Abstract
DNA interstrand cross-linking agents have been widely used in chemotherapeutic treatment of cancer. The majority of interstrand cross-links (ICLs) in mammalian cells are removed via a complex process that involves the formation of double strand breaks at replication forks, incision of the ICL, and subsequent error-free repair by homologous recombination. How double strand breaks effect the removal of ICLs and the downstream homologous recombination process is not clear. Here, we describe a plasmid-based recombination assay in which one copy of the CFP gene is inactivated by a site-specific psoralen ICL and can be repaired by gene conversion with a mutated homologous donor sequence. We found that the homology dependent recombination (HDR) is inhibited by the ICL. However, when we introduced a double strand break adjacent to the site of the ICL, the removal of the ICL was enhanced and the substrate was funneled into a HDR repair pathway. This process was not dependent on the nucleotide excision repair pathway, but did require the ERCC1-XPF endonuclease and REV3. In addition, both the Fanconi anemia pathway and the mismatch repair protein MSH2 were required for the recombinational repair processing of the ICL. These results suggest that the juxtaposition of an ICL and a DSB stimulates repair of ICLs through a process requiring components of mismatch repair, ERCC1-XPF, REV3, Fanconi anemia proteins, and homologous recombination repair factors.
Keywords: interstrand cross-links, mismatch repair, translesion bypass, homologous recombination
1. Introduction
DNA interstrand cross-links (ICLs) are among the most deleterious of DNA lesions in that they effectively prevent DNA strand unwinding and separation, thus blocking DNA transactions such as transcription and replication [1–3]. DNA interstrand cross-linking agents such as cyclophosphamide and mitomycin C are widely used as chemotherapeutic agents in the treatment of a broad spectrum of cancers. Nonetheless, the molecular mechanisms of ICL repair still remains largely unresolved in mammalian cells. In prokaryotic cells and yeast, the repair of ICLs involves incision by the nucleotide excision repair (NER) pathway on one strand of the cross-linked DNA and a subsequent gap filling step mediated either by homologous recombinational proteins [4,5], or by a translesion bypass DNA polymerase [6,7]. A second round of NER action removes the remaining monoadduct. In yeast, a third poorly understood pathway involving SNM1 may also be present [8,9].
In mammalian cells, two pathways of ICL repair have been identified. The minor homologous recombination independent pathway appears analogous to the E. coli pathway in that it requires processing by NER and translesion bypass by REV3 [10–12]. This pathway has also proved to be highly mutagenic in response to ICL reagents. The existence of the major homologous recombination dependent pathway is evidenced by the hypersensitivity of mutants defective in genes such as XRCC2, XRCC3, and RAD51C to ICL-inducing agents [13]. Other mutants that are hypersensitive to ICL-inducing agents include the NER genes ERCC1 and XPF [14,15], REV3 [10,16–18], and genes found mutated in the human disease Fanconi anemia [19]. This major pathway is not dependent upon NER, since, mutants in components of the NER pathway other than ERCC1 and XPF exhibit only mild sensitivity to ICL agents, and have been shown to be proficient in the unhooking step of nitrogen mustard ICLs in vivo [20]. In all eukaryotic cells examined double-strand breaks have been observed as intermediates of ICL repair, and the repair of these breaks requires the homologous recombination machinery [1]. However, the relationship between double strand break formation and the direct processing or uncoupling of ICLs remains unclear.
In the study presented here, we investigated the effect of a site-specific psoralen ICL on homology dependent recombination (HDR) in vivo using a plasmid-based assay. We found that an ICL stimulates homologous recombination only when a DSB is present near the ICL site. This process was not dependent on the nucleotide excision repair pathway, but did require the ERCC1-XPF endonuclease, MSH2, REV3, and Fanconi anemia, and homologous recombination proteins. These results indicate that a DSB stimulates the repair of ICLs, and therefore, that replication fork collapse may be an obligatory step in the efficient repair of these lesions. They also demonstrate that the ERCC1-XPF endonuclease and MSH2 are required for processing of the ICL prior to recombination-mediated repair.
2. Materials and methods
2.1. Cell lines and proteins
Human lymphoid cell lines were cultured in suspension with RPMI 1640 medium supplemented with 20% fetal calf serum (FCS). Human and hamster cell lines were cultured in minimal essential medium plus 10% FCS. HeLa cells were cultured in Joklit medium plus 10% FCS, and mouse embryonic fibroblasts were cultured in DMEM supplemented with 10% FCS, 0.3 mg/ml L-Glutamine, 1 mM sodium pyruvate.
2.2. Preparation of site-specific interstrand cross-links
The construction of a cross-linked duplex oligonucleotide involved the use of one self-complementary oligonucleotide with the sequence: 5′CCGGGCTAGCCCGGCACA. The letters in bold indicate nucleotide residues involved in the cross-link, and the underlined residues indicate a Nhe I restriction site. Annealing of this oligonucleotide with itself generates an identical 4-nucleotide sticky end at both ends of the duplex. These ends can be ligated to BstX I-digested sticky ends. Two hundred micrograms of this substrate were added to 4,5,8-trimethylpsoralen at 100 ng/μl in 5 mM Tris-HCL, 0.5 mM EDTA, 25 mM NaCl, and 2% ethanol. The sample was irradiated with 365-nm UV light (15 min at 12.5 mW/cm2) to create the interstrand cross-link. The reaction was incubated for 15 min to minimize damage to the oligonucleotide by UV irradiation, and to decrease the possibility of creating multiple adducts per duplex oligonucleotide. The cross-linked oligonucleotide was purified from non-cross-linked DNA by denaturing polyacrylamide gel electrophoresis [21].
2.3. Preparation of DNA Substrates
pECFP-Int-NtCFP was kindly provided by Dr. David Roth. The vector was digested with Pas I, blunt-ended and religated into the same vector to generate pECGP-Int-ReCFP which contains a reversed inactivated CFP gene as a donor for homologous recombination. A linker with the sequence 5′AGATCTCCAGTGTGCTGGTCTGCCCATCACACTGGATGCCCTCAGC containing two BstX I sites was inserted between the EcoR V and Bgl II sites of pECFP-Int-ReCFP to generate pECFP-BstX-ReCFP. After digestion with BstX I, the large fragment was recovered and ligated with oligonucleotides with or without cross-links. The ligation products were purified by CsCl-ethidium bromide gradient centrifugation to obtain both supercoiled and linear plasmids. Randomly cross-linked plasmid DNA was prepared by incubation of supercoiled or linearized pECFPHR with different concentrations of trimethylpsoralen in PBS with UVA irradiation. The cross-linked DNAs were detected by denaturation agarose gel analysis as previously described [21].
2.4. Homology dependent recombination (HDR) assay
Cells were seeded on 6 well plates or 35 mm dishes a day before transfection. DNA was transfected into the cells when confluency reached 50–70% using Fugene HD transfection reagent (Invitrogen). Transfections were carried out using a mixture of 1 μg homologous recombination substrate and 1 μg of pDsRed-N1 according to manufacture’s instructions. Thirty hours after transfection the cells were checked under a fluorescent microscope and then harvested for flow cytometry analysis.
2.5. Flow cytometry
Cells were harvested by trypsinization and fixed in PBS with 1% paraformaldehyde for 10 min at room temperature. Cells were subsequently resuspended in PBS and analyzed on a BD FACS Aria flow cytometer and 300,000 events were analyzed for each transfection using FlowJo software. The analysis compared CFP signal to FITC signal in order to distinguish CFP-positive cells from autofluorescent cells. Red fluorescent signal was also quantitated to adjust for differences in transfection efficiency between samples. The proportion of cyan fluorescent cells to red fluorescent cells represents the homologous recombination efficiency. The ratio of homologous recombination efficiency of linear plasmid with or without ICL was used to quantitate the stimulation of ICL on HDR.
2.6. Plasmid extraction
HEK293T cells were seeded in 10 cm plates and transfected with 10 μg homologous recombination substrates using Fugene HD, and harvested 48 hours post transfection. Cells were lysed in 0.8 ml Hirt buffer (0.6% SDS, 0.01M EDTA, pH7.4) [22]. Then 0.2 ml of 5M NaCl were added and the lysate chilled on ice for at least one hour, and subsequently spun for 30 min at 12000g at 4°C. The supernatant was removed and extracted with phenol/chloroform twice, and DNA was precipitated with ethanol. The DNA pellet was resuspended and digested with DpnII. After digestion, the DNA was extracted, precipitated, resuspended, and transformed into XL-Gold cells.
3. Results
3.1. ICLs inhibits spontaneous homologous recombination
It has been clearly established that DNA ICLs are repaired via homologous recombination [1–3]. To further assess the mechanism of ICL-dependent homologous recombination, we set up an in vivo plasmid-based assay. We inserted a small oligonucleotide containing a single site-specific psoralen ICL into the ECFP gene to block its expression. The removal of the ICL alone will not reactivate ECFP due to a frameshift mutation introduced by the inserted oligonucleotide. The ECFP gene can be reactivated only by first uncoupling of the ICL and subsequent repair of the frameshift mutation via homology dependent recombination (HDR). The donor sequence for HDR was provided by an inverted repeat of the ECFP gene lacking a promoter and start codon (Fig. 1A). This configuration does not allow repair of the ECFP gene by intramolecular single-strand annealing. The modified plasmid was termed pECFPHR, and by monitoring cyan fluorescence we were able to evaluate the efficiency of HDR. It should be noted that this plasmid contained the SV40 origin of DNA replication, and thus can replicate in cells expressing the large T antigen. Transfection of pECFPHR without an ICL into HeLa cells resulted in only a low level of spontaneous HDR of approximately 0.3%. Surprisingly, when the site-specific ICL was present in the plasmid, the HDR decreased to 0.17% indicating that the single ICL inhibited HDR events by about two-fold in cells unable to replicate the plasmid (Fig. 1B). To further confirm this result, we randomly introduced increasing numbers of ICLs into pECFPHR by exposing the plasmid to increasing concentrations of psoralen with constant UVA treatment (Fig. 1C). Upon transfection of these substrates into HeLa cells, we observed that increasing the number of ICLs per plasmid strongly depressed the degree of HDR (Fig. 1D). We conclude that ICLs actually inhibit spontaneous HDR in our assay system.
Fig. 1.
DNA ICLs inhibits spontaneous HDR. (A) Schematic showing the pECFPHR plasmid substrate. NtECFP indicates the amino terminal segment of the ECFP gene, and CtECFP indicates the carboxy terminal segment of the ECFP gene. CMV indicates the cytomegalovirus promoter. SV40 indicates the SV40 origin of DNA replication. The single site-specific psoralen ICL is indicated by a “X”. (B) Site-specific ICL inhibits HDR. pECFPHR with or without a site-specific ICL was transfected into HeLa cells and HDR quantitated by FACS. The “p value” is indicated. (C) Quantitation of average ICL number per molecule for substrates with random ICLs. The uncross-linked portion was quantitated and used to estimate average number of ICLs per molecule by the Poisson distribution. (D) Random ICLs inhibit HDR. The pECFPHR substrates shown in (C) were assayed for HDR after transfection into HeLa cells. The value for the untreated plasmids (0) were normalized to 100%.
3.2. ICLs stimulate HDR in the presence of DSBs
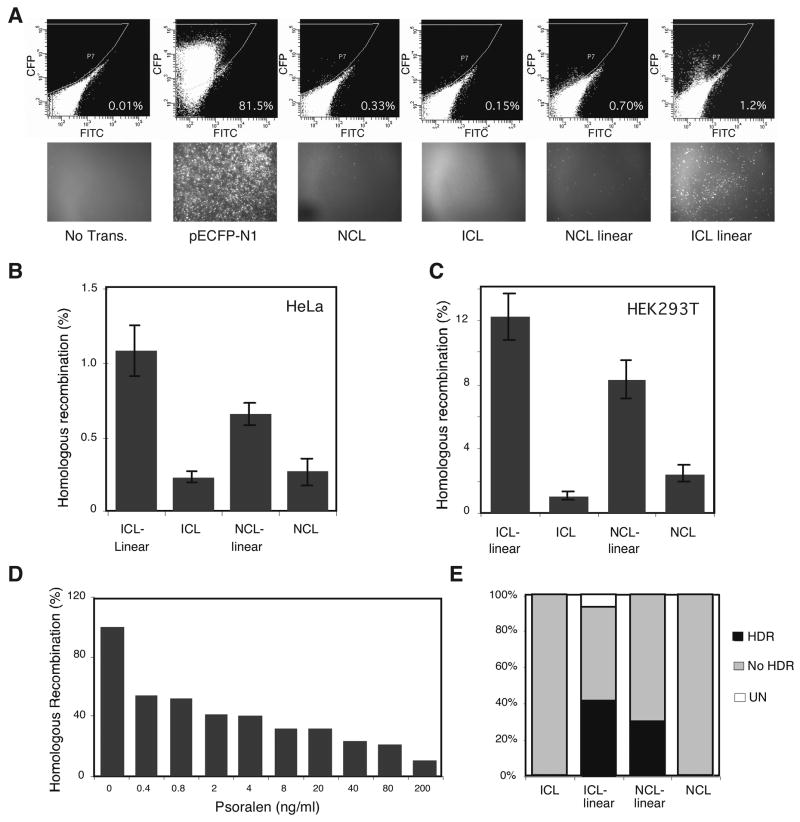
The results described above indicate that an ICL per se does not stimulate homologous recombination in our plasmid substrate. Nevertheless, ICLs do stimulate recombination in vivo possibly by causing collapse of the replication fork and the resulting creation of DSBs. To test this model, we linearized the pECFPHR plasmid near the site of the cross-link (20 bp) in order to mimic a collapsed replication fork at an ICL, and then examined the HDR efficiency of this substrate. Compared to supercoiled, noncross-linked plasmid, introduction of a DSB stimulated HDR up to 2.5 fold in HeLa cells, and in the cross-linked substrates the DSB stimulated HDR approximately 5 fold (Fig. 2A, B). Thus, in an intact plasmid that is not replicated ICLs inhibited HDR, whereas, when placed near a DSB ICLs were found to be stimulatory to HDR. The same general pattern was also observed in HEK293T cells, although the overall levels of HDR were much higher in this cell line presumably due to replication of repaired plasmids (Fig. 2C). We also tested the primary human fibroblast cell line IMR90 and similar results were observed (data not shown). In the above experiments the ICL was placed 20 bp from the DSB and changing this distance to 11 bp did not affect the stimulation of the ICL on HDR (data not shown). In order to determine if the placement of the ICL near the DSB was important for the observed stimulation of HDR, we examined whether random introduction of ICLs would have a similar affect. As shown (Fig. 2D), random introduction of ICLs into a linear molecule also led to a reduction in HDR, although to a somewhat greater extent than observed in the supercoiled substrate (Fig. 1D). To further address this issue, we used Hirt extraction [22] to rescue transfected plasmids from HEK293T cells, and performed a digestion with Dpn II to remove replicated molecules. This latter step was undertaken so that replication of the repaired plasmids would not bias the final outcome. The samples were then transfected into E. coli and DNAs from resulting colonies were examined for the presence of diagnostic Bgl II and Nhe I restriction enzyme sites in the inserted oligonucleotide by gel electrophoresis. The absence of such sites and the fact that the sizes of the plasmids were as expected indicated repair by HDR, while the presence of these sites suggested repair by NER and translesion bypass synthesis [10–12]. As shown (Fig. 2E), plasmids repaired by HDR were only observed after linearization, and usage of this pathway was increased by the presence of the ICL. Taken together, these results indicate that the proximity of the ICL near the DSB was critical for the observed increase of HDR, suggesting that repair processing of the ICL stimulates the formation of a recombinagenic substrate.
Fig. 2.
ICLs stimulate HDR in the presence of DSBs (A) Flow cytometry profile and fluorescent microscopic images of HeLa cells without transfection, or transfection with pECFP-N1, pECFPHR (NCL), pECFPHR with a site-specific ICL (ICL), linear pECFPHR (NCL linear), and linear pECFPHR with a site-specific ICL (ICL linear). The numbers in the lower right hand corner of the upper panels indicate the percentage of CFP positive cells. (B,C) ICLs stimulate HDR in presence of DSBs in HeLa and HEK293T cells. Linear or supercoiled pECFPHR with or without a site-specific ICL were assayed by FACS for HDR. (D) Random ICLs inhibit HDR in linearized pECFPHR. Linearized pECFPHR was treated with the indicated concentrations of trimethypsoralen and UVA irradiation and assayed for HDR. (E) Analysis of ICL repair products recovered from HEK293T cells upon transfection of the indicated substrates (described in (A)). The percentages of the products derived from homology dependent recombination (HDR), or exhibit no recombination (no HDR), or from an unknown mechanism (UN) are indicated. A total of 20 clones were analyzed for each substrate.
3.3. Role of HR and NER/Translesion Bypass in ICL-mediated stimulation of HDR
Since we have shown above that an ICL in close proximity to a DSB stimulates homologous recombination, it was of interest to determine the factors that are involved in mediating this reaction. We first examined two hamster cell lines, Irs1 and Irs1SF, that are defective in the XRCC2 and XRCC3 genes, respectively [23]. These genes are homologues of RAD51 and are known to be involved in homologous recombination. In both mutant cell lines no stimulation of HDR by the ICL was observed, while it was observed in wild-type cell lines (Fig. 3A). A RAD51D knockout cell line and its complemented cell line were also examined, and similar results were observed (Fig. 3A). It is interesting to note that significant background recombination (approximately 25% of wild-type) was observed in the mutant cell lines indicating that other pathways such as intermolecular single-strand annealing may occur in these cells.
Fig. 3.
Identification of factors involved in the DSB-stimulated processing of ICLs in the homologous recombination pathway. (A) Effect of homologous recombination factors on HDR assay. HDR was determined in the XRCC2-deficient cell line Irs1 and its parental cell line V79, the XRCC3-deficient cell line Irs1SF and its parental cell line AA8, a RAD51D knockout cell line and the RAD51D knockout cell line complemented with an artificial chromosome (SAC) carrying the RAD51D gene. A value of “1” indicates the level of HDR in the NCL pECFPHR substrate. (B) Effect of nucleotide excision repair pathway on HDR assay. HDR was determined in AA8, the ERCC1-deficient cell line UV20, the XPF-deficient cell line UV41, the XPB-deficient cell line UV24, and the XPG-deficient cell line UV135. (C) Effect of Rev3 on HDR. HDR determined in two rev3− − MEF clones, 1.2 and 1.3, and a rev3+/+ MEF clone. (D) Effect of the Fanconi anemia pathway on HDR. HDR determined in FANCD2-, FANCG-, and FANCA-deficient cell lines and their complemented cell lines. (E) Effect of mismatch repair pathway on HDR. HDR was determined in the MSH2-deficient cell lines Lovo and Hec59, the MLH1-deficient cell lines SW48 and A2780/CP70, the MSH6-deficient cell line DLD1, and a Hec59 cell line complemented with chromosome 2.
It has been established in mammalian cells that the NER pathway is required for the removal of ICLs via a recombination-independent pathway that also requires translesion bypass synthesis mediated by REV3 [10–12]. This mechanism is considered to be a minor pathway that occurs during the G1 phase of the cell cycle. However, NER-deficient cells in general are not highly sensitive to cross-linking agents, and current evidence indicates that the NER pathway is not required for homologous recombination-dependent ICL repair that occurs during S phase [20]. The ERCC1-XPF endonuclease, however, is known to be essential for processing of ICLs both in vivo and in vitro [20,21,24,25]. To determine the requirement for NER factors in our plasmid-based assay, we examined several cell lines that are deficient in NER. Consistent with previous results, we found that the Chinese hamster cell lines UV24 and UV135, which are deficient in XPB and XPG, respectively, were proficient in ICL-stimulated HDR (Fig. 3B). Furthermore, the cell lines UV20 and UV41 that are defective in ERCC1 and XPF, respectively, are deficient in the assay. These results indicate that the ERCC1-XPF heterodimer, but not the intact NER pathway is required for the ICL-induced stimulation of HDR.
Rev3 cells have also been shown to be highly sensitive to ICL-inducing agents [16–18]. We therefore examined MEF cells that contain a homozygous disruption of Rev3 in our assay. Compared to control cells, the mutant cells exhibited a dramatic reduction in ICL-stimulated HDR (Fig. 3C). Taken together, these findings are consistent with previous in vitro and in vivo findings on the role of NER, translesion bypass, and homologous recombination in ICL repair, and thus validate our assay as representative of repair of this class of lesions.
3.4. Role of the Fanconi anemia pathway in ICL-mediated stimulation of HDR
Fanconi anemia (FA) is a multigenic recessive cancer susceptibility syndrome [19]. FA cells are hypersensitive to DNA interstrand cross-linking reagents, which indicates the importance of these proteins in the processing of ICL lesions. To assess the role of the FA pathway in ICL-stimulated HDR, assays were carried out in FANCA, FANCG, and FANCD2 cells and their respective complemented cells. In each case the complemented cells exhibited an increase in ICL-stimulated HDR (Fig. 3D) indicating that the FA pathway is involved in the repair of ICLs in our plasmid-based assay.
3.5. Role of mismatch repair proteins in ICL-mediated stimulation of HDR
Recently, we and others have shown that components of the mismatch repair pathway play an important role in the processing of DNA ICLs [21,26,27]. We, therefore, examined several mismatch repair-deficient cell lines in our HDR assay. As shown (Fig. 3E), two cell lines defective for MSH2 (Hec 59 and Lovo) did not exhibit stimulation by the ICL in the HDR assay, while cell lines defective for MSH6 (DLD1 and A2780/CP70) or MLH1 (SW48) exhibited ICL stimulation. These results are consistent with our previous biochemical findings showing that MutSβ, but not MutSα or MLH1, were required for processing of psoralen ICLs [21].
4.0. Discussion
The mechanisms by which interstrand cross-links are repaired in mammalian cells are incompletely understood. This is particularly true of the early stages of repair in which these lesions are recognized and incised or uncoupled. A clear role for ERCC1-XPF in the uncoupling step has been established both in vitro and in vivo [20,21,25,28–31], however, it is unlikely that this complex can carry out this step without the assistance of additional factors. We previously showed a requirement for the mismatch repair factor MutSβ in the early stage of ICL processing using an in vitro assay termed CRS (for cross-link induced repair synthesis) [21], and several other reports have demonstrated a role for mismatch repair components in ICL removal in vivo [26,27,32,33]. In addition, MSH2 and ERCC1-XPF have been shown to interact by co-IP experiments [26]. We have also shown an involvement of RPA, WRN, and a four protein complex composed of PRP19/PSO4, CDC5L, SPF27, and PLRG1 in ICL repair processing using the CRS assay mentioned above [34]. The CRS assay measures the initial recognition and processing of the ICL, and thus the factors required for this assay are involved in the uncoupling stage of repair. In addition, however, ICLs cause the formation of DSBs in the genome presumably as a result of replication fork collapse. The stabilization and repair of the collapsed fork is currently thought to require the Fanconi anemia and other homologous recombination proteins [35]. Cells deficient in the translesion bypass polymerase REV3 are also highly sensitive to ICL inducing agents suggesting that this enzyme also has a role in the recombination-dependent pathway of ICL repair [16–18].
In our work reported here, we have investigated the affect of ICLs on an in vivo assay measuring HDR in a plasmid-based system. Surprisingly, the insertion of a site-specific ICL into the reporter gene in a circular plasmid actually reduced the level of background recombination, and this depression of HDR was further suppressed by the introduction of increasing numbers of random ICLs. Thus, in a circular plasmid that is not replicated, ICLs are inhibitory to recombination, and would be removed by the NER/translesion bypass mechanism [10–12]. In vivo, collapsed forks likely contain an ICL in close proximity to the DSB, we therefore introduced a DSB in our plasmid substrate near the site of the ICL to mimic this structure. In this configuration the ICL was found to stimulate recombination, while randomly introduced ICLs were inhibitory to recombination in the linearized plasmid. Thus, the combination of a DSB and an ICL in close proximity produced a highly recombinagenic substrate.
Using this assay we examined a number of mutants known to be sensitive to ICL-inducing agents. Genes required for homologous recombinational repair of DSBs such as XRCC2, XRCC3, FANCA, FANCD2, and FANCG were all found to be deficient in ICL-induced stimulation of HDR. Mutants defective in ERCC1 and XPF genes were also deficient in the assay, while mutants deficient in other NER genes such as XPB and XPG were shown to have a normal level of HDR stimulation. Also, a mutant cell line defective in REV3 was also highly deficient in the assay. These results are consistent with the response of these various mutants to cross-linking agents, and thus validate our assay as being representative of ICL repair. In addition, we also examined a number of mutants defective in mismatch repair. Consistent with our previous biochemical findings [21], mutants defective in MSH2, but not MSH6 or MLH1, failed to show ICL-induced stimulation of HDR. Taken together, our findings indicate that components of mismatch repair and NER, and the Fanconi anemia and homologous recombination pathways cooperate in the repair of ICLs at collapsed replication forks.
Our findings thus both confirm and extend knowledge about the mechanisms of ICL repair. Two processes appear to occur at ICLs during S phase. Due to the presence of the lesion the fork first stalls and then collapses with the formation of a DSB. The collapse of the fork has been shown to be dependent upon MUS81-EME1 [36]. In addition, there is repair processing of the ICL to effect its uncoupling. The relationship between these two processes is not completely clear at present. While the function of ERCC1-XPF has been shown not to be required for the formation of DSBs, it is required for the resolution of these breaks [29], suggesting that fork collapse does not depend on uncoupling. Whether uncoupling depends on fork collapse is currently unknown. Our findings, reported here demonstrate that the mismatch repair factor MSH2 acts, presumably in concert with ERCC1-XPF, to uncouple the ICL [21]. Our previous findings have also implicated RPA, WRN, and a complex composed of PRP19, CDC5L, PLRG1, and SPF27 in the uncoupling process [34,37]. Subsequent to the uncoupling of the ICL, REV3 would act to bypass the remaining monoadduct. These initial processings of the ICL would allow for the subsequent fork restoration brought about by recombination reactions carried out by Fanconi anemia and homologous recombination proteins. Since the NER pathway is not required for recombination in our substrate, monoadduct removal may not be a required step, and may be circumvented by a second translesion bypass after fork restoration. A model depicting these considerations is shown in Fig. 4. The plasmid substrate described here contains an ICL in close proximity to a DSB, and appears to recapitulate, at least for repair processing, the structure of a collapsed replication fork stalled at an ICL in genomic DNA. It should, therefore, be useful as an assay for further investigations into the mechanisms of this complex repair process.
Figure 4.
Model depicting repair of ICLs at a stalled replication fork.
Acknowledgments
This work was supported by NCI grants CA075160 and CA097175. DNA sequencing resources were supported by the Cancer Center Support (Core) Grant CA16672. We thank Frank Culajay and Jia Liu for providing technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dronkert ML, Kanaar R. Repair of DNA interstrand cross-links. Mutat Res. 2001;486:217–247. doi: 10.1016/s0921-8777(01)00092-1. [DOI] [PubMed] [Google Scholar]
- 2.Legerski RJ, Richie C. Mechanisms of repair of interstrand crosslinks in DNA. Cancer Treat Res. 2002;112:109–128. doi: 10.1007/978-1-4615-1173-1_6. [DOI] [PubMed] [Google Scholar]
- 3.McHugh PJ, Spanswick VJ, Hartley JA. Repair of DNA interstrand crosslinks: molecular mechanisms and clinical relevance. Lancet Oncol. 2001;2:483–490. doi: 10.1016/S1470-2045(01)00454-5. [DOI] [PubMed] [Google Scholar]
- 4.Cole RS, Sinden RR. Repair of cross-linked DNA in Escherichia coli. Basic Life Sci. 1975;5B:487–495. doi: 10.1007/978-1-4684-2898-8_10. [DOI] [PubMed] [Google Scholar]
- 5.Sinden RR, Cole RS. Repair of cross-linked DNA and survival of Escherichia coli treated with psoralen and light: effects of mutations influencing genetic recombination and DNA metabolism. J Bacteriol. 1978;136:538–547. doi: 10.1128/jb.136.2.538-547.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berardini M, Foster PL, Loechler EL. DNA polymerase II (polB) is involved in a new DNA repair pathway for DNA interstrand cross-links in Escherichia coli. J Bacteriol. 1999;181:2878–2882. doi: 10.1093/gao/9781884446054.article.t031385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berardini M, Mackay W, Loechler EL. Evidence for a recombination-independent pathway for the repair of DNA interstrand cross-links based on a site-specific study with nitrogen mustard. Biochemistry. 1997;36:3506–3513. doi: 10.1021/bi962778w. [DOI] [PubMed] [Google Scholar]
- 8.Grossmann KF, Ward AM, Matkovic ME, Folias AE, Moses RE. S. cerevisiae has three pathways for DNA interstrand crosslink repair. Mutat Res. 2001;487:73–83. doi: 10.1016/s0921-8777(01)00106-9. [DOI] [PubMed] [Google Scholar]
- 9.Grossmann KF, Ward AM, Moses RE. Saccharomyces cerevisiae lacking Snm1, Rev3 or Rad51 have a normal S-phase but arrest permanently in G2 after cisplatin treatment. Mutat Res. 2000;461:1–13. doi: 10.1016/s0921-8777(00)00035-5. [DOI] [PubMed] [Google Scholar]
- 10.Shen X, Jun S, O’Neal LE, Sonoda E, Bemark M, Sale JE, Li L. REV3 and REV1 play major roles in recombination-independent repair of DNA interstrand cross-links mediated by monoubiquitinated proliferating cell nuclear antigen (PCNA) J Biol Chem. 2006;281:13869–13872. doi: 10.1074/jbc.C600071200. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Peterson CA, Zheng H, Nairn RS, Legerski RJ, Li L. Involvement of nucleotide excision repair in a recombination-independent and error-prone pathway of DNA interstrand cross-link repair. Mol Cell Biol. 2001;21:713–720. doi: 10.1128/MCB.21.3.713-720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng H, Wang X, Legerski RJ, Glazer PM, Li L. Repair of DNA interstrand cross-links: interactions between homology-dependent and homology-independent pathways. DNA Repair (Amst) 2006;5:566–574. doi: 10.1016/j.dnarep.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Collins AR. Mutant rodent cell lines sensitive to ultraviolet light, ionizing radiation and cross-linking agents: a comprehensive survey of genetic and biochemical characteristics. Mutat Res. 1993;293:99–118. doi: 10.1016/0921-8777(93)90062-l. [DOI] [PubMed] [Google Scholar]
- 14.Andersson BS, Sadeghi T, Siciliano MJ, Legerski R, Murray D. Nucleotide excision repair genes as determinants of cellular sensitivity to cyclophosphamide analogs. Cancer Chemother Pharmacol. 1996;38:406–416. doi: 10.1007/s002800050504. [DOI] [PubMed] [Google Scholar]
- 15.Hoy CA, Thompson LH, Mooney CL, Salazar EP. Defective DNA cross-link removal in Chinese hamster cell mutants hypersensitive to bifunctional alkylating agents. Cancer Res. 1985;45:1737–1743. [PubMed] [Google Scholar]
- 16.McHugh PJ, Sones WR, Hartley JA. Repair of intermediate structures produced at DNA interstrand cross-links in Saccharomyces cerevisiae. Mol Cell Biol. 2000;20:3425–3433. doi: 10.1128/mcb.20.10.3425-3433.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niedzwiedz W, Mosedale G, Johnson M, Ong CY, Pace P, Patel KJ. The Fanconi anaemia gene FANCC promotes homologous recombination and error-prone DNA repair. Mol Cell. 2004;15:607–620. doi: 10.1016/j.molcel.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Nojima K, Hochegger H, Saberi A, Fukushima T, Kikuchi K, Yoshimura M, Orelli BJ, Bishop DK, Hirano S, Ohzeki M, Ishiai M, Yamamoto K, Takata M, Arakawa H, Buerstedde JM, Yamazoe M, Kawamoto T, Araki K, Takahashi JA, Hashimoto N, Takeda S, Sonoda E. Multiple repair pathways mediate tolerance to chemotherapeutic cross-linking agents in vertebrate cells. Cancer Res. 2005;65:11704–11711. doi: 10.1158/0008-5472.CAN-05-1214. [DOI] [PubMed] [Google Scholar]
- 19.D’Andrea AD, Grompe M. The Fanconi anaemia/BRCA pathway. Nat Rev Cancer. 2003;3:23–34. doi: 10.1038/nrc970. [DOI] [PubMed] [Google Scholar]
- 20.De Silva IU, McHugh PJ, Clingen PH, Hartley JA. Defining the roles of nucleotide excision repair and recombination in the repair of DNA interstrand cross-links in mammalian cells. Mol Cell Biol. 2000;20:7980–7990. doi: 10.1128/mcb.20.21.7980-7990.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang N, Lu X, Zhang X, Peterson CA, Legerski RJ. hMutSbeta is required for the recognition and uncoupling of psoralen interstrand cross-links in vitro. Mol Cell Biol. 2002;22:2388–2397. doi: 10.1128/MCB.22.7.2388-2397.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967;26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- 23.Liu N, Lamerdin JE, Tebbs RS, Schild D, Tucker JD, Shen MR, Brookman KW, Siciliano MJ, Walter CA, Fan W, Narayana LS, Zhou ZQ, Adamson AW, Sorensen KJ, Chen DJ, Jones NJ, Thompson LH. XRCC2 and XRCC3, new human Rad51-family members, promote chromosome stability and protect against DNA cross-links and other damages. Mol Cell. 1998;1:783–793. doi: 10.1016/s1097-2765(00)80078-7. [DOI] [PubMed] [Google Scholar]
- 24.Li L, Peterson CA, Lu X, Wei P, Legerski RJ. Interstrand cross-links induce DNA synthesis in damaged and undamaged plasmids in mammalian cell extracts. Mol Cell Biol. 1999;19:5619–5630. doi: 10.1128/mcb.19.8.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang N, Zhang X, Peterson C, Li L, Legerski R. Differential processing of UV mimetic and interstrand crosslink damage by XPF cell extracts. Nucleic Acids Res. 2000;28:4800–4804. doi: 10.1093/nar/28.23.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lan L, Hayashi T, Rabeya RM, Nakajima S, Kanno S, Takao M, Matsunaga T, Yoshino M, Ichikawa M, Riele H, Tsuchiya S, Tanaka K, Yasui A. Functional and physical interactions between ERCC1 and MSH2 complexes for resistance to cis-diamminedichloroplatinum(II) in mammalian cells. DNA Repair (Amst) 2004;3:135–143. doi: 10.1016/j.dnarep.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Wu Q, Christensen LA, Legerski RJ, Vasquez KM. Mismatch repair participates in error-free processing of DNA interstrand crosslinks in human cells. EMBO Rep. 2005;6:551–557. doi: 10.1038/sj.embor.7400418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Busch DB, van Vuuren H, de Wit J, Collins A, Zdzienicka MZ, Mitchell DL, Brookman KW, Stefanini M, Riboni R, Thompson LH, Albert RB, van Gool AJ, Hoeijmakers J. Phenotypic heterogeneity in nucleotide excision repair mutants of rodent complementation groups 1 and 4. Mutat Res. 1997;383:91–106. doi: 10.1016/s0921-8777(96)00048-1. [DOI] [PubMed] [Google Scholar]
- 29.Niedernhofer LJ, Odijk H, Budzowska M, van Drunen E, Maas A, Theil AF, de Wit J, Jaspers NG, Beverloo HB, Hoeijmakers JH, Kanaar R. The structure-specific endonuclease Ercc1-Xpf is required to resolve DNA interstrand cross-link-induced double-strand breaks. Mol Cell Biol. 2004;24:5776–5787. doi: 10.1128/MCB.24.13.5776-5787.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sijbers AM, van der Spek PJ, Odijk H, van den Berg J, van Duin M, Westerveld A, Jaspers NG, Bootsma D, Hoeijmakers JH. Mutational analysis of the human nucleotide excision repair gene ERCC1. Nucleic Acids Res. 1996;24:3370–3380. doi: 10.1093/nar/24.17.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian M, Shinkura R, Shinkura N, Alt FW. Growth retardation, early death, and DNA repair defects in mice deficient for the nucleotide excision repair enzyme XPF. Mol Cell Biol. 2004;24:1200–1205. doi: 10.1128/MCB.24.3.1200-1205.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aquilina G, Ceccotti S, Martinelli S, Hampson R, Bignami M. N-(2-chloroethyl)-N′-cyclohexyl-N-nitrosourea sensitivity in mismatch repair-defective human cells. Cancer Res. 1998;58:135–141. [PubMed] [Google Scholar]
- 33.Fiumicino S, Martinelli S, Colussi C, Aquilina G, Leonetti C, Crescenzi M, Bignami M. Sensitivity to DNA cross-linking chemotherapeutic agents in mismatch repair-defective cells in vitro and in xenografts. Int J Cancer. 2000;85:590–596. doi: 10.1002/(sici)1097-0215(20000215)85:4<590::aid-ijc23>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 34.Zhang N, Kaur R, Lu X, Shen X, Li L, Legerski RJ. The Pso4 mRNA splicing and DNA repair complex interacts with WRN for processing of DNA interstrand cross-links. J Biol Chem. 2005;280:40559–40567. doi: 10.1074/jbc.M508453200. [DOI] [PubMed] [Google Scholar]
- 35.Thompson LH, Hinz JM, Yamada NA, Jones NJ. How Fanconi anemia proteins promote the four Rs: replication, recombination, repair, and recovery. Environ Mol Mutagen. 2005;45:128–142. doi: 10.1002/em.20109. [DOI] [PubMed] [Google Scholar]
- 36.Hanada K, Budzowska M, Modesti M, Maas A, Wyman C, Essers J, Kanaar R. The structure-specific endonuclease Mus81-Eme1 promotes conversion of interstrand DNA crosslinks into double-strands breaks. Embo J. 2006;25:4921–4932. doi: 10.1038/sj.emboj.7601344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang N, Lu X, Legerski RJ. Partial reconstitution of human interstrand cross-link repair in vitro: characterization of the roles of RPA and PCNA. Biochem Biophys Res Commun. 2003;309:71–78. doi: 10.1016/s0006-291x(03)01535-3. [DOI] [PubMed] [Google Scholar]