Abstract
The effects of Dactylella oviparasitica strain 50 applications on sugarbeet cyst nematode (Heterodera schachtii) population densities and plant weights were assessed in four agricultural soils. The fungus was added to methyl iodide-fumigated and nonfumigated portions of each soil. The soils were seeded with Swiss chard. Four weeks later, soils were infested with H. schachtii second-stage juveniles (J2). Approximately 1,487 degree-days after infestation, H. schachtii cyst, egg and J2 numbers and plant weights were assessed. In all four fumigated soils, D. oviparasitica reduced all H. schachtii population densities and increased most of the plant weights compared to the nonamended control soils. In two of the nonfumigated soils (10 and SC), D. oviparasitica reduced H. schachtii population densities and increased most plant weight values compared to the nonamended control soils. For the other two nonfumigated soils (44 and 48), which exhibited pre-existing levels of H. schachtii suppressiveness, fungal applications had relatively little impact on H. schachtii population densities and plant weights. The results from this study combined with those from previous investigations suggest that D. oviparasitica strain 50 could be an effective biological control agent.
Keywords: biological control, Dactylella oviparasitica, Heterodera schachtii, soil, sugarbeet cyst nematode, suppressiveness
Achieving effective biological control of soil-borne pests or pathogens is a difficult task. It typically requires that the organism(s) be applied or established such that they are present and active at the appropriate locations and times and in sufficient population densities. In addition, the organisms need to have an effective means of inhibiting, inactivating or eliminating the target pest or pathogen or of inducing resistance in the plant. Finding combinations of organisms and application strategies that produce effective results has been and will continue to be a considerable challenge, especially given that the organisms need to function in highly complex and diverse ecosystems.
One potential source of effective biological control organisms is soil with natural suppressiveness to pests or pathogens (Cook and Baker, 1983). If the suppressiveness is caused by microorganisms, such soils provide a unique opportunity for discovering biological control agents because the causal organism(s) have already demonstrated the ability to function in soil. If these organisms can be identified and isolated, they could become important components of effective pest management strategies. Organisms involved in such suppressiveness have been described for several soil systems (Weller et al., 2002).
Prior research from our laboratories has identified two fungi that appear to play key roles in sugarbeet cyst nematode (Heterodera schachtii) suppressiveness in 9E field soil at the University of California-Riverside's Agricultural Research Station. Dactylella oviparasitica and Fusarium oxysporum were common colonizers of H. schachtii cysts in this soil (Westphal and Becker, 2001). Subsequent molecular population studies showed that D. oviparasitica and F. oxysporum rRNA gene levels positively correlated with high and minimal to moderate levels of suppressiveness, respectively (Yin et al., 2003). When strains of these fungi were added to fumigated 9E soil re-infested with H. schachtii juveniles in greenhouse trials, D. oviparasitica strain 50 reduced H. schachtii population densities to those in the naturally suppressive soil, while F. oxysporum strain 471 did not significantly reduce the nematode populations (Olatinwo et al., 2006b). However, in longer-term field microplot trials, both fungal strains suppressed H. schachtii numbers (Olatinwo et al., 2006a). The objective of the study described below was to assess the potential of D. oviparasitica strain 50 as a biological control agent in four different agricultural soils.
Materials and Methods
Fungus production: The isolation and identification of the D. oviparasitica strain 50 used in this study was previously described (Yin et al., 2003; Olatinwo et al., 2006b). Ten-cm diameter petri plates containing potato dextrose agar (PDA) were inoculated with individual 5-mm diam. agar plugs of D. oviparasitica strain 50. Cultures were grown at room temperature for approximately 21 d. After the incubation period, the fungal culture and PDA medium from each plate were separately transferred to a Sunbeam 6 Speed Blender (Model 4142, Sunbeam Products Inc., Boca Raton, FL) containing 50 ml sterile water. The components were blended for 30 sec using the “blend” setting. A 0.5-ml portion of each mixture was used to estimate the inoculum concentrations by determining the number of colony forming units (CFU) from a dilution series on PDA after incubation at room temperature for 7 d. The remaining portions of the fungal homogenates were added to soil (within 1 hr of blending the fungi) as described below.
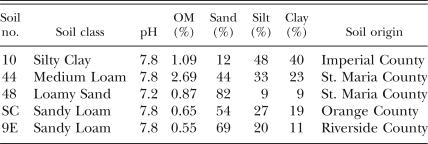
Soils used in this study: The soils used in this study were collected from agricultural fields located in southern California (Table 1). Random samples from the upper 25 cm of an approximately 0.5-ha area were collected, pooled and thoroughly mixed. Three of the soils (10, 44, and 48) had a cropping history that included host plants of the sugarbeet cyst nematode. At the time of collection, H. schachtii was detected in all soils but SC. Soils 10, 44, 48 and 9E contained 2.3, 1.4, 0.1 and 0.9 H. schachtii eggs/g soil, respectively. None of the soils had a significant infestation with any other plant-parasitic nematode. The 9E soil from the University of California Agricultural Experimental Station served as a suppressive control and enabled comparison with previous investigations (Olatinwo et al., 2006b).
Table 1.
Physiocochemical characteristics of the soils used in this study.
Soil physiocochemical analyses were performed at the University of California ANR Analytical Laboratory (Davis, CA) (Table 1). Soil pH was determined by the saturated paste extract method, organic matter content by potassium dichromate reduction of organic carbon and subsequent spectrophotometric measurement (Nelson and Sommers, 1982), and soil texture by particle size analysis (Sheldrick and Wang, 1993).
Greenhouse trials: Two replicate greenhouse trials were conducted to evaluate the ability of D. oviparasitica strain 50 to suppress H. schachtii populations when added to four different agricultural soils. The treatments are described in Table 2. After large aggregates were removed from the soil using a sieve with 6-mm apertures, portions (∼18,000 cm3) of the sieved soils were fumigated with 2 ml of methyl iodide (DSM Fine Chemicals, Saddle Brook, NJ) in 19-liter polyethylene buckets for 4 d (Becker et al., 1998). Fumigated and nonfumigated soils were each mixed 10:1 with silica sand to facilitate water drainage during the greenhouse trials. The amount of soil per pot (∼1,600 cm3) was standardized by filling 15-cm diam. pots to 2.5 cm below the top. Fungal suspensions from one petri plate (described above) and soil from each pot were thoroughly mixed in plastic bags and returned to the pots. The numbers of D. oviparasitica strain 50 CFU/pot were 3.5 x 107 and 3.0 x 107 in trials 1 and 2, respectively. Each pot was sown with three seeds of Swiss chard (Beta vulgaris cv. Large White Ribbed). All pots were placed in a greenhouse under natural light at 23 ± 3°C in a randomized complete block design with five replicates. Plants were watered daily as needed with tap water. Soil temperature was monitored for degree-day estimations (base temperature 8°C) (Caswell and Thomason, 1991) using HOBO® Temp devices (Onset Computer Corporation, Bourne, MA) buried in the soil. After emergence, seedlings were thinned to one per pot and fertilized with 6 g slow-release fertilizer (Sierra 17–6-10 plus Minors, Scotts-Sierra Horticultural Products Company, Marysville, OH). Four weeks after seeding, each pot was infested with H. schachtii by pipetting an aqueous suspension of 10,000 freshly hatched second-stage juveniles (J2) into three holes (∼3.0 cm deep and 1.5 cm wide) in the soil near the base of the plants. The J2 inoculum was collected using a zinc chloride hatching protocol (Shepherd, 1970). Fifteen weeks after infestation (∼1,477 degree-days for trial 1 and ∼1,497 for trial 2), the experiment was ended. This time period allowed for approximately three nematode generations (Caswell and Thomason, 1991). Plant tops were cut off at soil level, and roots were removed from soil. Fresh and dry shoot and fresh root weights were measured. Cysts were extracted from 350-g subsamples of the soils, using the modified Fenwick flotation can method (Caswell et al., 1985) and then counted. These cysts were broken open in a tissue homogenizer to release the eggs, which were counted using standard procedures. Because this method used a glycerol egg-cleaning step, which reduces egg hatch, cysts from additional 350-g subsamples were extracted with a Fenwick can for J2 enumeration. These cysts were placed on modified Baerman funnels in 0.4% zinc chloride to stimulate egg hatching. After a 5-d incubation at 26°C, H. schachtii J2 were counted. Because the procedure used to extract eggs for their enumeration breaks some open, J2 numbers were frequently greater than egg numbers.
Table 2.
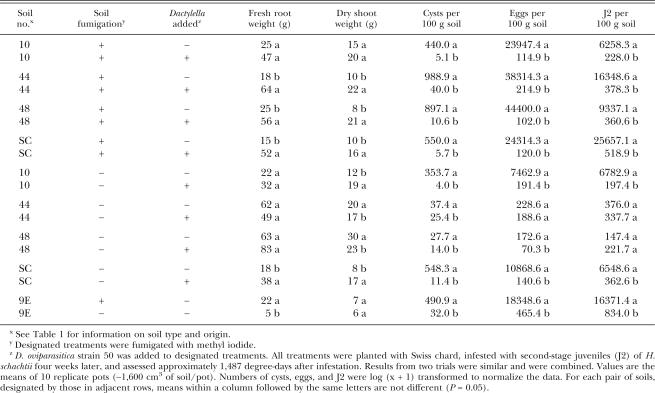
Effect of Dactylella oviparasitica strain 50 applications on Heterodera schachtii population densities and plant weights in four different agricultural soils.
Data analysis: The numbers of cysts, eggs, and J2 were log (x + 1) transformed to normalize the data. The transformed cyst, egg and J2 data and the plant weights were subjected to ANOVA and Fisher's least significant difference (LSD) tests (SAS Institute, Cary, NC).
Results
The four southern Californian soils examined in this study possessed different physiocochemical characteristics (Table 1). The results from the two greenhouse trials were similar; therefore, the data were combined for presentation (Table 2).
When D. oviparasitica was added to the fumigated soils, H. schachtii egg, cyst and J2 numbers in all four soils were reduced compared to the nonamended control soils. When D. oviparasitica was added to nonfumigated soils, H. schachtii egg, cyst and J2 numbers for soils 10 and SC were reduced compared to the nonamended control soils. In the other two nonfumigated soils, cyst numbers for soils 44 and 48 and egg numbers for 48 were reduced compared to the nonamended control soils.
When D. oviparasitica was added to the fumigated soils 44, 48 and SC, all plant weights were increased compared to the nonamended control soils. Dactylella oviparasitica did not significantly alter plant weights in fumigated soil 10. When D. oviparasitica was added to nonfumigated soil SC, all plant weights were increased compared to the nonamended control soil. When D. oviparasitica was added to the other nonfumigated soils (10, 44, 48), there was an increase in dry shoot weights for soil 10 and a decrease in dry shoot weights for soils 44 and 48 compared to the nonamended control soils.
H. schachtii egg, cyst and J2 numbers were significantly higher in fumigated 9E soil than nonfumigated 9E soil. These soils were included as controls in the greenhouse trials, as their abilities to influence H. schachtii populations in such assays have been previously reported (Westphal and Becker, 1999).
Discussion
This study examined the effect of D. oviparasitica applications on H. schachtii population densities and plant weights in four different agricultural soils. When D. oviparasitica was added to the fumigated soils, H. schachtii cyst, egg and J2 numbers were reduced in all four soils. Application of this fungus to the fumigated soils also produced an increase in most of the plant weights. These results suggest that D. oviparasitica strain 50 is capable of suppressing H. schachtii in soils with a variety of physiocochemical characteristics.
Dactylella oviparasitica was less effective in suppressing H. schachtii numbers in two of the nonfumigated soils (44 and 48) than in their fumigated counterparts. However, for both of these soils, pre-existing nematode suppressiveness was detected. Such suppressiveness can be assessed by comparing the abilities of the fumigated and nonfumigated soils to suppress nematode population densities (Table 2). Since these soils exhibited high levels of suppressiveness, it is not surprising that D. oviparasitica applications had relatively little effect on H. schachtii populations.
For the nonfumigated soils that did not show any pre-existing suppressiveness (10 and SC), D. oviparasitica applications reduced H. schachtii cyst, egg and J2 numbers. However, only in the SC soil did the nematode reductions consistently coincide with greater plant weights. For the nonfumigated soil 10, application of the fungus produced greater dry shoot weights but it did not significantly alter fresh root or fresh shoot weights (data not shown). This result is similar to the analysis of the fumigated soil 10, where all of the H. schachtii numbers were reduced by D. oviparasitica, yet none of the plant weights were significantly increased. These results could be attributed to characteristics of this particular soil, which may have influenced the ability of the nematode to damage the host or of the host to tolerate the nematode. However, given the limited number of soils tested in this study, further analysis would be needed to assess this issue. The discrepancy between the H. schachtii population densities and plant weights with soil 10 could also have resulted from experimental procedures such as the type and timing of the H. schachtii infestation as well as the relatively short-term duration of the experiments.
The effectiveness of D. oviparasitica in reducing H. schachtii population densities in the four different soils may be associated with its ability to colonize plant roots and to infect late developmental stages of the nematode. In our prior studies, D. oviparasitica was shown to be one of the most abundant fungi in H. schachtii cysts from the suppressive 9E soil (Westphal and Becker, 2001; Yin et al., 2003). In studies from other investigators, several D. oviparasitica isolates were shown to be efficient parasites of Meloidogyne eggs (Stirling and Mankau, 1979). This fungus has also been shown to infect Meloidogyne females (Stirling et al., 1979) and to grow and sporulate on peach roots (Stirling, 1979). In addition, Meloidogyne eggs from the rhizosphere were more likely to be parasitized by D. oviparasitica than eggs from nonrhizosphere soil, suggesting a higher population density and or activity of this fungus near the roots (Stirling et al., 1979). The ability of D. oviparasitica to colonize the rhizoplane may lead to frequent contact between the fungus and fourth-stage juveniles and female nematodes, which have broken through the root surface but remain attached to the roots. Such interactions could result in high levels of parasitized nematodes and therefore to effective H. schachtii control. Finally, if D. oviparasitica’s ability to survive and compete with other microorganisms is largely determined by its ability to inhabit both the nematodes and the rhizoplane of host plants, instead of soil, this may explain why this fungus was effective in reducing H. schachtii population densities in a variety of soil types.
The results from this study combined with those from previous investigations suggest that D. oviparasitica strain 50 could be an effective biological control agent and an important component of H. schachtii management strategies. Application of this fungus to nonsuppressive soil produced the same level of H. schachtii population suppressiveness as the naturally suppressive 9E soil in short-term (two nematode generations) greenhouse trials (Olatinwo et al., 2006b). In addition, a single application of this fungus to nonsuppressive soil produced stable suppressiveness over two cropping cycles and at least six nematode generations in combined field microplot and greenhouse studies (Olatinwo et al., 2006a). Finally, in this study, we showed that D. oviparasitica strain 50 reduced H. schachtii population densities in four soils possessing different physioco- chemical characteristics.
Footnotes
The authors thank John Darsow for his technical assistance. This research was supported by the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service, grant number 2003–35316–13824.
This manuscript was edited by Brian Kerry.
Literature Cited
- Becker JO, Ohr HD, Grech NM, McGiffen ME, Jr., Sims JJ. Evaluations of methyl Iodide as a soil fumigant in container and small field plot studies. Pesticide Science. 1998;52:58–62. [Google Scholar]
- Caswell EP, Thomason IJ. A model of egg production by Heterodera schachtii (Nematoda: Heteroderidae) Canadian Journal of Zoology. 1991;69:2085–2088. [Google Scholar]
- Caswell EP, Thomason IJ, McKinney HE. Extraction of cysts and eggs of Heterodera schachtii from soil with an assessment of extraction efficiency. Journal of Nematology. 1985;17:337–340. [PMC free article] [PubMed] [Google Scholar]
- Cook RJ, Baker KF. The nature and practice of biological control of plant pathogens. St. Paul: APS Press; 1983. [Google Scholar]
- Nelson DW, Sommers LE. Total carbon, organic carbon and organic matter. In: Page AL, Miller RH, Keeney DR, editors. Methods of soil analysis: Part 2. Chemical and microbiological properties. Madison, Wisconsin: American Society of Agronomy; 1982. pp. 539–579. [Google Scholar]
- Olatinwo R, Borneman J, Becker JO. Induction of beet-cyst nematode suppressiveness by the fungi Dactylella oviparasitica and Fusarium oxysporum in field microplots. Phytopathology. 2006a;96:855–859. doi: 10.1094/PHYTO-96-0855. [DOI] [PubMed] [Google Scholar]
- Olatinwo R, Yin B, Becker JO, Borneman J. Suppression of the plant-parasitic nematode Heterodera schachtii by the fungus Dactylella oviparasitica . Phytopathology. 2006b;96:111–114. doi: 10.1094/PHYTO-96-0111. [DOI] [PubMed] [Google Scholar]
- Sheldrick BH, Wang C. Particle-size distribution. In: Carter MR, editor. Soil sampling and methods of analysis. Ann Arbor, Michigan: Canadian Society of Soil Science. Lewis Publishers; 1993. pp. 499–511. [Google Scholar]
- Shepherd AM. Extraction and estimation of Heterodera. In: Southey JF, editor. Laboratory methods for work with plant and soil nematodes. London: Her Majesty's Stationery Office; 1970. pp. 23–33. [Google Scholar]
- Stirling GR. Techniques for detecting Dactylella oviparasitica and evaluating its significance in field soils. Journal of Nematology. 1979;11:99–100. [PMC free article] [PubMed] [Google Scholar]
- Stirling GR, Mankau R. Mode of parasitism of Meloidogyne and other nematode eggs by Dactylella oviparasitica . Journal of Nematology. 1979;11:282–288. [PMC free article] [PubMed] [Google Scholar]
- Stirling GR, McKenry MV, Mankau R. Biological control of root-knot nematodes (Meloidogyne spp.) on peach. Phytopathology. 1979;69:806–809. [Google Scholar]
- Weller DM, Raaijmakers JM, McSpadden-Gardener BB, Thomashow LS. Microbial population responsible for specific soil suppressiveness to plant pathogens. Annual Review of Phytopathology. 2002;40:309–348. doi: 10.1146/annurev.phyto.40.030402.110010. [DOI] [PubMed] [Google Scholar]
- Westphal A, Becker JO. Biological suppression and natural population decline of Heterodera schachtii in a California field. Phytopathology. 1999;89:434–440. doi: 10.1094/PHYTO.1999.89.5.434. [DOI] [PubMed] [Google Scholar]
- Westphal A, Becker JO. Components of soil suppressiveness against Heterodera schachtii. Soil Biology and Biochemistry. 2001;33:9–16. [Google Scholar]
- Yin B, Valinsky L, Gao X, Becker JO, Borneman J. Identification of fungal rDNA associated with soil suppressiveness against Heterodera schachtii using oligonucleotide fingerprinting. Phytopathology. 2003;93:1006–1013. doi: 10.1094/PHYTO.2003.93.8.1006. [DOI] [PubMed] [Google Scholar]