analysis of Covariance, Chapter 18. Florida: Chapman & Hall/CRC Press; 2002. [Google Scholar]
- de Nooij MP, Mook JH. Interactions with organisms other than plants. In: Kuiper PJC, Bos M, editors. Plantago: a multidisciplinary study. Ecological Studies 89. New York: Springer-Verlag; 1992. pp. 52–68. [Google Scholar]
- Roberts DP, Lohrke SM, Meyer SLF, Buyer JS, Bowers JH, Baker CJ, Li W, de Souza JT, Lewis JA, Chung S. Biocontrol agents applied individually and in combination for suppression of soilborne diseases of cucumber. Crop Protection. 2005;24:141–155. [Google Scholar]
- Roberts DP, Sheets CJ, Hartung JS. Evidence for proliferation of Enterobacter cloacae on carbohydrates in cucumber and pea spermosphere. Canadian Journal of Microbiology. 1992;38:1128–1134. [Google Scholar]
- Samuelsen AB. The traditional uses, chemical constituents and biological activities of Plantago major L. A review. Journal of Ethnopharmacology. 2000;71:1–21. doi: 10.1016/S0378-8741(00)00212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner RH. Cultivar and environmental effects on freezing tolerance of narrow-leaf plantain. Crop Science. 2005;45:2330–2336. [Google Scholar]
- Uzun E, Sariyar G, Adsersen A, Karakoc B, Ötük G, Oktayoglu E, Pirildar S. Traditional medicine in Sakarya province (Turkey) and antimicrobial activities of selected species. Journal of Ethnopharmacology. 2004;95:287–296. doi: 10.1016/j.jep.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Whittington DP, Zehr EI. Populations of Criconemella xenoplax on peach interplanted with certain herbaceous plants. Supplement to the Journal of Nematology. 1992;24:688–692. [PMC free article] [PubMed] [Google Scholar]