Abstract
Melanin-concentrating hormone (MCH) is a hypothalamic neuropeptide that has been implicated in energy homeostasis. Pharmacological studies with MCH and its receptor antagonists have suggested additional behavioral roles for the neuropeptide in the control of mood and vigilance states. These suggestions have been supported by a report of modified sleep in the MCH-1 receptor knockout mouse. Here we found that MCH knockout (MCH−/−) mice slept less during both the light and dark phases under baseline conditions. In response to fasting, MCH−/− mice exhibited marked hyperactivity, accelerated weight loss and an exaggerated decrease in rapid eye movement (REM) sleep. Following a 6-h period of sleep deprivation, however, the sleep rebound in MCH−/− mice was normal. Thus MCH−/− mice adapt poorly to fasting, and their loss of bodyweight under this condition is associated with behavioral hyperactivity and abnormal expression of REM sleep. These results support a role for MCH in vigilance state regulation in response to changes in energy homeostasis and may relate to a recent report of initial clinical trials with a novel MCH-1 receptor antagonist. When combined with caloric restriction, the treatment of healthy, obese subjects with this compound resulted in some subjects experiencing vivid dreams and sleep disturbances.
Keywords: locomotor activity, lateral hypothalamus, NGD-4715, orexin, hypocretin
Melanin-concentrating hormone (MCH) is a neuropeptide produced by cell bodies located exclusively in the lateral hypothalamic area (Bittencourt et al., 1992). MCH neurons project widely throughout the neuraxis, including dense innervations of the cholinergic and monoaminergic arousal centers that drive the forebrain signs of wakefulness (Bittencourt et al., 1992). The distributions of MCH neurons and receptors largely overlap those of the orexin (hypocretin) system, including innervations of the arousal centers (Trivedi et al., 1998, Saito et al., 2001), though MCH exerts neuroinhibitory effects (Gao and van den Pol, 2001, 2002) whereas orexin is neuroexcitatory (de Lecea et al., 1998). Since orexins are known to regulate sleep and wakefulness, the anatomical similarities between the MCH and orexin systems suggested that MCH might also affect vigilance. In fact, intracerebroventricular (icv) administration of MCH was found to enhance sleep (Verret et al., 2003), and indirect evidence indicated that MCH neurons were more active during rapid eye movement (REM) sleep (Verret et al., 2003, Modirrousta et al., 2005). Recently, two novel MCH-1 receptor antagonists have been shown to decrease sleep, including REM sleep, while increasing wakefulness in the rat (Ahnaou et al., 2008). In contrast, REM sleep is increased in the MCH-1 receptor knockout (MCH-1R−/−) mouse (Adamantidis et al., 2008). Inconsistent behavioral results have also been reported in other studies. Anxiolytic effects, for example, have been observed under conditions of both increased (Monzon and De Barioglio, 1999, Kela et al., 2003) and decreased (Borowsky et al., 2002, Chaki et al., 2005, Roy et al., 2006, Smith et al., 2006) MCH signaling.
Metabolic status was not controlled in these studies, however, despite the established importance of MCH in the modulation of energy balance. MCH knockout (MCH−/−) mice have reduced body weight and increased metabolic rate (Shimada et al., 1998), mirroring in some respects an anorectic syndrome induced by lesions of the lateral hypothalamus. We have previously described a role for the orexin system in promoting vigilance and locomotor activity in response to fasting (Yamanaka et al., 2003), and we hypothesized that metabolic challenge could also influence the actions of MCH on arousal and vigilance. We therefore characterized sleep and wakefulness in normally fed and fasting MCH−/− mice using electroencephalography/electromyography (EEG/EMG). Locomotor activity was also evaluated under both of these conditions. Our results show that MCH−/− mice express a profound inability to adapt appropriately to fasting, manifested as sleep deficits, behavioral disinhibition and accelerated weight loss. As a corollary of the known role of MCH in energy homeostasis, we therefore demonstrate that MCH also regulates behavioral arousal and vigilance states during adaptation to changes in energy balance.
EXPERIMENTAL PROCEDURES
Production of MCH null mice
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center and were strictly in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Independent targeting of the MCH allele to produce MCH null mice has been previously described (Shimada et al., 1998). The first filial (C57BL6/J:129SvEv F1) generation of these mice was backcrossed to C57BL6/J for 5 generations (N5), and experiments were performed using F2 and F3 generations of the N5 line. Mice survived until weaning at approximately the expected Mendelian frequency, and experienced mothers exhibited similar fertility, producing litters of comparable size in which 5–7 offspring typically survived. Male mice were weaned at 21–22 days of age, genotyped, and housed 3–5 mice per cage with ad libitum access to water and standard mouse chow (11% fat), unless otherwise noted. The genotypes was confirmed by polymerase chain reaction (PCR) amplification of genomic DNA, performed with primers as previously described (Shimada et al., 1998).
EEG/EMG studies
For vigilance state determination, male mice (18–20 weeks old) were anesthetized and surgically implanted for long-term EEG/EMG monitoring as previously described (Chemelli et al., 1999). Throughout the study, mice were housed under a 12 h light:dark cycle (07:00 –19:00) at 25°C, and were habituated to the recording conditions for 2 weeks before experiments started. EEG/EMG monitoring began when the mice were 20–23 weeks of age. EEG/EMG signals were amplified (Grass Model 78; Grass Instruments, West Warwick, RI), filtered (EEG: 0.3–10 Hz, EMG: 30–300 Hz), digitized at a sampling rate of 250 Hz, and displayed using custom polygraph software. After being archived for off-line analysis, EEG/EMG records were visually scored in 20 sec epochs according to standard criteria of rodent sleep (Radulovacki et al., 1984). Vigilance state parameters were determined over hourly intervals and 12 h blocks; REM sleep latency was determined as the time from the end of the preceding wakefulness to the onset of an episode of REM sleep.
For the fasting study, mice were recorded in matched groups of wild-type (N = 8) and MCH−/− mice (N = 8), beginning at lights-on. Food and water were replenished as necessary at lights-off each day during the baseline recording under ad libitum feeding conditions, but the mice were not otherwise disturbed. Following completion of 2 × 24 h periods of continuous baseline recording, the cages were changed with fresh bedding at lights-on and the remaining food was then removed at the successive lights-off, at which time vigilance state recording began under fasting conditions. This recording continued for a period of 24 h when mice were re-fed. For display, the average of the two 24 h baseline recordings was compared with the data from the 24 h recorded when food was absent; hourly results for both conditions are displayed from lights-off.
A similar protocol was followed for the sleep deprivation study, for which separate groups of wild-type (N = 4) and MCH−/− (N = 4) male mice were prepared for EEG/EMG monitoring and habituated to the recording conditions for 2 weeks. They were then recorded in matched groups for 5 days beginning with 48 h of continuous undisturbed baseline recording from lights-on. On the third day, while EEG/EMG monitoring continued, mice were allowed to sleep normally for the first 6 h, and then kept awake for the second 6 h of the light phase using the gentle handling technique. At the completion of sleep deprivation, at lights-off, mice were left undisturbed for the subsequent 36 h. For analysis, genotypes were compared for the 36 h of recovery sleep, with results displayed for 48 h of recording and thus including the 6 h sleep deprivation period. The 24 h baseline vigilance state distributions in these groups of mice were not significantly different from the baseline averages recorded for the fasting study.
EEG spectral analysis
For spectral analysis, the frequency distribution of the EEG was determined by power spectral analysis (i.e., fast Fourier transform, FFT) in 1 Hz bins from 1 to 32 Hz. For derivation of the power spectra, FFT data for 60 min of wakefulness or non-rapid eye movement (NREM) sleep were extracted from the EEG recordings, beginning at the onset of the dark phase. Any epochs with artifacts were excluded from the analysis. These results were averaged for each mouse before being normalized to a spectral density function by dividing each 1 Hz bin by the total average power of all epochs for that mouse during the baseline period. This allowed derivation of the mean spectral density for each genotype and comparison across animals. We compared the EEG spectral density function between the genotypes during wakefulness while the mice were being normally fed and during fasting. We also examined EEG spectral δ (1 – 4 Hz) power during (NREM) sleep prior to, and following sleep deprivation in both genotypes. The depth of NREM sleep is correlated with δ power which is increased by sleep deprivation (Borbely et al., 1984).
Locomotor activity
Locomotor activity was determined from infrared beam breaks within an open field apparatus, subdivided into 8″ × 8″ activity chambers by Plexiglas dividers (VersaMax Animal Activity Monitor, AccuScan Instruments, Inc., Columbus, OH), and modified to allow ad libitum access to food and water, except as noted below. Litter covered the floor of the activity chambers throughout. Individual mice (N = 18, wild-type; N = 12, MCH−/−) were placed in the clean activity chamber at the midpoint of the light phase and were monitored continuously for the subsequent 78 h. The first 6 h of data collection constituted a period of habituation reflecting responses to a novel environment and are not reported here. Subsequently, data were collected for a period of 72 h beginning at lights-out, initially during a baseline 24 h period with normal access to food. This was followed by a 24 h period of fasting after food was removed, and then under re-fed conditions for 24 h. Mice were weighed and food was removed or replaced, as appropriate, at transitions to lights-out, but the animals were not otherwise disturbed. Raw beam break data were collected on-line to a computer and subsequently analyzed to calculate locomotor activity as total distance traveled/h. The weights for each mouse during the baseline period, and the loss of weight during the 24 h period of fasting were recorded. Food was also weighed at the beginning and end of the baseline 24 h period and, similarly, at the beginning and end of the 24 h re-feeding period to calculate food consumption under normal and re-fed conditions.
Body composition analysis
Fat mass and lean body mass were evaluated in conscious 21-week old mice maintained on standard chow (N = 17, wild-type; N = 12, MCH−/− ) by low-resolution nuclear magnetic resonance (NMR) scanning analysis (Minispec mq10, Bruker Optics, Inc., Billerica, MA). Briefly, the mouse was weighed and restrained in a thin wall glass cylinder (inside diameter 4.5 cm) which was then inserted into the scanner. After about 1.5 min, when the scan was completed and the results stored, the mouse was returned to the home cage. The application of NMR to body composition analysis has been previously described (Mitchell et al., 1991). The method relies on the differences in the NMR absorption spectrum generated by fat, muscle and body fluid and, as applied here, was fully automated.
Statistical analysis
Sleep parameters and activity data were analyzed with 2-factor analysis of variance (ANOVA) using the mean of the 2 baseline recording periods for the vigilance state data. ANOVA was followed by Tukey post-hoc analysis as appropriate Body composition analysis, hourly sleep data comparisons and spectral data were analyzed with the t-test, and the χ2 –test was used for the REM sleep latency distributions. We used both JMP 5.1 (SAS Institute Inc., Cary, NC) and Prism 5.0 for statistical analysis (GraphPad, San Diego, CA).
RESULTS
MCH deficiency increases wakefulness
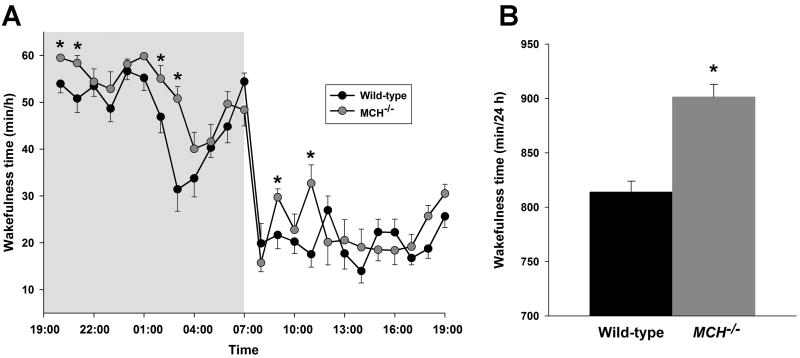
We characterized the sleep and wakefulness of MCH−/− mice from EEG/EMG records. The overall organization of vigilance states in MCH−/− mice during normal feeding resembled that of C57Bl6/J wild-type mice: both genotypes exhibited long, consolidated bouts of wakefulness, especially during the dark phase. Sleep episodes were similarly consolidated, particularly during the light phase. However, MCH−/− mice spent about 90 min more time awake compared with wild-type mice over the 24 h period (Table 1, Figure 1; F[1, 28] = 12.59, P = 0.03), but especially during the dark phase (F[1, 28] = 21.92, P < 0.0001). Hourly analysis (cf. Figure 1A) showed that the maximal increase in wakefulness occurred during the eighth hour from the onset of the dark phase (51 ± 3 min versus 31 ± 5 min, MCH−/− and wild-type mice respectively; t(14) = 3.63, P < 0.005). This time corresponds to the period of mid-dark phase rest in wild-type mice (Dudley et al., 2003). MCH−/− mice also tended to exhibit longer wakefulness episode durations during the dark phase (Table 1) and thus fewer wakefulness episodes. This result indicated that the increased wakefulness was not an indirect consequence of vigilance state fragmentation. Furthermore, time awake in the MCH−/− mice was greater than that in wild-type mice over the first four hours of the light phase (101 ± 5 min versus 79 ± 4 min, MCH−/− and wild-type mice respectively; t(14) = 3.84, P < 0.001); thereafter this parameter was not different between the genotypes (Figure 1A). Hence the nocturnal arousal of the MCH−/− mice was not associated with a diurnal rebound. No EEG spectral differences were found between the MCH−/− and wild-type mice under baseline conditions (data not shown).
Table 1.
Sleep and wakefulness parameters (mean ± SEM) for wild-type and MCH−/− mice during 24 h periods of normal feeding and fasting. Significant differences between the genotypes (P < 0.05) are designated by an asterisk.
| Ad libitum feeding | Fasting | ||||
|---|---|---|---|---|---|
| Wild-type | MCH−/− | Wild-type | MCH−/− | ||
| 24 h | |||||
|
| |||||
| REM sleep | Total time (min) | 51.7 ± 1.6 | 47.6 ± 1.5 | 31.5 ± 5.2 | 12.8 ± 6.1* |
| Episode duration (sec) | 67.5 ± 2.5 | 62.4 ± 2.8 | 89.7 ± 5.0 | 59.2 ± 7.5* | |
| REM sleep latency (min) | 9.6 ± 0.7 | 9.8 ± 0.7 | 13.6 ± 0.9 | 17.5 ± 1.9 | |
| Inter-REM interval (min) | 29.0 ± 1.5 | 28.2 ± 1.8 | 55.6 ± 10.8 | 43.7 ± 12.4 | |
| NREM sleep | Total time (min) | 574.5 ± 11.1 | 491.0 ± 11.2* | 540.2 ± 22.0 | 450.7 ± 44.2* |
| Episode duration (sec) | 312.9 ± 24.3 | 283.7 ± 23.4 | 397.7 ± 15.6 | 369.5 ± 28.2 | |
| Wakefulness | Total time (min) | 813.8 ± 10.1 | 901.4 ± 11.5* | 868.3 ± 25.1 | 976.5 ± 46.7* |
| Episode duration (sec) | 565.7 ± 48.7 | 674.0 ± 36.5 | 708.0 ± 57.8 | 845.3 ± 69.5 | |
| 12 h dark period | |||||
|
| |||||
| REM sleep | Total time (min) | 6.9 ± 1.1 | 4.8 ± 1.1 | 3.8 ± 0.9 | 1.8 ± 0.6 |
| Episode duration (sec) | 54.9 ± 5.6 | 49.6 ± 5.2 | 62.3 ± 5.2 | 38.8 ± 4.1* | |
| REM sleep latency (min) | 9.0 ± 0.4 | 8.4 ± 1.3 | 15.8 ± 1.7 | 11.7 ± 3.4 | |
| Inter-REM interval (min) | 75.7 ± 20.4 | 106.1 ± 28.4 | 30.7 ± 3.7 | 23.0 ± 8.8 | |
| NREM sleep | Total time (min) | 142.9 ± 5.8 | 86.8 ± 10.1* | 123.3 ± 8.5 | 101.0 ± 9.1 |
| Episode duration (sec) | 285.0 ± 24.5 | 225.6 ± 28.3 | 473.1 ± 24.3 | 395.0 ± 27.1 | |
| Wakefulness | Total time (min) | 570.2 ± 5.7 | 628.4 ± 11.1* | 592.9 ± 8.7 | 617.3 ± 8.9 |
| Episode duration (sec) | 1304.9 ± 201.1 | 1724.6 ± 125.6 | 2462.6 ± 286.5 | 2686.9 ± 426.2 | |
| 12 h light period | |||||
|
| |||||
| REM sleep | Total time (min) | 44.8 ± 1.7 | 42.8 ± 1.4 | 27.7 ± 4.8 | 11.0 ± 5.7* |
| Episode duration (sec) | 69.6 ± 2.5 | 63.6 ± 2.9 | 95.9 ± 5.1 | 69.5 ± 8.2* | |
| REM sleep latency (min) | 9.7 ± 0.8 | 9.8 ± 0.7 | 13.5 ± 1.1 | 9.4 ± 3.2 | |
| Inter-REM interval (min) | 18.0 ± 1.1 | 17.4 ± 0.9 | 47.1 ± 9.3 | 24.2 ± 4.7 | |
| NREM sleep | Total time (min) | 431.6 ± 9.9 | 404.2 ± 12.0 | 416.8 ± 27.7 | 349.8 ± 43.6 |
| Episode duration (sec) | 326.6 ± 26.2 | 304.8 ± 28.1 | 381.6 ± 18.0 | 366.5 ± 36.3 | |
| Wakefulness | Total time (min) | 243.6 ± 9.7 | 273.0 ± 12.1 | 275.4 ± 30.9 | 359.3 ± 46.5 |
| Episode duration (sec) | 246.2 ± 12.4 | 286.2 ± 25.8 | 298.0 ± 57.5 | 407.4 ± 69.6 | |
Figure 1. MCH deficiency promotes wakefulness.
(A) Mean wakefulness times for each genotype displayed on an hourly basis (min/h ± SEM) during 24 h of ad libitum feeding. (B) The total times awake over 24 h (min/24 h) under these conditions. MCH−/− mice spent more time awake during the dark phase and over 24 h. A significant difference between wild-type and MCH−/− mice is indicated by an asterisk.
Inactivation of MCH causes hyperactivity in response to fasting
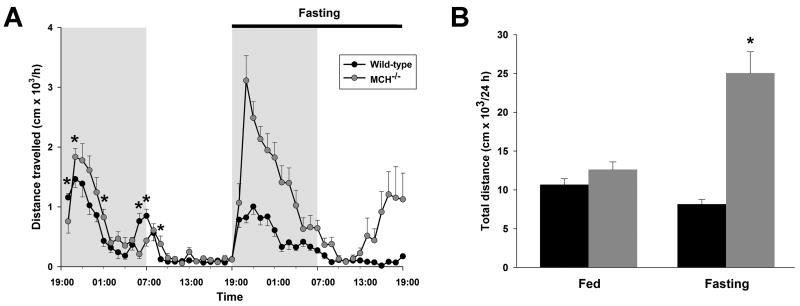
Under baseline conditions, the 24 h locomotor activity of MCH−/− mice did not differ significantly from wild-type mice (Figure 2B), though, on an hourly basis, MCH−/− mice showed a slightly different pattern of activity, especially during the dark phase (Figure 2A). This variation may be related to the differences in time awake under these conditions. During 24 h of food deprivation, the activity of fasting wild-type mice was not different from that recorded during baseline (Figure 2B). In contrast, fasting MCH−/− mice were profoundly hyperactive when compared with both their basal activity (24 h, F[1,56] = 47.78, P < 0.00001) and fasting wild-type mice (24 h, P < 0.00001). Hourly analysis revealed two peaks of hyperactivity in fasting MCH−/− mice: the first began shortly after the onset of fasting at the beginning of the dark phase; the second occurred towards the end of the successive light phase and may therefore be in anticipation of the subsequent dark phase (Figure 2A).
Figure 2. MCH deficiency induces marked hyperactivity when food is unavailable.
(A) Mean hourly distance traveled in the open field (103 cm/h ± SEM) displayed for each genotype during 24 h of ad libitum feeding, followed by 24 h of fasting. Note the pattern of the increase in activity of the MCH−/− mice during fasting: hyperactivity occurs during an initial phase at the onset of fasting and this is followed by a second phase towards the end of the subsequent light phase. (B) The mean total distance traveled in the open field (103 cm/24 h ± SEM) during ad libitum feeding and fasting, integrated over 24 h for each genotype. A significant difference between wild-type and MCH−/− mice is indicated by an asterisk. For clarity of display, significant differences on the hourly plot during the 24 h of fasting are not shown (all hourly values are significantly different except hours 20:00, 10:00, 11:00, 12:00).
MCH−/− mice exhibit a more pronounced reduction in REM sleep in response to fasting
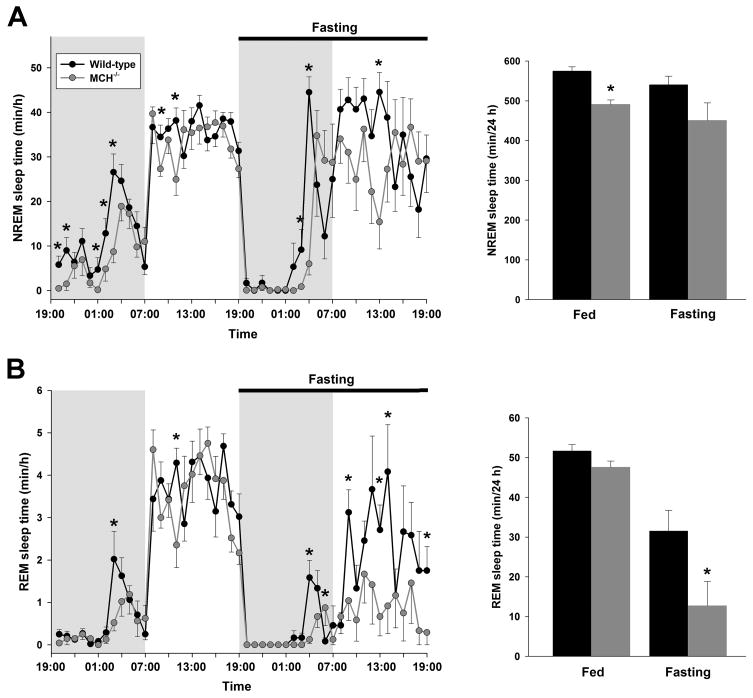
Food craving and metabolic disequilibrium induced by fasting provide motivational and emotional stimuli that alter levels of arousal (Fadel and Deutch, 2002, Saper et al., 2002). Adaptation to conditions of fasting depends on a balance between food-seeking locomotor activity and energy conservation, including sleep (Willie et al., 2001). Consistent with the previously described effects in rodents (Borbely, 1977, Dewasmes et al., 1989, Yamanaka et al., 2003), fasting wild-type mice responded with more wakefulness and less NREM sleep time during both the light and dark phases (Table 1, Figure 3A). During fasting, MCH−/− mice exhibited a similar increase in wakefulness and decrease in NREM sleep time (6 % decrease in 24 h NREM sleep time versus 8 %, wild-type and MCH−/− mice, respectively) (Table 1, Figure 3A). Thus, similar to the difference in NREM sleep time between MCH−/− and wild-type mice under baseline conditions (F[1,28] = 11.12, P = 0.03), NREM sleep time was also less in MCH−/− mice during the 24 h of fasting (P = 0.02).
Figure 3. MCH deficiency decreases REM sleep during fasting.
Time spent in NREM sleep (A) and REM sleep (B), on an hourly basis (min/h ± SEM) and the total times in these states over 24 h (min/24 h), displayed for each genotype during 24 h of ad libitum feeding, followed by 24 h fasting. Although MCH−/− mice respond similarly to wild-type mice during fasting with a reduction NREM sleep, the effect on REM sleep is disproportionately greater in MCH−/− mice. See text and table for details. A significant difference between wild-type and MCH−/− mice is indicated by an asterisk.
There was no significant difference between the two genotypes in their response to fasting as determined by EEG spectral power for any vigilance state nor for any frequency band (data not shown). We noted, however, increased awake EEG spectral power in the θ(5 –9Hz) frequency range during fasting in each genotype (t[7] = 2.53, P = 0.04, MCH−/− fasting versus MCH−/− baseline; t[7] = 3.40, P = 0.01, wild-type fasting versus wild-type baseline).
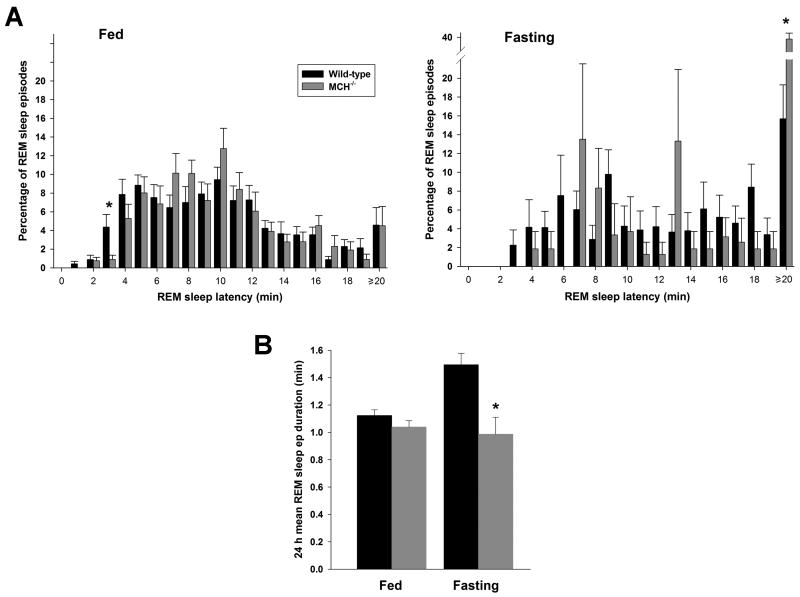
REM sleep time was also significantly reduced by fasting in both genotypes during the light phase (Table 1, Figure 3B; F[1,28] = 5.85, P = 0.004, wild-type; P < 0.00001, MCH−/− mice). However, we noted a disproportionate reduction in REM sleep time in MCH−/− mice (Figure 3B) (38 % versus 74 %, wild-type and MCH−/− mice, respectively). Indeed, two (of 8) MCH−/− mice completely lacked REM sleep throughout the 24 h fasting period and an additional 2 mice had either no REM sleep or only one episode during the 12 h light phase. This compares with a mean of 17 REM sleep episodes during the 12 h light phase in the wild-type mice during fasting. In view of the possibility that the 4 MCH−/− mice with exceptionally limited REM sleep expression during fasting were biasing the data, we repeated the analysis after excluding the results of these mice. The remaining 4 mice exhibited a 55% reduction in REM sleep time during fasting, of which 19.3 ± 7.5 min was in the 12 h light period (cf. Table 1). This was not different from the data for all 8 MCH−/− mice (P = 0.2). However, as well as the reduced REM sleep time, wild-type mice also displayed a significant increase in mean REM sleep episode duration during fasting (F[1,25] = 10.32, P = 0.005). In contrast, the mean REM sleep episode duration remained unchanged in MCH−/− mice (Table 1, Figure 4B; P = 0.4). Furthermore, the distribution of REM sleep latencies revealed a significantly greater shift towards latencies longer than 20 min in MCH−/− mice while fasting, reflecting fewer episodes of this state under these conditions (Figure 4A; χ2 [17] = 45.4, P = 0.0002, wild-type versus MCH−/− mice). The only bin that was significantly different between the genotypes under this condition was the ≥ 20 min bin (t[12] = 3.63, P < 0.005). During ad libitum feeding, the overall distribution of REM sleep latencies did not differ between the genotypes (P > 0.9), though the 3 min bin was different (t[14] = 2.38, P = 0.03). Taken together, these results show a marked deficit in the expression of REM sleep during fasting in MCH−/− mice, in contrast to baseline conditions when this vigilance state did not differ from that recorded in wild-type mice.
Figure 4. Increase of REM sleep latency in fasting MCH−/− mice.
The reduction in REM sleep time in MCH−/− mice during fasting reflects both an excessive reduction in the number of episodes of REM sleep combined with a failure to increase the mean episode duration. (A) Histograms of the distributions of REM sleep latencies for both genotypes, comparing both ad libitum feeding and fasting conditions. These distributions are different only during fasting, reflecting the shift to long latencies (≥20 min) in MCH−/− mice. Under ad libitum feeding, although the overall distributions are not different, a deficit in the occurrence of shorter REM sleep latencies (i.e., ≤ 3 min) is apparent in the MCH−/− mice. (B) The mean REM sleep episode duration (min + SEM) displaying the increase in this parameter in wild-type mice during fasting in contrast with the lack of change in the MCH−/− mice. A significant difference between wild-type and MCH−/− mice is indicated by an asterisk.
Accelerated weight loss of MCH−/− mice during fasting correlates with behavioral abnormalities
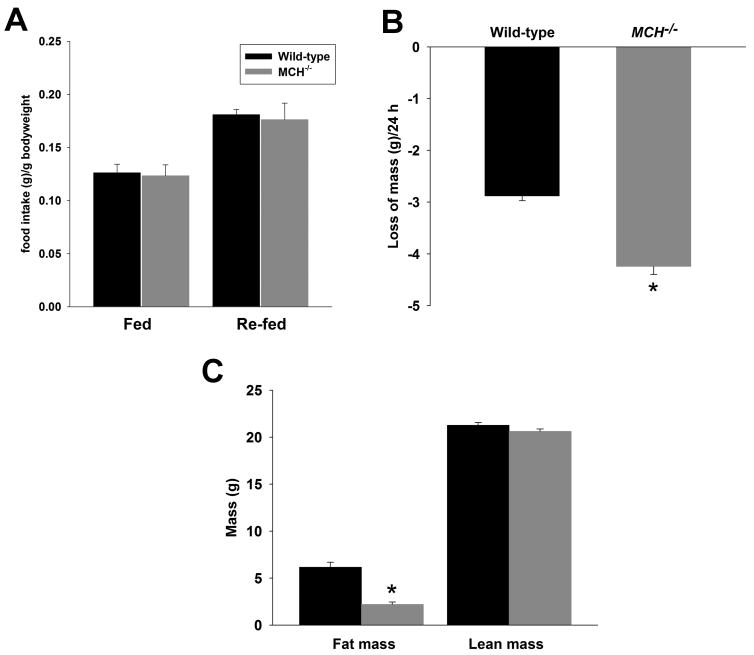
When monitored during measurement of locomotor activity, food intake of MCH−/− mice was not different from wild-type mice under either normal feeding or re-feeding after 24 h of fasting (i.e., during the same experimental session as Figure 2) (Figure 5A). The rate of weight loss during fasting reflects rates of energy utilization through basal metabolism, muscle activity, and non-shivering thermogenesis. As expected from their markedly increased locomotor activity during fasting, MCH−/− mice lost weight at an accelerated rate: fasting wild-type mice lost 10% bodyweight/24 h versus 16% of bodyweight in the MCH−/− mice (Figure 5B; t[27] = 7.94, P < 0.00001). NMR body composition analysis showed that MCH−/− mice were leaner than controls under baseline conditions: 21 week old MCH−/− mice had 64% less adiposity than wild-type mice (t[27] = 5.80, P < 0.00001) (Figure 5C).
Figure 5. Accelerated loss of bodyweight in fasting MCH−/− mice.
(A) During a 24 h period in the open field with ad libitum access to chow, MCH−/− and wild-type mice consumed similar amounts of food (g/24 h/g bodyweight + SEM) (B) During a subsequent 24 h period in the open field without access to food, MCH−/− mice lost more weight (cf. Figure 2) (C) MCH−/− mice exhibited reduced adiposity as determined NMR body mass analysis under baseline conditions. A significant difference between wild-type and MCH−/− mice is indicated by an asterisk.
The sleep homeostat is unaffected by MCH deficiency
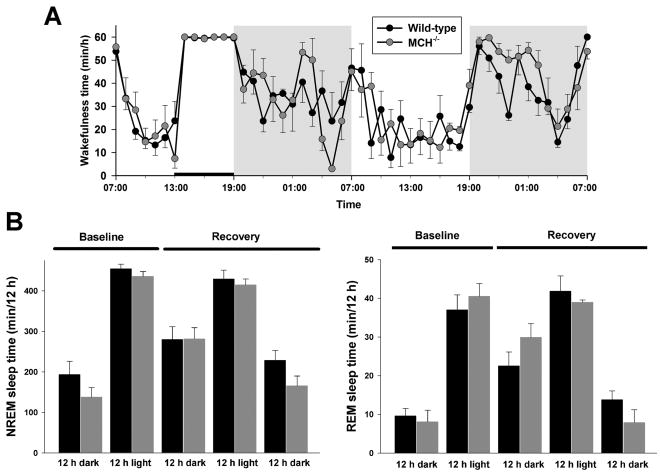
To examine if the abnormal response to fasting in the MCH−/− mice reflected a more general failure to cope with homeostatic disequilibrium, we compared the response to 6 h of total sleep deprivation in this genotype with that of wild-type mice (Figure 6). The rebound in total NREM sleep time over the first 12 h after sleep deprivation was not different between MCH−/− and wild-type mice. Thus, during this period, the NREM sleep time was 281.7 ± 27.4 min versus 280.0 ± 31.4 min (P > 0.9), MCH−/− versus wild-type mice respectively (Figure 6B). Similarly, the rebound in REM sleep time was the same in both genotypes during the first 12 h after sleep deprivation: 29.9 ± 3.5 min versus 22.5 ± 3.6 min, MCH−/− and wild-type mice respectively (P = 0.2). As a measure of the depth of NREM sleep, we also compared δ (1 – 4 Hz) power in the EEG spectrum of NREM sleep recorded during the baseline period with that recorded immediately after sleep deprivation. The two genotypes did not differ in normalized (i.e., dimensionless) δ power either before (36.5 ± 1.1 versus 36.9 ± 1.5, wild-type and MCH−/− mice, respectively, P = 0.8), or after (52.0 ± 5.4 versus 48.3 ± 3.1, wild-type and MCH−/− mice, respectively, P = 0.6) sleep deprivation. Delta power was significantly and similarly increased by sleep deprivation in both genotypes: a 42% increase in wild-type mice (t[3] = 3.2, P < 0.05) and a 31% increase in MCH−/− mice (t[3] = 4.0, P < 0.03).
Figure 6. The sleep homeostat in MCH−/− mice is normal.
Mice were deprived of sleep by gentle handling for the second 6 h of the light phase, marked by the horizontal bar. (A) The mean hourly times spent awake (min/h ± SEM) in the two genotypes during the subsequent 36 h of recovery sleep time, beginning at the onset of the dark phase (cf. Figure 1). (B) The 12-h NREM and REM sleep times (min/12 h + SEM), during the recovery phase are not different.
DISCUSSION
Indirect evidence suggested that MCH might modulate vigilance states (Kilduff and de Lecea, 2001, Verret et al., 2003, van den Pol et al., 2004, Modirrousta et al., 2005), and MCH-1 receptor antagonists decrease sleep, including REM sleep, and increase wakefulness (Ahnaou et al., 2008). Here we have demonstrated for the first time that, under baseline conditions, MCH−/− mice exhibit consolidated, increased wakefulness during the dark phase without any rebound in the light phase. Our data thus provide behavioral support for indications from electrophysiological studies that MCH could inhibit the ascending arousal systems (van den Pol et al., 2004, Bayer et al., 2005). Notably, however, the increased wakefulness in MCH−/− mice was not associated with differences in EEG spectral power. In response to food deprivation, a stress that involves energy homeostasis, MCH−/− mice exhibited an excessive reduction in REM sleep time, without showing any EEG spectral differences relative to wild-type mice. During fasting, MCH−/− mice also rapidly became hyperactive, suggesting unmitigated food-seeking behavior. However, despite the baseline deficit in sleep in MCH−/− mice, their homeostatic sleep response to the stress of a limited period of sleep deprivation appeared essentially normal, in terms of both the recovery of NREM and REM sleep time and δ (1 – 4 Hz) power in the EEG during recovery NREM sleep.
The baseline deficit in sleep in MCH−/− mice is consistent with the effects on sleep of MCH-1 receptor antagonists (Ahnaou et al., 2008) and central administration of MCH (Verret et al., 2003), but must be reconciled with recent findings indicating that MCH-1R−/− mice show increased REM sleep (Adamantidis et al., 2008). However, despite evidence of only a single functional MCH receptor in this species, MCH−/− and MCH-1R−/− mice exhibit other phenotypic differences. MCH−/− mice are lean, hypermetabolic and relatively hypophagic (Shimada et al., 1998). In contrast, MCH-1R−/− mice are relatively hyperphagic but also lean, probably due to additional energy expenditure from increased locomotor activity (Marsh et al., 2002). Although differences in genetic background and experimental conditions could contribute to these observations, another explanation is that MCH−/− mice are generated as prepro-MCH null and two other possible neuropeptides encoded by this gene are therefore also lacking in this genotype (Marsh et al., 2002). The functional significance and receptor binding profiles of these products, neuropeptide E1 (NE1) and neuropeptide GE (NGE), are currently unknown. Comparing our results, which are based on the MCH−/− mice of Shimada et al. (1998), with the data of Adamantidis et al. (2008) suggest that NE1 and NGE may thus play a role in sleep regulation in addition to energy balance. Additional experiments are needed to investigate this possibility.
When MCH−/− mice were unable to feed, they exhibited a remarkable hyperactivity which, though peaking soon after the removal of food, continued throughout the active phase. This apparently maladaptive behavior correlated with accelerated weight loss, suggesting an inability to balance the search for food with energy conservation through rest (Willie et al., 2001). Thus MCH gene products may function to damp excessive food-seeking behavior when appropriate in favor of energy conservation: MCH−/− mice would therefore be expected to succumb to starvation more rapidly than normal mice. However, MCH−/− mice have reduced fat mass under baseline conditions and hence less reserves to deal with a period of fasting than wild-type mice. Therefore an alternative explanation for the marked hyperactivity is that the MCH−/− mice would have a more urgent need, or a stronger drive to search for food under conditions of negative energy balance. This drive would likely be expressed as accelerated and excessive food seeking combined with hyperactivity, and further studies are needed to confirm this possibility. For example, the bodyweights and adiposity of both genotypes could be matched either by feeding the MCH−/− mice a high fat diet, or the wild-type mice a calorically restricted diet, prior to the onset of the fasting period. However, such a study could exacerbate confounding differences between the genotypes such as differential hedonistic taste preferences or activation of the hypothalamic-pituitary-adrenal (HPA) axis under high fat feeding conditions (Tannenbaum et al., 1997, Ziotopoulou et al., 2000). Conversely, the potential chronic effects of a calorically restricted diet on sleep and behavior in the wild-type mice would need to be considered. Our results, despite these reservations, are therefore best viewed as a specific behavioral response in MCH−/− mice under a defined set of experimental conditions, which were chosen to correspond to a period when mice naturally initiate maximal feeding. Under such circumstances, we note a very rapidly increasing rate of hyperactivity beginning within 2 hours of the onset of a fast and, importantly, this is combined with specific REM sleep changes.
Increased wakefulness, abnormal suppression of REM sleep and hyperactivity in the MCH−/− mice in response to the stress of fasting could also indicate a more general inability to adapt to stressful stimuli in this genotype. Although we found no difference in the homeostatic sleep response to mild sleep deprivation, additional studies might reveal differences in the ability to cope with stress. Such studies, for example, could employ a more prolonged and invasive technique of sleep deprivation or use other paradigms to apply exogenous stressors. However, in this regard we note that baseline plasma corticosterone is normal in MCH−/− mice (Shimada et al., 1998), and several studies have shown that increases in MCH lead to higher levels of plasma corticosterone (Jezova et al., 1992, Kennedy et al., 2003, Smith et al., 2006). Nevertheless, results at variance with the latter finding have also been reported (Bluet-Pajot et al., 1995, Ludwig et al., 1998), indicating that at least under some circumstances MCH acts to suppress the responsiveness of the HPA axis and reduce plasma corticosterone levels, though circadian factors are likely to be important when interpreting such results. Multiple interactions between appetitive and stress factors also make any interpretation difficult and, like the potential role of decreased fat mass, will require further experimentation. Indeed, fat mass and the response of the HPA axis to stress are themselves related since circulating leptin and corticosterone levels are linked (Ahima et al., 1996, Jeong et al., 2004). But MCH acts as a downstream central mediator of energy balance through both plasma glucose levels (Burdakov et al., 2005) and the leptin signal (Segal-Lieberman et al., 2003). Hence lack of MCH is more likely to be critical for an inappropriate central response to a change in metabolic status, including glucocorticoid levels, rather than vice versa.
Under baseline conditions, MCH−/− mice spent less time in NREM sleep and this was associated with normal sleep and wakefulness bout durations. But, during fasting, despite a reduction in NREM sleep time that corresponded to that of wild-type mice, MCH−/− mice exhibited a disproportionate decrease in REM sleep time. This decrease in REM sleep resulted from a reduced number of REM sleep bouts. Indeed, some MCH−/− mice exhibited no or very few REM sleep episodes throughout the 24 h fasting period. Furthermore, when REM sleep was present in fasting MCH−/− mice, the episode duration was unchanged. In contrast, fasting wild-type mice showed a significant increase in REM sleep mean bout length during fasting, consistent with a compensatory consolidation of this state into fewer and longer episodes. Taken together, these results suggest that when MCH−/− mice are fasting they are unable to initiate normally or maintain a REM sleep episode. This may be another facet of the limited ability of MCH−/− mice to balance energy expenditure since REM sleep is energetically relatively expensive (Ramm and Frost, 1983, 1986). Interestingly, Ahnaou et al. (2008) also reported a reduction in REM sleep episode duration after pharmacological antagonism of the MCH-1 receptor, though vigilance state fragmentation was not limited to REM sleep under these conditions and the mice were fed normally. Overall, the data are consistent with a role of MCH in sleep state maintenance, most likely in relation to metabolic status. The differences between our study and that of Ahnaou et al. (2008) in vigilance state episode duration may therefore relate to the lack of the other prepro-MCH gene products in the MCH−/− mice, as noted above.
The relationship, if any, between behavioral hyperactivity and the change in REM sleep that is evident during fasting in MCH−/− mice remains to be determined. However, the fact that both result from MCH inactivation is additional evidence to suggest that the neuropeptide may play a role in modifying behavior, including the expression of REM sleep, on the basis of metabolic status. In fact, previous pharmacological data, as noted above, have consistently linked the neuropeptide with the expression of REM sleep (Verret et al., 2003, Ahnaou et al., 2008) and the activity of MCH neurons is increased with REM sleep during sleep recovery (Modirrousta et al., 2005). Furthermore, though in a contrasting direction, the increased REM sleep time observed in MCH-1R−/− mice also relates MCH to the expression of REM sleep (Adamantidis et al., 2008). However, these prior reports do not address the potentially important specificity of this relationship to energy balance. Hence, a recently released report of results from Phase I clinical trials of a novel MCH-1 receptor antagonist (NGD-4715) may be more directly relevant to the present study. MCH receptor antagonists are currently being investigated for their potential anti-obesity properties (Handlon and Zhou, 2006). NGD-4715 exhibited this profile as it was found to reduce food intake in rats without increasing locomotor activity. In the most recent clinical trial, the compound was administered to healthy obese subjects (8 treated, 4 placebo) under conditions of caloric restriction (http://phx.corporateir.net/phoenix.zhtml?c=96629&p=irol-newsArticle&ID=1093057&highlight). Unlike an earlier trial of the same compound when the subjects were placed under a high caloric diet, half the treated subjects on the restricted diet reported vivid dreams and interrupted sleep during the first week of the trial. Without a corresponding polysomnographic evaluation, the sleep changes that caused these subjective reports remain unknown. However, we speculate that the combination of MCH blockade and food restriction, as observed in the MCH−/− mice, may have resulted in REM sleep abnormalities. Indeed, the reports from the subjects are similar to those recorded during previous human studies under conditions of increased need for REM sleep or the inability to express normal amounts of REM sleep (Cartwright and Monroe, 1968, Schlosberg and Benjamin, 1978).
In conclusion, our data reveal that the link between energy homeostasis and behavioral adaptation, including vigilance state regulation, involves MCH. Additional studies will be required if MCH-1 receptor antagonists are to be used in the treatment of obesity.
Acknowledgments
We thank D. Sierra and D Koovakkattu for programming and data analysis, S.A. Dixon for technical support, and W.H. Koster and J.E. Krause, formerly of Neurogen Corporation, for unpublished results from preclinical and clinical studies with NGD-4715. M.Y. is an investigator of the Howard Hughes Medical Institute. J.T.W. was previously a fellow of the Medical Scientist Training program of the University of Texas Southwestern Medical Center at Dallas. This work has been supported in part by research grants from the Perot Family Foundation and Exploratory Research for Advanced Technology of Japan Science and Technology Corporation to M.Y.
Abbreviations
- EEG
electroencephalography
- EMG
electromyography
- FFT
fast Fourier transform
- HPA
hypothalamic-pituitary-adrenal
- icv
intracerebroventricular
- MCH
melanin-concentrating hormone
- NMR
nuclear magnetic resonance
- NREM
non-rapid eye movement
- PCR
polymerase chain reaction
- REM
rapid eye movement
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamantidis A, Salvert D, Goutagny R, Lakaye B, Gervasoni D, Grisar T, Luppi PH, Fort P. Sleep architecture of the melanin-concentrating hormone receptor 1-knockout mice. Eur J Neurosci. 2008;27:1793–1800. doi: 10.1111/j.1460-9568.2008.06129.x. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- Ahnaou A, Drinkenburg WH, Bouwknecht JA, Alcazar J, Steckler T, Dautzenberg FM. Blocking melanin-concentrating hormone MCH1 receptor affects rat sleep-wake architecture. Eur J Pharmacol. 2008;579:177–188. doi: 10.1016/j.ejphar.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Bayer L, Eggermann E, Serafin M, Grivel J, Machard D, Muhlethaler M, Jones BE. Opposite effects of noradrenaline and acetylcholine upon hypocretin/orexin versus melanin concentrating hormone neurons in rat hypothalamic slices. Neuroscience. 2005;130:807–811. doi: 10.1016/j.neuroscience.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- Bluet-Pajot MT, Presse F, Voko Z, Hoeger C, Mounier F, Epelbaum J, Nahon JL. Neuropeptide-E-I antagonizes the action of melanin-concentrating hormone on stress-induced release of adrenocorticotropin in the rat. J Neuroendocrinol. 1995;7:297–303. doi: 10.1111/j.1365-2826.1995.tb00761.x. [DOI] [PubMed] [Google Scholar]
- Borbely AA. Sleep in the rat during food deprivation and subsequent restitution of food. Brain Res. 1977;124:457–471. doi: 10.1016/0006-8993(77)90947-7. [DOI] [PubMed] [Google Scholar]
- Borbely AA, Tobler I, Hanagasioglu M. Effect of sleep deprivation on sleep and EEG power spectra in the rat. Behav Brain Res. 1984;14:171–182. doi: 10.1016/0166-4328(84)90186-4. [DOI] [PubMed] [Google Scholar]
- Borowsky B, Durkin MM, Ogozalek K, Marzabadi MR, DeLeon J, Lagu B, Heurich R, Lichtblau H, Shaposhnik Z, Daniewska I, Blackburn TP, Branchek TA, Gerald C, Vaysse PJ, Forray C. Antidepressant, anxiolytic and anorectic effects of a melanin-concentrating hormone-1 receptor antagonist. Nat Med. 2002;8:825–830. doi: 10.1038/nm741. [DOI] [PubMed] [Google Scholar]
- Burdakov D, Gerasimenko O, Verkhratsky A. Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ. J Neurosci. 2005;25:2429–2433. doi: 10.1523/JNEUROSCI.4925-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright RD, Monroe LJ. Relation of dreaming and REM sleep: the effects of REM deprivation under two conditions. J Pers Soc Psychol. 1968;10:69–74. doi: 10.1037/h0026277. [DOI] [PubMed] [Google Scholar]
- Chaki S, Funakoshi T, Hirota-Okuno S, Nishiguchi M, Shimazaki T, Iijima M, Grottick AJ, Kanuma K, Omodera K, Sekiguchi Y, Okuyama S, Tran TA, Semple G, Thomsen W. Anxiolytic- and antidepressant-like profile of ATC0065 and ATC0175: nonpeptidic and orally active melanin-concentrating hormone receptor 1 antagonists. J Pharmacol Exp Ther. 2005;313:831–839. doi: 10.1124/jpet.104.081711. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewasmes G, Duchamp C, Minaire Y. Sleep changes in fasting rats. Physiol Behav. 1989;46:179–184. doi: 10.1016/0031-9384(89)90252-7. [DOI] [PubMed] [Google Scholar]
- Dudley CA, Erbel-Sieler C, Estill SJ, Reick M, Franken P, Pitts S, McKnight SL. Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science. 2003;301:379–383. doi: 10.1126/science.1082795. [DOI] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Gao XB, van den Pol AN. Melanin concentrating hormone depresses synaptic activity of glutamate and GABA neurons from rat lateral hypothalamus. J Physiol. 2001;533:237–252. doi: 10.1111/j.1469-7793.2001.0237b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XB, van den Pol AN. Melanin-concentrating hormone depresses L-, N-, and P/Q-type voltage- dependent calcium channels in rat lateral hypothalamic neurons. J Physiol. 2002;542:273–286. doi: 10.1113/jphysiol.2002.019372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handlon AL, Zhou H. Melanin-concentrating hormone-1 receptor antagonists for the treatment of obesity. J Med Chem. 2006;49:4017–4022. doi: 10.1021/jm058239j. [DOI] [PubMed] [Google Scholar]
- Jeong KH, Sakihara S, Widmaier EP, Majzoub JA. Impaired leptin expression and abnormal response to fasting in corticotropin-releasing hormone-deficient mice. Endocrinology. 2004;145:3174–3181. doi: 10.1210/en.2003-1558. [DOI] [PubMed] [Google Scholar]
- Jezova D, Bartanusz V, Westergren I, Johansson BB, Rivier J, Vale W, Rivier C. Rat melanin-concentrating hormone stimulates adrenocorticotropin secretion: evidence for a site of action in brain regions protected by the blood-brain barrier. Endocrinology. 1992;130:1024–1029. doi: 10.1210/endo.130.2.1310274. [DOI] [PubMed] [Google Scholar]
- Kela J, Salmi P, Rimondini-Giorgini R, Heilig M, Wahlestedt C. Behavioural analysis of melanin-concentrating hormone in rats: evidence for orexigenic and anxiolytic properties. Regul Pept. 2003;114:109–114. doi: 10.1016/s0167-0115(03)00114-9. [DOI] [PubMed] [Google Scholar]
- Kennedy AR, Todd JF, Dhillo WS, Seal LJ, Ghatei MA, O’Toole CP, Jones M, Witty D, Winborne K, Riley G, Hervieu G, Wilson S, Bloom SR. Effect of direct injection of melanin-concentrating hormone into the paraventricular nucleus: further evidence for a stimulatory role in the adrenal axis via SLC-1. Journal of Neuroendocrinology. 2003;15:268–272. doi: 10.1046/j.1365-2826.2003.00997.x. [DOI] [PubMed] [Google Scholar]
- Kilduff TS, de Lecea L. Mapping of the mRNAs for the hypocretin/orexin and melanin- concentrating hormone receptors: networks of overlapping peptide systems. J Comp Neurol. 2001;435:1–5. doi: 10.1002/cne.1189. [DOI] [PubMed] [Google Scholar]
- Ludwig DS, Mountjoy KG, Tatro JB, Gillette JA, Frederich RC, Flier JS, Maratos-Flier E. Melanin-concentrating hormone: a functional melanocortin antagonist in the hypothalamus. Am J Physiol. 1998;274:E627–E633. doi: 10.1152/ajpendo.1998.274.4.E627. [DOI] [PubMed] [Google Scholar]
- Marsh DJ, Weingarth DT, Novi DE, Chen HY, Trumbauer ME, Chen AS, Guan XM, Jiang MM, Feng Y, Camacho RE, Shen Z, Frazier EG, Yu H, Metzger JM, Kuca SJ, Shearman LP, Gopal-Truter S, MacNeil DJ, Strack AM, MacIntyre DE, Van der Ploeg LH, Qian S. Melanin-concentrating hormone 1 receptor-deficient mice are lean, hyperactive, and hyperphagic and have altered metabolism. Proc Natl Acad Sci USA. 2002;99:3240–3245. doi: 10.1073/pnas.052706899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AD, Elsasser TH, Wang PC. Determination of fat and water content in vitro and in vivo by proton nuclear magnetic resonance. J Sci Food Agric. 1991;56:265–276. [Google Scholar]
- Modirrousta M, Mainville L, Jones BE. Orexin and MCH neurons express c-Fos differently after sleep deprivation vs. recovery and bear different adrenergic receptors. Eur J Neurosci. 2005;21:2807–2816. doi: 10.1111/j.1460-9568.2005.04104.x. [DOI] [PubMed] [Google Scholar]
- Monzon ME, De Barioglio SR. Response to novelty after i.c.v. injection of melanin-concentrating hormone (MCH) in rats. Physiol Behav. 1999;67:813–817. doi: 10.1016/s0031-9384(99)00117-1. [DOI] [PubMed] [Google Scholar]
- Ramm P, Frost BJ. Regional metabolic activity in the rat brain during sleep-wake activity. Sleep. 1983;6:196–216. doi: 10.1093/sleep/6.3.196. [DOI] [PubMed] [Google Scholar]
- Ramm P, Frost BJ. Cerebral and local cerebral metabolism in the cat during slow wave and REM sleep. Brain Res. 1986;365:112–124. doi: 10.1016/0006-8993(86)90728-6. [DOI] [PubMed] [Google Scholar]
- Roy M, David NK, Danao JV, Baribault H, Tian H, Giorgetti M. Genetic inactivation of melanin-concentrating hormone receptor subtype 1 (MCHR1) in mice exerts anxiolytic-like behavioral effects. Neuropsychopharmacology. 2006;31:112–120. doi: 10.1038/sj.npp.1300805. [DOI] [PubMed] [Google Scholar]
- Saito Y, Cheng M, Leslie FM, Civelli O. Expression of the melanin-concentrating hormone (MCH) receptor mRNA in the rat brain. J Comp Neurol. 2001;435:26–40. doi: 10.1002/cne.1191. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- Schlosberg A, Benjamin M. Sleep patterns in three acute combat fatigue cases. J Clin Psychiatry. 1978;39:546–549. [PubMed] [Google Scholar]
- Segal-Lieberman G, Bradley RL, Kokkotou E, Carlson M, Trombly DJ, Wang X, Bates S, Myers MG, Jr, Flier JS, Maratos-Flier E. Melanin-concentrating hormone is a critical mediator of the leptin-deficient phenotype. Proc Natl Acad Sci USA. 2003;100:10085–10090. doi: 10.1073/pnas.1633636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- Smith DG, Davis RJ, Rorick-Kehn L, Morin M, Witkin JM, McKinzie DL, Nomikos GG, Gehlert DR. Melanin-concentrating hormone-1 receptor modulates neuroendocrine, behavioral, and corticolimbic neurochemical stress responses in mice. Neuropsychopharmacology. 2006;31:1135–1145. doi: 10.1038/sj.npp.1300913. [DOI] [PubMed] [Google Scholar]
- Tannenbaum BM, Brindley DN, Tannenbaum GS, Dallman MF, McArthur MD, Meaney MJ. High-fat feeding alters both basal and stress-induced hypothalamic-pituitary-adrenal activity in the rat. Am J Physiol. 1997;273:E1168–E1177. doi: 10.1152/ajpendo.1997.273.6.E1168. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Acuna-Goycolea C, Clark KR, Ghosh PK. Physiological properties of hypothalamic MCH neurons identified with selective expression of reporter gene after recombinant virus infection. Neuron. 2004;42:635–652. doi: 10.1016/s0896-6273(04)00251-x. [DOI] [PubMed] [Google Scholar]
- Verret L, Goutagny R, Fort P, Cagnon L, Salvert D, Leger L, Boissard R, Salin P, Peyron C, Luppi PH. A role of melanin-concentrating hormone producing neurons in the central regulation of paradoxical sleep. BMC Neurosci. 2003;4:19. doi: 10.1186/1471-2202-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. 2001;24:429–458. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami K, Sugiyama F, Goto K, Yanagisawa M, Sakurai T. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- Ziotopoulou M, Mantzoros CS, Hileman SM, Flier JS. Differential expression of hypothalamic neuropeptides in the early phase of diet-induced obesity in mice. Am J Physiol Endocrinol Metab. 2000;279:E838–E845. doi: 10.1152/ajpendo.2000.279.4.E838. [DOI] [PubMed] [Google Scholar]