Abstract
The root-knot nematode Meloidogyne incognita is an obligate endoparasite of plant roots and stimulates elaborate modifications of selected root vascular cells to form giant cells for feeding. An Arabidopsis thaliana endoglucanase (Atcel1) promoter is activated in giant cells that were formed in Atcel1::UidA transgenic tobacco and Arabidopsis plants. Activity of the full-length Atcel1 promoter was detected in root and shoot elongation zones and in the lateral root primordia. Different 5’ and internal deletions of regions of the 1,673 bp Atcel1 promoter were each fused to the UidA reporter gene and transformed in tobacco, and roots of the transformants were inoculated with M. incognita to assay for GUS expression in giant cells and noninfected plant tissues. Comparison of the Atcel1 promoter deletion constructs showed that the region between −1,673 and −1,171 (fragment 1) was essential for Atcel1 promoter activity in giant cells and roots. Fragment 1 alone, however, was not sufficient for Atcel1 expression in giant cells or roots, suggesting that cis-acting elements in fragment 1 may function in consort with other elements within the Atcel1 promoter. Root-knot nematodes and giant cells developed normally within roots of Arabidopsis that expressed a functional antisense construct to Atcel1, suggesting that a functional redundancy in endoglucanase activity may represent another level of regulatory control of cell wall-modifying activity within nematode feeding cells.
Keywords: cellulase, cis-acting elements, giant cells, Nicotiana tabacum, parasitism, regulatory motif
The root-knot nematode, Meloidogyne incognita, is an obligate endoparasite of plant roots that has evolved a complex feeding relationship with its host (reviewed in Davis et al., 2004). The structure of giant cells induced by root-knot nematodes includes extensive cell wall modifications acting as specialized feeding sites to allow cellular expansion and solute uptake (Jones and North- cot, 1972; Hussey and Grundler, 1998; Goellner et al., 2001; Gheysen and Fenoll, 2002; Vercauteren et al., 2002). The complex morphological and physiological changes during the establishment of giant cells and other nematode feeding sites (NFS) are reflected by altered gene expression in affected root cells (Wilson et al., 1994; Gheysen et al., 1996; Williamson and Hussey, 1996; Favery et al., 1998). The coordinated temporal expression and localization of cell wall-modifying enzymes that promote wall loosening and expansion in giant cells, and concomitantly promote cell-wall thickening and extensive ingrowths at the interface of neighboring vascular elements, likely represent augmentation of regulatory machinery active during normal plant cell wall growth and maturation.
The plant cell wall is a network of cellulose microfibrils, hemicellulose, pectin, and proteins that undergoes extensive changes in architecture during plant growth and development (reviewed in Carpita and Gibeaut, 1993). During the growth process, plant cells respond to multiple internal and external signals. In many cases, the response is translated into the loosening of the cell wall to enable turgor-driven cell expansion (Crosgrove, 1999; Rose and Bennett, 1999). Targeted enzymatic digestion of cellulose microfibrils by endogenous plant endoglucanases (EGases) is a tightly regulated process that is one primary component of cell-wall loosening (Fry, 1995; Rose and Bennett, 1999). The plant EGase genes identified to date are usually expressed within different developmental stages of the plant such as elongation, ripening and abscission (Lashbrook et al., 1994; Shani et al., 1997; del Campillo, 1999; Levy et al., 2002).
The Arabidopsis thaliana EGase gene, Cel1 (Atcel1), is essential for normal plant cell growth and elongation, as it plays a role in cell wall deposition, cell differentiation, and cytogenesis (Tsabary et al., 2003). The primary activity of Atcel1 has been observed during cell elongation and within fast growing tissues (Shani et al., 1997; Nicol et al., 1998; del Campillo, 1999; Shani et al., 2000). Overexpression of Atcel1 resulted in accelerated growth of transgenic tobacco and poplar plants (Levy et al., 2002; Shani et al., 2004). Conversely, A. thaliana plants expressing Atcel1 antisense exhibit shorter stems and roots, a corrugated cell wall surface, and fewer xylem elements per bundle, and both xylem elements and the interfascicular fibers are significantly less lignified than in the wild type (Tsabary et al., 2003).
Recently published evidence shows that elevated plant EGase activity localized in NFS may be, in part, responsible for the dramatic cell wall modifications observed within NFS (Goellner et al., 2001; Mitchum et al., 2004). Increased expression of five Nicotiana tabacum EGase (Ntcel) genes was detected within NFS induced in tobacco roots by both root-knot and cyst nematodes, and differential expression levels of each of the upregulated Ntcel genes in NFS were observed by semi-quantitative PCR (Goellner et al., 2001). The full-length promoter of Atcel1 fused to GUS (Shani et al., 1997) was upregulated in giant cells induced by root-knot nematodes, but not within syncytia induced by cyst nematodes in either N. tabacum or A. thaliana host plants (Mitchum et al., 2004). The data suggest that differential expression of plant EGases gives rise to different NFS, but it is not clear how this activity may be regulated or which plant EGases are essential for proper formation of a given NFS. To this end, the activity of different deletions of the Atcel1 promoter upon plant infection by root-knot nematodes and the response of plants expressing antisense to the Atcel1 gene (Tsabary et al., 2003) to nematode infection have been investigated.
Materials and Methods
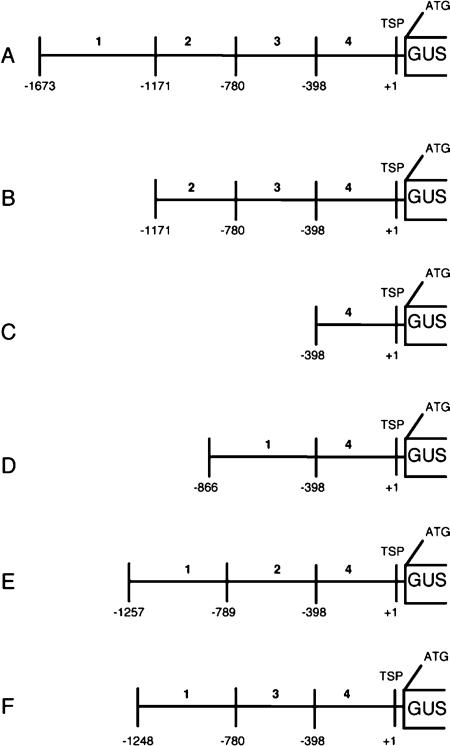
Atcel1 constructs and transgenic plants: Transgenic tobacco (N. tabacum SR1) and A. thaliana (Col-0 Ecotype) plants containing the full-length Atcel1 promoter fused to the β-glucuronidase (GUS) reporter gene were developed previously (Shani et al., 1997; Tsabary, 2003). The construct was a transcriptional fusion between 1.6 kilobases (kb) of the putative Atcel1 promoter region (bases 5–1,618; Genbank accession X98543) and the 5’ end of the β-glucuronidase gene (UidA) (Jefferson, 1987; Shani et al., 1997). The Atcel1 promoter was divided into four 382 to 468 bp fragments (Fig. 1A), and six promoter::GUS constructs containing one to three fragments each were developed.
Fig. 1.
Schematic representation of Atcel1 promoter-UidA constructs in transformed tobacco plants analyzed for tissue expression and response to nematode infection. A) The full-length 1,673 bp Atcel1 promoter, B-F) 5’ Atcel1 promoter deletion constructs harboring different lengths of the promoter (serial B and C, internal D, E, and F). Numbers indicate the length in bp of the respective promoter regions. Coding region of the β-glucuronidase gene. TSP transcription starting point, 1 to 4 indicate respective excised promoter regions.
To facilitate subcloning, each promoter segment was amplified using primers that contain restriction sites for the enzymes Hind III, Nde I and Sal I. The primers used were as follows: Fragment 1) 5’-AAAAAAGCTTACCTGCAGGTCAACGG-3’ and 5’-AAAACATATGTTCATTTAGTATATAACAAAATTCG-3’; Fragment 2) 5’-ATTTAAGCTTACACCATATGAAATGAACATTTGCTCTGATTTGG-3 and 5’-AAAACATATGATTATTATATACTTTTTTTTTTATAAAAG-3’; Fragment 3) 5’-AAAAAAGCTTAAAACATATGTATATAATAATTTACACTCGAATC-3’ and 5’- TGTGCATATGCTCAATAGTTGATTTTTGGAGG-3’; Fragment 4) 5’-AAAAAGTTAAATCATATGGAGATCAAAACACGTGTCGC-3’ and 5’-CCCCGTCGACGTCTCTTCTTTCTTGTGC-3’. The PCR reactions were performed using thermal cycling conditions of 94°C for 4 min, 30 cycles of 94°C for 10 sec, 55°C for 10 sec, and 72°C for 10 sec, and 72°C for 4 min using a buffer containing 1 Unit of DeepVent Taq polymerase (New England Biolabs, Inc., Beverly, MA), 20mM Tris-HCl pH 8.8, 10mM KCl, 10mM (NH4)2SO4, 2mM MgSO4, 0.1% Triton X-100, 200μM dNTPs, 10 pmol primer and 1ng DNA template. The plasmid pUC18, containing a Sal I/Eco RI genomic fragment harboring the Atcel1 promoter (Shani et al., 1997), was used as the template.
The fragments were purified, digested with different digestion enzymes (Fermentas Inc., Hanover, MD) and cloned, in various combinations, into pUC18 to create the promoter segment combinations shown in Figure 1. The promoter constructs were then digested with Hind III and Sal I and cloned into the binary vector pBinPlus, which contained the UidA.
The vectors were introduced into Agrobacterium tumefaciens using the freeze-thaw method (Erbert et al., 1988) and then transformed into tobacco. The constructs were then transformed into N. tabaccum-SR1 plants using the leaf-disc transformation as described previously (Horsch et al., 1985). Kanamycin-resistant plants were regenerated and confirmed by PCR. T2 homozygote plants were selected for further analysis.
Cel1 antisense Arabidopsis plants were previously developed and characterized by Tsabary et al. (2003). The construct contains bases 1 to 403 bp of the Atcel1 coding region inserted in reverse orientation into the vector pBI101.1 containing the CaMV 35S RNA promoter and the octopine polyadenylation site (Tsabary et al., 2003).
Seeds of transgenic Cel1-UidA tobacco and Arabidopsis, Cel1 antisense Arabidopsis, and nontransformed seeds were surface disinfected with 2.5% NaOCl and 0.5% sodium dodecyl sulfate (SDS) for 10 min, rinsed four times with sterile water, and then germinated and grown monoxenically in petri plates containing 0.8% Noble agar (Fisher Scientific, Pittsburgh, PA) supplemented with MS basal medium (Murashige and Skoog, 1962), pH 5.8, sucrose (30 g/liter), and kanamycin (50 ug/ml). Tobacco and Arabidopsis seedlings were grown in a controlled temperature growth chamber at 25°C with a 16-hr photoperiod. At least five independent kanamycin-resistant lines were analyzed for each transgenic construct.
Nematode Infection: The root-knot nematode, M. incognita, was propagated on roots of greenhouse-grown tomato (Lycopersicon esculentum cv. Rutgers). Meloidogyne incognita eggs were isolated from egg masses on tomato roots with 0.5% NaOCl (Hussey and Barker, 1973), surface disinfected in a solution of 0.02% sodium azide for 30 min, rinsed with water on a 25-μm-opening sieve, and hatched in water at 28°C on a Baermann pan (Mit-chum et al., 2004). Hatched M. incognita J2 were surface sterilized in 0.002% HgCl2, 0.002% NaN3, and 0.001% triton X-100 for 10 min, followed by five washes with sterile water. Surface-sterilized J2 were resuspended in 50 μl of 2 mM penicillin-G and 950 μl of 0.1% water agar immediately prior to inoculation of roots of plants grown on sterile nutrient agar. Five-microliter aliquots of M. incognita J2 were used to inoculate 10- to 12-day-old tobacco root tips and 10-day-old Arabidopsis root tips grown in monoxenic culture at a concentration of 15 J2/μl and 100 J2/5 μl, respectively. Penetration of roots by J2 was monitored using an inverted light microscope. Infected and noninfected transgenic root tissues were excised from petri dishes at specific time points after penetration of roots by J2. For all the time points examined, at least 100 infected and 30 uninfected transgenic roots were assayed for nematode infection and GUS expression. Promoter activity was also monitored in the root elongation zone and giant cells of control plants harboring the Δ0.6 TobRB7 promoter-UidA construct (Yamamoto et al., 1991) treated similarly as a positive control.
Histochemical GUS analysis: β-glucuronidase (GUS) activity was monitored by the method of Jefferson (1987) with some modifications (Yamaguchi et al., 2001). Fresh, excised root pieces were vacuum-infiltrated for 5 min with GUS substrate (2 mM 5-bromo-4-chloro-3- indolyl β-D-glucuronide [X-Gluc], 100 mM Tris, pH 7.0, 50 mM NaCl, 0.06% Triton X-100) and incubated 12 hr at 37°C. In leaf samples excised to confirm construct (GUS) expression in experimental lines, chlorophyll pigmentation was removed by incubation of the samples for approximately 1 hr in 90% (v/v) ethanol. Samples stained for GUS activity were placed in 70% ethanol for long-term storage at 4°C.
Tobacco and Arabidopsis plants harboring the Atcel: GUS construct were analyzed for GUS expression following infection of host roots by M. incognita at 3, 4, 7, 14, 21 and 28 dpi in five to seven independent transformed lines for which 10 to 15 seedlings were assayed.
Histology of nematode feeding cells: Prior to sectioning, stained root pieces were fixed at 4°C in 4% paraformaldehyde for 16 hr for Arabidopsis and 3 d for tobacco, washed twice in PBS for 15 min after fixation, dehydrated in a graded ethanol series (30 min each), incubated sequentially in Histoclear (National Diagnosis, Atlanta, GA):ethanol 25:75, 50:50, 75:25, and then in 100% Histoclear twice for 30 min each time (Goellner et al., 2001). The root pieces were incubated in Histoclear:Paraplast Plus (Fisher Scientific) 75:25 overnight at 60°C and then overnight in pure Paraplast at 60°C. The Paraplast-embedded root pieces were sectioned to a thickness of 30 μm for tobacco and 10 μm for Arabidopsis using an a rotary microtome (American Optical, Buffalo, NY) and adhered to Superfrost Plus microscope slides (Fisher Scientific) overnight at 40°C on a slide warmer. Three 15 min incubations in Histoclear were used to remove the Paraplast from sections adhered to slides, followed by rehydration in a graded ethanol series to water prior to mounting with Per-mount (Fisher Scientific). For each time point, 15 to 30 infected roots were analyzed for GUS staining, and an equal number of uninoculated roots were analyzed for comparison.
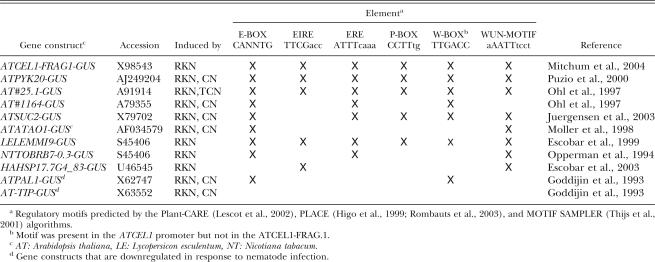
Computational analyses of the Atcel1 promoter sequence: For putative motif analysis of the Atcel1 promoter, we utilized the results of the Plant-CARE (Lescot et al., 2002) (http://oberon.fvms.ugent.be:8080/PlantCARE/index.html) and PLACE (Higo et al., 1999) (http://www.dna.affrc.go.jp/htdocs/PLACE/signalscan.html) algorithms as described by Rombauts et al. (2003). To detect novel common regulatory elements in multiple promoters, the MOTIF SAMPLER algorithm (Thijs et al., 2001) was used (http://www.esat.kuleuven.ac.be/∼thijs/Work/MotifSampler.html). Consensus motifs identified using MOTIF SAMPLER were subsequently compared with the regulatory sites described in the Plant-CARE and PLACE databases.
Cel1 antisense A. thaliana: The development of M. incognita females in roots of A. thaliana expressing the antisense Atcel1 construct (Tsabary et al., 2003) were compared to M. incognita development in roots of wild-type A. thaliana. Gross shoot and root morphology was compared to published descriptions to confirm the reported Atcel1 phenotype (Tsabary et al., 2003) in test plants. The cellular morphology of infection sites in nematode-infected antisense roots as compared to wild-type was also evaluated using the fixation, embedding, and sectioning procedures described above. Sections were stained using Johansen's safranin/fast green protocol (Johansen, 1940) with some modifications (Ruzin, 1999) to enhance observable differences among cells and tissues. Photomicrographs of specimens were taken using a Nikon eclipse E600 microscope (Nikon Instruments, Melville, NY) equipped with RT-color SPOT camera (Diagnostic Instruments, Sterling Heights, MI).
Results
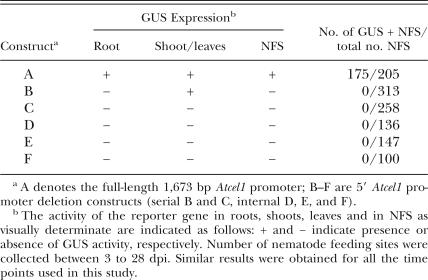
Impact of Atcel1 promoter deletions on tissue-specific and nematode-induced expression: Tobacco plants transformed with the full-length or a truncated Atcel1 promoter-UidA constructs were analyzed for promoter activity in uninfected plant tissue and within giant cells (Table 1). Five to seven independent transformed lines and at least 10 plants per transformed line were tested for each promoter construct. At least 100 tissue samples, including samples from each independent transformed line, were assayed for each promoter construct. Histochemical as-says of β-glucuronidase (GUS) expression were used to analyze the temporal and spatial characteristics of the Atcel1 promoter activity. During plant development, the expression of the full-length Atcel1 promoter-UidA construct was observed in shoot and root elongation zones of infected and uninfected plants (Construct A, Figs. 1,2A-B, Table 1). The expression of the full-length Atcel1 promoter-UidA construct was not induced by mechanical wounding of the roots or leaves (data not shown). Low expression of construct B (Figs. 1,2C-D, Table 1), harboring a 502 bp 5′ deletion (promoter fragment 1), was observed in tobacco shoot elongation zones, but not in the roots. There was no obvious variation in GUS expression patterns among the transformed lines containing the same promoter construct, although slight variations in expression intensity could be observed. GUS activity was never observed in plants with constructs C to F, even though constructs D to F contained promoter fragment 1 (Fig. 1, Table 1). GUS activity was observed in the root elongation zone and giant cells of control plants harboring the Δ0.6 TobRB7 promoter-UidA construct (Opperman et al., 1994), treated similarly as a positive control (data not shown).
Table 1.
Activity of the Atcel1 promoter-UidA constructs in roots, shoot, leaves and root-knot nematode feeding sites (NFS).
Fig. 2.
Histochemical staining for GUS activity in transgenic tobacco plants containing the Atcel1 promoter infected by the root-knot nematode, Meloidogyne incognita. A) Atcel1-driven GUS expression in a 7-day-old uninfected tobacco root. B) Atcel1-driven GUS expression in a shoot tip of a young uninfected tobacco seedling. C) Atcel1 promoter deletion construct B (harboring a 502 bp 5’ deletion, promoter fragment 1)-driven GUS expression in the shoot elongation zone of an uninfected tobacco plant. No activity is detectable in the roots. D) Whole-mount histochemical GUS assay of a Cel1-transgenic tobacco plant infected with M. incognita. Atcel1 activity is confined to the nematode feeding cells (not shown) and the plant elongation/ differentiation zones. A = shoot meristem (Construct B shown). E) Atcel1-driven GUS expression within M. incognita-induced giant cells four days post-inoculation of nematodes to an Atcel1-GUS transgenic tobacco root (construct A, Fig. 1). GUS activity is confined to the central region of the developing gall tissue. F) Sections (30 μm thick) through M. incognita-infected Atcel1-GUS tobacco roots after GUS staining. GUS expression is restricted to the giant cells induced by the nematode. N = nematode, GC = giant-cells,
We previously demonstrated that the full-length Atcel1 promoter could drive UidA gene expression within giant-cells three days after nematode infection of Arabidopsis plants by root-knot nematodes (Mitchum et al., 2004). Upregulation of the full-length Atcel1-UidA construct (construct A) was observed within giant cells induced by root-knot nematodes in tobacco plants (Table 1, Fig. 2E-F) between 4 to 28 dpi. Similar results were obtained for all the time points used in this study. Frequently, full-length Atcel1-driven GUS activity was also visible early in the lateral root primordia, even in roots distant from the nematode feeding site. In contrast to full-length Atcel1, in all deletions in the Atcel1 promoter assayed, including construct B, no detectable activity within the giant cells induced by M. incognita was observed (Fig. 1, Table 1).
Deletion of fragment 1 (−1,171 to −1,673) of the Atcel1 promoter abolished expression in both giant cells and uninfected roots in all constructs examined. To determine whether all the motifs needed for Atcel1 expression in roots and in NFS were in fragment 1 of the Atcel1 promoter (Fig. 1), several internal deletions between fragments 1 and 4 were examined (Fig. 1D-F; Table 1). No internal Atcel1 promoter deletions that included fragment 1 were sufficient for GUS expression in NFS or roots.
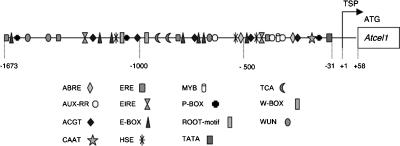
Identification of conserved sequence motifs in the Atcel1 promoter: Because the Atcel1 promoter is upregulated within giant cells induced by root-knot nematode, we analyzed the promoter for specific sequences that act as nematode responsive cis-acting elements that could be responsible for the observed GUS gene expression. Plant-CARE, PLACE, and MOTIF SAMPLER analyses of the 1,673 bp Atcel1 promoter sequence revealed a number of predicted functional motifs found in most eukaryotic promoters, in addition to several potential regulatory elements that have been shown to be functional in other plant promoters (Fig. 3). A typical TATA box was identified at position −31, and a CAAT box-like sequence was found at position −50 relative to its transcription start point (TSP), respectively. The upstream sequence relative to the TSP of the Atcel1 has several regions with over 80% A/T content (data not shown).
Fig. 3.
Putative cis-acting elements of the Atcel1 promoter as predicted by the Plant-CARE (Lescot et al., 2002), PLACE (Higo et al., 1999; Rombauts et al., 2003), and MOTIF SAMPLER (Thijs et al., 2001) algorithms. The transcription start point (TSP) is indicated with +1: transcription start point. Distances in bp are relative to the translation start codon.
To evaluate sequence motifs that may be common among promoters of NFS-expressed genes, we compared motifs found in the Atcel1 promoter to those of other genes known to be upregulated in NFS. Included were characterized promoters of five other Arabidopsis genes, in addition to Atcel1, that are known to be up- regulated in NFS, as well as three NFS-responsive promoters from other plant species (Table 2). In particular, the 300 bp ‘nematode box’ from the tobacco TobRB7 promoter and a 246 bp fragment from the Hahsp17.7G4 sunflower promoter were included because functional studies have definitively linked these minimal nematode-inducible sequences with NFS-specific expression. Two Arabidopsis promoters reported to be down regulated in NFS were included as negative controls.
Table 2.
Putative regulatory elements common between the Atcel1 promoter (accession X98543) and other plant promoters activated and downregulated in feeding cells induced by root-knot nematodes (RKN) and cyst nematodes (CN).
Analysis of the promoter regions using MOTIF SAMPLER revealed the presence of several putative regulatory elements that were previously reported in NFS upregulated genes, including E-BOX, AUX-RR, ROOT-MOTIF, and W-BOX (Fenoll et al., 1997; Escobar et al., 1999; Puzio et al., 2000; Mazarei et al., 2002; Thurau et al., 2003). All of these sequences were present in one or both of the promoter sequences from the NFS downregulated genes, suggesting that they do not, in fact, represent NFS-specific transcription factor binding sites. Four motifs (EIRE, ERE, P-BOX, and WUN-MOTIF) were present in the NFS upregulated promoter sequences but not in the downregulated ones (Table 2).
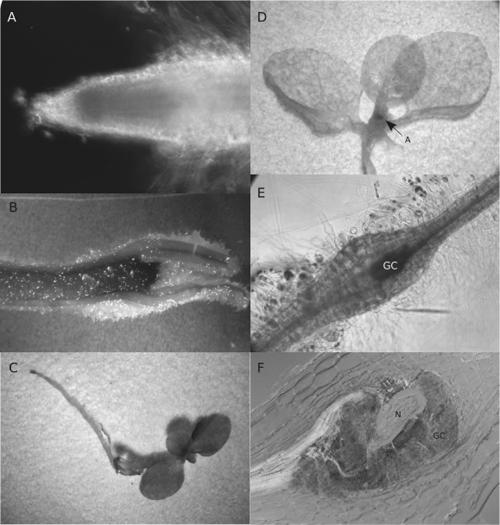
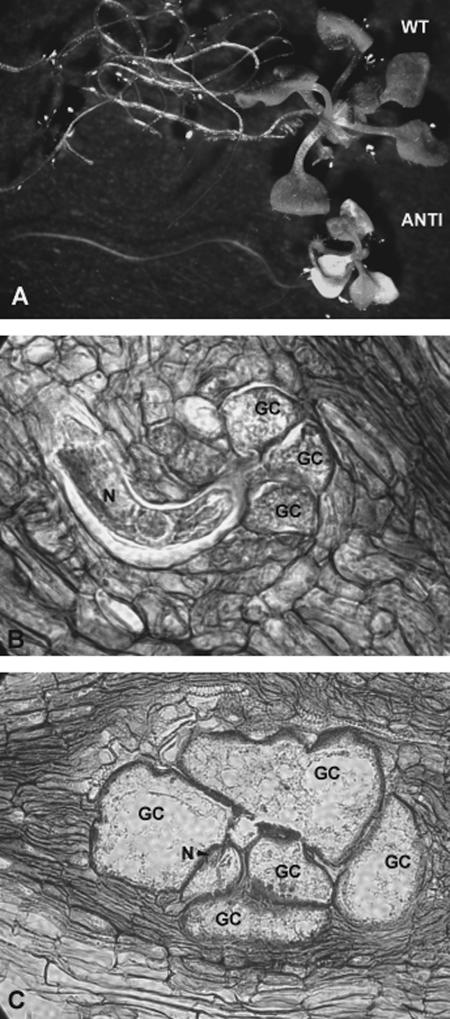
Nematode infection of antisense Atcel1 plants: To further investigate the potential function of Atcel1 during root-knot nematode infection, three Arabidopsis lines expressing an antisense Cel1 construct (Tsabary et al., 2003) were infected with root-knot nematode. Samples of infected and uninfected plant roots were collected at 3, 4, 7, 14, 21 and 28 dpi. Samples were fixed, sectioned, stained, and examined for general root morphology as well as the ability for M. incognita to induce giant-cell development. Uninfected A. thaliana plants containing the Atcel1 antisense construct exhibited the same alterations in shoot and root morphology as previously reported (Tsabary et al., 2003). The Atcel1 antisense plants had shorter stems and roots relative to the wild-type plants (Fig. 4A), indicating that the antisense construct was actively expressed.
Fig. 4.
Constitutive expression of antisense Atcel1 in transgenic A. thaliana and infection of these plant roots with the root-knot nematode, M. incognita. A) Both shoot and root development of Atcel1 antisense (ANTI) plants are compromised as compared to wild-type (WT) Arabidopsis. B) Giant cells (GC) form normally around the head of a developing root-knot nematode (N) in a (10-μm-thick) cross- section of a wild-type Arabidopsis root. C) Giant cell and nematode development in antisense Atcel1 Arabidopsis progress normally even as root and shoot development are compromised. Abbreviations are defined in the text and in Table 2.
Microscopic examination of stained root sections revealed a typical pattern of M. incognita infection and development, as well as typical giant cell formation (Fig. 4B,C). Similar patterns of nematode and giant-cell development were observed in both the wild-type and in the Atcel1 antisense constructs. Similar numbers of NFS developed in wild-type and in the Atcel1 antisense infected plants (1,151 galls/50 plants in wild type vs. 1,185 galls/50 plants in antisense plants; data from two repetitions). Because sections from both wild-type Arabidopsis (Fig. 4B) and Atcel1 antisense Arabidopsis (Fig. 4C) revealed that M. incognita J2 penetrated roots of both plant types equally and that giant cell and nematode development were comparable, we did not count numbers of adult female nematodes. However, we did observe that the nematodes completed their life cycle in the antisense plants.
Discussion
The observed upregulation of the Atcel1 promoter within giant cells induced in roots by root-knot nematodes and the lack of this activity within the feeding sites of cyst nematodes suggested potential transcriptional regulation of Atcel1 expression upon nematode infection (Mitchum et al., 2004). In this study, a comparative analysis of Atcel1 promoter deletion constructs demonstrates that the region between −1,673 and −1,171 (fragment 1) was essential to provide specificity of Atcel1 promoter expression in roots and giant cells. It is unclear if elements within fragment 1 of the Atcel1 promoter that are required for expression within roots are also required for expression within giant cells. Some analyses of gene expression within giant cells (Wilson et al., 1994; Gheysen and Fenoll, 2002) indicate that wild-type expression in roots was not a prerequisite for plant genes recruited during giant-cell formation. The expression of Atcel1 promoter construct B (containing a deletion of fragment 1) in shoots observed here, however, was uncoupled from expression in giant cells and roots. It has been demonstrated with promoter deletions of the TobRB7 gene of tobacco that elements that drive root-specific expression can be un-coupled from elements that can drive expression specifically within giant cells (Opperman et al., 1994). Similarly, the −83 to +163 region of the Hahsp17.7G4 gene in sunflower that contains heat shock element core sequences was sufficient to drive expression within giant cells (Escobar et al., 2003). Inclusion of fragment 1 of the Atcel1 promoter in the absence of internal regions within the Atcel1 promoter constructs analyzed here, however, indicates that the presence of a fragment 1 alone within Atcel1 is not sufficient to drive expression within giant cells. The effects may be due simply to the relative change in distance and conformation between upstream and downstream elements. In general, the function of a regulatory region is complex, involving a multiprotein complex interacting with the transcription factors bound to neighboring DNA sites. A supplementary layer of complexity is added by bringing the transcription factors together on the promoter and by adopting a three-dimensional configuration, enabling the interaction with other parts to activate the basal transcription machinery (Buratowski, 2000; Rombauts et al., 2003).
Alternatively, the activity of other functional elements within the Atcel1 promoter may also be required for both the root and giant-cell expression. A number of conserved sequence motifs that may represent transcription factor binding sites were found within the Atcel1 promoter as well as in promoter regions of other nematode induced genes. Interestingly, these motifs include WUN-motif (Van de Loecht et al., 1990; Washida, et al., 1999) and EIRE (Shah and Klessig, 1996; Fuduka, 1997), both of which have been reported in genes that are transcriptionally activated in response to pathogen- derived elicitors. The ethylene responsive element (ERE) is found in many pathogenesis-related (PR) genes that are activated during nematode infection (Schwechheimer et al., 1998; Mazarei et al., 2002). The ERE motif was also found in within the Atcel1 promoter, but not in the promoter regions of nematode-repressed genes. Whether these motifs have specific roles in transcriptional modulation of Atcel1 remains to be determined; however, they represent candidates for further functional analyses using directed mutagenesis and/or deletions.
Regulation of plant endoglucanase expression at the transcriptional level may be only one level of control of cell wall-modifying activity induced by nematodes within feeding cells. The inability of a functional Atcel1 antisense to affect giant-cell or nematode development here suggests that Atcel1 activity may not be essential for proper giant-cell formation and/or that functional redundancy in induced endoglucanase activity within NFS exists. In Arabidopsis, the EGase gene family comprises more than 20 members (del Campillo, 1999; Tsabary et al., 2003). Potential functional redundancy is supported by the upregulation of at least five tobacco endoglucanase genes within the feeding cells of both root-knot and cyst nematodes (Goellner et al., 2001). The tobacco endoglucanases upregulated in NFS are phylogenetically distinct, however, and may represent functional differences in both normal plant development and activity within NFS. It remains to be investigated whether an endoglucanase essential to the formation of NFS can be identified.
These results indicate that expression of the Atcel1 promoter in NFS is regulated by the combinatorial interactions of cis-acting regulatory elements in the promoter, including essential element in the distal region of the promoter. The multiple putative cis-acting elements (Fig. 3) of the Atcel1 promoter accommodate the argument that they may act as coupling elements that may function in different combinations to confer a diversity of tissue-specific, developmental, and stress-regulated patterns. The promoter deletions examined did not result in restricting activity to giant cells as observed with the Δ0.3 TobRB7 element (Opperman et al., 1994). Further work, including the generation of a fine- scale series of 5’ promoter deletions within fragment 1 in combination with linker scanning and or/site- directed mutagenesis will be required to precisely define, if possible, given cis-elements within the Atcel1 promoter that convey a specific response in NFS. This potential has important implications for strategies to engineer nematode resistance. Nematode-responsive promoters may be used to localize the expression of anti-nematode constructs that interfere with the development of the feeding site and/or nematode specifi-cally to nematode infection sites (Atkinson, 2003). When targeting plant endoglucanase genes for inhibition within NFS, one must consider potential functional redundancy of endoglucanase activity within NFS as well as whether the target gene is essential to the success of NFS formation.
Footnotes
This research was supported by the North Carolina-Israel Partnership/Binational Science Foundation under grant 1999011, the North Carolina Tobacco Research Commission under grant 666382, and the North Carolina Agricultural Research Service. We thank C. H. Opperman for supplying Δ0.6 TobRB7 tobacco and X. Wang for technical assistance. We also thank M. Thon and Jim Starr for review of this manuscript.
This paper was edited by: Andrea Skantar
Literatured Cited
- Atkinson HJ, Urwin PE, McPherson MJ. Engineering plants for nematode resistance. Annual Review of Phytopathology. 2003;41:615–639. doi: 10.1146/annurev.phyto.41.052002.095737. [DOI] [PubMed] [Google Scholar]
- Buratowski S. Snapshots of RNA polymerase II transcription initiation. Current Opinion in Cell Biology. 2000;12:320–325. doi: 10.1016/s0955-0674(00)00095-8. [DOI] [PubMed] [Google Scholar]
- Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants—consistency of molecular structure with the physical properties of the walls during growth. Plant Journal. 1993;3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. Enzymes and other agents that enhance cell wall extensibility. Annual Review of Plant Physiology and Plant Molecular Biology. 1999;50:391–417. doi: 10.1146/annurev.arplant.50.1.391. [DOI] [PubMed] [Google Scholar]
- Davis EL, Hussey RS, Baum TJ. Getting to the roots of parasitism by nematodes. Trends in Parasitology. 2004;20:134–141. doi: 10.1016/j.pt.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Del Campillo E. Multiple endo-1,4-beta-D-glucanase (cellulase) genes in Arabidopsis . Current Topic in Develomental Biology. 1999;46:39–61. doi: 10.1016/s0070-2153(08)60325-7. [DOI] [PubMed] [Google Scholar]
- Erbert PR, Mitra A, Ha SB. Binary vectors. In: Gelvin SB, Schilperoot RA, Verma DPS, editors. Plant Molecular Biology Manual. Dordrecht: Kluwer Academic Publishers; 1988. pp. 1–19. [Google Scholar]
- Escobar C, Barcala M, Portillo M, Almoguera C, Jordano J, Fenoll C. Induction of the Hahsp17.7G4 promoter by root-knot nematodes: Involvement of heat-shock elements in promoter activity in giant cells. Molecular Plant-Microbe Interactions. 2003;16:1062–1068. doi: 10.1094/MPMI.2003.16.12.1062. [DOI] [PubMed] [Google Scholar]
- Escobar C, De Meutter J, Aristizabal FA, Sanz-Alferez S, del Campo FF, Barthels N, Van der Eycken W, Seurinck J, Van Montagu M, Gheysen G, Fenoll C. Isolation of the LEMMI9 gene and promoter analysis during a compatible plant-nematode interaction. Molecular Plant-Microbe Interactions. 1999;12:440–449. doi: 10.1094/MPMI.1999.12.5.440. [DOI] [PubMed] [Google Scholar]
- Favery B, Lecomte P, Gil N, Bechtold N, Bouchez D, Dalmasso A, Abad P. RPE, a plant gene involved in early developmental steps of nematode feeding cells. EMBO Journal. 1998;17:6799–6811. doi: 10.1093/emboj/17.23.6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoll C, Aristizabal FA, Sanz-Alferez S, del Campo FF. Regulation of gene expression in feeding sites. In: Fenoll C, W Grundler FM, Ohl SA, editors. Cellular and Molecular Aspects of Plant-Nematode Interactions. Dordrecht: Kluwer Academic Publishers; 1997. pp. 133–149. [Google Scholar]
- Fry SC. Polysaccharide-modifying enzymes in the plant cell wall. Annual Review of Plant Physiology and Plant Molecular Biology. 1995;46:497–520. [Google Scholar]
- Fuduka Y. Interaction of tobacco nuclear protein with an elicitor-responsive element in the promoter of a basic class I chitinase gene. Plant Molecular Biology. 1997;34:81–87. doi: 10.1023/a:1005737128339. [DOI] [PubMed] [Google Scholar]
- Gheysen G, Fenoll C. Gene expression in nematode feeding sites. Annual Review of Phytopathology. 2002;40:191–219. doi: 10.1146/annurev.phyto.40.121201.093719. [DOI] [PubMed] [Google Scholar]
- Gheysen G, Van der Eycken W, Barthels N, Karimi M, Van Montagu M. The exploitation of nematode-responsive plant genes in novel nematode control methods. Pesticide Sciences. 1996;47:95–101. [Google Scholar]
- Goellner M, Wang X, Davis EL. Endo-β-1, 4- glucanase expression in compatible plant-nematode interactions. Plant Cell. 2001;13:2241–2255. doi: 10.1105/tpc.010219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Korenaga T. Plant cis-acting regulatory DNA elements (PLACE) database. Nucleic Acids Research. 1999;27:297–300. doi: 10.1093/nar/27.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsch RB, Fry JE, Hoffman NL, Eichholtz D, Rogers SG, Fraley RT. A simple and general-method for transferring genes into plants. Science. 1985;227:1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Hussey RS, Barker KR. A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Disease Reporter. 1973;57:1025–1028. [Google Scholar]
- Hussey RS, Grundler FM. Nematode parasitism of plants. In: Perry RN, Wright DJ, editors. The Physiology and Biochemistry of Free-living and Plant-Parasitic Nematodes. Wallingford: CABI Publishers; 1998. pp. 213–243. [Google Scholar]
- Jefferson RA. Assaying chimeric genes in plants: The GUS gene fusion system. Plant Molecular Biology Reporter. 1987;5:387–405. [Google Scholar]
- Johansen DA. Plant Microtechnique. New York: McGraw-Hill; 1940. Staining Procedures; pp. 65–94. [Google Scholar]
- Jones MGH, Northcot DH. Multinucleate transfer cells induced in coleus roots by the root-knot nematode, Meloidogyne arenaria . Protoplasma. 1972;75:381–395. [Google Scholar]
- Juergensen K, Scholz-Starke J, Sauer N, Hess P, van Bel AJE, Grundler FMW. The companion cell-specific Arabidopsis disaccharide carrier AtSUC2 is expressed in nematode-induced syncytia. Plant Physiology. 2003;131:61–69. doi: 10.1104/pp.008037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashbrook CC, Gonzales-Bosch C, Bennett AB. Two divergent endo-β-1,4-glucanase genes exhibit overlapping expression in ripening fruit and abscising flowers. Plant Cell. 1994;6:1485–1493. doi: 10.1105/tpc.6.10.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescot M, Déhais P, Moreau Y, De Moor B, Rouzé P, Rombauts S. PlantCARE: A database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Research. 2002;30:325–327. doi: 10.1093/nar/30.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy I, Shani Z, Shoseyov O. Modification of polysaccharides and plant cell wall by endo-1,4-glucanase and cellulose-binding domains. Biomolecular Engineering. 2002;19:17–30. doi: 10.1016/s1389-0344(02)00007-2. [DOI] [PubMed] [Google Scholar]
- Mazarei M, Puthoff DP, Hart JK, Rodermel SR, Baum TJ. Identification and characterization of a soybean ethylene-responsive element-binding protein gene whose mRNA expression changes during soybean cyst nematode infection. Molecular Plant-Microbe Interactions. 2002;15:577–586. doi: 10.1094/MPMI.2002.15.6.577. [DOI] [PubMed] [Google Scholar]
- Mitchum M, Sukno S, Shani Z, Shoseyov O, Davis EL. The promoter of the Arabidopsis thaliana cel1 Endo-1, 4-β-glucanase gene is differentially expressed in plant feeding cells induced by root-knot and cyst nematodes. Molecular Plant Pathology. 2004;5:175–181. doi: 10.1111/j.1364-3703.2004.00216.x. [DOI] [PubMed] [Google Scholar]
- Moller SG, McPherson MJ. Developmental expression and biochemical analysis of the Arabidopsis atao1 gene encoding an H2O2-generating diamine oxidase. The Plant Journal. 1998;1:781–791. doi: 10.1046/j.1365-313x.1998.00080.x. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog FK. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plan-tarum. 1962;15:473–497. [Google Scholar]
- Nicol F, His I, Jauneau A, Vernhettes S, Canut H, Hofte H. A plasma membrane-bound putative endo-1,4-beta-D- glucanase is required for normal wall assembly and cell elongation in Arabidopsis . EMBO Journal. 1998;17:5563–5576. doi: 10.1093/emboj/17.19.5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohl SA, van der Lee FM, Sijmons PC. Anti-feeding structure approaches to nematode resistance. Regulation of gene expression in feeding sites. In: Fenoll C, Grundler FMW, Ohl SA, editors. Cellular and Molecular Aspects of Plant-Nematode Interactions. Dordrecht: Kluwer Academic Publishers; 1997. pp. 250–261. [Google Scholar]
- Opperman CH, Taylor CG, Conkling MA. Root-knot nematode directed expression of a plant root-specific gene. Science. 1994;263:221–223. doi: 10.1126/science.263.5144.221. [DOI] [PubMed] [Google Scholar]
- Puzio PS, Lausen J, Heinen P, Grundler FM. Promoter analysis of pyk20, a gene from Arabidopsis thaliana . Plant Sciences. 2000;157:245–255. doi: 10.1016/s0168-9452(00)00287-9. [DOI] [PubMed] [Google Scholar]
- Rombauts S, Florquin K, Lescot M, Marchal K, Rouzé P, Van de Peer Y. Computational approaches to identify promoters and cis regulatory elements in plant genomes. Plant Physiology. 2003;132:1162–1176. doi: 10.1104/pp.102.017715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JKC, Bennett AB. Cooperative disassembly of the cellulose–xyloglucan network of plant cell walls: Parallels between cell expansion and fruit ripening. Trends in Plant Sciences. 1999;4:176–183. doi: 10.1016/s1360-1385(99)01405-3. [DOI] [PubMed] [Google Scholar]
- Ruzin SE. Plant Microtechnique and Microscopy. New York: Oxford University Press; 1999. Staining; pp. 87–120. [Google Scholar]
- Schwechheimer C, Zourelidou M, Bevan MW. Plant transcription factor studies. Annual Reviews of Plant Physiology and Molecular Biology. 1998;49:127–150. doi: 10.1146/annurev.arplant.49.1.127. [DOI] [PubMed] [Google Scholar]
- Shah J, Klessig DF. Identification of a salicylic acid- responsive element in the promoter of the tobacco pathogenesis-related β-1, 3- glucanase gene, PR-2d. Plant Journal. 1996;10:1089–1101. doi: 10.1046/j.1365-313x.1996.10061089.x. [DOI] [PubMed] [Google Scholar]
- Shani Z, Dekel M, Jensen CS, Tzfira T, Goren R, Altman A, Shoseyov O. Arabidopsis thaliana endo-β-1,4-glucanase (Cel1) promoter mediates UidA expression in elongating tissues of aspen (Populus tremula) Journal of Plant Physiology. 2000;156:118–120. [Google Scholar]
- Shani Z, Dekel M, Tsabary G, Goren R, Shoseyov O. Growth enhancement of transgenic poplar plants by overexpression of Arabidopsis thaliana endo-1, 4-ß-glucanase (cel1) Molecular Breeding. 2004;14:321–330. [Google Scholar]
- Shani Z, Dekel M, Tsabary G, Shoseyov O. Cloning and characterization of elongation specific endo-β-1,4-glucanase (cel1) from Arabidopsis thaliana. Plant Molecular Biology. 1997;34:837–842. doi: 10.1023/a:1005849627301. [DOI] [PubMed] [Google Scholar]
- Thijs G, Lescot M, Marchal K, Rombauts S, De Moor B, Rouzé P, Moreau Y. A higher order background model improves the detection of regulatory elements by Gibbs Sampling. Bioinformatics. 2001;17:1113–1122. doi: 10.1093/bioinformatics/17.12.1113. [DOI] [PubMed] [Google Scholar]
- Thurau T, Kifle S, Jung C, Cai D. The promoter of the nematode resistance gene Hs1pro-1 activates a nematode- responsive and feeding site-specific gene expression in sugar beet (Beta vulgaris L.) and Arabidopsis thaliana. Plant Molecular Biology. 2003;52:643–660. doi: 10.1023/a:1024887516581. [DOI] [PubMed] [Google Scholar]
- Tsabary G, Shani Z, Roiz L, Levy I, Riov J, Shoseyov O. Abnormal ‘wrinkled’ cell walls and retarded development of transgenic Arabidopsis thaliana plants expressing endo-1,4-betaglucanase (cel1) antisense. Plant Molecular Biology. 2003;51:213–224. doi: 10.1023/a:1021162321527. [DOI] [PubMed] [Google Scholar]
- Van de Loecht V, Meier I, Hahlbrock K, Somssich I. A 125 bp promoter fragment is sufficient for strong elicitor-mediated gene activation in parsley. EMBO Journal. 1990;9:2945–2950. doi: 10.1002/j.1460-2075.1990.tb07486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercauteren I, de Almeida Engler J, De Groodt R, Gheysen G. An Arabidopsis thaliana pectin acetylesterase gene is upregulated in nematode feeding sites induced by root-knot and cyst nematodes. Molecular Plant-Microbe Interactions. 2002;15:404–407. doi: 10.1094/MPMI.2002.15.4.404. [DOI] [PubMed] [Google Scholar]
- Washida H, Wu CY, Suzuki A, Yamanouchi U, Akihama T, Harada K, Takaiwa F. Identification of cis-regulatory elements required for endosperm expression of the rice storage protein glutelin gene GluB-1. Plant Molecular Biology. 1999;40:1–12. doi: 10.1023/a:1026459229671. [DOI] [PubMed] [Google Scholar]
- Williamson VM, Hussey RS. Nematode pathogenesis and resistance in plants. Plant Cell. 1996;8:1735–1745. doi: 10.1105/tpc.8.10.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, Bird DM, Vanderknapp E. A comprehensive subtractive cDNA cloning approach to identify nematode- induced transcripts in tomato. Phytopathology. 1994;84:299–303. [Google Scholar]
- Yamaguchi S, Kamiya Y, Sun TP. Distinct cell-specific expression patterns of early and late gibberellin biosynthetic genes during Arabidopsis seed germination. Plant Journal. 2001;28:443–453. doi: 10.1046/j.1365-313x.2001.01168.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto YT, Taylor CG, Acedo GN, Cheng CL, Conkling MA. Characterization of cis-acting sequences regulating root-specific gene expression in tobacco. Plant Cell. 1991;3:371–382. doi: 10.1105/tpc.3.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]