Summary
The Mre11 complex (Mre11, Rad50, and Nbs1) and Chk2 have been implicated in the DNA damage response, an inducible process required for the suppression of malignancy. The Mre11 complex is predominantly required for repair and checkpoint activation in S phase, while Chk2 governs apoptosis. We examined the relationship between the Mre11 complex and Chk2 in the DNA damage response via the establishment of Nbs1ΔB/ΔB Chk2−/− and Mre11ATLD1/ATLD1 Chk2−/− mice. Chk2 deficiency did not modify the checkpoint defects or chromosomal instability of Mre11 complex mutants; however, the double mutant mice exhibited synergistic defects in DNA damage-induced p53 regulation and apoptosis. Nbs1ΔB/ΔB Chk2−/− and Mre11ATLD1/ATLD1 Chk2−/− mice were also predisposed to tumors. In contrast, DNA-PKcs deficient mice, in which G1-specific chromosome breaks are present, did not exhibit synergy with Chk2−/− mutants. These data suggest that Chk2 suppresses the oncogenic potential of DNA damage arising during S and G2 phases of the cell cycle.
Keywords: Mre11, Nbs1, Rad50, Chk2, Atm, p53, apoptosis, tumorigenesis
Introduction
The Mre11 complex, consisting of Mre11, Rad50 and Nbs1 (Xrs2 in S. cerevisiae), plays a central role in the cellular response to DNA double-strand breaks (DSBs). In addition to promoting recombinational DNA repair (Bressan et al., 1999), the complex acts as a DSB sensor and modulates the activity of the primary transducing kinases Ataxia-telangiectasia mutated (ATM) and ATM and RAD3 related (ATR) (Lee and Paull, 2007; Petrini and Stracker, 2003). The Mre11 complex is required for the activation of ATM and also functions downstream of ATM to facilitate the phosphorylation of numerous substrates including Smc1 and Chk2 (Lee and Paull, 2007). ATM is required for checkpoint activation throughout the cell cycle (Shiloh, 2003) and controls the intra-S phase checkpoint via the effector kinases Chk1 and Chk2 as well as Nbs1 and the cohesin subunit SMC1, with the latter two proteins acting in a parallel pathway to Chk2 (Falck et al., 2002; Kitagawa et al., 2004).
ATM is required for p53 regulation (Kastan et al., 1992) and promotes apoptosis in response to diverse stimuli including DSBs (Xu and Baltimore, 1996), oncogene expression (ie. Myc and E2F1)(Powers et al., 2004; Pusapati et al., 2006; Rogoff et al., 2004), and genotoxic stress induced by the Rad50S allele (Morales et al., 2005). As a result, Atm−/− mice exhibit apoptotic defects in numerous tissues, including lymphoid organs and the central nervous system (CNS) (Herzog et al., 1998; Westphal et al., 1997; Xu and Baltimore, 1996).
Several lines of evidence support the view that the Mre11 complex exerts a significant influence on p53-dependent apoptosis via its functional interaction with ATM. First, the precipitous apoptotic attrition of hematopoietic and spermatogenic cells in Rad50S/S mice is acutely dependent on ATM; loss of a single ATM allele (in Rad50S/S Atm+/− mice) mitigates Rad50S/S-induced cellular attrition (Morales et al., 2005). We have recently shown that apoptosis is blocked in multiple tissues of Rad50S/S mice that express a C-terminally truncated Nbs1 variant lacking an ATM interaction domain (Nbs1ΔC) (Falck et al., 2005; Stracker et al., 2007; You et al., 2005), and that this C-terminal Nbs1 domain is required for ATM-dependent apoptosis in response to ionizing radiation (IR) in lymphoid cells (Stracker et al., 2007).
Chk2 also contributes to the regulation of p53-dependent apoptosis. Chk2−/− mice exhibit defects in p53 phosphorylation, stabilization, and p53-dependent transcriptional events in response to damaging agents (Hirao et al., 2002; Hirao et al., 2000; Takai et al., 2002). Accordingly, apoptosis is attenuated in the brain, thymus and other tissues of Chk2−/− mice (Hirao et al., 2002; Takai et al., 2002). Chk2 phosphorylates p53 on S20 (Chehab et al., 2000) (S23 in the mouse) and mice harboring the p53S23A allele showed an apoptotic phenotype similar to that of Chk2−/− mice (MacPherson et al., 2004). Although Chk2−/− mice are not predisposed to spontaneous tumors (Hirao et al., 2002), a non-null Chk2 allele predisposes to tumorigenesis in transgenic mice (Kwak et al., 2006) and several Chk2 mutations have been identified as low penetrance alleles in human malignancies including breast and prostate cancer (Nevanlinna and Bartek, 2006).
Single gene disorders arising from mutations in the ATM, MRE11, or NBS1 genes present with extensive phenotypic overlap (Ataxia-telangiectasia, A-T, ATM deficiency; Ataxia-telangiectasia like disorder, A-TLD, Mre11 hypomorphism; and Nijmegen breakage syndrome, NBS, NBS1 hypomorphism) (Shiloh, 2003; Stracker et al., 2004). Cell lines from these human patients, or from murine models of the human diseases (Figure 1A), exhibit defective responses to DNA double-strand break inducing agents, including cell cycle checkpoint defects, chromosomal instability, and increased sensitivity (Barlow et al., 1996; Theunissen et al., 2003; Williams et al., 2002; Xu and Baltimore, 1996). Given the functional interdependence of the Mre11 complex and ATM, and the fact that ATM regulates both Chk2 and p53, it has been suggested that defects in the activation of Chk2 is among the pathologic consequences of these disorders (Bartek et al., 2001).
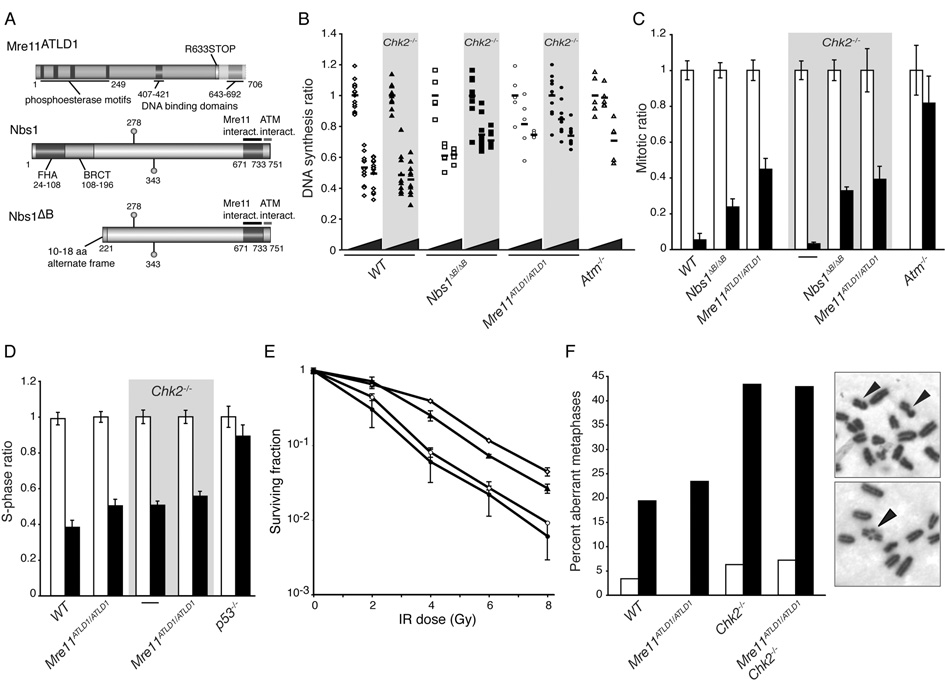
Figure 1. Loss of Chk2 does not exacerbate the checkpoint defects, damage sensitivity, or chromosome instability conferred by Mre11 complex hypomorphism.
(A) Diagram of the hypomorphic Mre11ATLD1 and Nbs1ΔB alleles (reviewed in Stracker et al., 2004).
(B) Intra-S phase checkpoint response of WT (◇), Chk2−/− (◆), Nbs1ΔB/ΔB (□), Nbs1ΔB/ΔBChk2−/− (■), Mre11ATLD1/ATLD1 (○), Mre11ATLD1/ATLD1Chk2−/− (●) and Atm−/− (△) fibroblasts. The DNA synthesis ratios (untreated and IR treated cultures, normalized to untreated samples) are plotted. Wedge denotes increasing IR dose (0, 10, or 20 Gy) and horizontal bars indicate the average value of replicate experiments. Chk2−/− cultures are not significantly different than WT (P=0.33 at 10 and 0.82 at 20 Gy, WT vs. Chk2−/−) and loss of Chk2 does not exacerbate the defect of Mre11ATLD1/ATLD1 (P=0.88 at 10 and 0.55 at 20 Gy, Mre11ATLD1/ATLD1 vs. Mre11ATLD1/ATLD1Chk2−/−). However, Nbs1ΔB/ΔBChk2−/− cultures showed increased RDS compared to Nbs1ΔB/ΔB (P=1.5×10−3 at 10 and 2.5×10−3 at 20 Gy, Nbs1ΔB/ΔB vs. Nbs1ΔB/ΔBChk2−/−). Wilcoxon rank sum test, 2-sided.
(C) G2/M checkpoint responses of exponentially growing MEFs. The mitotic ratios (mock or IR treated normalized to mock treated) of mock (white) or 10 Gy IR (black) treated cells at 1 hr post treatment are presented. Results are the average of 3–6 experiments performed in triplicate for each genotype. Error bars denote standard deviation.
(D) G1/S checkpoint in early passage MEF cultures. The S-phase ratios (%BrdU positive of irradiated or unirradiated/ average %BrdU positive unirradiated) are plotted. Results are the average of 2–4 experiments performed in triplicate for each genotype. Error bars denote standard deviation.
(E) IR sensitivity determined by clonogenic survival assay. WT (◇), Chk2−/− (▲), Mre11ATLD1/ATLD1 (○), and Mre11ATLD1/ATLD1Chk2−/− (●) SV40 transformed MEF cultures were exposed to IR at the indicated doses and plated for colony formation. Results from 2 representative experiments performed in triplicate are shown. Error bars denote standard deviation.
(F) Genomic instability in mock and irradiated primary proliferating splenocytes. Examples of metaphases from IR treated Mre11ATLD1/ATLD1 Chk2−/− spreads are shown: 2 chromatid breaks (top) and a rearrangement (bottom) are indicated. The number of spreads analyzed and aberrations identified are described in Supplementary Table S1.
To test this idea, and to further define the relative disposition of Chk2 and the Mre11 complex in the DSB response, we generated Nbs1ΔB/ΔB Chk2−/− and Mre11ATLD1/ATLD1 Chk2−/− mice. Loss of Chk2 did not strongly affect the checkpoint phenotypes, damage sensitivity, or chromosomal instability resulting from Mre11 complex hypomorphism. However, both Nbs1ΔB/ΔB Chk2−/− and Mre11ATLD1/ATLD1Chk2−/− double mutant animals exhibited apoptotic defects and were predisposed to a wide spectrum of tumors. Tumors were not evident in Prkdcscid/scid Chk2−/− double mutant mice in which DSBs generated in G0/G1 phase lymphoid cells are stabilized (Roth et al., 1992). These results suggest that Chk2 is required to suppress the oncogenic potential of DNA replication-associated DNA damage.
Results
Chk2 deficiency does not exacerbate the cell cycle checkpoint and DNA repair defects of Mre11 complex hypomorphs
Chk2 activation appears to require phosphorylation by ATM (Ahn et al., 2000; Falck et al., 2001; Melchionna et al., 2000). ATM activity is attenuated in Nbs1ΔB/ΔB and Mre11ATLD1/ATLD1 cells; this is correlated with decreased Chk2 phosphorylation upon IR treatment in those mutants (Theunissen et al., 2003). Were Mre11 complex hypomorphism to abolish Chk2 activation, Chk2 deficiency would not be expected to exacerbate the phenotypes of Mre11ATLD1/ATLD1 and Nbs1ΔB/ΔB cells. Conversely, if those mutant settings retain some degree of Chk2 function, then phenotypic additivity would be observed in Chk2 deficient Mre11 complex mutants. To test these predictions, mice were intercrossed to generate Nbs1ΔB/ΔB Chk2−/− and Mre11ATLD1/ATLD1 Chk2−/− mice and the ability of double mutant cells mount a DNA damage response was assessed.
The intra-S phase checkpoint suppresses the rate of DNA synthesis in response to DNA damage. Whereas DNA synthesis was suppressed following IR in Chk2−/− murine embryo fibroblasts (MEFs) to the same extent as WT cells, it was suppressed to a lesser extent in Nbs1ΔB/ΔB and Mre11ATLD1/ATLD1 cells as previously reported (Figure 1B) (Takai et al., 2002; Theunissen et al., 2003; Williams et al., 2002). Loss of Chk2 increased the severity of the intra-S phase defect in Nbs1ΔB/ΔB Chk2−/− (DNA synthesis suppression of 26%) compared to Nbs1ΔB/ΔB (DNA synthesis suppression of 38%) after 10 Gy but did not exacerbate the phenotype of Mre11ATLD1/ATLD1 Chk2−/− cells (Figure 1B).
Inhibition of the G2/M transition following DNA damage (the G2/M checkpoint), which is defective in Nbs1ΔB/ΔB and Mre11ATLD1/ATLD1 cultures, occurred normally in Chk2−/− MEFs 1 hour after IR treatment (Figure 1C) (Takai et al., 2002; Theunissen et al., 2003; Williams et al., 2002). Chk2 deficiency did not enhance the G2/M checkpoint defects associated in Mre11 complex hypomorphism in Nbs1ΔB/ΔB Chk2−/− and Mre11ATLD1/ATLD1 Chk2−/− cultures (Figure 1C).
DNA damage-induced cell cycle arrest at the G1/S transition (the G1/S checkpoint) is primarily governed by p53 (Kuerbitz et al., 1992), and is partially impaired by ATM deficiency (Xu et al., 1998). Although, like ATM, Chk2 regulates p53, differing results regarding its role in the G1/S checkpoint have been reported (Cao et al., 2006; Hirao et al., 2002; Jack et al., 2002; Takai et al., 2002). To examine the G1/S checkpoint in Chk2 deficient Mre11 complex mutants, cells were exposed to IR and the percentage of cells in S phase 14hrs post treatment was measured by BrdU incorporation. Whereas greater than 90% of p53−/− cells had entered S phase, only 38% of WT had resumed cell cycle (Figure 1D). Chk2−/− and Mre11ATLD1/ATLD1 Chk2−/− were both mildly defective in the G1/S arrest with 50–60% of cells in S phase 14 hrs post IR (Figure 1D). These data indicate that neither Mre11ATLD1/ATLD1 nor Chk2 deficiency exerts a pronounced effect on the G1/S checkpoint.
Consistent with the lack of additivity in DNA damage dependent cell cycle checkpoint responses, Chk2 deficiency did not modify the IR sensitivity of either Nbs1ΔB/ΔB (data not shown) or Mre11ATLD1/ATLD1 (Figure 1E). Similarly, Chk2−/− splenocytes did not show increased spontaneous or IR induced chromosomal lesions and both Mre11ATLD1/ATLD1 and Mre11ATLD1/ATLD1 Chk2−/− cells showed similar levels of chromosomal instability, primarily chromatid type breaks (Figure 1F and Supplemental Table S1). Together these data demonstrate that loss of Chk2 does not exacerbate the checkpoint deficiency, DSB sensitivity or chromosomal instability conferred by the hypomorphic Mre11ATLD1/ATLD1 or Nbs1ΔB/ΔB alleles.
The Mre11 complex and Chk2 effect p53-dependent apoptosis through parallel pathways
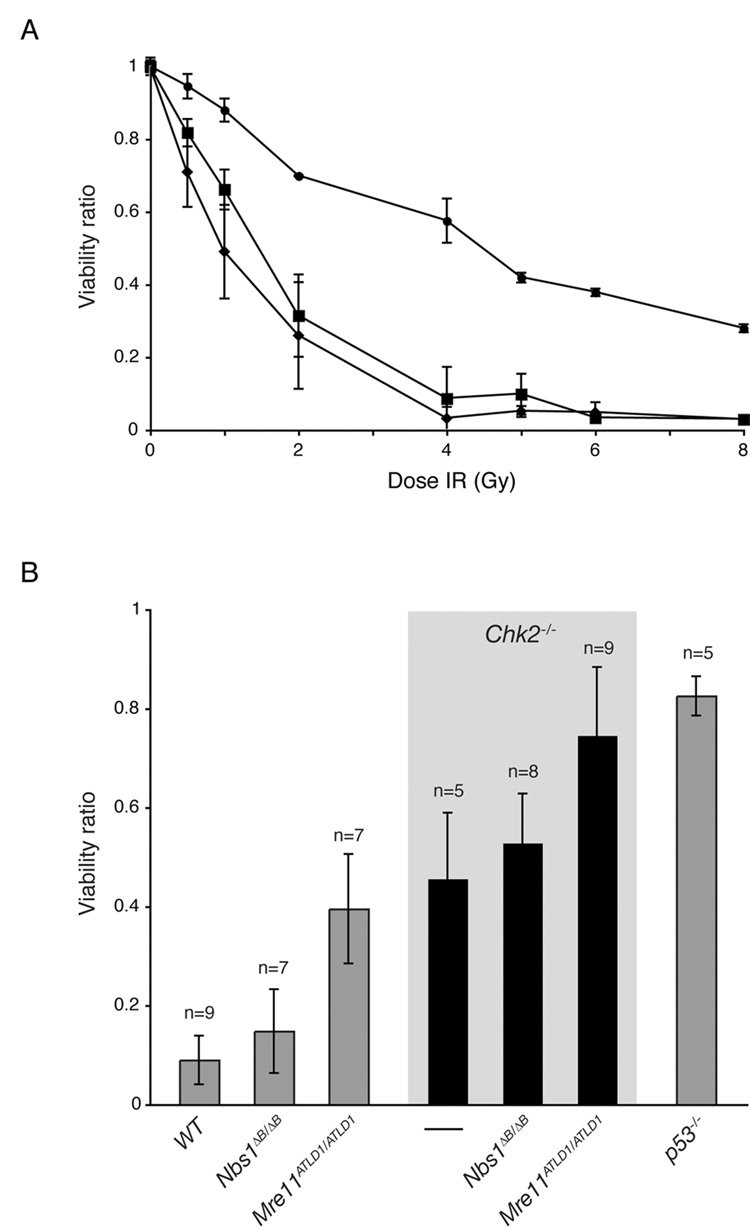
We previously showed that deficiencies in ATM and Chk2 confer additive apoptotic defects in IR-treated thymocytes, suggesting that they act in parallel to effect p53-dependent apoptosis (Stracker et al., 2007). That ATM activity is impaired in Nbs1ΔB/ΔB and Mre11ATLD1/ATLD1 predicts that Mre11 complex hypomorphism would also be additive with Chk2 deficiency for apoptotic defects. Whereas Mre11ATLD1/ATLD1 exhibited apoptotic defects at IR doses ranging from 0.5 to 8 Gy, Nbs1ΔB/ΔB was indistinguishable from WT over the same range (Figure 2A). The apoptotic defect in Mre11ATLD1/ATLD1 was markedly enhanced by Chk2 deficiency, as thymocytes from Mre11ATLD1/ATLD1 Chk2−/− were as defective as p53−/− thymocytes for IR-induced apoptosis (Figure 2B). In contrast, Nbs1ΔB/ΔB Chk2−/− was not significantly different than that of Chk2−/− (Figure 2B). No differences in dexamethasone induced apoptosis, which is p53 independent, were evident in any of the genotypes (Supplemental Figure S1).
Figure 2. The Mre11 complex and Chk2 regulate apoptosis in parallel pathways.
(A) Apoptosis in irradiated WT (◆), Nbs1ΔB/ΔB (■), and Mre11ATLD1/ATLD1 (●) thymocytes 20 hours post exposure to the indicated IR dose. Viability ratios are plotted for each dose (%viable cells mock or IR treated/ % viable cells mock treated). Triplicate results of 2 representative experiments are plotted. Error bars denote standard deviation.
(B) Thymocytes from the indicated genotypes were mock treated or exposed to 5 Gy of IR in culture and analyzed 20 hours post treatment. Viability ratios are plotted for each dose and experiments were performed in triplicate with n indicating the number of animals represented and error bars denoting standard deviation. The viability ratio differences between Nbs1ΔB/ΔB and WT, or Chk2−/− and Nbs1ΔB/ΔB Chk2−/− are not statistically significant (Wilcoxon rank sum test, 2-sided).
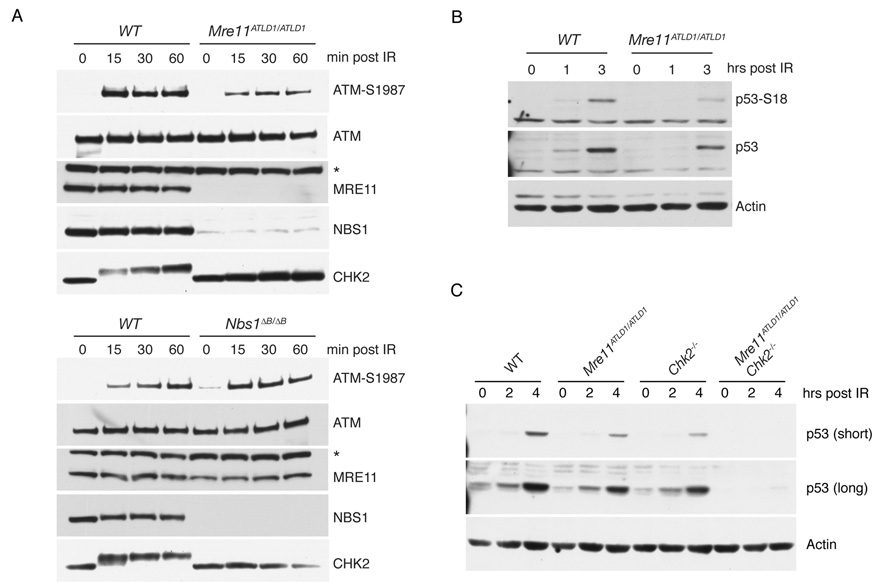
Despite their differing effects on apoptosis, IR-induced Chk2 phosphorylation was markedly impaired in both Mre11ATLD1/ATLD1 and Nbs1ΔB/ΔB thymocytes (Figure 3A). The Chk2 phosphorylation defect was not correlated with defects in ATM activation (inferred from S1987 phosphorylation (Bakkenist and Kastan, 2003)), as ATM autophosphorylation was impaired in Mre11ATLD1/ATLD1 thymocytes but unaffected in Nbs1ΔB/ΔB (Figure 3A) (Morales et al., 2005). Hence, both hypomorphic Mre11 complex alleles impair the modification of ATM substrates such as Chk2 (Figure 3A) and the pro-apoptotic effector BID (Supplemental Figure S2)—a reflection of ATM activity—whereas the Mre11ATLD1/ATLD1 allele also affects ATM activation.
Figure 3. The Mre11 complex and Chk2 regulate p53 in parallel pathways.
(A) Western blot of thymocyte lysates from the indicated genotypes. Membranes were probed with antibodies for ATM-S1981, ATM, Nbs1, Mre11, and Chk2 at various times following 5 Gy of IR treatement. A non-specific band is indicated by *.
(B) Western blot of thymocyte lysates probed with antibodies for p53-S18, p53 and Actin (loading control) at the indicated times following 5 Gy of IR.
(C) Western blot of thymocyte lysates probed for p53 or Actin (loading control) at the indicated times post 5 Gy of IR.
The effects of Nbs1ΔB/ΔB and Mre11ATLD1/ATLD1 on p53 phosphorylation support this interpretation. Phosphorylation and stabilization of p53 is influenced by both ATM and Chk2 (Barlow et al., 1997; Hirao et al., 2002; Kastan et al., 1992; Takai et al., 2002). We observed reduced levels of S18-phosphorylated (ATM consensus site) and total p53 in Mre11ATLD1/ATLD1 thymocytes after IR treatment (Figure 3B), whereas p53 phosphorylation and stabilization was normal in Nbs1ΔB/ΔB cells (Supplemental Figure S3) (Morales et al., 2005). These data suggest that impaired ATM activation in Mre11ATLD1/ATLD1 accounts for reduced p53 phosphorylation and may further account for the additive defects of Mre11ATLD1/ATLD1 and Chk2−/− in apoptotic induction.
p53 responses in Mre11ATLD1/ATLD1 Chk2−/− mice
The apoptotic defect in Mre11ATLD1/ATLD1 Chk2−/− thymocytes is correlated with the virtually complete loss of DNA damage induced-p53 stabilization. Stabilization of p53 was slightly diminished in each of the single mutants at 2 and 4 hrs post IR, but p53 was barely detectable at those time points in Mre11ATLD1/ATLD1 Chk2−/− double mutants (Figure 3C). Loss of DNA damage-dependent p53 regulation, as opposed to basal p53 expression, underlies the observed defect as Mre11ATLD1/ATLD1 Chk2−/− thymocytes treated with the proteasome inhibitor MG132 exhibited similar levels of p53 as WT cells (Supplemental Figure S4).
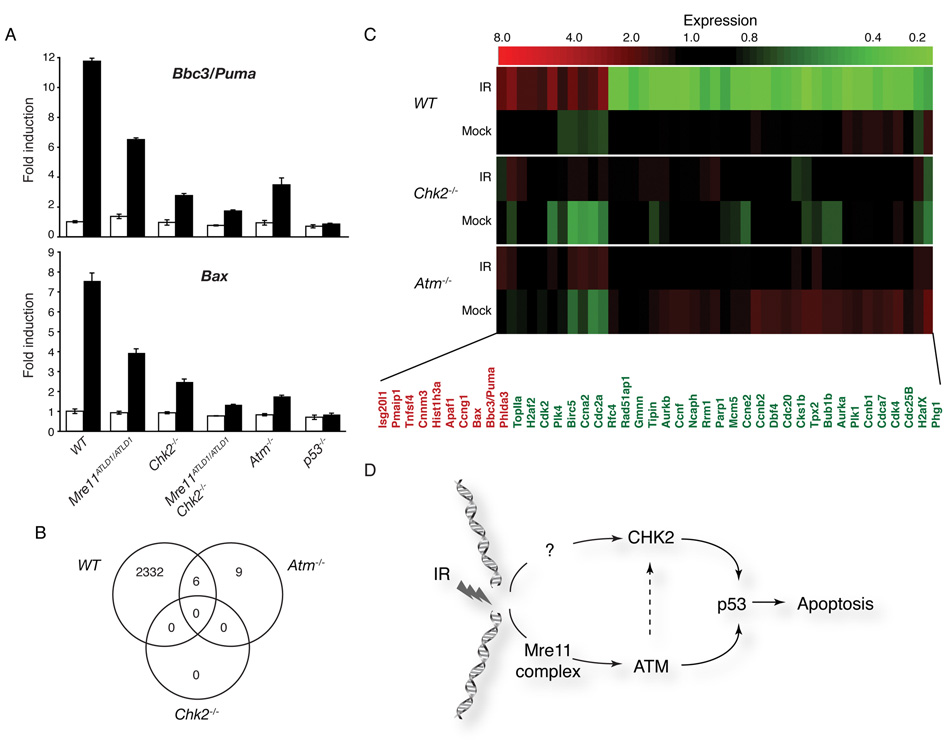
Central to p53’s influence on the DNA damage response and tumor suppression is its role in transcriptional regulation. Using quantitative PCR (Q-PCR), the induction of the proapoptotic genes Bbc3/Puma and Bax in irradiated thymocytes was analyzed. In WT cells these p53 target genes are induced approximately 12 and 8 fold respectively, and unchanged by IR in p53−/− cells as expected (Figure 4A). Whereas their induction was 30–50% of WT in Chk2−/−, Mre11ATLD1/ATLD1, and Atm−/− single mutants, Bbc3/Puma and Bax induction was similar to p53−/− in Mre11ATLD1/ATLD1 Chk2−/− double mutants, consistent with the observed p53 stabilization defect (Figure 4A).
Figure 4. The Mre11 complex-ATM and Chk2 regulate global p53 transcriptional responses.
(A) Quantitative PCR analysis of p53 target genes Bbc3/Puma and Bax. Thymocytes were harvested 8 hours after exposure to 5 Gy of IR. Representative experiments performed in triplicate are shown: mock (white) or IR treated (black).
(B) Analysis of microarray data for the indicated genotypes. 2338 probe sets showed statistically significant expression changes following IR treatment in WT thymocytes. No changes were significant in Chk2−/− and only 15 in Atm−/− (listed in Supplementary Table S2). The number of common genes altered in both WT and Atm−/− are indicated by the Venn diagram (GEO accession number pending).
(C) Transcriptional regulation of genes involved in the cell cycle and apoptosis are impaired in both Atm−/− and Chk2−/− thymocytes. The heatmap indicates the induction (red) or repression (green) of selected genes with known roles in cell cycle regulation or apoptosis following IR treatment.
(D) Model of p53-dependent apoptotic signaling. The Mre11 complex is required for activation of ATM and this function is attenuated in Mre11ATLD1/ATLD1 thymocytes. While both ATM and the Mre11 complex influence Chk2 phosphorylation, the apoptotic defects of Mre11ATLD1/ATLD1 or Atm−/− are additive with loss of Chk2 indicating that they function through a largely parallel signaling pathway.
The transcriptional effects on Bbc3/Puma and Bax seen by Q-PCR in Chk2−/− and Atm−/− were a reflection of global effects on DNA damage-induced transcriptional responses in these mutants. Microarray analysis of transcriptome changes in WT, Atm−/− and Chk2−/− thymocytes following IR treatment was carried out. When compared to WT cells, few statistically significant changes that were specific to Chk2−/− or Atm−/− were noted in 2338 probe sets that were induced or repressed after IR treatment (Figure 4B, 4C, Supplemental Table S2) Together these data indicate that Chk2 and the ATM/Mre11 complex pathway have independent, global effects on p53 transcriptional programs and apoptosis and argue against p53 transcriptional targets that are specifically regulated by either arm of the apoptotic response (Figure 4D).
Chk2 is required to suppress tumorigenesis in diverse tissues of animals expressing hypomorphic Mre11 complex alleles
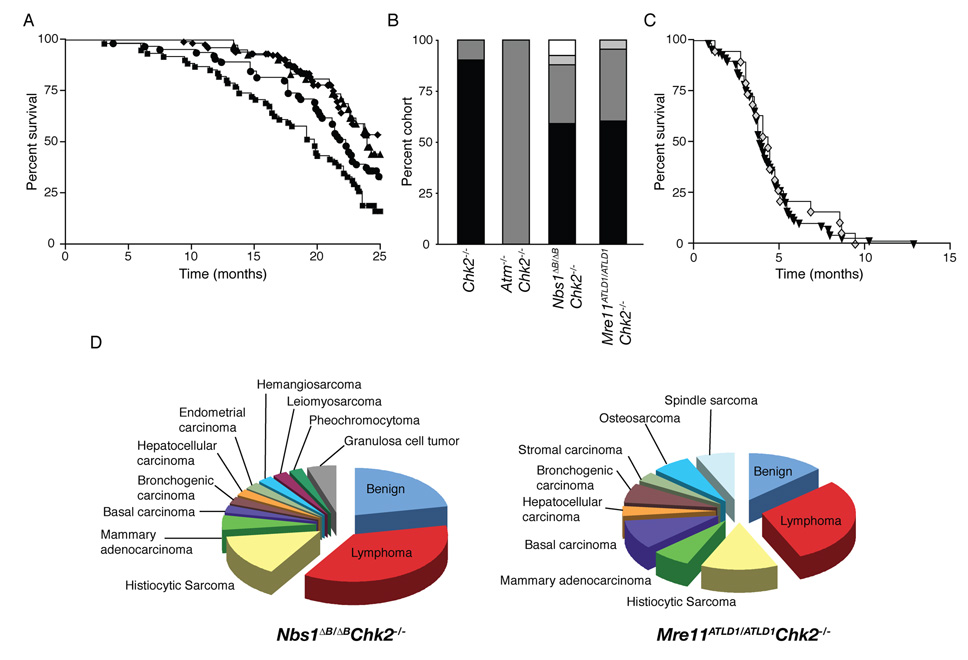
Whereas none of the single mutants were predisposed to malignancy (Supplemental Figure S5, S6 and Takai et al., 2002; Theunissen et al., 2003; Williams et al., 2002) survival of both Nbs1ΔB/ΔB Chk2−/− and Mre11ATLD1/ATLD1 Chk2−/− was reduced, with roughly 40% of each cohort succumbing to malignancy within 2 years (Figure 5A, 5B, Supplemental Figure S6 and Supplemental Tables S3, S4). In contrast, no reduction in the tumor latency or spectrum seen in Atm−/− was evident in Atm−/− Chk2−/− (Figure 5C).
Figure 5. Nbs1ΔB/ΔB Chk2−/− and Mre11ATLD1/ATLD1 Chk2−/− double mutants are predisposed to tumorigenesis.
(A) Cohort survival of WT, Mre11ATLD1ATLD1 and Nbs1ΔB/ΔB (*combined n=172, ◆), Chk2−/− (n=41, ▲), Nbs1ΔB/ΔB Chk2−/− (n=66, ■), and Mre11ATLD1ATLD1 Chk2−/− (n=67, ●) is shown on a Kaplan Meier curve. *Individual survival curves, tumor free survival curves, and statistical analysis are included in Supplemental Figure S5, S6, and Table S3.
(B) Animals succumbing to tumors are shown as a percentage of their cohort: tumor free (black), 1 tumor (dark gray), 2 tumors (light gray), more than 2 tumors (white).
(C) Survival of Atm−/− (n=71, ▼) and Atm−/− Chk2−/− (n=19, ◇) cohorts plotted on a Kaplan Meier curve.
(D) The percentage of tumor bearing animals with a specific tumor type is plotted for the indicated genotypes.
Contrary to expectations based on the defects in p53 regulation observed in Nbs1ΔB/ΔB Chk2−/− nor Mre11ATLD1/ATLD1 Chk2−/− mice, neither displayed the penetrant, early onset lymphoma characteristic of p53−/− or Atm−/− animals (Barlow et al., 1996; Donehower et al., 1992; Xu et al., 1996). Instead, both double mutants developed a wide variety of tumor types. Atm−/− (or Atm−/− Chk2−/−) developed only T-cell lymphomas, whereas Mre11ATLD1/ATLD1 Chk2−/− and Nbs1ΔB/ΔB Chk2−/− mice developed lymphomas that expressed T, B, or macrophage markers (histiocytic sarcoma) as well as biphenotypic lymphoblastic lymphomas that were positive for both T and B cell markers (CD3 and B220- Supplemental Figure S7). Mre11ATLD1/ATLD1 Chk2−/− and Nbs1ΔB/ΔB Chk2−/− mice also developed a wide range of highly proliferative solid tumors including carcinomas and sarcomas in multiple tissues in which apoptosis was absent or detected to a very minimal extent (Figure 5D, Supplemental Figure S7, Supplemental Table S4, and data not shown).
To determine whether tumorigenesis in Nbs1ΔB/ΔB Chk2−/− and Mre11ATLD1/ATLD1 Chk2−/− mice was associated with p53 inactivation, DNA or RNA from 13 representative tumors was used for sequence analysis of p53. For genomic DNA samples, exons 5–10 of p53, encompassing the p53 DNA binding domain that contains the nucleotides most frequently mutated in tumors (UMD p53 database 2007_R1; http://p53.free.fr/; (Soussi and Wiman, 2007)), was sequenced and from cDNA preparations we analyzed the entirety of the p53 coding sequence (Exons 2–11). These analyses revealed no p53 mutations in any of the tumor samples analyzed (Table 1). As mutations in p19ARF could also reduce the selective pressure for p53 mutations, we amplified and sequenced p19ARF from cDNA prepared from 7 tumors. In all cases, p53 was wild type and in 5 of 7 tumors p19ARF was wild type (Table 1) and was not amplifiable from the remaining 2 (presumably due to mutation or deletion). These results are consistent with a diminished selective pressure against p53 function during tumorigenesis in the context of Chk2 deficiency and impaired Mre11 complex activity.
Table 1. Analysis of p53 and p19ARF sequence in tumor samples.
Exons 5–10 of p53 genomic DNA and exons 2–11 of p53 cDNA were PCR amplified and sequenced. The coding sequence of p19ARF was amplified and sequenced (exons 1–3) from cDNA samples. All sequencing was performed multiple times and PCR products were sequenced in both directions.
| p53 exon amplification from genomic DNA | ||||||||
|---|---|---|---|---|---|---|---|---|
| ID | genotype | tumor type | Ex. 5 | Ex. 6 | Ex.7 | Ex. 8 | Ex. 9 | Ex. 10 |
| 22790 | M-C2 | basal carcinoma | WT | WT | WT | WT | WT | WT |
| 23186 | N-C2 | adeno/squamous carcinoma | WT | WT | WT | WT | WT | WT |
| 23182 | M-C2 | osteosarcoma | WT | WT | WT | WT | WT | WT |
| 23828 | N-C2 | granulosa cell tumor | WT | WT | WT | WT | WT | WT |
| 21872 | N-C2 | histiocytic sarcoma | WT | WT | WT | WT | WT | WT |
| 23646 | N-C2 | lymphoma | WT | WT | WT | WT | WT | WT |
| 23649 | N-C2 | mammary carcinoma | WT | WT | WT | WT | WT | ND |
| 24098 | N-C2 | basal carcinoma | WT | WT | WT | WT | WT | WT |
| p53 and p19ARF amplification from cDNA | ||||
|---|---|---|---|---|
| ID | tumor type |
p53 Cdna exons 2–11 |
p19ARF cDNA exons 1–3 |
|
| 23902 | M-C2 | basal carcinoma | WT | NA |
| 24071 | N-C2 | lymphoma | WT | NA |
| 23828 | N-C2 | granulosa cell tumor | WT | WT |
| 23129 | M-C2 | histiocytic sarcoma | WT | WT |
| 23186 | N-C2 | adeno/squamous carcinoma | WT | WT |
| 25094 | N-C2 | uterine leiomyosarcoma | WT | WT |
| 23646 | N-C2 | lymphoma | WT | WT |
(M-C2=Mre11ATLD1/ATLD1 Chk2−/−, N-C2=Nbs1ΔB/ΔB Chk2−/−, WT=wild type, ND=not determined, NA=not amplifiable)
The tumor suppressive effects of Chk2 are specific for replication associated chromosome instability
As chromosomal instability arises predominantly during S-phase in Mre11 complex hypomorphs, the tumor predispostion of Nbs1ΔB/ΔB Chk2−/− and Mre11ATLD1/ATLD1 Chk2−/− mice indicates that Chk2 suppresses the oncogenic potential of replication-associated damage. To determine if Chk2 was also responsive to lesions arising in G1, we generated mice doubly deficient for Chk2 and the catalytic subunit of DNA-PK (DNA-PKcs), encoded by the Prkdc gene. DSBs induced in G1/G0 by the RAG proteins during antigen receptor gene assembly are stabilized in Prkdcscid/scid animals (Roth et al., 1992). This triggers p53-dependent apoptosis in developing B and T lymphocytes, resulting in severe combined immune deficiency (Guidos et al., 1996). p53 deficiency partially mitigates the attrition of lymphocytes in Prkdcscid/scid mice, and Prkdcscid/scid p53−/− mice exhibit early onset B and T lymphoma (Guidos et al., 1996; Nacht et al., 1996).
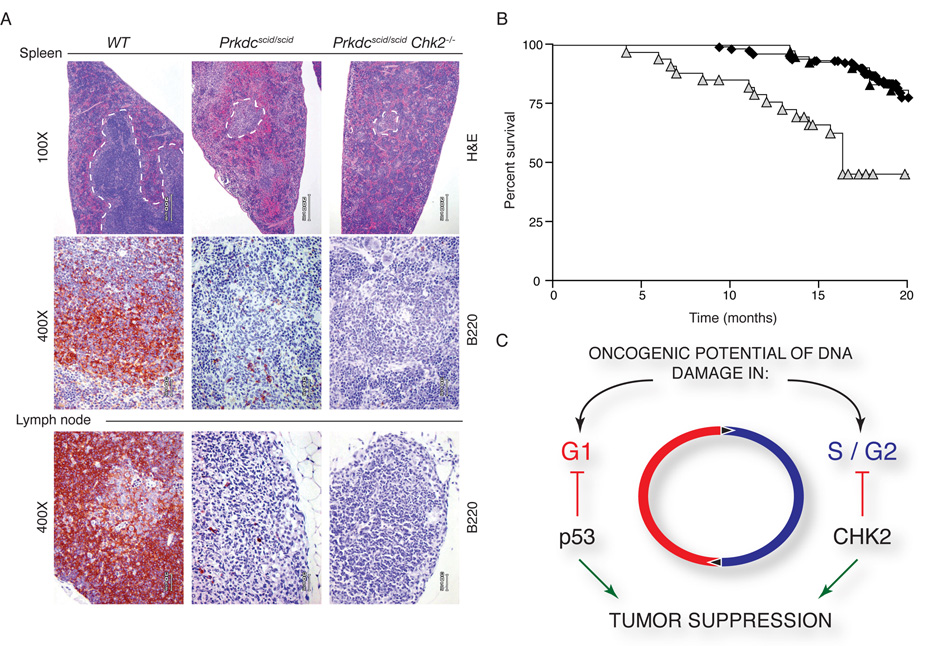
In contrast, Chk2 does not appear to respond to RAG-induced DSBs, as no rescue of lymphoid organ cellularity was evident (thymus, spleen, and lymph nodes), and although survival was reduced due to severe immunodeficiency, no tumors developed in Prkdcscidscid Chk2−/− mice (Figure 6A, 6B, and data not shown). These results suggest that Chk2 activity is not required for the DSB response in G1 cells, and support the interpretation that its function is critical for reducing the oncogenic potential of replication associated DNA damage (Figure 6C).
Figure 6. Prkdcscid/scid Chk2−/− animals are not tumor prone.
(A) Immunohistochemical staining of the spleen and lymph nodes of WT, Prkdcscid/scid and Prkdcscid/scid Chk2−/− mice. White pulp (WP) of the spleen is outlined in broken lines (top panels). Regions of WP are smaller and contain few lymphocytes in Prkdcscid/scid and Prkdcscid/scid Chk2−/−. B220 staining of B-cells in the spleen (middle panels) and lymph nodes (bottom panels). In Prkdcscid/scid and Prkdcscid/scid Chk2−/− mice, both tissues have few B220 positive cells compared to WT.
(B) Survival of WT (◆, n=62), Chk2−/− (▲, n=41), and Prkdcscid/scid Chk2−/− ( , n=34) is plotted on a Kaplan Meier curve. Survival of Prkdcscid/scid Chk2−/− animals was decreased due to infections and related pathology but no tumors were evident.
, n=34) is plotted on a Kaplan Meier curve. Survival of Prkdcscid/scid Chk2−/− animals was decreased due to infections and related pathology but no tumors were evident.
(C) Model for the role of Chk2 in tumor suppression. Chk2 is required for suppressing the oncogenic potential of DNA-replication associated breaks, such as those arising in Nbs1ΔB/ΔB or Mre11ATLD1ATLD1 mutants, but not for breaks in G0/G1 phase cells resulting from defects associated with the Prkdcscid/scid mutation.
Discussion
Genetic Interactions Between the Mre11 Complex and Chk2
We previously established evidence that Nbs1 and Chk2 acted in parallel pathways to regulate the intra S-phase checkpoint (Falck et al., 2002). This conclusion is supported by enhancement of the Nbs1ΔB/ΔB intra-S phase checkpoint defect by Chk2 deficiency reported here (Figure 1B). Other data suggest that the Mre11 complex and Chk2 act in the same pathway; Chk2 activation appears to require ATM-dependent phosphorylation of T68 (Ahn et al., 2000; Melchionna et al., 2000) that is dependent upon the Mre11 complex (Carson et al., 2003) and Chk2 phosphorylation is reduced in cells established from NBS and A-TLD patients (Uziel et al., 2003) and murine mutants that model Mre11 complex hypomorphism (Theunissen et al., 2003).
To examine this issue genetically, we generated Nbs1ΔB/ΔB Chk2−/− and Mre11ATLD1/ATLD1 Chk2−/− double mutant mice and examined several phenotypic outcomes in cell cultures derived from them. The data demonstrate that Chk2 has substantial effects on apoptosis that are independent of the Mre11 complex and ATM, as double mutants exhibited apoptotic defects that far exceeded that of the single mutants (Figure 2B and (Stracker et al., 2007)). Further, Nbs1ΔB/ΔB Chk2−/− and Mre11ATLD1/ATLD1 Chk2−/− mice developed a diverse spectrum of tumors, indicating that the penetrance of potentially oncogenic lesions arising in Mre11 complex mutants is suppressed by Chk2. Because Chk2 deficiency had a limited effect on cell cycle checkpoint, damage sensitivity, and chromosomal instability phenotypes in Nbs1ΔB/ΔB and Mre11ATLD1/ATLD1 mice (Figure 1), a parsimonious interpretation of this outcome is that the apoptotic function of Chk2 is the primary determinant of its tumor suppressive effect.
Our genetic data clearly demonstrates that Chk2 is functional for apoptosis in settings where its hyperphosphorylation is compromised (Atm−/−, Nbs1ΔB/ΔB and Mre11ATLD1/ATLD1). Reconstitution of Chk2−/− animals with mutant Chk2 (7 SQ/TQ sites mutated to alanine) is insufficient to complement the apoptotic defect, implicating phosphorylation as a prerequisite for Chk2 apoptotic activity (Hirao et al., 2002). Chk2 may be activated by other ATM-related kinases, such as ATR or DNA-PKcs that have been demonstrated to target Chk2 in vitro (Li and Stern, 2005; Matsuoka et al., 2000; Wang et al., 2006). As ATM and ATR show differences in their preference for Chk2 target residues (Matsuoka et al., 2000), this alternative mode of activation may involve the utilization of phosphorylation sites that do not produce the slow-migrating species present in wild type cells. Alternatively, Chk2 may have kinase-independent roles in apoptotic signaling. Previous biochemical analysis demonstrated that Chk2 kinase activity is severely impaired in the absence of ATM (Falck et al., 2001; Falck et al., 2002) and our genetic results indicate that it is largely functional for apoptosis in that context (Stracker et al., 2007).
Chk2 and the DNA damage response
In Nbs1ΔB/ΔB and Mre11ATLD1/ATLD1, DNA replication-associated chromosome instability is evident at the cytologic level, and is indicated genetically by the effect of these mutations on p53+/− mice in which tumor latency and spectrum are shifted to resemble p53−/− (Theunissen et al., 2003). Despite the chromosome instability and the intra-S and G2/M checkpoint defects caused by Mre11 complex hypomorphism, Nbs1ΔB/ΔB and Mre11ATLD1/ATLD1 are not predisposed to malignancy (Theunissen et al., 2003; Williams et al., 2002). On this basis, we proposed that a high rate of chromosome breakage-induced cell death may contribute to suppressing the penetrance of potentially oncogenic chromosome rearrangements (Petrini and Theunissen, 2004).
The occurrence of multiple and diverse tumors in Nbs1ΔB/ΔB Chk2−/− and Mre11ATLD1/ATLD1 Chk2−/− mice clearly demonstrates that Chk2 also contributes substantially to suppressing the oncogenic potential of Mre11ATLD1/ATLD1 and Nbs1ΔB/ΔB. The phenotypic presentation of cells established from Chk2−/− mice is not consistent with the view that Chk2 plays a significant role in DSB-induced cell cycle checkpoint regulation (Hirao et al., 2002; Jack et al., 2002; Takai et al., 2002). This is somewhat paradoxical given the primary role of the yeast Chk2 orthologs Rad53 (S. cerevisiae) and Cds1 (S. pombe) in affecting checkpoint functions in their respective contexts (Allen et al., 1994; Boddy et al., 1998; Lindsay et al., 1998; Paulovich and Hartwell, 1995; Zheng et al., 1993). However, in both yeast and mice, these orthologous proteins influence DNA damage-dependent transcriptional changes (Allen et al., 1994). The apparent cell cycle specificity of Chk2 tumor suppressive functions also may provide some reconciliation of this issue. Like Rad53 and Cds1, Chk2 may indeed affect the S-phase DNA damage response, but in mammals, its role in the response to DNA replication-associated chromosome breakage would be confined to tumor suppression and apoptosis.
This interpretation is supported by the phenotypes of both Brca1Δ11/Δ11 Chk2−/− and Prkdcscid/scid Chk2−/− mice. Like the Mre11 complex, Brca1 functions in promoting homologous recombination mediated repair and contributes to S and G2/M phase checkpoint responses (Boulton, 2006). Brca1Δ11/Δ11 Chk2−/− mice developed tumors with a similar latency as Nbs1ΔB/ΔB Chk2−/− and Mre11ATLD1/ATLD1 Chk2−/− double mutants, but exhibited a more circumscribed tumor spectrum (Cao et al., 2006). Similarly, conditional deletion of Brca1 in the thymus or mammary gland of Chk2−/− mice predisposed to tumorigenesis (McPherson et al., 2004).
The PRKDC gene product, DNA-PKcs, is required for the rejoining of DSBs created during antigen receptor gene assembly (V(D)J) recombination (Bassing and Alt, 2004). The induction of these breaks is restricted to the G1 phase of the cell cycle (Lin and Desiderio, 1995), and failure to rejoin them in a timely manner results in p53 activation and apoptotic death of the developing B and T cells. In Prkdcscid/scid p53−/− double mutant mice, early onset (7–12 weeks; earlier than p53−/−) pro-B and immature-T lymphoma is observed. This, as well as studies utilizing both Artemis and Ligase 4 deficient mice, indicates that p53 suppresses the oncogenic potential of V(D)J-associated DSBs (Gao et al., 1998; Guidos et al., 1996; Nacht et al., 1996; Rooney et al., 2004). In contrast, Chk2 deficiency had no effect on the attrition of Prkdcscid/scid B and T cell progenitors and Prkdcscid/scid Chk2−/− double mutant mice are not tumor prone (Figure 6 and data not shown). These data demonstrate that DSBs induced in G1 lymphocytes do not trigger Chk2-dependent apoptosis, and that the oncogenic potential of DSBs in G1 cells is not suppressed by Chk2.
Apoptosis and Checkpoints in Tumor Suppression
Chk2 deficiency strongly attenuated DNA damage-induced p53-dependent transcriptional changes (Figure 4) (Hirao et al., 2002; Takai et al., 2002). Given this result, the lack of overt cancer predisposition in Chk2 null mice was surprising. This is likely to reflect a broader role in the DNA damage response for p53 than Chk2 with respect to the types of genotoxic stress it responds to or particular DNA lesions such as those generated during V(D)J recombination (Guidos et al., 1996).
An alternative explanation is that the G1/S checkpoint, which remains intact in Chk2−/−, may play a significant role in tumor suppression. The significance of the G1/S checkpoint in this regard has been previously suggested from the analysis of mice expressing the p53515C/515C (R172P) allele (Liu et al., 2004). In these mice, the G1/S checkpoint is relatively normal but apoptotic induction is severely compromised. p53515C/515C mice do not present with early onset lymphomas, and instead develop sarcomas and other malignancies at 7 to 13 months of age. Tumors that arise in p53515C/515C do not exhibit the aneuploidy characteristic of p53−/−. Attenuation of the G1/S checkpoint in these mice by deletion of the p21 gene decreases tumor latency and enhances aneuploidy in the resulting tumors (Barboza et al., 2006). These data suggest that the G1/S checkpoint is critical for maintenance of euploidy and suppression of certain types of malignancy, and demonstrate that apoptotic induction and tumor suppression can be uncoupled.
Thus, it is conceivable that the absence of tumors in Prkdcscid/scid Chk2−/− mice may be partially attributable to the tumor suppressive effect of the G1/S checkpoint. The relatively long latency of tumorigenesis in Nbs1ΔB/ΔB Chk2−/− and Mre11ATLD1/ATLD1 Chk2−/− mice may similarly be attributed to the fact that the G1/S checkpoint is largely intact in those double mutants.
Multiple roles of the Mre11 complex in apoptosis
The influence of the Mre11 complex on apoptosis can be parsed into two general categories: i. promoting events at the site of DNA damage that facilitate ATM activity; ii. promoting DSB-induced ATM activation. Our data indicated that both of these molecular events are required for the promotion of Mre11 complex-dependent apoptosis. We observed a deficit in IR-induced ATM activation in Mre11ATLD1/ATLD1 mice (Figure 3A), which is correlated with reduced phosphorylation of p53 and Chk2 in response to DNA damage. As is true for Mre11ATLD1/ATLD1 Chk2−/−, mice expressing p53 alleles in which the ATM and CHK2 consensus sites at S18 and S23 are altered (p53S18A/S23A) have a substantial apoptotic defect, fail to fully stabilize p53 in thymocytes following IR, and develop a broad spectrum of malignancies with a long latency period (Chao et al., 2006). These data support the interpretation that reduced ATM activation associated with Mre11ATLD1/ATLD1 contributes to the apoptotic defects observed. Conversely, Nbs1ΔC/ΔC mice exemplify the effect of attenuating ATMs access to its pro-apoptotic substrates. In these mice, ATM activation is unaffected, but ATM activity and apoptosis is defective, and this is associated with a reduction in the phosphorylation of certain ATM substrates (Stracker et al., 2007).
Chk2 hypomorphism and deficiency
The data presented herein clearly support of a global role for Chk2 as a tumor suppressor; however, Chk2 deficiency alone does not appreciably increase spontaneous tumor risk. Mice expressing either a kinase dead allele of Chk2 (D347A) or the equivalent of the human CHEK2*1100delC allele, identified in several types of human cancers, have been established (Bahassi el et al., 2007; Kwak et al., 2006; Nevanlinna and Bartek, 2006). Unlike Chk2 deficient mice, mice expressing the Chk2- D347A allele from the MMTV promoter in the spleen and mammary gland developed spontaneous tumors (Kwak et al., 2006). Analogously, checkpoint defects not apparent in Chk2−/− mice or cell lines with acute Chk2 deletion have been described in human or mouse cells expressing hypomorphic Chk2 alleles or in which siRNA-mediated depletion has been induced (Bahassi el et al., 2007; Falck et al., 2001; Falck et al., 2002; Jallepalli et al., 2003; Matsuoka et al., 1998; Takai et al., 2002). These data are consistent with the idea that the protein products of certain Chk2 alleles may exert an inhibitory effect on DNA damage responses. These observations are also consistent with the possibility that compensating mutations arise during development of Chk2−/− mice and may suppress some aspects of Chk2 deficiency.
This study demonstrates that the Mre11 complex and ATM function together to promote apoptosis in a pathway that parallels Chk2. The Mre11 complex is required for both ATM activation and to facilitate access of the active kinase to its substrates (Lee and Paull, 2007). In addition, the study elucidates a mechanism by which the oncogenic potential of DNA replication-associated chromosome breakage is suppressed. We show that Chk2 plays a heretofore, unrecognized role in responding to DNA replication-associated DNA damage. Chk2’s influence in that context comprises apoptosis as well as tumor suppression, supporting the view that Chk2 is a tumor suppressor.
This interpretation resonates with the observation that the DNA damage response is activated in pre-malignant lesions in humans; this is indicated by several parameters, including the phosphorylation of Chk2 (Bartkova et al., 2005; Gorgoulis et al., 2005). Tumor progression correlates with the attenuation of DNA damage response (DDR) markers, indicating that the damage response acts as an inducible barrier to tumorigenesis. Our data is consistent with this view and demonstrate that the loss of multiple facets of the DDR results in the predisposition to spontaneous tumorigenesis in diverse tissues. In this regard, the data presented define a mechanistic basis for such a barrier to be effected in response to DNA replication stress and genome instability.
Experimental Procedures
Mice
Chk2−/−, p53−/−, and Atm−/− mice were obtained from T. Mak, T. Jacks, and T. Wynshaw Boris, and Prkdc+/scid were purchased (Jackson Laboratories). Nbs1ΔB and Mre11ATLD1 mice were described (Theunissen et al., 2003; Williams et al., 2002). Mice were raised in a pathogen free facility and genotyped by PCR (details upon request).
Immunoreagents and Western Blotting
Antibodies and Western blotting techniques are described in the Supplemental Data.
Cellular Assays
The generation of MEFs, checkpoint assays and analysis of chromosome spreads were performed as described (Theunissen and Petrini, 2006). Sensitivity of SV40 transformed MEFs to IR was assessed using a colony formation assay as described (Williams et al., 2002).
Apoptosis Assays
Thymi were dissected from 6–9 wk old animals and 1×106 cells were irradiated using a Cs-137 source (dose rate 220 cGy/min- Shepherd Mark-1) or treated with 1mM dexamethasone (Sigma). Viable (double negative, DN) cells were identified by flow cytometry after propidium iodide and anti-AnnexinV-FITC antibody (BD Biosciences) staining. A viability ratio (%DN treated or mock/%DN mock) is plotted.
Pathology, Histology, and Immunohistochemistry
Moribund mice were sacrificed, tissues fixed in 10% neutral buffered formalin, and processed for immunohistochemistry (IHC) (described in Supplemental Data).
Sequencing of p53 and p19ARF
For sequencing of p53 or Arf, genomic DNA or cDNA was amplified by PCR (Primers described in Supplemental Data). Genomic DNA was prepared from tumors by digestion with proteinase-K, phenol/chloroform extraction and DNA precipitation. Some samples were isolated using a PixCell laser capture microscopy (LCM) system (Arcturus) or from paraffin by macro-dissection of tumor-enriched lesions. DNA was isolated from LCM samples using the Pico Pure DNA extraction kit (Arcturus). RNA was extracted from tumor tissue using Trizol reagent (Invitrogen) and cDNA was prepared using the Superscript RT-PCR kit (Invitrogen).
Microarray analysis
Thymocytes were cultured in vitro and treated with RNAlater (Ambion) 12 hours post-IR. Whole RNA was extracted using RNAeasy columns (Qiagen). Generation of cDNA, labeling and hybridization to MOE430A_2 gene arrays (Affymetrix) was performed in the Sloan-Kettering Genomics Core Facility. Analysis of microarray data is described in the Supplemental Data.
Supplementary Material
Acknowledgements
We thank S. Shelly, H. Hussein and A. Baker for diligent maintenance of our animal colony. Thanks to N. Draghi and P. Savage for advice on B-cell proliferation, J. Theunissen for technical assistance and discussions, G. Lozano, S. Post, and R. Maser for p53 or p19ARF primer and sequencing information, A. Viale and N. Socci for microarray analysis, and A. Koff for critical reading of the manuscript. J.H.J.P was 22 funded by grants from the NIH and the Joel and Jean Smilow Initiative. T.H.S. was funded by a NIH National Research Service Award and is a Leukemia and Lymphoma Society Special Fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn JY, Schwarz JK, Piwnica-Worms H, Canman CE. Threonine 68 phosphorylation by ataxia telangiectasia mutated is required for efficient activation of Chk2 in response to ionizing radiation. Cancer Res. 2000;60:5934–5936. [PubMed] [Google Scholar]
- Allen JB, Zhou Z, Siede W, Friedberg EC, Elledge SJ. The SAD1/RAD53 protein kinase controls multiple checkpoints and DNA damage-induced transcription in yeast. Genes & Dev. 1994;8:2401–2415. doi: 10.1101/gad.8.20.2401. [DOI] [PubMed] [Google Scholar]
- Bahassi el M, Penner CG, Robbins SB, Tichy E, Feliciano E, Yin M, Liang L, Deng L, Tischfield JA, Stambrook PJ. The breast cancer susceptibility allele CHEK2* 1100delC promotes genomic instability in a knock-in mouse model. Mutat Res. 2007;616:201–209. doi: 10.1016/j.mrfmmm.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- Barboza JA, Liu G, Ju Z, El-Naggar AK, Lozano G. p21 delays tumor onset by preservation of chromosomal stability. Proc Natl Acad Sci U S A. 2006;103:19842–19847. doi: 10.1073/pnas.0606343104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow C, Brown KD, Deng CX, Tagle DA, Wynshaw-Boris A. Atm selectively regulates distinct p53-dependent cell-cycle checkpoint and apoptotic pathways. Nat Genet. 1997;17:453–456. [Google Scholar]
- Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley JN, Ried T, Tagle D, Wynshaw-Boris A. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996;86:159–171. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Bartek J, Falck J, Lukas J. CHK2 kinase--a busy messenger. Nat Rev Mol Cell Biol. 2001;2:877–886. doi: 10.1038/35103059. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- Bassing CH, Alt FW. The cellular response to general and programmed DNA double strand breaks. DNA Repair (Amst) 2004;3:781–796. doi: 10.1016/j.dnarep.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Boddy MN, Furnari B, Mondesert O, Russell P. Replication checkpoint enforced by kinases Cds1 and Chk1. Science. 1998;280:909–912. doi: 10.1126/science.280.5365.909. [DOI] [PubMed] [Google Scholar]
- Boulton SJ. Cellular functions of the BRCA tumour-suppressor proteins. Biochem Soc Trans. 2006;34:633–645. doi: 10.1042/BST0340633. [DOI] [PubMed] [Google Scholar]
- Bressan DA, Baxter BK, Petrini JH. The Mre11-Rad50-Xrs2 protein complex facilitates homologous recombination-based double-strand break repair in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:7681–7687. doi: 10.1128/mcb.19.11.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Kim S, Xiao C, Wang RH, Coumoul X, Wang X, Li WM, Xu XL, De Soto JA, Takai H, et al. ATM-Chk2-p53 activation prevents tumorigenesis at an expense of organ homeostasis upon Brca1 deficiency. Embo J. 2006;25:2167–2177. doi: 10.1038/sj.emboj.7601115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson CT, Schwartz RA, Stracker TH, Lilley CE, Lee DV, Weitzman MD. The Mre11 complex is required for ATM activation and the G(2)/M checkpoint. Embo J. 2003;22:6610–6620. doi: 10.1093/emboj/cdg630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao C, Herr D, Chun J, Xu Y. Ser18 and 23 phosphorylation is required for p53-dependent apoptosis and tumor suppression. Embo J. 2006;25:2615–2622. doi: 10.1038/sj.emboj.7601167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehab NH, Malikzay A, Appel M, Halazonetis TD. Chk2/hCds1 functions as a DNA damage checkpoint in G(1) by stabilizing p53. Genes Dev. 2000;14:278–288. [PMC free article] [PubMed] [Google Scholar]
- Donehower LA, Harvey M, Slagle BL, Mc Arthur MJ, Montgomery CAJ, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaenous tumours. Nature (London) 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Falck J, Coates J, Jackson SP. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature. 2005;434:605–611. doi: 10.1038/nature03442. [DOI] [PubMed] [Google Scholar]
- Falck J, Mailand N, Syljuasen RG, Bartek J, Lukas J. The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature. 2001;410:842–847. doi: 10.1038/35071124. [DOI] [PubMed] [Google Scholar]
- Falck J, Petrini JH, Williams BR, Lukas J, Bartek J. The DNA damage-dependent intra--S phase checkpoint is regulated by parallel pathways. Nat Genet. 2002;30:290–294. doi: 10.1038/ng845. [DOI] [PubMed] [Google Scholar]
- Gao Y, Sun Y, Frank KM, Dikkes P, Fujiwara Y, Seidl KJ, Sekiguchi JM, Rathbun GA, Swat W, Wang J, et al. A critical role for DNA end-joining proteins in both lymphogenesis and neurogenesis. Cell. 1998;95:891–902. doi: 10.1016/s0092-8674(00)81714-6. [DOI] [PubMed] [Google Scholar]
- Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, Venere M, Ditullio RA, Jr., Kastrinakis NG, Levy B, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- Guidos CJ, Williams CJ, Grandal I, Knowles G, Huang MTF, Danska JS. V(D)J recombination activates a p53-dependent DNA damage checkpoint in scid lymphocytes. Genes & Dev. 1996;10:2038–2054. doi: 10.1101/gad.10.16.2038. [DOI] [PubMed] [Google Scholar]
- Herzog KH, Chong MJ, Kapsetaki M, Morgan JI, McKinnon PJ. Requirement for Atm in ionizing radiation-induced cell death in the developing central nervous system. Science. 1998;280:1089–1091. doi: 10.1126/science.280.5366.1089. [DOI] [PubMed] [Google Scholar]
- Hirao A, Cheung A, Duncan G, Girard PM, Elia AJ, Wakeham A, Okada H, Sarkissian T, Wong JA, Sakai T, et al. Chk2 is a tumor suppressor that regulates apoptosis in both an ataxia telangiectasia mutated (ATM)-dependent and an ATM-independent manner. Mol Cell Biol. 2002;22:6521–6532. doi: 10.1128/MCB.22.18.6521-6532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao A, Kong YY, Matsuoka S, Wakeham A, Ruland J, Yoshida H, Liu D, Elledge SJ, Mak TW. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science. 2000;287:1824–1827. doi: 10.1126/science.287.5459.1824. [DOI] [PubMed] [Google Scholar]
- Jack MT, Woo RA, Hirao A, Cheung A, Mak TW, Lee PW. Chk2 is dispensable for p53-mediated G1 arrest but is required for a latent p53-mediated apoptotic response. Proc Natl Acad Sci U S A. 2002;99:9825–9829. doi: 10.1073/pnas.152053599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jallepalli PV, Lengauer C, Vogelstein B, Bunz F. The Chk2 tumor suppressor is not required for p53 responses in human cancer cells. J Biol Chem. 2003;278:20475–20479. doi: 10.1074/jbc.M213159200. [DOI] [PubMed] [Google Scholar]
- Kastan MB, Zhan Q, El-Diery WS, Carrier F, Jacks T, Walsh WV, Plunkett BS, Vogelstein B, Fornace AJ. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Kitagawa R, Bakkenist CJ, McKinnon PJ, Kastan MB. Phosphorylation of SMC1 is a critical downstream event in the ATM-NBS1-BRCA1 pathway. Genes Dev. 2004;18:1423–1438. doi: 10.1101/gad.1200304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuerbitz SJ, Plunkett BS, Walsh WV, Kastan MB. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci USA. 1992;89:7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak EL, Kim S, Zhang J, Cardiff RD, Schmidt EV, Haber DA. Mammary tumorigenesis following transgenic expression of a dominant negative CHK2 mutant. Cancer Res. 2006;66:1923–1928. doi: 10.1158/0008-5472.CAN-05-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Paull TT. Activation and regulation of ATM kinase activity in response to DNA double-strand breaks. Oncogene. 2007;26:7741–7748. doi: 10.1038/sj.onc.1210872. [DOI] [PubMed] [Google Scholar]
- Li J, Stern DF. Regulation of CHK2 by DNA-dependent protein kinase. J Biol Chem. 2005;280:12041–12050. doi: 10.1074/jbc.M412445200. [DOI] [PubMed] [Google Scholar]
- Lin WC, Desiderio S. V(D)J recombination and the cell cycle. Immunol Today. 1995;16:279–289. doi: 10.1016/0167-5699(95)80182-0. [DOI] [PubMed] [Google Scholar]
- Lindsay HD, Griffiths DJ, Edwards RJ, Christensen PU, Murray JM, Osman F, Walworth N, Carr AM. S-phase-specific activation of Cds1 kinase defines a subpathway of the checkpoint response in Schizosaccharomyces pombe. Genes Dev. 1998;12:382–395. doi: 10.1101/gad.12.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Parant JM, Lang G, Chau P, Chavez-Reyes A, El-Naggar AK, Multani A, Chang S, Lozano G. Chromosome stability, in the absence of apoptosis, is critical for suppression of tumorigenesis in Trp53 mutant mice. Nat Genet. 2004;36:63–68. doi: 10.1038/ng1282. [DOI] [PubMed] [Google Scholar]
- MacPherson D, Kim J, Kim T, Rhee BK, Van Oostrom CT, DiTullio RA, Venere M, Halazonetis TD, Bronson R, De Vries A, et al. Defective apoptosis and B-cell lymphomas in mice with p53 point mutation at Ser 23. Embo J. 2004;23:3689–3699. doi: 10.1038/sj.emboj.7600363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S, Huang M, Elledge SJ. Linkage of ATM to Cell Cycle Regulation by the Chk2 Protein Kinase. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- Matsuoka S, Rotman G, Ogawa A, Shiloh Y, Tamai K, Elledge SJ. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc Natl Acad Sci U S A. 2000;97:10389–10394. doi: 10.1073/pnas.190030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson JP, Lemmers B, Hirao A, Hakem A, Abraham J, Migon E, Matysiak-Zablocki E, Tamblyn L, Sanchez-Sweatman O, Khokha R, et al. Collaboration of Brca1 and Chk2 in tumorigenesis. Genes Dev. 2004;18:1144–1153. doi: 10.1101/gad.1192704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchionna R, Chen XB, Blasina A, McGowan CH. Threonine 68 is required for radiation-induced phosphorylation and activation of Cds1. Nat Cell Biol. 2000;2:762–765. doi: 10.1038/35036406. [DOI] [PubMed] [Google Scholar]
- Morales M, Theunissen JW, Kim CF, Kitagawa R, Kastan MB, Petrini JH. The Rad50S allele promotes ATM-dependent DNA damage responses and suppresses ATM deficiency: implications for the Mre11 complex as a DNA damage sensor. Genes Dev. 2005;19:3043–3054. doi: 10.1101/gad.1373705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacht M, Strasser A, Chan YR, Harris AW, Schlissel S, Bronson RT, Jacks T. Mutations in the p53 and SCID genes cooperate in tumorigenesis. Genes & Dev. 1996;10:2055–2066. doi: 10.1101/gad.10.16.2055. [DOI] [PubMed] [Google Scholar]
- Nevanlinna H, Bartek J. The CHEK2 gene and inherited breast cancer susceptibility. Oncogene. 2006;25:5912–5919. doi: 10.1038/sj.onc.1209877. [DOI] [PubMed] [Google Scholar]
- Paulovich AG, Hartwell LH. A checkpoint regulates the rate of progression through S phase in S. cerevisiae in response to DNA damage. Cell. 1995;82:841–847. doi: 10.1016/0092-8674(95)90481-6. [DOI] [PubMed] [Google Scholar]
- Petrini JH, Stracker TH. The cellular response to DNA double-strand breaks: defining the sensors and mediators. Trends Cell Biol. 2003;13:458–462. doi: 10.1016/s0962-8924(03)00170-3. [DOI] [PubMed] [Google Scholar]
- Petrini JH, Theunissen JW. Double Strand Break Metabolism and Cancer Susceptibility: Lessons from the Mre11 Complex. Cell Cycle. 2004;3 doi: 10.4161/cc.3.5.835. [DOI] [PubMed] [Google Scholar]
- Powers JT, Hong S, Mayhew CN, Rogers PM, Knudsen ES, Johnson DG. E2F1 uses the ATM signaling pathway to induce p53 and Chk2 phosphorylation and apoptosis. Mol Cancer Res. 2004;2:203–214. [PubMed] [Google Scholar]
- Pusapati RV, Rounbehler RJ, Hong S, Powers JT, Yan M, Kiguchi K, McArthur MJ, Wong PK, Johnson DG. ATM promotes apoptosis and suppresses tumorigenesis in response to Myc. Proc Natl Acad Sci U S A. 2006;103:1446–1451. doi: 10.1073/pnas.0507367103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogoff HA, Pickering MT, Frame FM, Debatis ME, Sanchez Y, Jones S, Kowalik TF. Apoptosis associated with deregulated E2F activity is dependent on E2F1 and Atm/Nbs1/Chk2. Mol Cell Biol. 2004;24:2968–2977. doi: 10.1128/MCB.24.7.2968-2977.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney S, Sekiguchi J, Whitlow S, Eckersdorff M, Manis JP, Lee C, Ferguson DO, Alt FW. Artemis and p53 cooperate to suppress oncogenic N-myc amplification in progenitor B cells. Proc Natl Acad Sci U S A. 2004;101:2410–2415. doi: 10.1073/pnas.0308757101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth DB, Menetski JP, Nakajima PB, Bosma MJ, Gellert M. V(D)J recombination: broken DNA molecules with covalently sealed (hairpin) coding ends in scid mouse thymocytes. Cell. 1992;70:983–991. doi: 10.1016/0092-8674(92)90248-b. [DOI] [PubMed] [Google Scholar]
- Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- Soussi T, Wiman KG. Shaping genetic alterations in human cancer: the p53 mutation paradigm. Cancer Cell. 2007;12:303–312. doi: 10.1016/j.ccr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Stracker TH, Morales M, Couto SS, Hussein H, Petrini JH. The carboxy terminus of NBS1 is required for induction of apoptosis by the MRE11 complex. Nature. 2007 doi: 10.1038/nature05740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracker TH, Theunissen JW, Morales M, Petrini JH. The Mre11 complex and the metabolism of chromosome breaks: the importance of communicating and holding things together. DNA Repair (Amst) 2004;3:845–854. doi: 10.1016/j.dnarep.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Takai H, Naka K, Okada Y, Watanabe M, Harada N, Saito S, Anderson CW, Appella E, Nakanishi M, Suzuki H, et al. Chk2-deficient mice exhibit radioresistance and defective p53-mediated transcription. Embo J. 2002;21:5195–5205. doi: 10.1093/emboj/cdf506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen JW, Kaplan MI, Hunt PA, Williams BR, Ferguson DO, Alt FW, Petrini JH. Checkpoint failure and chromosomal instability without lymphomagenesis in Mre11(ATLD1/ATLD1) mice. Mol Cell. 2003;12:1511–1523. doi: 10.1016/s1097-2765(03)00455-6. [DOI] [PubMed] [Google Scholar]
- Theunissen JW, Petrini JH. Methods for studying the cellular response to DNA damage: influence of the Mre11 complex on chromosome metabolism. Methods Enzymol. 2006;409:251–284. doi: 10.1016/S0076-6879(05)09015-4. [DOI] [PubMed] [Google Scholar]
- Uziel T, Lerenthal Y, Moyal L, Andegeko Y, Mittelman L, Shiloh Y. Requirement of the MRN complex for ATM activation by DNA damage. Embo J. 2003;22:5612–5621. doi: 10.1093/emboj/cdg541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XQ, Redpath JL, Fan ST, Stanbridge EJ. ATR dependent activation of Chk2. J Cell Physiol. 2006;208:613–619. doi: 10.1002/jcp.20700. [DOI] [PubMed] [Google Scholar]
- Westphal CH, Rowan S, Schmaltz C, Elson A, Fisher DE, Leder P. atm and p53 cooperate in apoptosis and suppression of tumorigenesis, but not in resistance to acute radiation toxicity. Nat Genet. 1997;16:397–401. doi: 10.1038/ng0897-397. [DOI] [PubMed] [Google Scholar]
- Williams BR, Mirzoeva OK, Morgan WF, Lin J, Dunnick W, Petrini JH. A murine model of nijmegen breakage syndrome. Curr Biol. 2002;12:648–653. doi: 10.1016/s0960-9822(02)00763-7. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ashley T, Brainerd EE, Bronson RT, Meyn MS, Baltimore D. Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes & Dev. 1996;10:2411–2422. doi: 10.1101/gad.10.19.2411. [DOI] [PubMed] [Google Scholar]
- Xu Y, Baltimore D. Dual roles of ATM in the cellular response to radiation and in cell growth control. Genes Dev. 1996;10:2401–2410. doi: 10.1101/gad.10.19.2401. [DOI] [PubMed] [Google Scholar]
- Xu Y, Yang EM, Brugarolas J, Jacks T, Baltimore D. Involvement of p53 and p21 in cellular defects and tumorigenesis in Atm−/− mice. Mol Cell Biol. 1998;18:4385–4390. doi: 10.1128/mcb.18.7.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Z, Chahwan C, Bailis J, Hunter T, Russell P. ATM activation and its recruitment to damaged DNA require binding to the C terminus of Nbs1. Mol Cell Biol. 2005;25:5363–5379. doi: 10.1128/MCB.25.13.5363-5379.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Fay DS, Burton J, Xiao H, Pinkham JL, Stern DF. SPK1 is an essential S-phase-specific gene of Saccharomyces cerevisiae that encodes a nuclear serine/threonine/tyrosine kinase. Mol Cell Biol. 1993;13:5829–5842. doi: 10.1128/mcb.13.9.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.