Abstract
Mechanical forces participate in morphogenesis from the level of individual cells to whole organism patterning. This manuscript reviews recent research that has identified specific roles for mechanical forces in important developmental events. One well-defined example is that dynein-driven cilia create fluid flow that determines left-right patterning in the early mammalian embryo. Fluid flow is also important for vasculogenesis, and evidence suggests that fluid shear stress rather than fluid transport is primarily required for remodeling the early vasculature. Contraction of the actin cytoskeleton, driven by nonmuscle myosins and regulated by the Rho family GTPases, is a recurring mechanism for controlling morphogenesis throughout development, from gastrulation to cardiogenesis. Finally, novel experimental approaches suggest critical roles for the actin cytoskeleton and the mechanical environment in determining differentiation of mesenchymal stem cells. Insights into the mechanisms linking mechanical forces to cell and tissue differentiation pathways are important for understanding many congenital diseases and for developing regenerative medicine strategies.
Keywords: Mechanotransduction, cytoskeleton, shear stress, embryonic development, stem cells
Introduction
If you wonder how mechanical forces can regulate the highly orchestrated process of morphogenesis, you have only to look at the tips of your fingers and ask: Are the fingerprints of identical twins also identical?
The people of Stevenage, England still celebrate their most famous identical twins, Albert Ebenezer and Ebenezer Albert Fox, who lived from 1857–1926.1 For their habit of roaming freely on the land while hunting, the Fox twins managed to accumulate 150 convictions for poaching between them. Despite the convictions, their enthusiasm for mocking the local police force never flagged. Inevitably, they complained, the police always managed to convict the wrong twin for the crime. They always hunted alone and they could always provide alibis for each other. Perhaps tired of the international comedic attention to the routine,2 Edward R. Henry, chief commissioner of the London Metropolitan Police, decided to resolve the issue with his new system for identification -- the fingerprint, which he claimed was unique for everyone, including identical twins.
Routine forensic DNA analysis is useless in the case of identical twins, which has in fact led to several cases of modern-day twins who blame each other for the crime.3–6 Yet the fingerprints of identical twins are not identical. Some characteristics, such as pattern type and ridge count, are similar between twins.7 However, the detailed “minutiae” -- where skin ridges meet, end, or bifurcate -- are different even between identical twins.
The fingertip skin ridges are thought to form as compressive stresses develop in the dermal cell layer.8, 9 Like the buckling of landmasses under compression, regular ridges form to relieve the stress.10 Where the skin is flat, uninteresting parallel ridges develop. But primates have raised pads on their fingertips at this stage of the embryo. With the addition of curvature, the ridges are no longer formed in parallel lines; instead, they form along lines of equal stress. Surrounding the highest point of the raised pad, ridges form in concentric circles, like a contour plot of elevation surrounding a hill.
Just as the ridges are forming, the pads are regressing, and the relative timing of the two processes leads to differences in fingerprint pattern.8, 10 Where the pads remain high longer, ridges form regular concentric circles or “whorls”. Largely regressed pads lead to a simple “arch” pattern. Most commonly, both processes overlap, yielding an intermediate “loop” pattern. The fingerprint ridges are therefore shaped by the combined effects of cell proliferation and mechanical forces.
However, the ridges are not always regularly spaced: there are ridges that divide, and ridges that suddenly end. These irregularities are easily changed by small variations in their local environment, so they are not predetermined. Even monozygotic twins will have different positions in the uterus and experience a slightly different environment. In genetically identical twins, then, subtle differences in the mechanical environment of the embryo in utero are sufficient to drive a developing system towards different morphogenic outcomes.
Mechanical forces regulate not only dermal ridge patterns, but also fundamental aspects of morphogenesis.11–13 In this review, we present several examples of recent research that have shed light on how mechanical processes can control development (Figure 1).
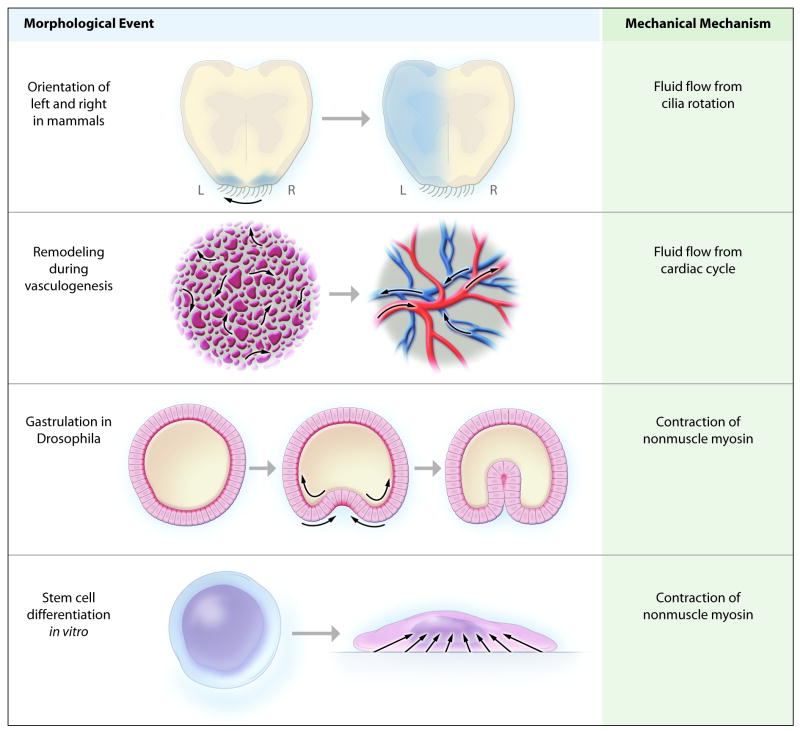
Figure 1.
Schematic overview of morphological events demonstrating the involvement of mechanical forces. (Top row) Early mammalian embryos determine their right from their left by creating fluid flow in one direction. Cilia located in a specialized node at the midline propel flow to the left. The embryo rapidly senses the fluid flow and upregulates the signaling molecule, nodal, on the left side only (shown in blue). Inherited defects in cilia motor proteins result in randomization of left-right patterning. In humans, this leads to Kartegener’s syndrome in 50% of patients. (Second row) During embryonic vasculogenesis, remodeling of immature vessels requires shear stress created by fluid flow. Before the beginning of fluid flow, embryonic mesodermal cells form “blood islands” outside the embryo. After the embryonic heart tube begins to function, fluid begins to flow and rapidly reshapes the blood islands into mature branched arteries and veins. This process specifically requires fluid shear stress. (Third row) Gastrulation, the first major morphological change in the embryo of the fruit fly D. melanogaster, is controlled by contraction of nonmuscle myosin. Before gastrulation, the embryo forms a single layer of cells arranged in a cylindrical egg (shown in cross-section, left panel), and myosin is localized to the inner surface. At the start of gastrulation, myosin relocalizes to the outer surface of a few cells, and its contraction pulls the outer portions of these cells together. This compresses the inner portions of the cells, pushing them inwards and creating a bulge (middle panel). Soon the cell layer has folded within itself (right panel). (Bottom row) Differentiation of mesenchymal stem cells is controlled by the level of nonmuscle myosin contraction inside the cell and the stiffness of its surroundings. Culturing cells on hard substrates will activate nonmuscle myosin contraction and promote differentiate towards osteoblasts. Culturing cells on soft substrates, or minimizing contact with hard surfaces, promotes differentiation towards adipocytes and chondrocytes.
The mechanisms organisms use to determine right from left is a clear case of cells using mechanical forces to control embryonic development: throughout evolution, molecular motors are required. Mammals require dynein, a motor protein complex that drives movement of cilia. The cilia generate flow in only one direction, allowing the embryo to know right from left (figure 1, top row).14 The fruit fly (D. melanogaster) does not require cilia or flow to determine the handedness of its gut loops, but it does require a different molecular motor: a nonmuscle myosin (type ID).15, 16 The nonmuscle myosins are a large family of motor proteins, expressed in every cell, that generate mechanical forces through contraction of the actin cytoskeleton. Both fluid flow and regulation of actin cytoskeletal contraction are recurring themes in mechanical control of morphogenesis.
Control of Morphogenesis by Fluid Flow
Cilia Determine Left vs. Right in Mammals by Creating Leftward Flow
The classic description of patients with Kartagener’s syndrome has been a puzzling combination: left-right reversal of the major visceral organs (situs inversus) accompanied by mucus-blocked airways in the lungs (bronchiectasis) and chronic sinusitis.17 Afzelius later observed that male patients with the syndrome were infertile due to defects in sperm flagella motility. Putting this together with the airway defects, he demonstrated that the primary defect was in cilia function. The cilia lacked the dynein motor proteins, leading to lack of airway clearance, frequent respiratory tract infections, and immotile spermatozoa.18 The relationship between cilia function and left-right patterning, however, remained a mystery for many years. Finally Nonaka et al., investigating a mutation that caused randomization of left-right patterning in mice, noticed at a spot on the midline of the early embryo where the epithelium had cilia, and postulated that cilia-driven “nodal flow” was the cause of left-right patterning.19
Genetic studies have confirmed that mutations in ciliary dynein motor proteins are responsible. Mutations in dynein axonemal intermediate chain 1 (DNAI1)20, 21 and dynein heavy chain 522 have been identified in human cohorts. Notably, loss of cilia function does not always lead to the full Kartagener’s syndrome: only 50% have situs inversus. Left-right patterning still occurs; it is just left to random chance which side will be which.
Leftward fluid flow has since been convincingly established as the causal event in left-right patterning through a series of experiments in mouse embryos. Artificially imposing rightward flow during gestation causes reversal of the left-right pattern in wildtype mice. Furthermore, imposing an artificial leftward flow returns left-right patterning to normal in mice with defective nodal cilia.23 But how do rotating cilia cause flow across the surface in one direction? The cilia are not perpendicular to the surface – they are sufficiently tilted in the posterior direction so that they brush against the posterior surface and fail to generate much force during this part of their rotation.24, 25 The net result is that fluid is driven in only one direction, although at a low velocity.
Soon after flow begins, the TGF-family signaling molecule, nodal, is expressed more strongly on the left side of the node, leading to a cascade that rapidly induces nodal and other factors throughout the left side of the embryo.26 However, the mechanism the cells use to sense flow and differentially induce nodal remains unresolved. One possibility is that the directional flow redistributes morphogens released from the node, such as retinoic acid or sonic hedgehog, to the adjacent cells on the left.27 However, there is another intriguing hypothesis. The node has a separate set of cilia that are nonmotile, and there is evidence these cilia are specifically designed to sense the shear stress created by fluid flow.28, 29 The cilia are coupled to a calcium channel, so fluid shear may lead to a rise in calcium ion concentration which then triggers the induction of nodal. In support of this hypothesis, mutations leading to polycystic kidney disease have recently implicated the same type of cilia and calcium channel in sensing fluid flow during morphogenesis of the kidneys.30
Fluid shear stress is critical for remodeling vessels during vasculogenesis
One of the most critical effects of fluid flow on morphogenesis is its control of vasculogenesis, which refers to the development of new blood vessels directly from precursor cells. While the importance of fluid flow has long been postulated, only recently has it emerged that fluid shear stress is specifically required for remodeling in vasculogenesis.
In contrast, the process of angiogenesis in adults, in which new blood vessels sprout off from existing vasculature, was classically thought to be independent of fluid flow.31 Endothelial cells can form tubular structures in the absence of fluid flowing through them. Angiogenesis seems largely controlled by signaling integrated through the hypoxia-induced factors,32 and is stimulated by low local oxygen tension as sensed by the prolyl hydroxylases.33
The importance of fluid flow for proper vascular function in adults is also well recognized.34, 35 Mature endothelial cells respond rapidly to fluid flow by multiple mechanisms, and disturbed fluid flow promotes endothelial dysfunction.36 Furthermore, fluid flow is required for formation of the lymphatic vessels. The relatively low velocity of fluid flow in the extracellular space allows flow-induced gradients of growth factors such as VEGF to form, and these gradients promote the alignment of lymphatic vessels in the direction of the flow.37, 38
A major site of vasculogenesis during mouse development is outside the embryo itself, where cells migrate to form the “blood islands” of the yolk sac primary vascular plexus. Soon after the heart begins to produce a fetal circulation (8.5–9.5 dpc), these islands must be remodeled from largely undirected passages into a vascular tree with branching arteries and veins (Fig. 1, second row).39, 40 Initially, it was not clear whether precursor cells formed an artery or a vein based on their environment or whether this was genetically predetermined. Eichmann and colleagues answered this question by demonstrating that stopping flow to the yolk sac prevents remodeling even though the blood islands continue to grow.41
Fluid flow is important for both transport and mechanical processes. One might expect that stopping fluid flow prevents vascular remodeling by preventing transport of nutrients, oxygen, or other morphogenic factors. Surprisingly, Lucitti et al. have recently shown that fluid shear stess is the critical requirement.42 By physically sequestering the erythroblasts, the hematocrit is greatly reduced, and the pre-existing yolk sac vessels do not mature into an organized vascular tree (Figure 2D–F). However, artificially increasing the fluid viscosity by adding starch rescues blood vessel formation (Figure 2G–I), demonstrating that it is shear stress and not transport that is required for vascular remodeling during vasculogenesis.
Figure 2.
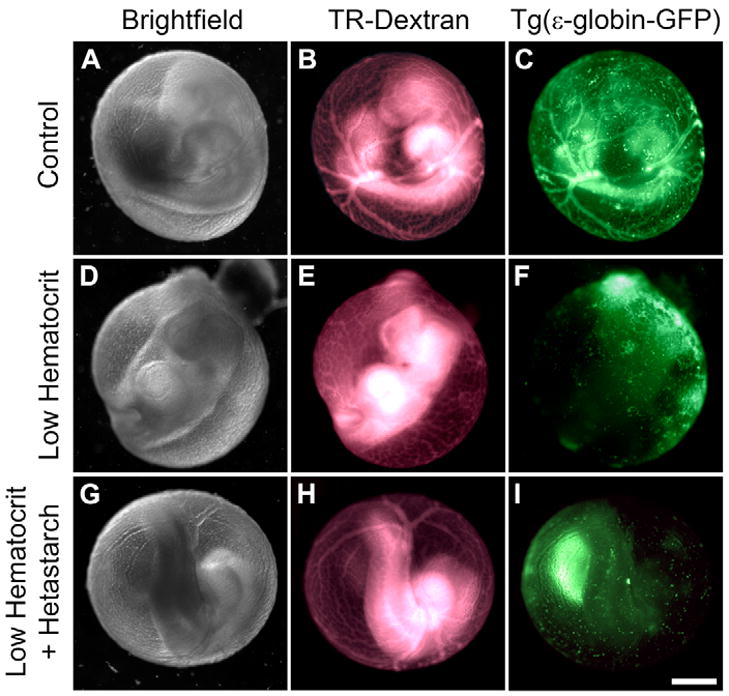
Vessel remodeling during vasculogenesis requires fluid shear stress. In the mouse, vasculogenesis begins largely outside the embryo in the yolk sac. The yolk sac vessels mature from largely undirected passages into branched arteries and veins soon after the beginning of the fetal heart function (A–C). The normal mature vessels can be seen both by injection of fluorescent-labeled dextran (B, “TR-Dextran”) and by imaging erythroblasts genetically labeled with green fluorescent protein (C, “Tg(e-globin-GFP)”). Physically sequestering the erythroblasts outside the embryo completely prevents remodeling of yolk sac vessels (D–F, “Low hematocrit”). However, increasing the viscosity of the fluid by adding starch rescues vasculogenesis, even though the erythroblasts remain sequestered (H–I, “Low Hematocrit + Hetastarch”). The results demonstrate that heart function initiates remodeling by fluid shear stress, not by transport of oxygen or other factors in the blood. Reproduced from Lucitti et al.42 with permission of the Company of Biologists.
Recent in vitro studies have also suggested an important role for fluid flow in differentiation of mesenchymal precursor cells into endothelial cells. In a mesenchymal progenitor cell line (C3H/10T1/2), cells plated on a collagen-based gel and exposed to fluid flow could spontaneously form into tubular structures.43 As the sharp distinction between embryonic vasculogenesis and adult angiogenesis becomes more blurred, it has also become evident that angiogenesis involves more than just sensing oxygen levels. Fluid flow may also play a role in some circumstances. However, there are other mechanical inputs that are known to be important: angiogenesis is directed in part through contraction of the actin cytoskeleton driven by nonmuscle myosins.44
Regulation of Morphogenesis by Cellular Contractility
Nonmuscle Myosin Causes Shape Change in Gastrulation
Control of actin cytoskeleton contractility is a fundamental mechanism for molding shape changes in development. A well-studied example of this effect is gastrulation in the fruit fly, D. melanogaster. Gastrulation is the first major shape change of the developing embryo, and cell-directed mechanical forces have a critical role.11 Before gastrulation, the middle of the D. melanogaster embryo appears roughly as a cylindrical tube composed of a single layer of cells. Gastrulation begins when a furrow appears across the length of the tube, and the furrow deepens until it has created a new layer of cells by folding within itself (Figure 1, third row).
The appearance of the furrow, it had long been hypothesized, would be neatly explained as a purely mechanical event: just like the formation of the dermal ridges, increasing compression in the cell layer could cause it to buckle in the direction with the least resistance.45 Here, instead of buckling upwards to form a ridge, the cells buckle inwards to form a furrow. This raises two questions: how does the cell layer generate compression, and how does it control the direction in which the layer buckles?
Early biologists proposed that the compression was created as cells divided and grew while being restrained from expanding in size.46 However, compressive forces created by cell proliferation alone cannot determine whether the tissue folds inwards or outwards. To ensure the layer folds inwards, the inner side of the cell layer must be less stiff than the outer side, so there must be a mechanism to regulate stiffness. Eventually, it was realized that the primary regulator of mechanical forces in the cell layer is not cell proliferation, but contraction of the actin cytoskeleton by the type II nonmuscle myosins.
The role of muscle-specific type II myosin motors in generating muscle contractions is well known. However, all cells contain related type II myosins, referred to as the nonmuscle myosins, that bind to actin and control contraction of the cytoskeleton. Initially found to be required for cell division, the roles of nonmuscle myosins continue to expand into many aspects of cell biology.47
In Drosophila, the requirement of the nonmuscle myosins for early events in embryogenesis was first demonstrated by characterization of flies with deletion of the nonmuscle myosin II heavy chain gene.48 Flies have only one isoform of nonmuscle myosin II heavy chain, and the deletion is lethal due to failure of an event late in gastrulation known as dorsal closure. Gene deletion, however, does not capture the importance of myosin for the earliest events in morphogenesis, since the embryos carry over enough maternal myosin to make it through most of gastrulation without synthesizing new myosin.
Visualizing the location of nonmuscle myosin by immunostaining strongly but indirectly suggests the role of myosin in regulating tension and compression during gastrulation. Before gastrulation begins, myosin is located in a sharp ring around the inner circumference of the cells. Just as the initial furrow appears, myosin disappears from the inner surface and relocates to the outer surface precisely where the furrow is forming.49 The presumed mechanical effect is that myosin is causing the outer (apical) surface to contract, creating compression of the cells simultaneously with the loss of tension around the inner surface. This causes the cell layer to buckle inwards and deepens the furrow on the outside, leading to invagination.11, 50
Further evidence for control of gastrulation by nonmuscle myosins is that the gastrulation requires signaling through the Rho family of GTPases, which control myosin contractile activity. Nonmuscle myosin II, unlike skeletal and cardiac myosin II, is regulated by phosphorylation of one of the two myosin light chains. One of the major kinases that phosphorylates myosin light chain and leads to increased myosin activity is Rho-associated coiled-coil protein kinase (Rock). Rock is regulated by association with the small GTPase RhoA, and Rho GTPases are regulated by guanine nucleotide exchange factors (RhoGEFs).47, 51 In Drosophila, all 3 levels of control are required for gastrulation: gene deletion of the homologs for Rock, RhoA, and RhoGEF2 each prevent gastrulation.49, 52, 53
Contraction of the apical side of epithelial cells by relocalization of RhoGEFs, Rho family GTPases, and nonmuscle myosin is a fundamental theme for morphogenesis that recurs frequently in subsequent epithelial cell shape changes.12, 54 Depending on the pattern of activation and the mechanical properties of the surroundings, apical contraction can lead to shape change, buckling, folding, or pit formation.12, 54
Dorsal Closure: Visualizing the Role of Myosin Contraction
Towards the end of gastrulation in Drosophila, two sheets of epithelial cells on the outer surface of the embryo move towards each other and meet at the dorsal midline, where they neatly fuse together. This event, known as dorsal closure, is a major test of the organism’s ability to produce and regulate the mechanical forces produced by the cells. As noted above, flies with null mutations in nonmuscle myosin heavy chain are unable to complete dorsal closure. More generally, of gene deletions that are lethal to embryos, failure to complete dorsal closure is a frequent cause of death for a surprisingly wide range of genes. Furthermore, the events of dorsal closure may provide insights into the regenerative processes involved in wound healing.55
Dorsal closure has thus become an important model for understanding the role of myosin contraction, its relative importance and its regulation. Studying the precise role of myosin contraction in vivo is challenging since there are few ways to directly visualize the forces produced by myosin in any particular cell. However, unlike the initial steps of gastrulation, dorsal closure involves only epithelial sheets on the outer surface, so the events are relatively easy to visualize. Recently, innovative genetic and engineering approaches have been applied to directly observing and measuring the mechanical forces involved in dorsal closure.
The importance of the nonmuscle myosin II heavy chain homolog (zipper) is visually clear in transgenic flies expressing nonmuscle myosin-GFP (Figure 3). The epithelial cell layer first meets at either side, forming an eye-shaped gap. Myosin-GFP is localized to the leading edge of the cells, suggesting that it acts as a drawstring that pulls the edges of the cells together as it contracts. However, to directly visualize the tension generated by nonmuscle myosin contraction, Franke et al. took advantage of a fortuitous observation in a myosin-null embryo that was also transgenic for overexpression of myosin-GFP.56 Although the transgenic myosin-GFP is driven by the same promoter in all cells, a few cells do not express any visible myosin-GFP and therefore remain null for myosin. The motions of these cells during dorsal closure were then followed by realtime fluorescence imaging. As the “eye” closes up, gaps are seen where non-expressing cells are located on the leading edge of the drawstring. As closure progresses, the gaps become larger -- visual evidence that myosin contraction creates tension that pulls on the adjacent cells in a ring around the opening.
Figure 3.
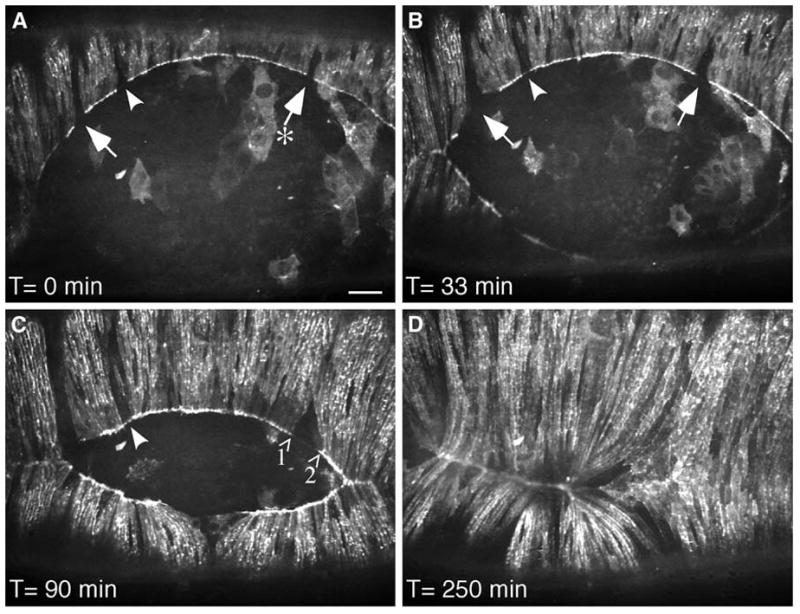
Tension created by myosin contraction is observed during dorsal closure of the epithelium in the fruit fly. In dorsal closure, two sheets of epithelial cells move towards each other and meet at the dorsal midline, leaving an eye-shaped opening. The epithelial sheets pull themselves together by three mechanisms: “zipping” inwards from the corners of the eye; constricting around the leading edge (like pulling on a drawstring); and using adhesion forces from the underlying cells. In flies with deletion of the homolog of vertebrate nonmuscle myosin heavy chain, dorsal closure fails and the embryos die. Transgenic overexpression of a GFP-myosin fusion protein in the myosin-null flies restores their ability to complete dorsal closure. However, in these flies, a few cells do not express the transgene and therefore do not express any myosin. On live fluorescent imaging of the flies during dorsal closure, these non-expressing cells are seen as gaps in the drawstring surrounding the opening between the epithelial cell sheets (A, arrows). As closure progresses, the space between GFP-myosin expressing cells clearly lengthens (A, arrow labeled with an asterisk; C, arrows labeled 1 and 2), visually demonstrating that contraction of myosin in the drawstring pulls on the neighboring cells to direct dorsal closure. Reproduced from Franke et al.56 with permission from Elsevier.
Thus, nonmuscle myosin drives contraction of the purse-string at the leading edge, and this contractile force is required for closure. But myosin plays other roles in cells, and there are other sources for the forces driving closure. A clear understanding of the process requires quantifying the relative roles of all these forces. Remarkably, Hutson et al. accomplished this feat; they measured forces by making incisions in the epithelium with a laser and observing how far and how quickly the cells snap back.57 This empirical data was then used to create a model for the relative contributions of various forces. Indeed, contraction of the leading edge myosin provided most of the forces for dorsal closure. However, the most astonishing aspect of the process is the extent of mechanical redundancy. Even without the contraction of myosin at the corners of the eye, adhesion forces from the underlying cell layer are enough to guide closure of the opening. This result echoes the conclusions of Franke et al. that expression of nonmuscle myosin in either the leading edge cells, or the cells in the underlying layer, is sufficient for dorsal closure.56
Cardiogenesis in Vertebrates
While nonmuscle myosin is also required for cardiogenesis, identifying the specific roles of mechanical forces in a mechanically functional tissue is more challenging than the previous examples. Nevertheless, understanding these roles has clinical implications since abnormal development of mechanical forces may be a major contributor to congenital heart defects.
The interplay of developmental form and mechanical function during cardiogenesis is remarkable. As the heart tube begins to function, the heart experiences both mechanical deformations and fluid flow. At the same time, developmental processes are coordinating major changes in form. Beginning as a linear tube in which the ventricles are upstream of the future atria, the tube loops around, fuses, and reconnects itself -- all while continuing to function as a pump.58, 59
Targeted gene deletion in mice shows that nonmuscle myosin is required for cardiogenesis. Unlike Drosophila, vertebrates express three different genes for nonmuscle myosin II heavy chain (NMHC2 A, B, and C).47 Most cells express both the A and B isoforms. In cardiomyocytes, however, expression is largely restricted to the B isoform. Mice with deletion of Nmhc2b can gastrulate but die as embryos due to failure of cardiogenesis.60 The mice have multiple cardiac malformations including ventricular septal defects, aortic root malformation, and an inability to form organized cardiac muscle. Thus, even though cardiomyocytes have normal amounts of the myosin that produces contraction of cardiac muscle, nonmuscle myosin is still required for normal patterning and morphogenesis of the heart.
The development of the heart endocardial cushions is particularly important for investigators of congenital heart defects. The endocardial cushions are the fore-runners of the heart valves as well as parts of the septae. Endocardial epithelial cells transdifferentiate into mesenchymal cells and migrate into bulges in the cardiac jelly to form the cushions.61, 62 Yet the forces involved in cushion development have resisted simple characterization. One major question has been whether the forces of fluid flow are required for inducing the endocardial cushions, or whether they develop through an independent program. Evidence supporting both views has recently been observed.58, 63–65
Hove and colleagues mechanically blocked flow through the early zebrafish heart tube and demonstrated that without fluid flow, the cushions failed to develop.63 Although blocking flow may change local fluid pressures as well, blocking flow either into the heart tube or out of the heart tube produced similar defects, suggesting that the lack of fluid shear stress was the critical factor. However, blocking flow also prevented cardiac looping, an earlier event in heart development, so it is possible that the lack of endocardial cushions was secondary to generally defective cardiogenesis.
On the other hand, Bartman and colleagues used a pharmacological inhibitor of myosin ATPase activity and observed that the endocardial cushion could indeed form even in the absence of a heart beat.64 At the same time, they convincingly showed by analysis of zebrafish contractile/cytoskeletal mutations that myocardial function is required for cushion formation. But the line between inhibiting myocardial function and inhibiting fluid flow may not have been clear enough to rule out the role of fluid flow. Higher doses of the ATPase inhibitor stopped contractile function, the heart beat, and cushion development. At the intermediate doses where cushions formed in the absence of a heart beat, it is possible that the remaining contractile function was still sufficient to produce fluid shear stress on the endocardium.58
Although the specific roles of forces such as shear stress continue to be debated, it seems clear that many steps in cardiogenesis can be viewed as an integrated feedback loop: alterations in shape change heart function, which changes the forces experienced by the cells, stimulating them to modify heart function both by secreting matrix and by continuing to change the heart shape.64, 66 Such biomechanical feedback loops are common throughout the development of the load-bearing and force-generating tissues.67
In vitro Cell Differentiation Is Controlled by Nonmuscle Myosins in Multiple Lineages
A final example of developmental processes controlled by myosin contractility is the in vitro differentiation of stem and precursor cells. Despite the nonphysiological environment,68 a major lesson of in vitro cell culture is that the mechanical properties of the materials surrounding the precursor cells are tightly linked to Rho family GTPase signaling and nonmuscle myosin contraction within the cell. Remarkably, manipulating any one of these three variables can be sufficient to change the differentiation pathway of a precursor cell. These insights are likely critical for guiding development of better tissue engineering and stem cell therapeutics.
It has been known since the 1970s that differentiation of precursor cells into chondrocytes and adipocytes in vitro is promoted by high cell density. For example, embryonic mesodermal cells isolated from chick limb buds will spontaneously undergo chondrogenesis when cultured at a very high density.69, 70 Similarly, subsets of 3T3 fibroblastic cells will spontaneously accumulate lipid when kept in high-density confluent monolayers.71
Interestingly, disrupting the actin cytoskeleton by treatment with cytochalasin also promotes differentiation of precursor cells into chondrocytes and adipocytes.71, 72 What mechanism links both cytoskeletal contractility and high cell density to cell differentiation? One might assume that the answer is simply related to cell-cell contact: by disrupting actin or culturing at high cell density, cells are prevented from spreading, instead forming rounder cells with more cell-cell contacts.
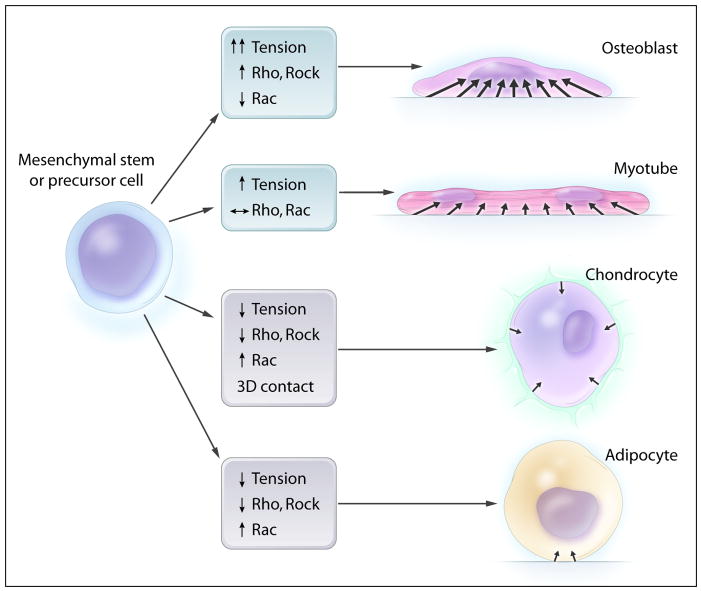
Recent experiments suggest that cell-cell contact is not the answer. Using micropatterning techniques to precisely manipulate the area available for cells to attach to a substrate, in the absence of any cell-cell contact, McBeath et al. showed that isolated human mesenchymal stem cells can change their differentiation pathway based solely on whether they are allowed to attach to a small fibronectin area (32 × 32 μm) or a large one (100 × 100 μm square).73 Cultured in the same mixture of adipogenic and osteogenic factors, stem cells grown on small fibronectin islands remain round and differentiate into adipocytes, while stem cells grown on the large fibronectin islands spread out over the entire island surface, form stress fibers, and differentiate into osteoblasts. At least for several mesenchymal cell lineages, myosin contraction of the actin cytoskeleton, the area of interaction with hard substrates, and cell shape seem to play primary roles in differentiation (Figure 4).
Figure 4.
Cytoskeletal contraction can direct differentiation of precursor cells in vitro. When cultured on a hard surface, cells spread out and cytoskeletal contraction generates high levels of tensile forces that pull on the surface. These changes promote differentiation of stem cells towards the osteoblast lineage. Overexpression of either Rho or Rho-associated kinase (Rock), which both stimulate contraction of the actin cytoskeleton, also promotes osteoblastic differentiation. On the other hand, cells can be kept round by preventing them from spreading, inhibiting actin polymerization, increasing cell density, or encapsulating them in a soft gel. All of these conditions decrease the area of hard surface that the cell is in contact with and prevent the cell from generating tension through contraction of the actin cytoskeleton. These conditions promote differentiation towards chondrocytes and adipocytes. In addition, overexpression of Rac, which opposes the actions of Rho and Rock, also inhibits cytoskeletal contraction and promotes differentiation towards chondrocytes and adipocytes. Differentiation into muscle cell lineages is promoted by an intermediate set of signals: some cytoskeletal contraction is required initially, but optimal conditions vary with each stage of differentiation into myotubes.
These experiments leave two major possibilities for the mechanism controlling differentiation in vitro. One possibility is that differentiation could depend on recapitulating the cell’s normal three-dimensional round shape. Indeed primary chondrocytes stop producing cartilage matrix when cultured in vitro, but maintain their phenotype when kept round either by treating with cytochalasin or by resuspending them in a soft gel.72, 74 Cell shape could affect spatial patterns of signaling through integrin or cadherin complexes on the cell surface. A second major possibility is that there could be a largely mechanical effect on differentiation: high cell density and round shape both minimize contact with the hard tissue culture surfaces, reducing the area available for stimulating formation of focal adhesions, stress fibers, and myosin contraction.
Further supporting the role of myosin contraction is that signaling through the Rho family GTPases also controls differentiation in a consistent manner. Overexpression of RhoA or Rock1, which stimulate myosin contraction, promotes osteogenesis but inhibits adipogenesis and chondrogenesis. Conversely, overexpression of Rac, which tends to oppose RhoA action, promotes differentiation into chondrocytes and adipocytes.73, 75
Why might cells use formation of intracellular mechanical stress to control differentiation? Is this just an artifact of in vitro culture or is there a reason for myosin contractility to control differentiation in vivo? It is not related to the need to resist mechanical stresses as a differentiated cell: chondrocytes and osteoblasts both become part of load-bearing structures, yet myosin contraction drives precursor cell differentiation in opposite directions.
The normal developmental program for making endochondral bone suggests one reason for the opposed effects of myosin contraction on differentiation into chondrocytes and osteoblasts. Chondrocytes first form directly from coalescing embryonic mesodermal cells in the absence of significant matrix or mechanical loading. The chondrocytes then synthesize a matrix, hypertrophy, and die by apoptosis.76 It is to the already hardened matrix that osteoblasts migrate and mature.77 This suggests the hypothesis that precursor cells might use the stiffness of their surroundings as a critical cue for determining differentiation in vivo.
Supporting this hypothesis, several investigators have reported that cellular phenotypes do indeed change in response to the stiffness of their physiological surroundings.78–84 For example, fibroblasts form stress fibers when in contact with stiff surroundings, regardless of whether they are grown on two-dimensional tissue culture surfaces or seeded in three-dimensional collagen gels.85 This in vitro behavior may recapitulate the physiological response of fibroblasts in healing skin wounds: as the wound begins to heal, tension is generated, which promotes differentiation of fibroblasts into myofibroblasts and generates more contraction in turn.78, 79
Engler et al. have recently tested this hypothesis more broadly by investigating whether stem cell differentiation can be regulated by the stiffness of the surroundings in vitro.86 Human mesenchymal stem cells were cultured as a monolayer on collagen-I-coated gels with varying stiffnesses. Under identical culture conditions except for the stiffness of the gel, stem cells were induced to express early markers of differentiation into neurons (for the softest gel, with an elastic modulus of 0.1–1 kPa), myocytes (8–17 kPa), or osteoblasts (25–40 kPa). The myosin contractile state of the cells adapted to the stiffness of the gel: on the hardest surface, the cells spread out the most, and developed focal adhesions. Importantly, Engler et al. provide evidence that the stiffness promoting stem cell differentiation into each lineage corresponded to the conditions normally present during differentiation of that lineage. For example, the gel stiffness that maximized expression of osteoblast markers was similar to the stiffness of matrix laid down by hypertrophic chondrocytes.
Further investigation of how the mechanical environment changes during muscle development may explain aspects of the less straightforward results regarding differentiation of mesenchymal stem cells into myotubes. Differentiation of myoblasts into myotubes in vitro is prevented by cytochalasin87 and requires integrin-mediated phosphorylation of focal adhesion kinase, RhoA signaling, and myosin contraction.88–92 On the other hand, RhoA or Rock1 can also inhibit myogenesis by preventing cell fusion and myotube formation.93–95 Myogenesis probably requires a balance of signals regulating contractility that changes during the multi-step differentiation process. Similarly, the differentiation of stem cells into cardiomyocytes requires a balance of Rho and Rac signaling that varies with time.96, 97
In vivo, primary myoblasts are induced directly from mesodermal cells in the absence of significant mechanical stresses.98 In contrast, after the myoblasts migrate to the site of muscle formation and fuse into myotubes, the developing muscles attach to tendons and are subjected to significant loads. Therefore, secondary myogenesis, as well as adult myogenesis from satellite cells, will experience an entirely different mechanical environment, and may require different programs for regulating contractile state during maturation.99, 100
While the hypothesis that cells use myosin contraction to adjust their developmental pathway as a function of extracellular substrate stiffness is attractive as a unifying concept, its full extent is not yet clear. It will be interesting to test this hypothesis range in a wider range of differentiation pathways and situations. Also, basic questions about the pathway from sensing matrix stiffness to myosin contraction and Rho signaling remain unanswered. Extracellular matrix stiffness may be sensed by signaling through integrin or cadherin-catenin events that activate Rho signaling and then myosin contraction.68 On the other hand, intracellular myosin contractile forces must be balanced by forces from the extracellular matrix. Therefore matrix stiffness must limit myosin contraction directly as well, since soft surroundings physically do not support much contraction.101 Most likely, as with most mechanotransduction signaling pathways, multiple pathways participate, and feedback mechanisms play important roles.
Acknowledgments
Sources of Funding: R. T. Lee is supported in part by NIH grant R01-HL081404; P. Patwari is supported in part by NIH grant K25-HL081523 and a Lerner Cardiovascular Award.
Footnotes
Disclosures: none
References
- 1.Ashby M. Stevenage Past. Chichester; Phillimore: 1995. [Google Scholar]
- 2.Twin Poachers Mixed Up. New York Times. Feb 9, 1913. p. C5.
- 3.Saltzman J. After three trials, twin is found guilty in 2001 rape; previous assault sealed the case. The Boston Globe. Mar 23, 2006. p. B4.
- 4.Stevenson J. Man’s murder charge dismissed because DNA couldn’t tell him from identical twin; A genetic loophole and the suspects’ silence made it impossible to determine who was involved in the July 2000 beating death. The Herald-Sun (Durham, NC) Feb 14, 2001. p. C1.
- 5.Twins’ DNA muddles rape trials; Boston circumstances similar to case in Grand Rapids. Grand Rapid Press (Michigan) Sep 4, 2005. p. A7.
- 6.Tilghman A. Rape trial dilemma: DNA points to two men; As the second of identical twins heads to court, prosecutors focus on his confession. The Houston Chronicle. Jun 2, 2005. p. B6.
- 7.Reed T, Viken RJ, Rinehart SA. High heritability of fingertip arch patterns in twin-pairs. Am J Med Genet. 2006;140A:263–271. doi: 10.1002/ajmg.a.31086. [DOI] [PubMed] [Google Scholar]
- 8.Hale AR. Morphogenesis of volar skin in the human fetus. Am J Anat. 1952;91:147–81. doi: 10.1002/aja.1000910105. [DOI] [PubMed] [Google Scholar]
- 9.Misumi Y, Akiyoshi T. Epidermal ridge formation in the human fetus: A correlation to the appearance of basal cell heterogeneity and the expression of epidermal growth factor receptor and cytokeratin polypeptides in the epidermis. Am J Anat. 1991;191:419–428. doi: 10.1002/aja.1001910409. [DOI] [PubMed] [Google Scholar]
- 10.Kucken M. Models for fingerprint pattern formation. Forensic Sci Int. 2007;171:85–96. doi: 10.1016/j.forsciint.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 11.Keller R, Davidson LA, Shook DR. How we are shaped: The biomechanics of gastrulation. Differentiation. 2003;71:171–205. doi: 10.1046/j.1432-0436.2003.710301.x. [DOI] [PubMed] [Google Scholar]
- 12.Lecuit T, Lenne P. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol. 2007;8:633–44. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- 13.Ingber DE. Mechanical control of tissue morphogenesis during embryological development. Int J Dev Biol. 2006;50:255–66. doi: 10.1387/ijdb.052044di. [DOI] [PubMed] [Google Scholar]
- 14.Schweickert A, Weber T, Beyer T, Vick P, Bogusch S, Feistel K, Blum M. Cilia-Driven Leftward Flow Determines Laterality in Xenopus. Curr Biol. 2007;17:60–66. doi: 10.1016/j.cub.2006.10.067. [DOI] [PubMed] [Google Scholar]
- 15.Spéder P, Adám G, Noselli S. Type ID unconventional myosin controls left-right asymmetry in Drosophila. Nature. 2006;440:803–7. doi: 10.1038/nature04623. [DOI] [PubMed] [Google Scholar]
- 16.Hozumi S, Maeda R, Taniguchi K, Kanai M, Shirakabe S, Sasamura T, Spéder P, Noselli S, Aigaki T, Murakami R, Matsuno K. An unconventional myosin in Drosophila reverses the default handedness in visceral organs. Nature. 2006;440:798–802. doi: 10.1038/nature04625. [DOI] [PubMed] [Google Scholar]
- 17.Kartagener M, Stucki P. Bronchiectasis with situs inversus. Arch Pediatr. 1962;79:193–207. [PubMed] [Google Scholar]
- 18.Eliasson R, Mossberg B, Camner P, Afzelius BA. The immotile-cilia syndrome. A congenital ciliary abnormality as an etiologic factor in chronic airway infections and male sterility. N Engl J Med. 1977;297:1–6. doi: 10.1056/NEJM197707072970101. [DOI] [PubMed] [Google Scholar]
- 19.Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–37. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- 20.Guichard C, Harricane MC, Lafitte JJ, Godard P, Zaegel M, Tack V, Lalau G, Bouvagnet P. Axonemal dynein intermediate-chain gene (DNAI1) mutations result in situs inversus and primary ciliary dyskinesia (Kartagener syndrome) Am J Hum Genet. 2001;68:1030–5. doi: 10.1086/319511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pennarun G, Escudier E, Chapelin C, Bridoux AM, Cacheux V, Roger G, Clément A, Goossens M, Amselem S, Duriez B. Loss-of-function mutations in a human gene related to Chlamydomonas reinhardtii dynein IC78 result in primary ciliary dyskinesia. Am J Hum Genet. 1999;65:1508–19. doi: 10.1086/302683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olbrich H, Häffner K, Kispert A, Völkel A, Volz A, Sasmaz G, Reinhardt R, Hennig S, Lehrach H, Konietzko N, Zariwala M, Noone PG, Knowles M, Mitchison HM, Meeks M, Chung EMK, Hildebrandt F, Sudbrak R, Omran H. Mutations in DNAH5 cause primary ciliary dyskinesia and randomization of left-right asymmetry. Nat Genet. 2002;30:143–4. doi: 10.1038/ng817. [DOI] [PubMed] [Google Scholar]
- 23.Nonaka S, Shiratori H, Saijoh Y, Hamada H. Determination of left-right patterning of the mouse embryo by artificial nodal flow. Nature. 2002;418:96–99. doi: 10.1038/nature00849. [DOI] [PubMed] [Google Scholar]
- 24.Brokaw CJ. Computer simulation of flagellar movement IX. Oscillation and symmetry breaking in a model for short flagella and nodal cilia. Cell Motil Cytoskel. 2005;60:35–47. doi: 10.1002/cm.20046. [DOI] [PubMed] [Google Scholar]
- 25.Nonaka S, Yoshiba S, Watanabe D, Ikeuchi S, Goto T, Marshall WF, Hamada H. De novo formation of left-right asymmetry by posterior tilt of nodal cilia. PLoS Biol. 2005;3:e268. doi: 10.1371/journal.pbio.0030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen MM. Nodal signaling: developmental roles and regulation. Development. 2007;134:1023–1034. doi: 10.1242/dev.000166. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka Y, Okada Y, Hirokawa N. FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left-right determination. Nature. 2005;435:172–177. doi: 10.1038/nature03494. [DOI] [PubMed] [Google Scholar]
- 28.McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two Populations of Node Monocilia Initiate Left-Right Asymmetry in the Mouse. Cell. 2003;114:61–73. doi: 10.1016/s0092-8674(03)00511-7. [DOI] [PubMed] [Google Scholar]
- 29.Tabin CJ, Vogan KJ. A two-cilia model for vertebrate left-right axis specification. Genes Dev. 2003;17:1–6. doi: 10.1101/gad.1053803. [DOI] [PubMed] [Google Scholar]
- 30.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AEH, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 31.Folkman J, Haudenschild C. Angiogenesis in vitro. Nature. 1980;288:551–556. doi: 10.1038/288551a0. [DOI] [PubMed] [Google Scholar]
- 32.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–84. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 33.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 34.Gimbrone MA. Vascular endothelium, hemodynamic forces, and atherogenesis. Am J Pathol. 1999;155:1–5. doi: 10.1016/S0002-9440(10)65090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee RT, Huang H. Mechanotransduction and arterial smooth muscle cells: new insight into hypertension and atherosclerosis. Ann Med. 2000;32:233–5. doi: 10.3109/07853890009011765. [DOI] [PubMed] [Google Scholar]
- 36.Gimbrone MA, Topper JN, Nagel T, Anderson KR, Garcia-Cardeña G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann N Y Acad Sci. 2000;902:230–9. doi: 10.1111/j.1749-6632.2000.tb06318.x. discussion 239–40. [DOI] [PubMed] [Google Scholar]
- 37.Boardman KC, Swartz MA. Interstitial Flow as a Guide for Lymphangiogenesis. Circ Res. 2003;92:801–808. doi: 10.1161/01.RES.0000065621.69843.49. [DOI] [PubMed] [Google Scholar]
- 38.Rutkowski JM, Swartz MA. A driving force for change: interstitial flow as a morphoregulator. Trends Cell Biol. 2007;17:44–50. doi: 10.1016/j.tcb.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–4. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt A, Brixius K, Bloch W. Endothelial precursor cell migration during vasculogenesis. Circ Res. 2007;101:125–36. doi: 10.1161/CIRCRESAHA.107.148932. [DOI] [PubMed] [Google Scholar]
- 41.le Noble F, Moyon D, Pardanaud L, Yuan L, Djonov V, Matthijsen R, Breant C, Fleury V, Eichmann A. Flow regulates arterial-venous differentiation in the chick embryo yolk sac. Development. 2004;131:361–375. doi: 10.1242/dev.00929. [DOI] [PubMed] [Google Scholar]
- 42.Lucitti JL, Jones EAV, Huang C, Chen J, Fraser SE, Dickinson ME. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134:3317–3326. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang H, Riha GM, Yan S, Li M, Chai H, Yang H, Yao Q, Chen C. Shear stress induces endothelial differentiation from a murine embryonic mesenchymal progenitor cell line. Arterioscler Thromb Vasc Biol. 2005;25:1817–23. doi: 10.1161/01.ATV.0000175840.90510.a8. [DOI] [PubMed] [Google Scholar]
- 44.Ingber DE. Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res. 2002;91:877–87. doi: 10.1161/01.res.0000039537.73816.e5. [DOI] [PubMed] [Google Scholar]
- 45.Gudernatsch JF. Concerning the mechanism and direction of embryonic foldings. Anat Rec. 1913;7:411–431. [Google Scholar]
- 46.Holtfreter J. A study of the mechanics of gastrulation. Journal of Experimental Zoology. 1944;95:171–212. [Google Scholar]
- 47.Conti MA, Adelstein RS. Nonmuscle myosin II moves in new directions. J Cell Sci. 2008;121:11–18. doi: 10.1242/jcs.007112. [DOI] [PubMed] [Google Scholar]
- 48.Young PE, Richman AM, Ketchum AS, Kiehart DP. Morphogenesis in Drosophila requires nonmuscle myosin heavy chain function. Genes Dev. 1993;7:29–41. doi: 10.1101/gad.7.1.29. [DOI] [PubMed] [Google Scholar]
- 49.Dawes-Hoang RE, Parmar KM, Christiansen AE, Phelps CB, Brand AH, Wieschaus EF. folded gastrulation, cell shape change and the control of myosin localization. Development. 2005;132:4165–4178. doi: 10.1242/dev.01938. [DOI] [PubMed] [Google Scholar]
- 50.Dassow MV, Davidson LA. Variation and robustness of the mechanics of gastrulation: The role of tissue mechanical properties during morphogenesis. Birth Def Res C. 2007;81:253–269. doi: 10.1002/bdrc.20108. [DOI] [PubMed] [Google Scholar]
- 51.Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from Rho to the Actin Cytoskeleton Through Protein Kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- 52.Halsell SR, Chu BI, Kiehart DP. Genetic Analysis Demonstrates a Direct Link Between Rho Signaling and Nonmuscle Myosin Function During Drosophila Morphogenesis. Genetics. 2000;155:1253–1265. doi: 10.1093/genetics/155.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Padash Barmchi M, Rogers S, Häcker U. DRhoGEF2 regulates actin organization and contractility in the Drosophila blastoderm embryo. J Cell Biol. 2005;168:575–85. doi: 10.1083/jcb.200407124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Settleman J. Rac ‘n Rho: the music that shapes a developing embryo. Dev Cell. 2001;1:321–31. doi: 10.1016/s1534-5807(01)00053-3. [DOI] [PubMed] [Google Scholar]
- 55.Martin P, Parkhurst SM. Parallels between tissue repair and embryo morphogenesis. Development. 2004;131:3021–3034. doi: 10.1242/dev.01253. [DOI] [PubMed] [Google Scholar]
- 56.Franke JD, Montague RA, Kiehart DP. Nonmuscle Myosin II Generates Forces that Transmit Tension and Drive Contraction in Multiple Tissues during Dorsal Closure. Curr Biol. 2005;15:2208–2221. doi: 10.1016/j.cub.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 57.Hutson MS, Tokutake Y, Chang M, Bloor JW, Venakides S, Kiehart DP, Edwards GS. Forces for morphogenesis investigated with laser microsurgery and quantitative modeling. Science. 2003;300:145–9. doi: 10.1126/science.1079552. [DOI] [PubMed] [Google Scholar]
- 58.Bartman T, Hove J. Mechanics and function in heart morphogenesis. Dev Dyn. 2005;233:373–81. doi: 10.1002/dvdy.20367. [DOI] [PubMed] [Google Scholar]
- 59.Srivastava D, Olson EN. A genetic blueprint for cardiac development. Nature. 2000;407:221–6. doi: 10.1038/35025190. [DOI] [PubMed] [Google Scholar]
- 60.Tullio AN, Accili D, Ferrans VJ, Yu ZX, Takeda K, Grinberg A, Westphal H, Preston YA, Adelstein RS. Nonmuscle myosin II-B is required for normal development of the mouse heart. Proc Natl Acad Sci U S A. 1997;94:12407–12. doi: 10.1073/pnas.94.23.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Armstrong EJ, Bischoff J. Heart Valve Development: Endothelial Cell Signaling and Differentiation. Circ Res. 2004;95:459–470. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Markwald RR, Fitzharris TP, Manasek FJ. Structural development of endocardial cushions. Am J Anat. 1977;148:85–119. doi: 10.1002/aja.1001480108. [DOI] [PubMed] [Google Scholar]
- 63.Hove JR, Koster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421:172–177. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- 64.Bartman T, Walsh EC, Wen K, McKane M, Ren J, Alexander J, Rubenstein PA, Stainier DYR. Early myocardial function affects endocardial cushion development in zebrafish. PLoS Biol. 2004;2:E129. doi: 10.1371/journal.pbio.0020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mironov V, Visconti RP, Markwald RR. On the role of shear stress in cardiogenesis. Endothelium. 12:259–61. doi: 10.1080/10623320500476708. [DOI] [PubMed] [Google Scholar]
- 66.Butcher JT, McQuinn TC, Sedmera D, Turner D, Markwald RR. Transitions in early embryonic atrioventricular valvular function correspond with changes in cushion biomechanics that are predictable by tissue composition. Circ Res. 2007;100:1503–11. doi: 10.1161/CIRCRESAHA.107.148684. [DOI] [PubMed] [Google Scholar]
- 67.Estes BT, Gimble JM, Guilak F. Mechanical Signals as Regulators of Stem Cell Fate. Stem Cells in Development and Disease. 2004 doi: 10.1016/S0070-2153(04)60004-4. [DOI] [PubMed] [Google Scholar]
- 68.Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix--cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- 69.Ahrens PB, Solursh M, Reiter RS. Stage-related capacity for limb chondrogenesis in cell culture. Dev Biol. 1977;60:69–82. doi: 10.1016/0012-1606(77)90110-5. [DOI] [PubMed] [Google Scholar]
- 70.Umansky R. The effect of cell population density on the developmental fate of reaggregating mouse limb bud mesenchyme. Dev Biol. 1966;13:31–56. doi: 10.1016/0012-1606(66)90048-0. [DOI] [PubMed] [Google Scholar]
- 71.Green H, Kehinde O. Sublines of mouse 3T3 cells that accumulate lipid. Cell. 1974;1:113–116. [Google Scholar]
- 72.Benya PD, Brown PD, Padilla SR. Microfilament modification by dihydrocytochalasin B causes retinoic acid-modulated chondrocytes to reexpress the differentiated collagen phenotype without a change in shape. J Cell Biol. 1988;106:161–70. doi: 10.1083/jcb.106.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell Shape, Cytoskeletal Tension, and RhoA Regulate Stem Cell Lineage Commitment. Developmental Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 74.Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 75.Woods A, Beier F. RhoA/ROCK signaling regulates chondrogenesis in a context-dependent manner. J Biol Chem. 2006;281:13134–40. doi: 10.1074/jbc.M509433200. [DOI] [PubMed] [Google Scholar]
- 76.Hunziker EB. Mechanism of longitudinal bone growth and its regulation by growth plate chondrocytes. Microscopy Research and Technique. 1994;28:505–519. doi: 10.1002/jemt.1070280606. [DOI] [PubMed] [Google Scholar]
- 77.Olsen BR, Reginato AM, Wang W. Bone development. Annu Rev Cell Dev Biol. 2000;16:191–220. doi: 10.1146/annurev.cellbio.16.1.191. [DOI] [PubMed] [Google Scholar]
- 78.Arora PD, Narani N, McCulloch CAG. The Compliance of Collagen Gels Regulates Transforming Growth Factor-{beta}1 Induction of {alpha}-Smooth Muscle Actin in Fibroblasts. Am J Pathol. 1999;154:871–882. doi: 10.1016/s0002-9440(10)65334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol. 2001;159:1009–20. doi: 10.1016/S0002-9440(10)61776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994;124:401–4. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Georges PC, Miller WJ, Meaney DF, Sawyer ES, Janmey PA. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys J. 2006;90:3012–8. doi: 10.1529/biophysj.105.073114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Genes NG, Rowley JA, Mooney DJ, Bonassar LJ. Effect of substrate mechanics on chondrocyte adhesion to modified alginate surfaces. Arch Biochem Biophys. 2004;422:161–7. doi: 10.1016/j.abb.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 83.Pelham RJ, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proceedings of the National Academy of Sciences. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vickers SM, Squitieri LS, Spector M. Effects of cross-linking type II collagen-GAG scaffolds on chondrogenesis in vitro: dynamic pore reduction promotes cartilage formation. Tissue Eng. 2006;12:1345–55. doi: 10.1089/ten.2006.12.1345. [DOI] [PubMed] [Google Scholar]
- 85.Halliday NL, Tomasek JJ. Mechanical properties of the extracellular matrix influence fibronectin fibril assembly in vitro. Exp Cell Res. 1995;217:109–17. doi: 10.1006/excr.1995.1069. [DOI] [PubMed] [Google Scholar]
- 86.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 87.Delain D, Wahrmann JP. Is fusion a trigger for myoblast differentiation? Exp Cell Res. 1975;93:495–498. doi: 10.1016/0014-4827(75)90480-2. [DOI] [PubMed] [Google Scholar]
- 88.Osses N, Brandan E. ECM is required for skeletal muscle differentiation independently of muscle regulatory factor expression. Am J Physiol Cell Physiol. 2002;282:C383–394. doi: 10.1152/ajpcell.00322.2001. [DOI] [PubMed] [Google Scholar]
- 89.Charrasse S, Meriane M, Comunale F, Blangy A, Gauthier-Rouviere C. N-cadherin-dependent cell-cell contact regulates Rho GTPases and {beta}-catenin localization in mouse C2C12 myoblasts. J Cell Biol. 2002;158:953–965. doi: 10.1083/jcb.200202034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bryan BA, Mitchell DC, Zhao L, Ma W, Stafford LJ, Teng B, Liu M. Modulation of Muscle Regeneration, Myogenesis, and Adipogenesis by the Rho Family Guanine Nucleotide Exchange Factor GEFT. Mol Cell Biol. 2005;25:11089–11101. doi: 10.1128/MCB.25.24.11089-11101.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fluck M, Carson JA, Gordon SE, Ziemiecki A, Booth FW. Focal adhesion proteins FAK and paxillin increase in hypertrophied skeletal muscle. Am J Physiol Cell Physiol. 1999;277:C152–162. doi: 10.1152/ajpcell.1999.277.1.C152. [DOI] [PubMed] [Google Scholar]
- 92.Swailes NT, Colegrave M, Knight PJ, Peckham M. Non-muscle myosins 2A and 2B drive changes in cell morphology that occur as myoblasts align and fuse. J Cell Sci. 2006;119:3561–3570. doi: 10.1242/jcs.03096. [DOI] [PubMed] [Google Scholar]
- 93.Nishiyama T, Kii I, Kudo A. Inactivation of Rho/ROCK Signaling Is Crucial for the Nuclear Accumulation of FKHR and Myoblast Fusion. J Biol Chem. 2004;279:47311–47319. doi: 10.1074/jbc.M403546200. [DOI] [PubMed] [Google Scholar]
- 94.Charrasse S, Comunale F, Fortier M, Portales-Casamar E, Debant A, Gauthier-Rouvière C. M-cadherin activates Rac1 GTPase through the Rho-GEF trio during myoblast fusion. Mol Biol Cell. 2007;18:1734–43. doi: 10.1091/mbc.E06-08-0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Castellani L, Salvati E, Alemà S, Falcone G. Fine regulation of RhoA and Rock is required for skeletal muscle differentiation. J Biol Chem. 2006;281:15249–57. doi: 10.1074/jbc.M601390200. [DOI] [PubMed] [Google Scholar]
- 96.Hakuno D, Takahashi T, Lammerding J, Lee RT. Focal adhesion kinase signaling regulates cardiogenesis of embryonic stem cells. J Biol Chem. 2005;280:39534–44. doi: 10.1074/jbc.M505575200. [DOI] [PubMed] [Google Scholar]
- 97.Puceat M, Travo P, Quinn Fort P. A Dual Role of the GTPase Rac in Cardiac Differentiation of Stem Cells. Mol Biol Cell. 2003;14:2781–2792. doi: 10.1091/mbc.E02-09-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Linker C, Lesbros C, Stark MR, Marcelle C. Intrinsic signals regulate the initial steps of myogenesis in vertebrates. Development. 2003;130:4797–4807. doi: 10.1242/dev.00688. [DOI] [PubMed] [Google Scholar]
- 99.Wigmore PM, Dunglison GF. The generation of fiber diversity during myogenesis. Int J Dev Biol. 1998;42:117–25. [PubMed] [Google Scholar]
- 100.Zhao P, Hoffman EP. Embryonic myogenesis pathways in muscle regeneration. Dev Dyn. 2004;229:380–92. doi: 10.1002/dvdy.10457. [DOI] [PubMed] [Google Scholar]
- 101.Georges PC, Janmey PA. Cell type-specific response to growth on soft materials. J Appl Physiol. 2005;98:1547–1553. doi: 10.1152/japplphysiol.01121.2004. [DOI] [PubMed] [Google Scholar]