Abstract
Accurate segregation of chromosomes, essential for the stability of genome, depends on ‘biorientation’ – simultaneous attachment of each individual chromosome to both poles of the mitotic spindle1. On bioriented chromosomes, kinetochores (macromolecular complexes that attach the chromosome to the spindle) reside on the opposite sides of chromosome's centromere2. In contrast, sister kinetochores shift toward one side of the centromere on ‘syntelic’ chromosomes that erroneously attach to one spindle pole with both sister kinetochores. Syntelic attachments often arise during spindle assembly and must be corrected to prevent chromosome loss3. It is assumed that restoration of proper centromere architecture occurs automatically due to elastic properties of the centromere1, 2. Here we test this assumption by combining laser microsurgery and chemical biology assays. We find that kinetochores of syntelic chromosomes remain juxtaposed upon detachment from spindle microtubules. These findings reveal that correction of syntelic attachments involves an extra step that has previously been overlooked: external forces must be applied to move sister kinetochores to the opposite sides of the centromere. Further, we demonstrate that shape of the centromere is important for spindle assembly, as bipolar spindles do not form is cells lacking centrosomes when multiple chromosomes with juxtaposed kinetochores are present. Thus, proper architecture of the centromere makes an important contribution to achieving high fidelity of chromosome segregation.
Kinetochores on bioriented chromosomes are positioned on the opposite sides of the centromere2. However, during mitotic spindle formation both sister kinetochores sometimes attach to the same spindle pole becoming ‘syntelic’. Under this condition, microtubule-dependent forces shift sister kinetochores to the same side of the centromere. As syntelic attachment would lead to aneuploidy, this configuration is not stable4,5. Kinetochore fibres (K-fibres) on syntelic chromosomes depolymerize so that the chromosome moves to the spindle pole where at least one of the two kinetochores detaches from microtubules6-8. Detached kinetochores can then connect to microtubules from the opposite spindle pole to achieve proper bi-orientation. However, for this mechanism to work properly the shape of the centromere must be restored such that sister kinetochores return to opposite sides of the centromere. It is generally assumed that this occurs automatically due to elasticity of chromatin9,10. However, this assumption has not been tested.
Cells treated with monastrol, a small-molecule inhibitor of the molecular motor Eg5 (kinesin-5), arrest in mitosis with monopolar spindles11. Up to 70% of chromosomes in these cells are syntelic11,12. We reasoned that if restoration of proper centromere architecture occurs automatically then kinetochores on syntelic chromosomes should spring back to the opposite sides of the centromere if microtubules are rapidly depolymerized. However, kinetochores will remain juxtaposed under these conditions if their repositioning on the centromere requires microtubule-based forces (Fig.1A).
Figure 1. Juxtaposed sister kinetochores on syntelic chromosomes do not return to the opposite sides of the centromere in the absence of microtubules.
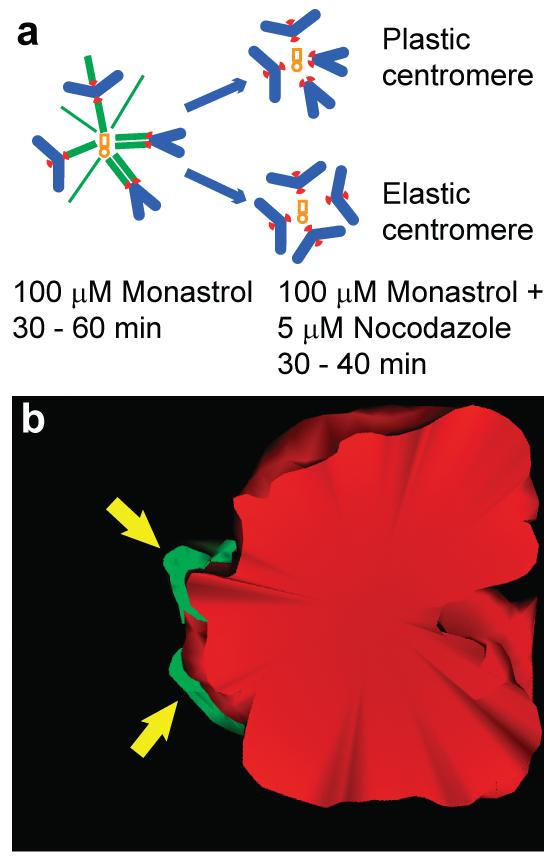
(a) A schematic representation of the experiment. Cells treated with monastrol form monopolar spindles with high incidence of syntelic chromosomes. Both sister kinetochores (red) on these chromosomes are positioned side-by-side and connected to the centrosomes (light brown) via microtubule bundles (green). If microtubules are depolymerized in monastrol-treated cells, kinetochores should remain juxtaposed if chromosome's centromere is malleable (or plastic) (top diagram) or return to the opposite sides if the centromere is spring-like (i.e., elastic) (bottom diagram). (b) Surface-rendered serial-section chromosome reconstruction from a cell assayed as described in (b). Despite complete lack of microtubules both sister kinetochores (arrows) remain juxtaposed (EM sections are presented in Fig.S1).
We treated PtK1 cells that constitutively express a γ-tubulin-GFP13 with 100-μM monastrol for 30-60 min. Mitotic cells with monopolar spindles were visualized by differential-interference contrast (DIC) microscopy and followed for 10-15 min (1 image every 30-60 s). Then, in addition to monastrol, the cells were treated with 5-μM nocodazole which completely depolymerizes microtubules in mitotic cells in ∼3 min (Fig.S1). The cells were imaged for another 30-45 min and then fixed for Electron Microscopy (EM). This approach ensured that EM analyses were conducted on cells exposed to nocodazole only after they had formed monopolar spindles and accumulated syntelic chromosomes in the presence of monastrol.
Serial-section EM reconstructions revealed that sister kinetochores on many chromosomes resided on the same side of the centromere (Figs.1B, S2). The frequency of chromosomes with juxtaposed kinetochores was estimated via 3-D fluorescence microscopy (Fig.2). Visual inspection revealed that sister kinetochores were positioned on the same side of the centromere (within ∼90° segment) on ∼50% of chromosomes in cells with monopolar spindles. This frequency did not change in cells that were additionally treated with nocodazole, suggesting that once displaced to the same side of the centromere (due to syntelic attachment), kinetochores do not return to the opposite sides of the centromere upon microtubule depolymerization. Further, the average distance between sister kinetochores measured in 3-D fluorescence LM revealed that sister kinetochores resided closer to one another in monopolar spindles than in prophase cells and this distance did not change upon microtubule depolymerization (Fig.2). Thus, restoration of proper centromere organization during correction of syntelic attachments is not achieved through elastic recoil, but requires external forces.
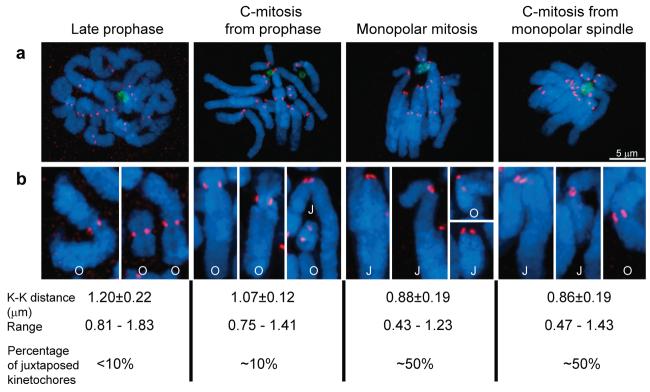
Figure 2. Frequencies of chromosomes with juxtaposed sister kinetochores observed under different experimental conditions.
(a) Maximal-intensity projection spanning the entire volume of the cell and (b) Individual chromosomes from cells shown in (a) presented at 2X additional magnification. During prophase in untreated cells sister kinetochores are positioned on the opposite sides of the centromere and separated by ∼1.2 μm. When microtubules are depolymerized during prophase-prometaphase sister kinetochores on most chromosomes remain opposed, although occasionally they can be seen on the same side of the centromere (cf. chromosomes marked “O” vs. “J”). In cells with monopolar spindles at least 50% of kinetochores are on the same side of the centromere (juxtaposed). This change in the organization of the centromere is reflected by the decrease in the average distance between sister kinetochores (the “C-mitosis” and “Monopolar mitosis” populations are different with >99.99% confidence in two-tailed Student's test). The percentage of chromosomes and average sister kinetochore separation does not change when microtubules in cells with monopolar spindles are depolymerized with 5-μM nocodazole. Centrosomes are shown in the green (γ-tubulin-GFP), kinetochores in the red (CREST), and DNA in the blue (Hoechst 33343).
Forces responsible for straightening the sister kinetochore-centromere axis can be generated when one kinetochore on a formerly syntelic chromosome captures astral microtubules from one spindle pole while its sister connects to the other pole. The formation of astral microtubules depends on centrosomes. Therefore, we tested if correction of syntelic attachments occurs in the absence of centrosomes by ablating these organelles with a laser microbeam14,15 (N=12). Astral microtubules disappear in 3-5 min after centrosome ablation15. Upon monastrol washout in cells without microtubule asters chromosomes remained disorganized, moving intermittently in random directions for ∼1 hr (Fig.3A). Immunofluorescence analyses (N=20) revealed a highly disorganized microtubule pattern (Fig. 3B). Prominent bundles of microtubules (K-fibres) emanated from the chromosomes and converged on numerous small foci (5-10 foci per cell). These foci were not γ-tubulin-positive but contained highly concentrated NuMA – a large protein responsible for spindle pole focusing16. Despite the lack of an organized mitotic spindle, ∼1 hr after centrosome ablation and monastrol washout sister chromatids separated and exhibited short directed movements resembling anaphase motion. The extent of these movements was limited to 2-3 μm along different directions. As a result, chromatids remained in a single group and ultimately reconstituted a single nucleus.
Figure 3. Monopolar spindles do not bipolarize and sister kinetochores remain juxtaposed upon monastrol washout in the absence of centrosomes.
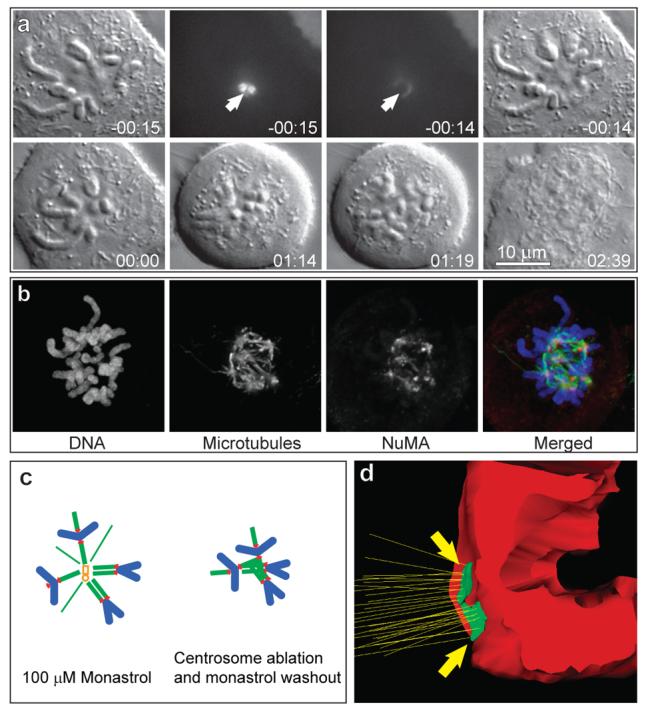
(a) Both centrosomes in a monastrol-induced monopolar mitosis were ablated (arrows in −00:15 and −00:14 frames), then monastrol was washed out and the cell followed by time-lapse DIC microscopy. The cell did not organize a bipolar spindle for more than an hour at which point cohesion between sister chromatids was lost (01:14) and the cell exited mitosis. Although individual sister chromatids attempted to move moved away from one another the extent of their movement was not sufficient to form discrete chromosome groups (01:19). A single daughter cell with one nucleus was formed upon completion of mitosis (02:39). Time in hours:minutes. (b) Distribution of microtubules, chromosomes, and NuMA in acentrosomal cells shortly before anaphase onset (45-60 min after monastrol washout). Prominent bundles of microtubules (K-fibres) were associated with all chromosomes. These K-fibres were not organized in a bipolar spindle but converged on multiple small centres that contained elevated amounts of the spindle-pole protein NuMA. All images are maximal-intensity projections through the entire cell. (c) Schematic representation of the spindle reorganization that occurs in monastrol-induced monopolar mitoses after ablation of the centrosome and monastrol washout. (d) Surface-rendered model of a centromere from a cell fixed 60 min after monastrol washout. Sister kinetochores remained juxtaposed and attached to prominent microtubule bundles that terminated inside the kinetochore (original EM data are presented in Fig.S2).
Serial-section EM analyses of 4 acentrosomal cells fixed 1 hr after monastrol washout revealed that sister kinetochores often remained on the same side of the centromere. K-fibres attached to these kinetochores were oriented parallel to each other (Figs.3D, S3). All kinetochores, whether juxtaposed or properly positioned, were associated with prominent K-fibres. Thus, in the absence of centrosomes restoration of proper centromere organization and spindle bipolarization are impeded. In sharp contrast, monastrol-induced monopolar spindles consistently bipolarize in cells with centrosomes in ∼1 hr6,12.
Kinetochores can attach to spindle microtubules either by capturing centrosome-generated astral microtubules17 or by developing their own K-fibres12,18,19. In the latter case, K-fibres formed by juxtaposed sister kinetochores would be oriented parallel to one another while K-fibres formed by sister kinetochores on a properly organized centromere extend in opposite directions. Thus, the shape of the centromere can be a major factor in spindle formation: proper centromere organization should promote bipolarity, while juxtaposed sister kinetochores should impede it. The effects of centromere architecture on spindle formation should be particularly prominent in the absence of centrosomes, when changes in the shape of centromeres induced by syntelic attachments cannot be restored.
To test this idea we examined whether a functional bipolar spindle can form via acentrosomal pathways in cells with multiple juxtaposed sister kinetochores. We treated PtK1 cells with monastrol to accumulate syntelic chromosomes, then ablated both centrosomes and depolymerized microtubules with nocodazole14,15. Nine of 12 cells failed to assemble a bipolar mitotic spindle when both drugs were washed out (Fig.4A, Table 1). In contrast, if centrosomes were ablated during prophase and cells were treated with monastrol and nocodazole before NEB (when the vast majority of sister kinetochores are on opposite sided of the centromere, Fig.2), they formed a functional spindle upon drug washout (Fig.4B, Table 1). For these two types of experiments cells were treated with the same combination of drugs. Nevertheless, the results were dramatically different, indicating that the effect was specific to the difference in the architecture of centromeres. Further, centrosomal cells consistently formed a bipolar spindle after consecutive treatment with monastrol and nocodazole, indicating that restoration of properly shaped centromeres allows chromosomes to achieve bipolarization (Fig.S4, Table 1).
Figure 4. Proper organization of centromere is required for successful spindle formation in the absence of centrosomes.
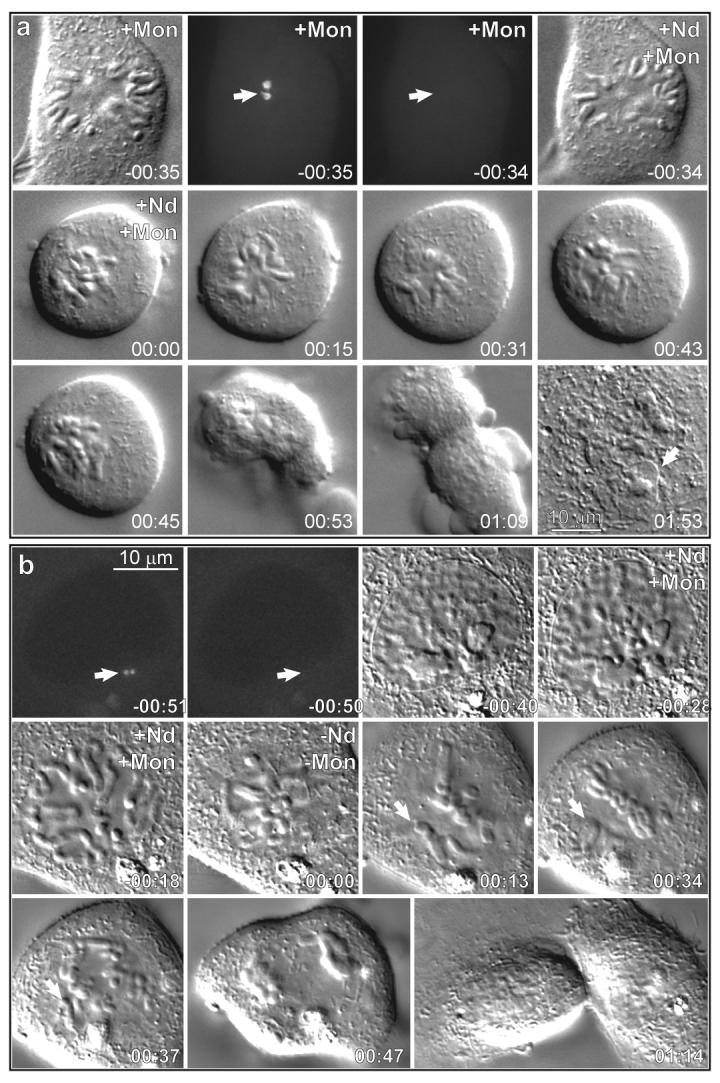
(a) This PtK1 cell was pre-treated with monastrol for 30 min. Both centrosomes were ablated (arrows in −00:35 and −00:34), after which point the cell was treated with 5-μM nocodazole in addition to monastrol. 30 min after addition of nocodazole both drugs were washed out. Time-lapse DIC microscopy revealed that chromosomes remained disorganized in the cytoplasm experiencing short erratic movements for approximately 1 hr. Then cohesion between sister chromatids was lost (cf. 00:43 and 00:45). Although the cell attempted to undergo cytokinesis, furrowing activity was disorganized (00:53-01:09) and all furrows ultimately failed so that mitosis resulted in a single daughter cell with a complexly-shaped nucleus (arrow in 01:53). (b) Mitosis in a cell where both centrosomes were ablated during late G2-early prophase (arrows in −00:51 and −00:50). Then, during late prophase the cell was treated with 5-μM nocodazole and 100-μM monastrol (−00:28 – 00:00). The cell entered mitosis 10 min after addition of the drugs (−00:10) and chromosomes become scattered in the cytoplasm. Upon washout of the drugs (00:00) the cell assembled a bipolar mitotic spindle (00:13-00:34) and successfully divided into 2 daughter cells (00:37-01:14). However, notice that anaphase was initiated in the presence of 2 syntelic chromosomes (arrows in 00:13 and 00:34). Maximal intensity projections of 3-D GFP fluorescence datasets and selected frames from the DIC time-lapse recording. Time in hours:minutes.
Table 1.
Frequency of successful mitotic spindle formation in centrosomal and acentrosomal cells upon different experimental conditions. See text for details.
| Outcome | Functional spindle |
Failed spindle |
|---|---|---|
| Experimental conditions | ||
| Centrosome ablation before NEB | >90%* | <10%* |
| Nocodazole -> C-mitosis -> centrosome ablation -> drug washout | 90% (9/10)** |
10% (1/10) |
| Monastrol -> monopolar spindle -> drug washout | >90%*** | <10%*** |
| Monastrol -> monopolar spindle -> + Nocodazole -> C-mitosis -> drug washout | 100% (10/10) |
0% (0/10) |
| Monastrol -> monopolar spindle -> centrosome ablation -> drug washout | 0% (0/12) |
100% (12/12) |
| Monastrol -> monopolar spindle -> centrosome ablation -> + Nocodazole -> C- mitosis -> drug washout |
25% (3/12) |
75% (9/12) |
| Centrosome ablation (in G2) -> Monastrol + Nocodazole (added before NEB) -> C-mitosis -> drug washout |
90% (8/9)**** |
10% (1/9) |
Although, cells with properly organized centromeres were able to form functional mitotic spindles in the absence of centrosomes many of these cells contained syntelic chromosomes at anaphase onset (Fig.4B, Table 1). Syntelic chromosomes were also consistently present at anaphase onset if cells were treated with nocodazole alone, then the centrosomes were ablated and nocodazole washed out (Fig.S5, Table 1). These observations are consistent with our hypothesis that correction of syntelic attachments is impeded in the absence of astral microtubules.
Intriguingly, the presence of multiple syntelic chromosomes did not prevent mitotic exit. It is unlikely that the centromere is under tension when sister kinetochores are juxtaposed as the distance between them does not change upon loss of microtubule attachments. These observations support the notion that in contrast to lower eukaryotes20,21, the spindle assembly checkpoint in mammals is satisfied in the absence of tension as long as all kinetochores are attached to microtubules21-24. Consistent with this hypothesis, immunofluorescence analyses demonstrated that while the checkpoint protein Mad2 was present on multiple kinetochores in acentrosomal cells soon after monastrol washout but gradually disappeared before the cells exited mitosis in spite of insufficient sister kinetochore separation (Fig.S6).
Intuitively, it seems that flexible centromeres would be disadvantageous to the cell. However, this feature can be important for promoting chromosome congression. Most mono-oriented chromosomes congress to the spindle equator within minutes after nuclear envelope breakdown. However, sometimes mono-oriented chromosomes are seen remaining in the vicinity of the pole for up to several hours24,25. EM analyses demonstrated that these persistently-monooriented chromosomes are ‘monotelic’ – they are attached to the proximal spindle pole with one kinetochore while the second kinetochore is positioned on the opposite side of the centromere25,26. In this configuration chances of the unattached kinetochore to encounter microtubules coming from the distal pole are negligible. In contrast, mono-oriented chromosomes with juxtaposed sister kinetochores can efficiently congress to the spindle equator via sliding on K-fibres of other already bioriented chromosomes27. During this type of congression the leading kinetochore is oriented toward the distal spindle pole maximizing its chances to capture an astral microtubule and become attached. In this respect, syntelic chromosome attachments may serve as an efficient intermediate step toward bi-orientation.
Achieving chromosome biorientation depends upon a complex interplay between mechanisms intrinsic to the centromere and those that act externally. The former include Aurora kinase/kinesin-13-mediated destabilization of K-fibres and release of erroneously attached chromosomes6-8. Activation of these mechanisms is a necessary first step in the correction of chromosome mal-orientation. However, we demonstrate here that in the absence of external forces needed to restore centromere architecture centromere-intrinsic mechanisms are not sufficient. Our findings imply that mechanical properties and the shape of the centromere play an important role in the fidelity of chromosome segregation.
METHODS SUMMARY
Detailed layout of our laser microscopy workstation has been described elsewhere28. In brief 8-ns pulses of 532-nm light from Nd:YAG laser (Diva II, Thales, Paris, France) were focused with the same 100X 1.4 PlanApo objective lens that was used for observations. It takes ∼10-20 pulses to completely destroy the centrosome in PtK cells during mitosis. Fluorescence images presented in the manuscript are maximal-intensity projections of complete Z-series through the cell. DIC images are single Z-planes.
Supplementary Material
Acknowledgements
We thank Drs. Bruce McEwen, Conly Rieder, and Valentin Magidson (Wadsworth Center) for fruitful discussions and critical reading of the manuscript. This work was supported by National Institutes of Health grants GM59363 (to A.K.) and GM65933 (to T.M.K.). Assembly of our laser microsurgery system was supported in part by a Nikon/MBL fellowship (to A.K.). We acknowledge the use of Wadsworth Centre's EM Core facility.
Abbreviations
- K-fibr
kinetochore fibre
- LM
Light microscopy
- EM
electron microscopy
Footnotes
Supplementary Information accompanies the paper on www.nature.com/nature.
Competing Interests statement: The Authors declare no competing financial interests.
Full Methods and any associated references are available in the online version of the paper at www.nature.com/nature
REFERENCES
- 1.Nicklas RB. How cells get the right chromosomes. Science. 1997;275:632–637. doi: 10.1126/science.275.5300.632. [DOI] [PubMed] [Google Scholar]
- 2.Rieder CL, Salmon ED. The vertebrate kinetochore and its roles during mitosis. Trends in Cell Biology. 1998;8:310–318. doi: 10.1016/s0962-8924(98)01299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cimini D, Degrassi F. Aneuploidy: a matter of bad connections. Trends in Cell Biology. 2005;8:442–451. doi: 10.1016/j.tcb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Ault JG, Nicklas RB. Tension, microtubule rearrangements, and the proper distribution of chromosomes in mitosis. Chromosoma. 1989;98:33–39. doi: 10.1007/BF00293332. [DOI] [PubMed] [Google Scholar]
- 5.Nicklas RB, Ward SC. Elements of error correction in mitosis: microtubule capture, release, and tension. J. Cell Biol. 1994;126:1241–1253. doi: 10.1083/jcb.126.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lampson MA, Renduchitala K, Khodjakov A, Kapoor TM. Correcting improper chromosome–spindle attachments during cell division. Nature Cell Biology. 2004;6:232–237. doi: 10.1038/ncb1102. [DOI] [PubMed] [Google Scholar]
- 7.Kline-Smith SL, Khodjakov A, Hergert P, Walczak CE. Depletion of Centromeric MCAK Leads to Chromosome Congression and Segregation Defects Due to Improper Kinetochore Attachments. Molec. Biol. of the Cell. 2004;15:1146–1159. doi: 10.1091/mbc.E03-08-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrews PD, et al. Aurora B regulates MCAK at the mitotic centromere. Developmental Cell. 2004;6:253–268. doi: 10.1016/s1534-5807(04)00025-5. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan KF, Shelby RD. Using time-lapse confocal microscopy for analysis of centromere dynamics in human cells. Meth. Cell Biol. 1999;58:183–202. [PubMed] [Google Scholar]
- 10.Poirier MG, Marko JF. Micromechanical studies of mitotic chromosomes. Current Topics in Developmental Biology. 2003;55:75–141. doi: 10.1016/s0070-2153(03)01002-0. [DOI] [PubMed] [Google Scholar]
- 11.Kapoor TM, Mayer TU, Coughlin ML, Mitchison TJ. Probing spindle assembly mechanisms with monastrol, a small molecule inhibitor of the mitotic kinesin, Eg5. J. Cell Biol. 2000;150:975–988. doi: 10.1083/jcb.150.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khodjakov A, Copenagle L, Gordon MB, Compton DA, Kapoor TM. Minus-end capture of preformed kinetochore fibers contributes to spindle morphogenesis. J. Cell Biol. 2003;160:671–683. doi: 10.1083/jcb.200208143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khodjakov A, Rieder CL. The sudden recruitment of g-tubulin to the centrosome at the onset of mitosis and its dynamic exchange throughout the cell cycle, do not require microtubules. J. Cell Biol. 1999;146:585–596. doi: 10.1083/jcb.146.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khodjakov A, Cole RW, Oakley BR, Rieder CL. Centrosome-independent mitotic spindle formation in vertebrates. Curr. Biol. 2000;10:59–67. doi: 10.1016/s0960-9822(99)00276-6. [DOI] [PubMed] [Google Scholar]
- 15.Khodjakov A, Rieder CL. Centrosomes enhance the fidelity of cytokinesis in vertebrates and are required for cell cycle progression. J. Cell Biol. 2001;153:237–242. doi: 10.1083/jcb.153.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Compton DA. Focusing on spindle poles. J. Cell Sci. 1998;111:1477–1481. doi: 10.1242/jcs.111.11.1477. [DOI] [PubMed] [Google Scholar]
- 17.Rieder CL, Alexander SP. Kinetochores are transported poleward along a single astral microtubule during chromosome attachment to the spindle in newt lung cells. J. Cell Biol. 1990;110:81–95. doi: 10.1083/jcb.110.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maiato H, Rieder CL, Khodjakov A. Kinetochore-driven formation of kinetochore fibers contributes to spindle assembly during animal mitosis. J. Cell Biol. 2004;167:831–840. doi: 10.1083/jcb.200407090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tulu US, Fagerstrom C, Ferenz NP, Wadsworth P. Molecular requirements for kinetochore-associated microtubule formation in mammalian cells. Curr. Biol. 2006;16:536–541. doi: 10.1016/j.cub.2006.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Indjeian VB, Stern BM, Murray AW. The centromeric protein Sgo1 is required to sense lack of tension on mitotic chromosomes. Science. 2005;307:130–133. doi: 10.1126/science.1101366. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Nicklas RB. Mitotic forces control a cell-cycle checkpoint. Nature. 1995;373:630–632. doi: 10.1038/373630a0. [DOI] [PubMed] [Google Scholar]
- 22.Waters JC, Chen R-H, Murray AW, Salmon ED. Localization of mad2 to kinetochores depends on microtubule attachment, not tension. J. Cell Biol. 1998;141:1181–1191. doi: 10.1083/jcb.141.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cimini D, et al. Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells. J. Cell Biol. 2001;153:517–528. doi: 10.1083/jcb.153.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rieder CL, Cole RW, Khodjakov A, Sluder G. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J. Cell Biol. 1995;130:941–948. doi: 10.1083/jcb.130.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rieder CL, Schultz A, Cole R, Sluder G. Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J. Cell Biol. 1994;127:1301–1310. doi: 10.1083/jcb.127.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cassimeris L, Rieder CL, Salmon ED. Microtubule assembly and kinetochore directional instability in vertebrate monopolar spindles: implications for the mechanism of chromosome congression. J. Cell Sci. 1994;107:285–297. doi: 10.1242/jcs.107.1.285. [DOI] [PubMed] [Google Scholar]
- 27.Kapoor TM, et al. Chromosomes can congress to the metaphase plate before biorientation. Science. 2006;311:388–391. doi: 10.1126/science.1122142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magidson V, Loncarek J, Hergert P, Rieder CL, Khodjakov A. Laser microsurgery in the GFP era: A cell biologist's perspective. In: Berns MW, Greulich KO, editors. Laser Manipulations of Cells and Tissues. Elsevier; 2007. pp. 237–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.