Abstract
The ability to site-specifically methylate DNA in vivo would have wide applicability to the study of basic biomedical problems as well as enable studies on the potential of site-specific DNA methylation as a therapeutic strategy for the treatment of diseases. Natural DNA methyltransferases lack the specificity required for these applications. Nomura and Barbas [1] have reported that an engineered DNA methyltransferase comprised of fragments of M.HhaI methyltransferase and zinc finger proteins has very high specificity for the chosen target site. Our analysis of this engineered enzyme shows that the fusion protein methylates target and non-target sites with similar efficiency.
The ability to site-specifically methylate DNA in vivo would have wide applicability to the study of basic biomedical problems as well as enable studies on the potential of site-specific DNA methylation as a therapeutic strategy for the treatment of diseases that involve abnormal hypomethylation of DNA [2-4]. A site specific DNA methyltransferase would be useful (1) as a tool for studying DNA methylation and the spread of methylation patterns, (2) as a molecular biological tool for silencing genes of interest, and (3) as a potential gene-therapy agent for the selective silencing of genes. The molecular tools to carry out truly site-specific control of DNA methylation do not exist. Existing approaches involving the end-to-end fusion of methyltransferases and DNA-binding domains at best create enzymes with preferences for methylation at target sites [4-9] presumably because the methyltransferases remain active in the absence of the DNA-binding domain binding to its target site.
We sought to create site-specific DNA methyltransferases by splitting a DNA methyltransferase into two fragments that (1) were unable to associate and form an active enzyme by themselves and (2) would assemble into active enzyme only at the target site for methylation when fused to zinc fingers whose DNA-binding sites flanked the target site (Figure 1A). As a first step in this direction we sought to identify fragment pairs of DNA methyltransferase M.HhaI that required appended leucine zipper domains for assembly into an active enzyme [10]. We utilized a combinatorial protein engineering method for identifying such fragment pairs. We had previously used this method to identify fragment pairs of aminoglycoside phosphotransferase IIa (Neo) that required appended leucine zippers for enzyme activity [11]. However, unlike Neo, no fragment pairs of M.HhaI that required fusion to dimerization domains for activity were identified. Instead several fragment pairs were identified that did not require leucine zipper dimerization for activity. One of these pairs, P5, could fully protect plasmid DNA from digestion with HhaI endonuclease when expressed in E. coli [10] – strong evidence that the P5 fragments assemble unassisted into an efficient, active enzyme.
Fig. 1.
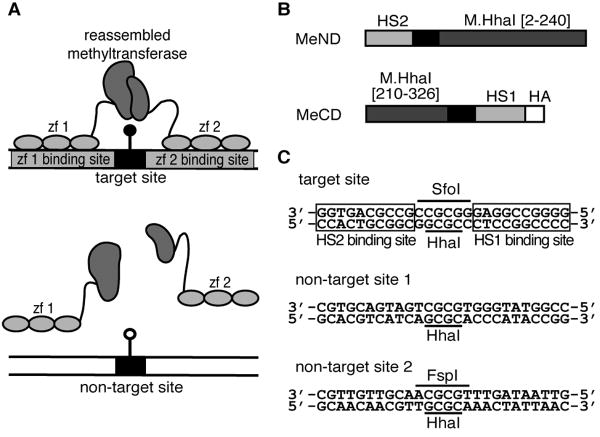
(A) Schematic illustration of a reassembled site-specific DNA methyltransferase–zinc finger (zf) fusion protein capable of methylating at the target site flanked by zinc finger binding sites but unable to reassemble and methylate in the absence of binding to zinc finger sites. (B) Schematic of the MeND and MeCD proteins consisting of a fusion between the zinc fingers HS1 and HS2 and the indicated M.HhaI fragments [1]. The linker sequence is shown in black. (C) Target [1] and two non-target DNA sequences containing restrictions sites HhaI, SfoI and FspI. SfoI and FspI cannot digest their cognate sites if the site is methylated. The HS1 and HS2 binding sites in the target sequence are boxed.
Based on the properties of P5, we predicted that fusion of the two P5 fragments to zinc fingers would not create a site-specific methyltransfase since the enzyme would not require the zinc finger domains to bind DNA for enzyme activity. At best, such a construct would exhibit a bias towards methylation at the target site, as has been seen with non-split DNA methyltransferases fused to DNA-targeting domains [4-9]. We were thus intrigued by the recent report by Nomura and Barbas that fusion of the P5 fragments to zinc finger domains created a DNA methyltransferase that methylated only a site between the zinc finger's DNA binding site and failed to methylate other M.HhaI sites, even when the protein was expressed at high levels [1]. The authors did not address how fusion of the P5 fragments to zinc fingers prevented methyltransferase activity in the absence of binding of the zinc finger domains to their cognate DNA site. In order to confirm this important result, we have characterized the DNA methylation specificity of this construct (MeND/MeCD, Figure 1B) in greater detail. Our evidence indicates that the protein is not a highly site-specific DNA methyltransferase. MeND/MeCD methylates target sites and non-target sites with similar efficiency.
Materials and Methods
Construction of pDIM-N7-MeND/MeCD expression vector
Restriction enzyme digestions and ligations were preformed as recommended by the manufacturer (New England Biolabs, Beverly, MA). Agarose gel electrophoresis and PCR were performed essentially as described [12]. The published protein sequences for the HS1 and HS2 zinc fingers with the corresponding linkers [1] were converted to a DNA sequence optimized for E. coli expression using the Gene Designer software [13]. The DNA encoding these fragments was synthesized by Integrated DNA Technologies (Coralville, IA).
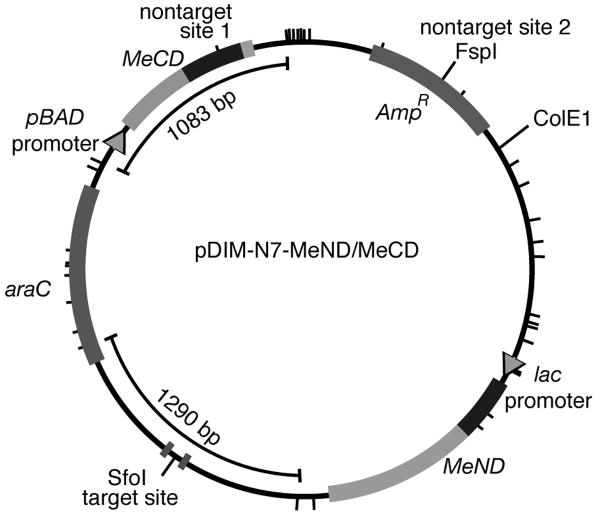
Plasmid pDIM-N7-MeND/MeCD (Fig. 2) was derived from pDIM-N7-MHhaI[1-302] and pAR-MHhaI[29-327] [10]. The pDIM-N7 vector first had a SalI site silently mutated for cloning purposes, and two HhaI sites silently mutated so as to facilitate target site methylation analysis by digestion with HhaI. The target site for methylation (Fig 1c) was created by inserting annealed oliognucletoides ZF insert-for and ZF insert-rev (see Supplementary Information for a table of oligonucleotide sequences used in this study) into pDIM-N7 digested with XmaI and EcoRI. The gene encoding MeND was assembled by overlap extension PCR. The HS2 zinc finger and linker segment was amplified by PCR using primers HS2-for and HS2-rev. The N-terminal M.HhaI fragment was amplified using primers HhaI[2]-for and HhaI[240]-rev. The gene encoding MeND was assembled from these two PCR products using primers HS2-for and HhaI[240]-rev, digested with NdeI and SalI and inserted into the similarly-digested pDIM-N7 vector downstream from the lac promoter to create pDIM-N7-MeND.
Fig. 2.
Scaled drawing of the pDIM-N7-MeND/MeCD plasmid. Plasmid contains the ColE1 origin of replication, araC gene and ampicillin resistance gene (AmpR). The MeND gene was cloned downstream of the lac promoter, and the MeCD gene was cloned downstream of the pBAD promoter. Tick marks around the plasmid indicate the position of the 36 HhaI sites. The location of the target site (flanked by the two zinc finger binding sites) and non-target site 2 overlapping a FspI site (Fig. 1C) are marked. Also indicated are the 1290 bp diagnostic fragment that occurs during HhaI digestion when the target site is methylated and another large fragment (1083 bp) that occurs when non-target site 1 in the gene encoding MeCD is methylated.
The pAR plasmid first had its NcoI site replaced with an NdeI site. The HS1 zinc finger and linker segment was amplified using HS1-for and HS1-rev. DNA encoding the C-terminal M.HhaI fragment was amplified using primers HhaI[210]-for and HhaI[326]-rev. These two segments were combined by overlap extension PCR using primers HhaI[210]-for and HS1-rev. This was followed by two rounds of PCR with a forward primer HhaI[210]-for and reverse primers of HA add1-rev and HA add2-rev to add on DNA encoding the HA epitope tag that is part of MeCD. The MeCD fragment was digested and ligated into the pAR vector downstream from the arabinose promoter using the NdeI and SpeI sites to create plasmid pAR-MeCD.
Plasmid pAR-MeCD was digested with EcoRI and SpeI. The fragment containing the methyltransferase gene, pBAD promoter and AraC gene was isolated and inserted into the EcoRI/SpeI digested pDIM-N7-MeND plasmid to create pDIM-N7-MeND/MeCD. The DNA sequence encoding MeND and MeCD was verified by DNA sequencing.
Restriction endonuclease protection assays
For experiments with E. coli strain ER2267 (New England Biolabs, Beverly, MA) 10 mL of LB media supplemented with 100 μg/mL ampicillin in 25 ml culture tubes was inoculated from frozen cell stocks harboring pDIM-N7-MeND/MeCD. Some cultures also included the following: 0.2% w/v glucose to repress expression, 1 mM IPTG to induce the lac promoter and 0.05% w/v arabinose to induce the arabinose promoter. For experiments using E. coli strain SURE, the culturing conditions of Nomura and Barbas [1] were followed. Plasmid pDIM-N7-MeND/MeCD was transformed into SURE cells. Colonies were culture in 3 mls of SB media supplemented with 100 μg/mL ampicillin. Some cultures also included the following: 0.4% w/v glucose to repress expression, 1 mM IPTG to induce the lac promoter and 0.05% w/v arabinose to induce the arabinose promoter. For both ER2267 and SURE cultures, cultures were incubated overnight at 37°C and the plasmid DNA was isolated using QIAprep spin miniprep kit (Qiagen, Valencia, CA).
Plasmid DNA (1.25 μg) was incubated with 12.6 U HhaI for 2 hours at 37°C. After digestion, 1.25 μg of digested plasmid was analyzed by agarose gel electrophoresis using a 1.4% agarose gel. In addition, 500 ng of the plasmid was digested with either 12 U SfoI (overlaps target site) or 2 U FspI (overlaps a non-target site) at 37°C. After digestion, 500 ng of digested plasmid was analyzed by agarose gel electrophoresis using a 0.8% agarose gel.
Western Blotting
ER2267 cells harboring pDIM-N7-MeND/MeCD were cultured under the same conditions used in the restriction endonuclease protection assays. Cells were lysed by sonication and the soluble fractions recovered. Total protein concentrations were determined using the DC Protein Assay (Bio-Rad Laboratories, Hercules, CA). Equal total protein amounts for each growth condition were loaded on SDS-PAGE gels. Western blots were performed using anti-M.HhaI antibodies (a gift from Rich Roberts, New England BioLabs) and polyclonal anti-hemagglutinin (HA) rabbit antibodies (Thermo Scientific, Fremont, CA) as primary antibodies. The anti-HA antibody was used to detect the HA-tag on the C-terminal fragment. The secondary antibody was a goat anti-rabbit HRP conjugate (Bio-Rad Laboratories, Hercules, CA). The Immun-Star WesternC Chemiluminescent kit (Bio-Rad Laboratories, Hercules, CA) was used for detection. The total protein loaded per lane for the western blot using anti-HA antibodies was twice that loaded for the western blot with anti-M.HhaI antibodies.
Bisulfite sequencing
Cultures of ER2267 containing the pDIM-N7-MeND/MeCD vector were grown in 10 mL LB, 100 μg/mL ampicillin, 1 mM IPTG and 0.05% w/v arabinose. Plasmid DNA was recovered and linearized by digesting with NcoI before conversion. Bisulfite conversion was done using the Epitect Bisulfite Kit (Qiagen, Valencia, CA) and performed using manufacturer's instructions. After the bisulfite conversion the target region (using primers target-for and target-rev) and the non-target region containing the FspI site and another HhaI site (using primers nontarget-for and nontarget-rev) were amplified by PCR using Qiagen's HotStarTaq DNA polymerase. The fragments were then digested with NdeI and NcoI and inserted into a similarly-digested pDIM-N7 plasmid and transformed into ER2267 cells. Random individual colonies were selected for sequencing. Sequencing was performed by Genewiz (South Plainfield, NJ). The relevant sections of the sequencing chromatograms are shown in the Supplementary Information.
Results and Discussion
Analysis of Nomura and Barbas' results
Nomura and Barbas reported that fusing zinc fingers to each of the two fragments of P5 resulted in an enzyme (MeND/MeCD) that methylated exclusively at a M.HhaI target site flanked by the zinc fingers' cognate DNA binding sites [1]. The activity of this enzyme was tested in experiments in which the protein fragments were expressed in E. coli SURE cells. The authors used a bicistronic method for expressing MeND and MeCD from an arabinose promoter. The authors presented two key experiments in support of their claim: (1) HhaI digestion assays of plasmid DNA showing protection from digestion at the target site and (2) bisulfite sequencing data. In these experiments, overnight cultures were grown and the plasmid DNA was purified and analyzed. Arabinose was not added to the growth media in these experiments. Production of the protein relied on low-level leaky expression from the arabinose promoter. However, the authors also reported that methylation was absent at non-target sites even when the protein was expressed at high levels, but presented no experimental data on the methylation profile produced under these conditions.
In Nomura and Barbas' HhaI protection assay, the lack of methylation should produce fragments of 1710, 1108, 390, 353, and 312 bp together with 15 fragments smaller than 300 bp when the plasmid is digested with HhaI. Methylation at the target site would result in the formation of a new fragment of 1461 bp, comprised of the 1108 and 353 bp fragments. A fragment of this size was observed in cells harboring the genes encoding MeND and MeCD [1]. However, the fraction of plasmids protected at this site is low. The fraction was not quantified but we estimate it to be <0.25. The authors do not state the size of new fragments that would form if non-targeted methylation were occurring. Furthermore, it is not clear that such fragments would be detectable given the low-level of methylation activity and the fact that the majority of fragments that would comprise such fragments are small in size and not visible or faint on the gel. The HhaI protection assay demonstrates partial protection at the target site, but the inclusion of a large size diagnostic fragment for non-targeted methylation would have more convincingly addressed whether methylation is occurring at non-target sites.
Nomura and Barbas also performed bisulfite sequencing on the plasmid at the target site (n=5) and non-target sites (n=1). They showed methylation at the target sites in all five clones and no methylation at three non-target sites from one clone. The frequency of clones with methylated target sites (5/5) obtained by bisulfite sequencing is much higher than the level of protection observed in their HhaI digestion experiment (<25%). Given the low-level of protection, the bisulfite sequencing of more than one clone would have more convincingly addressed whether methylation is occurring at non-target sites.
Since we found the evidence presented by Nomura and Barbas inconclusive as to whether MeCD and MeND had the desired high specificity, we constructed our own plasmid for expression of MeCD and MeND that was designed to more definitively address this protein's methylation specificity.
Plasmid design
We constructed the genes encoding the two fragments MeND and MeCD and placed them on the same plasmid, pDIM-N7-MeND/MeCD (Fig 2). Our genes encoded the exact same proteins described by Nomura and Barbas. Since the DNA sequence encoding the zinc fingers was not described by the authors, our DNA sequence for this region of the genes is likely different. The IPTG-inducible lac promoter controlled production of the N-terminal fragment (MeND) and the arabinose promoter controlled production of the C-terminal fragment (MeCD). Separate promoters afforded us the ability to vary the relative induction levels of the two fragments.
A target site (identical to that of Nomura and Barbas) and non-target sites for methylation (Figure 1C) were located on this same plasmid to facilitate analysis of plasmid methylation by digestion with HhaI endonuclease and other restriction enzymes that are blocked by methylation. This plasmid contained 36 HhaI sites. In the absence of methylation, digestion with restriction enzyme HhaI should produce 36 fragments, the largest of which is 858 bp. We designed the plasmid such that HhaI digestion of the plasmid methylated at the target site would produce a 1290 bp fragment that would be diagnostic for targeted methylation. In addition, we designed the plasmid such that HhaI digestion of the plasmid methylated at non-target site 1 would produce a 1083 bp fragment that would be diagnostic for non-target methylation. The inclusion of large size diagnostic fragments for both targeted and non-targeted methylation ensured that we could easily compare methylation specificity.
Restriction endonuclease protection assays
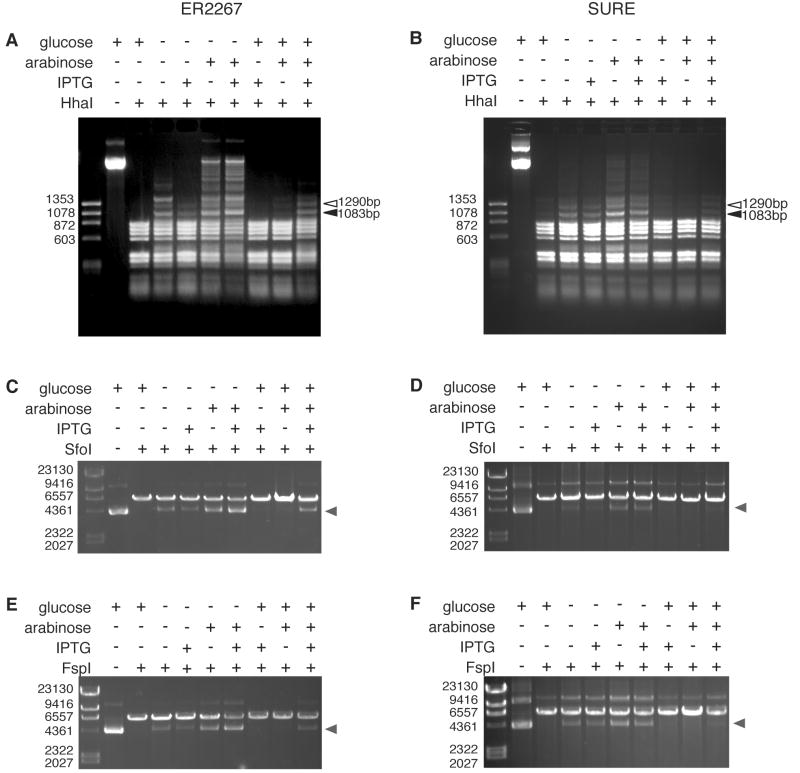
We transformed pDIM-N7-MeND/MeCD into E. coli strain ER2267, a methylation tolerant, recA− endA− strain. Since Nomura and Barbas reported targeted methylation at both low and high expression levels, we inoculated a series of cultures designed to test a variety of expression levels and ratios of the two fragments. Western blots confirmed that our growth conditions resulted in a wide range of expression levels of the two fragments, from barely detectable to relatively high (see Supplementary Information). Plasmid DNA was isolated from these overnight cultures, digested with HhaI and analyzed by agarose gel electrophoresis. Protection from HhaI digestion of DNA isolated from cells expressing MeND/MeCD was highest when both promoters were induced; however, full protection of the target site was not achieved (Figure 3A). A 1290 bp fragment diagnostic of target site methylation is visible; however, several bands consistent with methylation at non-target sites are also present at similar levels including the designed 1083 bp non-target fragment. Protection at non-target sites was observed in cells exhibiting a variety of production levels and ratios of the two fragments. Under no conditions do we observe evidence for a preference for methylation of the target site over non-target site. To better assess the relative protection frequency at the target and a non-target site in our plasmid we digested plasmid DNA with SfoI (overlaps the target site) or FspI (overlaps non-target site 2) (Figure 3C and 3E). Similar levels of protection are observed at both sites confirming the protection at non-target sites and the lack of significant specificity observed with HhaI digestions.
Fig. 3.
Restriction endonuclease protection assay for methylation detection. Plasmid DNA (pDIM-N7-MeND/MeCD) was isolated from ER2267 or SURE cells grown under different conditions as indicated and then incubated with restriction enzymes (A, B) HhaI, (C, D) SfoI, and (E, F) FspI. In the HhaI digestion, the open arrowhead indicates a unique band (1290 bp) that results from methylation at the target site, and the closed arrowhead indicates a unique band (1083 bp) that results from methylation at a non-target site. For the SfoI and FspI digestions, grey arrowheads indicate the migration location of protected DNA.
We next confirmed our results using the strain and growth media of Nomura and Barbas. We transformed pDIM-N7-MeND/MeCD into SURE cells (instead of ER2267 cells) and grew cultures from single colonies of freshly transformed cells in SB media (instead of LB media). We inoculated a series of cultures designed to test a variety of expression levels and ratios of the two fragments. We found that SURE cells required twofold higher glucose levels than ER2267 cells to achieve repression of protection. Restriction enzyme protection assays of plasmid DNA isolated from these cultures (Figure 3B, D, F) showed very similar protection patterns to that found in cultures of ER2267 cells (Figure 3A, C, E) but with less overall protection. We observed protection at non-target sites and no significant preference for protection at the target site.
Bisulfite sequencing
To confirm that the observed protection resulted from DNA methylation we performed bisulfite sequencing at both target (n=11) and non-target sites (n=8) for independent clones. Each clone examined for the non-target methylation contained two non-target sites, one of which was non-target site 2. The results confirm MeND/MeCD methylates target and non-target sites with similar efficiencies (see Supplementary Information for sequencing chromatograms). A total of 18% of the target sites (2/11) and 25% of the non-target sites (4/16) were methylated. This level of methylation is consistent with the fraction of DNA showing protection to restriction enzyme digestion (Figure 3A).
Conclusions
Our experiments provide no evidence that indicates that MeND/MeCD is a highly site-specific methyltransferase. To the contrary, our evidence indicates that the protein methylates target sites and non-target sites with similar efficiency. We did not perform experiments using the exact same expression system as Nomura and Barbas. However, given that we observe a lack of desired specificity over a wide range of production levels and ratios of the two fragments, we find it unlikely that there exists expression conditions that allow the protein to behave as a highly site-specific methyltransferase. We attribute our detection of non-target methylation to the design of a plasmid for HhaI protection assays that makes detection of non-target protection as easy as detection of target protection and the fact that we performed bisulfite sequencing on the number of clones necessary to detect methylation when the overall level of methylation is low.
Creation of a site-specific DNA methyltransferase remains a difficult challenge. The key challenge is to require binding of the zinc finger to its target site for DNA methylation to occur. We believe that engineering site-specific methyltransferases using fragmented methyltransferases is best approached starting with methyltransferase fragments that cannot associate into an active methyltransferase in vivo.
Supplementary Material
Acknowledgments
We thank Rich Roberts of New England Biolabs for the gift of polyclonal M.HhaI antibodies. This research was supported partially by grants from NIH (GM 072794 and GM 077291) and Maryland Stem Cell Research Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nomura W, Barbas CF., 3rd In vivo site-specific DNA methylation with a designed sequence-enabled DNA methylase. J Am Chem Soc. 2007;129:8676–8677. doi: 10.1021/ja0705588. [DOI] [PubMed] [Google Scholar]
- 2.Kress C, Thomassin H, Grange T. Local DNA demethylation in vertebrates: how could it be performed and targeted? FEBS Lett. 2001;494:135–140. doi: 10.1016/s0014-5793(01)02328-6. [DOI] [PubMed] [Google Scholar]
- 3.Szyf M. Towards a pharmacology of DNA methylation. Trends Pharmacol Sci. 2001;22:350–354. doi: 10.1016/s0165-6147(00)01713-2. [DOI] [PubMed] [Google Scholar]
- 4.Xu GL, Bestor TH. Cytosine methylation targetted to pre-determined sequences. Nat Genet. 1997;17:376–378. doi: 10.1038/ng1297-376. [DOI] [PubMed] [Google Scholar]
- 5.Carvin CD, Parr RD, Kladde MP. Site-selective in vivo targeting of cytosine-5 DNA methylation by zinc-finger proteins. Nucleic Acids Res. 2003;31:6493–6501. doi: 10.1093/nar/gkg853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McNamara AR, Hurd PJ, Smith AE, Ford KG. Characterisation of site-biased DNA methyltransferases: specificity, affinity and subsite relationships. Nucleic Acids Res. 2002;30:3818–3830. doi: 10.1093/nar/gkf501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carvin CD, Dhasarathy A, Friesenhahn LB, Jessen WJ, Kladde MP. Targeted cytosine methylation for in vivo detection of protein-DNA interactions. Proc Natl Acad Sci USA. 2003;100:7743–7748. doi: 10.1073/pnas.1332672100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li F, Papworth M, Minczuk M, Rohde C, Zhang Y, Ragozin S, Jeltsch A. Chimeric DNA methyltransferases target DNA methylation to specific DNA sequences and repress expression of target genes. Nucleic Acids Res. 2007;35:100–112. doi: 10.1093/nar/gkl1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith AE, Ford KG. Specific targeting of cytosine methylation to DNA sequences in vivo. Nucleic Acids Res. 2007;35:740–754. doi: 10.1093/nar/gkl1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choe W, Chandrasegaran S, Ostermeier M. Protein fragment complementation in M.HhaI DNA methyltransferase. Biochem Biophys Res Commun. 2005;334:1233–1240. doi: 10.1016/j.bbrc.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 11.Paschon DE, Patel ZS, Ostermeier M. Enhanced catalytic efficiency of aminoglycoside phosphotransferase (3′)-IIa achieved through protein fragmentation and reassembly. J Mol Biol. 2005;353:26–37. doi: 10.1016/j.jmb.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 12.Sambrook J, Russell D. Molecular Cloning: A Laboratory Manual. 3rd. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2001. [Google Scholar]
- 13.Villalobos A, Ness JE, Gustafsson C, Minshull J, Govindarajan S. Gene Designer: a synthetic biology tool for constructing artificial DNA segments. BMC Bioinformatics. 2006;7:285. doi: 10.1186/1471-2105-7-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.