Abstract
This study examined the antinociceptive (analgesic) efficacy of hydromorphone and hydromorphone-induced tolerance and regulation of μ-opioid receptor density. Initially s.c. hydromorphone’s time of peak analgesic (tail-flick) effect (45 min) and ED50 using standard and cumulative dosing protocols (0.22 mg/kg, 0.37mg/kg, respectively) were determined. The apparent analgesic efficacy (t) of hydromorphone was then estimated using the operational model of agonism and the irreversible μ-opioid receptor antagonist clocinnamox. Mice were injected with clocinnamox (0.32–25.6 mg/kg, i.p.) and 24 h later, the analgesic potency of hydromorphone was determined. The t value for hydromorphone was 35, which suggested that hydromorphone is a lower analgesic efficacy opioid agonist. To examine hydromorphone-induced tolerance, mice were continuously infused s.c. with hydromorphone (2.1–31.5mg/kg/day) for 7 days and then morphine cumulative dose response studies were performed. Other groups of mice were injected with hydromorphone (2.2-22 mg/kg/day) once, or intermittently every 24 h for 7 days. Twenty-four h after the last injection, mice were tested using morphine cumulative dosing studies. There was more tolerance with infusion treatments compared to intermittent treatment. When compared to higher analgesic efficacy opioids, hydromorphone infusions induced substantially more tolerance. Finally, the effect of chronic infusion (31.5mg/kg/day) and 7 day intermittent (22mg/kg/day) hydromorphone treatment on spinal cord μ-opioid receptor density was determined. Hydromorphone did not produce any change in μ-opioid receptor density following either treatment. These results support suggestions that analgesic efficacy is correlated with tolerance magnitude and regulation of μ-opioid receptors when opioid agonists are continuously administered. Taken together, these studies indicate that analgesic efficacy and treatment protocol are important in determining tolerance and regulation of μ-opioid receptors.
Keywords: hydromorphone, tolerance, μ-opioid receptor, efficacy, analgesia, receptor regulation, intermittent, continuous infusion
1. Introduction
The mechanisms that mediate tolerance to opioid agonists have been extensively studied. The results of many studies suggest that agonist efficacy may play an important role in the magnitude of tolerance (Duttaroy and Yoburn, 1995; Paronis and Holtzman, 1992; Pawar et al., 2007). For example, at equi-effective doses, a higher efficacy opioid agonist (e.g., etorphine) produces less tolerance than lower efficacy agonists (e.g., morphine, oxycodone) after chronic infusion treatment (Duttaroy and Yoburn, 1995; Stafford et al., 2001; Pawar et al., 2007). The regulation of μ-opioid receptor density also appears to be correlated with agonist efficacy. Higher efficacy agonists induce μ-opioid receptor internalization and downregulation in in vitro and in vivo studies (e.g., Patel et al., 2002; Whistler et al., 1999; Yoburn et al., 2004; Zaki et al., 2000); whereas, lower efficacy agonists are typically ineffective (e.g., Keith et al., 1996; Stafford et al., 2001; however see Haberstock-Debic et al., 2005). Nevertheless, while μ-opioid receptor downregulation is usually not observed in vivo with lower efficacy opioid agonists (Patel et al., 2002; Yoburn et al., 2004), downregulation contributes to the magnitude of tolerance (e.g., Stafford et al., 2001). Taken together, opioid agonist efficacy appears to play a role in both tolerance and opioid receptor regulation (Duttaroy and Yoburn, 1995; Paronis and Holtzman, 1992; Stevens and Yaksh, 1989; Walker and Young, 2001; Pawar et al., 2007).
Efficacy can be defined as the property of a drug that causes a receptor to change its behavior towards the host cell (Kenakin, 2002). Recent formulations of efficacy suggest that ligands acting at a given receptor can have multiple efficacies (e.g., Kenakin, 2007; Galandrin and Bouvier, 2006). In earlier studies examining the role of efficacy in tolerance and μ-opioid receptor regulation, opioid agonist efficacy had been considered as a parameter that characterizes the drug itself, rather than the drug and a particular effect (e.g., Stafford et al., 2001). Using the operational model of agonism in a previous study, morphine and oxycodone were found to have relatively low τ values for analgesia (i.e., antinociception), whereas etorphine was identified as a higher analgesic efficacy opioid (Pawar et al., 2007). This quantitative estimate of analgesic efficacy supported previous suggestions that efficacy can be used to predict μ-opioid receptor regulation and the magnitude of tolerance (e.g., Duttaroy and Yoburn, 1995; Paronis and Holtzman, 1992; Stafford et al., 2001).
In the present study, we used the irreversible μ-opioid receptor antagonist clocinnamox and the operational model of agonism (Black and Leff, 1983; Black et al., 1985; Leff et al., 1990) to estimate the analgesic efficacy of the opioid agonist hydromorphone. Hydromorphone is an opioid analgesic that is commonly used to manage pain and is abused (e.g., Cicero et al., 2005; Kumar and Lin, 2007; Murray and Hagen, 2005); and studying efficacy may enhance clinical effectiveness and lead to strategies to minimize tolerance, abuse and dependence. Based on the estimated low analgesic efficacy of this drug, we predicted that hydromorphone would produce substantial tolerance, but would not regulate the density of μ-opioid receptors. In addition, we have reported that the magnitude of tolerance was similar among several opioid analgesics when the drugs were administered intermittently rather than continuously infused (Duttaroy and Yoburn, 1995). In other words, opioid analgesic efficacy did not appear to be a major factor in predicting the magnitude of tolerance using an intermittent treatment protocol. Therefore, in this study we also examined tolerance and μ-opioid receptor regulation following intermittent as well as acute treatment with hydromorphone.
2. Materials and Methods
2.1 Subjects
Male Swiss Webster mice, weighing 22–30g, obtained from Taconic Farms (Germantown, NY) were used throughout. Animals were housed 10 per cage with food and water ad-libitum. Mice were used only once. All protocols and procedures were approved by the St. John’s University Institutional Animal Care and Use Committee.
2.2 Drugs and Chemicals
Hydromorphone HCl was obtained from Spectrum Chemicals Inc. (Gardena, CA). Morphine sulfate and placebo pellets were obtained from the Research Triangle Institute (Research Triangle Park, NC). Clocinnamox mesylate was obtained from Tocris Bioscience (Ellisville, MO). Placebo pellets were wrapped in nylon mesh before implantation. Hydromorphone and morphine were dissolved in 0.9% saline and doses are expressed as the free base. Clocinnamox was dissolved in dH2O with ≈4% DMSO added to enhance solubility. Clocinnamox dose is expressed as the salt. [3H] DAMGO was obtained from PerkinElmer Life Sciences (Boston, MA).
2.3 General Procedure
Initially, the time of peak analgesic (antinociceptive) effect (tail-flick) for hydromorphone was examined, followed by estimation of the analgesic ED50 at the time of peak effect using two dosing protocols (standard and cumulative, see below). Next, the analgesic efficacy of hydromorphone was determined using clocinnamox, an irreversible antagonist at μ-opioid receptors. Finally, infusion, acute and intermittent treatment studies were conducted to assess tolerance and changes in μ-opioid receptor binding.
2.4 Peak Analgesic Effect of Hydromorphone
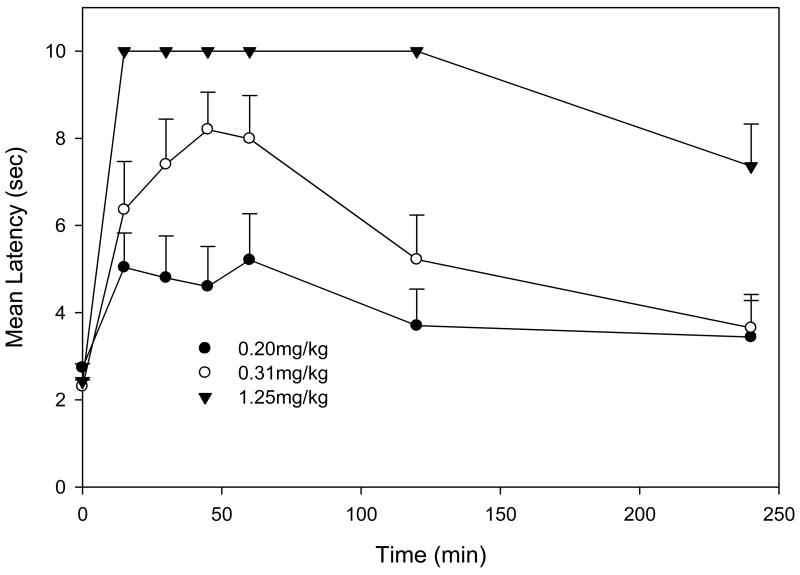
To determine the time of peak analgesic effect of hydromorphone, mice (N=5–9/group) were injected subcutaneously (s.c.) with 0.2 – 1.25mg/kg and tested for antinociception (tail-flick) 15–240 min following treatment. The approximate time at which the drug produced the greatest effect (mean latency) was defined as time of peak effect.
2.5 Hydromorphone Dose Response Studies
Dose response studies were performed using two methods: Standard dose response and cumulative dose response protocols. Standard dose response protocol: In this procedure, groups of mice (N=5–8/group), were injected s.c. (0.1–1.25mg/kg) and tested for antinociception using the tail-flick assay (see below) at 45min following hydromorphone. Mice were injected once and tested once. The standard dose protocol was used to estimate the ED50 of hydromorphone.
Cumulative dose response protocol
Mice (N=8) were injected s.c. with a starting dose of morphine (either 1.5 or 0.5mg/kg) or hydromorphone (0.15mg/kg) and tested for antinociception at the 30min or 45min, respectively. Mice that were not analgesic were given a second dose within 1min and retested for antinociception. This procedure was continued until all mice were analgesic (tail-flick latency > 10sec). The cumulative dosing sequence for morphine was based on previous studies (Duttaroy and Yoburn., 1995; Duttaroy et al., 1997). The cumulative dosing sequence for hydromorphone was determined in preliminary studies and was designed to approximate the ED50 calculated using the standard dosing protocol.
2.6 Efficacy Studies
To determine hydromorphone’s analgesic efficacy (see data analysis below), the irreversible μ-opioid receptor antagonist, clocinnamox was employed. Mice (N=6–10/dose) were injected i.p. with clocinnamox (0.32–25.6mg/kg). Controls were injected with saline. Cumulative dose response studies were performed using s.c. hydromorphone 24 h later.
2.7 Tolerance Studies
Mice (N=5–8/group) were implanted s.c. with an osmotic mini pump (Alzet model 2001, Durect Corporation, Curpertino, CA) that delivered hydromorphone at 2.1 – 31.5mg/kg/day which is ≈10–150 times the ED50, as determined in standard dosing studies. Other mice (N=8–10/group) were injected s.c. either once, or once every 24 h, for 7 days, with hydromorphone (2.2 – 22.0mg/kg/day, ≈10–100 times the ED50). Injection doses of hydromorphone higher than 100 times the ED50 could not be used due to lethality (≈20%). Infusion controls were implanted with an inert placebo pellet and injection controls were injected s.c. with saline. Pumps and pellets were implanted s.c. at the nape of the neck while mice were lightly anesthetized with oxygen: halothane (96:4) (Halocarbon Labs, River Edge, NJ). Following 7 days of treatment, pumps and pellets were removed and 16 h later, or 24 h after the last injection, mice were tested for tolerance using morphine cumulative dose response studies. Morphine was used as the test drug, as we have done previously (see Pawar et al., 2007), so that the magnitude of potency shifts induced by hydromorphone could be directly compared among opioid drugs.
2.8 Radioligand Binding Studies
Mice (N=10/treatment) were implanted s.c. with an osmotic mini pump that infused the highest dose of hydromorphone used in infusion tolerance studies (31.5mg/kg/day, ≈150 times the ED50). Controls were implanted with placebo pellets. Other groups of mice (N=8–10/treatment) were injected (s.c.) with saline or the highest intermittent dose of hydromorphone (22.0mg/kg/day, ≈100 times the ED50) for 7days. Mice were sacrificed either 16h (infusion) or 24h (intermittent) after the termination of treatment and spinal cords were rapidly removed, and placed in tubes containing 15 ml ice cold 50 mM Tris buffer (pH=7.4). Samples were homogenized (Brinkmann Polytron Homogenizer, Westbury, NY) at 20,000 rpm for 40 s. Homogenates were centrifuged at 15,000 rpm (≈ 26,000 x g) for 15 min at 3–9°C. The supernatant was discarded and the tissue pellets were collected and stored (−80°C) until analysis.
Tissue pellets were thawed, suspended in 15ml of ice cold Tris buffer, and centrifuged for 15min, supernatant was discarded and pellets were collected. The pellets were resuspended in 35ml Tris buffer and incubated for 30 min at 25°C. The samples were centrifuged again for 15 min and the supernatant was discarded and pellets collected. The pellets were resuspended in 20ml of ice cold 50 mM potassium phosphate buffer, pH 7.2. An aliquot of sample was collected for determination of protein concentration (Bradford, 1976). An aliquot (100 μl) of the homogenate was assayed in triplicate tubes containing 0.02–10nm [3H] DAMGO. Non specific binding was determined in the presence of 1000 nM levorphanol. The tubes were incubated for 90 min at 25°C. Incubation was terminated by the addition of ice cold phosphate buffer and the samples were filtered over glass fiber (GF/B) filters (Brandel, Gaithersburg, MD) using a cell harvester. Filters were washed three times with phosphate buffer; transferred into vials with liquid scintillation cocktail (Econo-safe, Research Products International Corporation, IL), and counted in a liquid scintillation counter (Packard A2300, Packard, Shelton, CT). Counts per minute were converted into disintegrations per minute, using the external standard method.
2.9 Analgesia Assay
Antinociception was determined using the tail-flick assay (Model TF6, Emdie Instrument Co., Maidens, VA) in which a beam of light was focused on the dorsal surface of the tail, approximately 2 cm from the tip of the tail. The intensity of the light was adjusted so that baseline tail flick latencies were typically in the range of 1–3 s. If a mouse failed to flick within 10sec following drug administration, it was defined as analgesic and the test was terminated. All tail-flick tests were conducted in a blind manner.
2.10 Data Analysis
Cumulative and standard dose response results were analyzed as quantal (percent analgesic) or graded (tail-flick latency) data. The estimation of agonist ED50 requires quantal data, while the calculation of agonist efficacy following receptor depletion is typically based on graded data. Quantal dose response data were analyzed using Probit Analysis (Finney, 1973) to calculate ED50 values, standard errors and 95% confidence intervals. Graded data were analyzed using nonlinear regression (four-parameter logistic equation; Prism version 4.03, GraphPad software, San Diego, CA); which estimates EC50 values, standard errors and 95% confidence intervals. Potency changes are based on the ratio of the ED50 or EC50 value in the treated groups relative to control. This change is referred to as the shift in the ED50 or EC50 value. Binding data were analyzed using GraphPad Prism (version 4.03, GraphPad, San Diego, CA) using nonlinear regression. All binding data were best fit by a one site model.
Efficacy of hydromorphone was analyzed using the operational model of agonism (Black and Leff, 1983) and the calculation methods of Zernig et al., (1995). In this approach to efficacy estimation, an irreversible μ-opioid receptor antagonist (e.g., clocinnamox) is used. τ is the operational definition of efficacy (Black and Leff 1983). τ can be defined as the ratio of the total receptor concentration [R0] and the concentration of agonist receptor complex necessary to produce a half maximal effect (KE), or:
q is the fraction of receptors still available to interact with the agonist following irreversible antagonist treatment and can be obtained by dividing the τ value of clocinnamox treated group with the τ value of control group (Zernig et al., 1996) and can be written as:
Rearranging, we get:
Black and Leff (1983) have proposed an equation for non-rectangular hyperbolic E/[A] curves (where E is the effect and [A] is the agonist concentration) as:
Where E in this case is the mean tail flick latency; Em is the maximum attainable antinociceptive response (10 s); [A] is the dose of hydromorphone, KA is the apparent dissociation constant, ‘n’ is the slope factor of the transducer function and τ is the transducer ratio (as described above). Rearranging the above equation into a semi-logarithmic form (Zernig et al., 1995), adding a new parameter ‘c’ which is the base line response (i.e. the response in the absence of μ-opioid agonist), and expressing τ as
we get:
The dose response curves were simultaneously fit to the above equation, using a nonlinear fitting routine developed by Zernig et al. (1995), and the general mathematical software package Mathematica Wolfram Research, Champaign, USA (Wolfram 1991).
3. Results
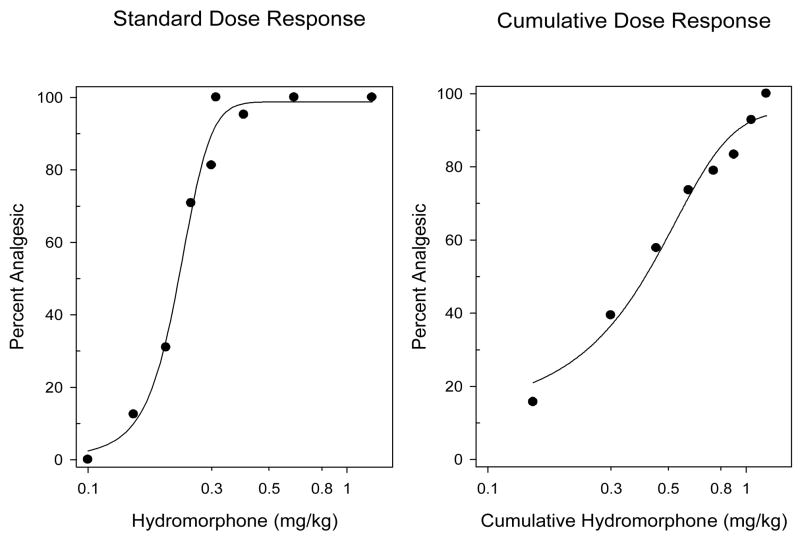
The time of peak analgesic effect for hydromorphone was estimated as 45 min (Fig. 1). Throughout the rest of this study, all testing was conducted at 45 min following hydromorphone administration. ED50 values for hydromorphone were determined using standard and cumulative dose response protocols (Fig. 2). The mean ED50 (95% CL) for the standard dosing protocol was estimated as 0.22 mg/kg (0.20–0.24 mg/kg), while that for the cumulative dosing protocol was estimated as 0.37mg/kg (0.31–0.43 mg/kg).
Fig. 1.
Mice (N=5–9/dose) were injected with hydromorphone (0.2–1.25 mg/kg s.c.) and tested for antinociception (tail flick) at various time points (15–240 min). Each time action profile was determined once except for the 0.3125mg/kg dose which was determined twice and the combined data (mean + S.E.M.) are presented. Hydromorphone’s time of peak analgesic effect was estimated as 45 min.
Fig. 2.
Dose response studies for hydromorphone were performed using standard (left panel) and cumulative dose response protocols (right panel) as described in the methods. For the standard dose response protocol, individual groups of mice (N=5–8/dose) were injected with a single dose of hydromorphone s.c. and tested for antinociception 45 min later. For the cumulative dose response protocol, mice (N=8) were injected with a starting dose of hydromorphone s.c. and tested for antinociception 45 min later. Mice that were not analgesic (i.e., tail flick latency< 10 s), were injected with another dose (see Methods) and retested. This cumulative dosing was continued until all mice were analgesic. The data presented are the combined results of five independent experiments for both cumulative and standard dosing protocols .The mean ED50 (95% CL) for the standard dosing experiments was 0.22 mg/kg (0.20–0.24 mg/kg). The mean ED50 (95% CL) for the cumulative dosing experiments was 0.37 mg/kg (0.31–0.43 mg/kg).
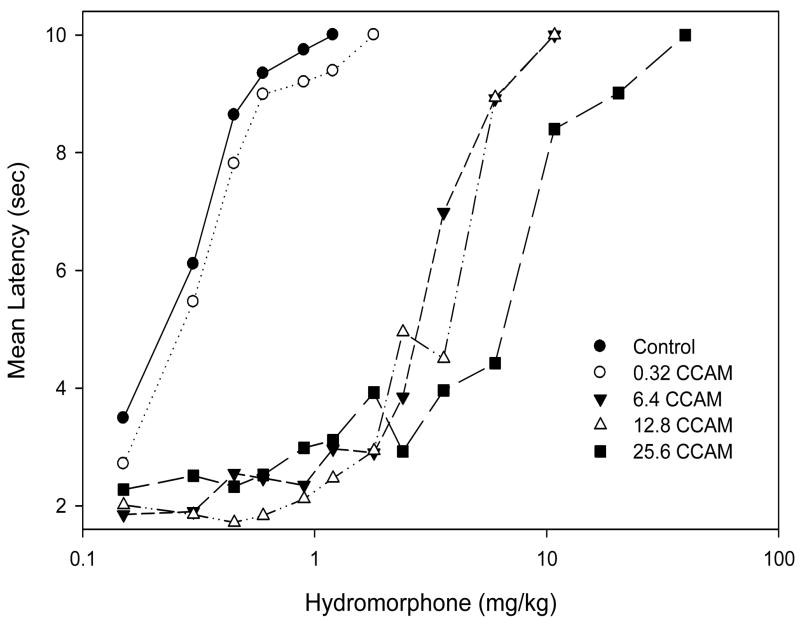
In order to estimate hydromorphone efficacy, mice were injected i.p. with clocinnamox (0.32, 6.4, 12.8, 25.6mg/kg) or saline and 24 h later cumulative hydromorphone dose response studies were conducted. There was a dose-dependent shift to the right for hydromorphone cumulative dose response functions following clocinnamox (Fig. 3). The EC50’s (95% CL) for hydromorphone following clocinnamox increased in a dose-dependent manner (Table 1). As determined by the operational model of agonism, increasing doses of clocinnamox produced a dose-dependent reduction in the q value, which is the fraction of receptors available for an agonist to interact with after irreversible antagonist treatment (Table 2). The apparent efficacy (t) value for hydromorphone was estimated as 35 (34–36, 95% CL); suggesting that hydromorphone is a relatively low efficacy agonist for analgesia (see discussion).
Fig. 3.
The effect of clocinnamox (CCAM) treatment on the analgesic potency of hydromorphone. Mice (N=6–10/treatment) were injected i.p. with CCAM or saline (control) and 24h later hydromorphone cumulative dose response studies (tail flick) were conducted. The data for control represent the mean of three experiments, while the CCAM treatment results are from one experiment for each dose. The data plotted are the mean tail flick latency as a function of cumulative dose. EC50 estimates were calculated for each treatment (see Table 1).
Table 1.
The analgesic EC50’s for hydromorphone 24 hr following CCAM treatment
| CCAM (mg/kg) | EC50 mg/kg (95% CL) |
|---|---|
| Control | 0.30 (0.23–0.39) |
| 0.32 | 0.32 (0.25–0.39) |
| 6.4 | 3.32 (2.77–3.97) |
| 12.8 | 3.77 (3.12–4.55) |
| 25.6 | 7.76 (6.35–9.50) |
The effect of clocinnamox (CCAM) treatment on the analgesic potency of hydromorphone. The EC50 value for control represents the mean of three experiments, while the CCAM treatment results are from one experiment for each dose.
Table 2.
Calculated q values for hydromorphone following treatment with CCAM
| CCAM (mg/kg) | q (95% CL) |
|---|---|
| 0.32 | 0.87 (0.82–0.91) |
| 6.4 | 0.11 (0.10–0.12) |
| 12.8 | 0.10 (0.09–0.11) |
| 25.6 | 0.07 (0.07–0.08) |
The effect of clocinnamox (CCAM) treatment on the q value for hydromorphone. The calculated q value represents the fraction of receptors available for an agonist to interact with μ-opioid receptors after CCAM treatment. q is determined as: τtreated / τcontrol (Zernig et al., 1996), see methods.
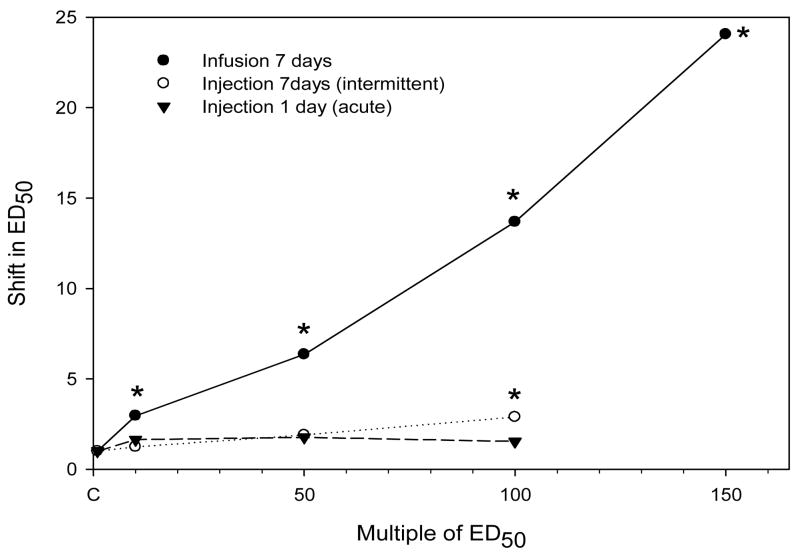
Next, the effect of chronic infusion or injection with hydromorphone on morphine’s analgesic potency was determined. Groups of mice were infused s.c. with hydromorphone (2.1–31.5mg/kg/day; ≈10–150 times the ED50) for 7 days. Pumps were removed at the end of treatment and 16 h later, a morphine cumulative dose response study was performed. Other groups of mice were injected s.c. with hydromorphone (2.2-22 mg/kg/day ≈10–100 times the ED50) once, or intermittently every 24 h for 7 days; and 24 h after the last injection, all mice were tested in morphine cumulative dosing studies. There was substantially more tolerance with infusion treatment compared to injection treatment (Fig. 4 and Table 3). All infusion treatments produced significant tolerance (p<0.05) whereas in injection studies, only the 7 day intermittent treatment at the highest hydromorphone dose produced significant tolerance.
Fig. 4.
The effect of hydromorphone treatment on morphine analgesic potency. For infusion studies, mice (N=5–8/dose) were infused for 7 days with hydromorphone (2.1, 10.5, 21, 31.5mg/kg/day; which is equivalent to ≈10 – 150 times the ED50 for hydromorphone). Controls (C) were implanted with placebo pellets. Pumps and pellets were removed at the end of treatment and 16hr later, a morphine cumulative dose response (tail flick) study was conducted. All infusion results are from one determination, except the data for 21 mg/kg/day which are the combined data from two experiments. For acute (one s.c. injection) and intermittent (7 daily s.c. injections) studies, mice (N=8–10/dose) were injected with hydromorphone (2.2–22mg/kg/day; equivalent to ≈10–100 times the ED50). Controls were injected with saline. Morphine cumulative dose response (tail flick) studies were conducted 24 hr after the last treatment. The data for acute and intermittent experiments represent the mean of three experiments. The shift in the ED50 relative to each individual control is presented as a function of the multiple of ED50 for hydromorphone (0.22mg/kg) as determined using the standard dosing protocol. (see Table 3). * significantly different from control (P<0.05).
Table 3.
Tolerance following treatment with hydromorphone
| Infusion
|
Injection
|
|||
|---|---|---|---|---|
| Shift in ED50 (±SEM) |
||||
| (Multiple of ED50) | Shift in ED50 (±se) | (Multiple of ED50) | 1 Day | 7 Days |
| 10 | 3.0 a (± 0.8) | 10 | 1.6 (± 0.1) | 1.2 (± 0.1) |
| 50 | 6.3 a (± 0.8) | 50 | 1.8 (±0.2) | 1.9 (± 0.5) |
| 100 | 13.7 a (± 0.9) | 100 | 1.5 (±0.3) | 2.9 a (± 0.6) |
| 150 | 24.1 a (± 0.8) | |||
Estimation of morphine tolerance following 7 day continuous infusion (left), and 1 or 7 day injections (right) with hydromorphone. At the end of hydromorphone treatment, morphine cumulative dose response studies were performed (see methods). Infusion studies present the shift in the ED50 (±se from Probit analysis) and are from a single determination, except for 100 times the ED50, which is the combined data from two experiments. Intermittent studies were repeated three times and the mean (± S.E.M.) is presented.
significantly different from 1.0 (P< 0.05).
Finally, we determined the effect of chronic infusion (≈150 times the ED50) and 7 day injection (≈100 times the ED50) with hydromorphone on μ-opioid receptor density in mouse spinal cord. Only the highest treatment dose of hydromorphone was examined for both intermittent and infusion protocols. Hydromorphone did not produce any significant changes in μ-opioid receptor density or ligand affinity following either treatment (Fig. 5).
Fig. 5.
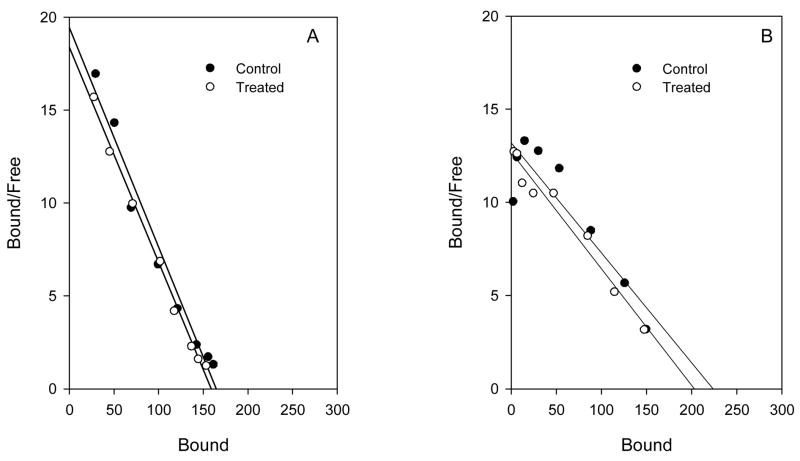
The effect of hydromorphone treatment on μ-opioid receptor density in mouse spinal cord. Panel A. Mice (N = 10) were injected s.c. for 7 days with hydromorphone, (22mg/kg/day; equivalent to ≈100 times the standard dose ED50). Controls (N = 10) were injected with saline. Twenty-four h after the last injection mice were sacrificed and spinal cords collected for saturation binding studies ([3H] DAMGO). The Bmax (95% CL) was 171 fmol/mg protein (163–179) and 161 (156–166) for control and hydromorphone groups, respectively. KD ‘s (95% CL) for control and treated groups were 1.0nM (0.8–1.2) and 1.0 (0.8–1.0), respectively. Similar results were found in 2 other experiments. Panel B. Mice (N=10) were infused s.c. with hydromorphone (31.5mg/kg/day; equivalent to ≈150 times the standard dose ED50) for 7 days. Controls (N=10) were implanted with placebo pellets. Pumps and pellets were removed at the end of treatment, and 16 h later, mice were sacrificed and spinal cords collected for ([3H]DAMGO) saturation binding studies. The Bmax (95% CL) was 191 fmol/mg protein (182–200) and 193 (181–206), for control and hydromorphone groups, respectively. KD’s (95% CL) for control and treated groups were 1.2nM (1.1–1.4) and 1.5 (1.2–1.7), respectively. Similar results were found in 2 other experiments. There were no significant differences (P > 0.05) between the groups for Bmax or KD for either treatment.
4. Discussion
It has been proposed that the magnitude of tolerance produced by opioid agonists after chronic infusion treatment is related to the analgesic efficacy of the agonist (e.g., Duttaroy and Yoburn, 1995; Pawar et al., 2007). Continuous infusions of lower analgesic efficacy opioid agonists (e.g., morphine, oxycodone) produce more tolerance compared to higher efficacy opioid agonists (e.g., etorphine) when these drugs are administered at equi-analgesic doses (Duttaroy and Yoburn, 1995; Paronis and Holtzman, 1992; Stevens and Yaksh, 1989; Walker and Young, 2001; Pawar et al., 2007). Regulation of μ-opioid receptor density is also related to analgesic efficacy, since higher efficacy agonists, but not lower efficacy agonists, are associated with μ-opioid receptor internalization and downregulation both in vivo and in vitro (Keith et al., 1996, 1998; Patel et al., 2002; Stafford et al., 2001; Yoburn et al., 2004; however see Haberstock-Debic et al., 2005).
To date, quantitative estimates of analgesic efficacy (i.e., τ ) have been confined to a relatively limited group of opioid agonists (e.g., Pawar et al., 2007; Pitts et al., 1998; Walker et al., 1998; Zernig et al., 1995). Furthermore, there are few studies directly examining the relationship between analgesic τ values, in vivo tolerance and μ-opioid receptor regulation (Pawar et al., 2007). In the current study, the analgesic efficacy of hydromorphone was estimated using the irreversible μ-opioid antagonist clocinnamox and the operational model of agonism (Black and Leff, 1983). Clocinnamox treatment produced a dose-dependent rightward shift in the analgesic dose response function for hydromorphone (Table 1, Fig. 3). The calculated τ value for hydromorphone was 35 which is similar to that for other lower analgesic efficacy opioid agonists such as oxycodone (τ = 20) and morphine (τ = 39) (Pawar et al., 2007); and lower than the τ value for etorphine (52), a high analgesic efficacy opioid. The characterization of hydromorphone as lower analgesic efficacy agrees with another report that used dose response studies and comparative Emax values in an in vitro assay (Peckham and Traynor, 2006). Taken together, these results indicate that hydromorphone, like oxycodone and morphine, is a lower efficacy agonist.
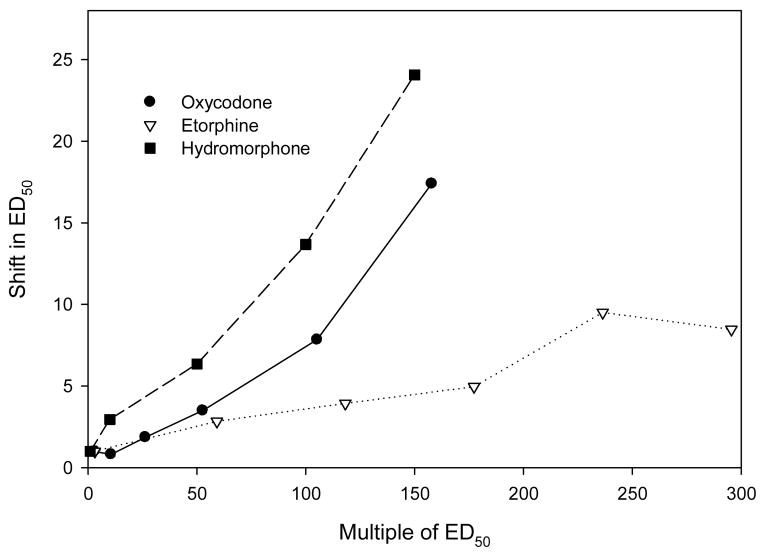
Based on previous studies (e.g., Duttaroy and Yoburn, 1995; Paronis and Holtzman, 1992; Stevens and Yaksh, 1989; Walker and Young, 2001) suggesting that opioids with lower analgesic efficacy produce more tolerance following equi-analgesic infusions, we hypothesized that hydromorphone infusion would induce more tolerance than higher efficacy agonists. Infusions of hydromorphone produced dose-dependent tolerance (Fig. 4, Table 3). When the magnitude of tolerance following hydromorphone infusion was compared to previously published data (Pawar et al., 2007), hydromorphone behaved similarly to that of another low efficacy opioid (oxycodone; Fig 6). Tolerance to morphine following hydromorphone infusions was substantially greater than that for equi-effective infusions of a higher efficacy agonist etorphine (Fig. 6). It was anticipated that since hydromorphone is a lower efficacy analgesic agonist that infusions would not downregulate μ-opioid receptor density. This was found to be the case (Fig. 5). Another study using cell culture has shown that hydromorphone was ineffective in inducing receptor internalization, which is an initial step in downregulation (Koch et al., 2005). The lack of hydromorphone induced μ-opioid receptor regulation contrasts with the substantial downregulation observed following etorphine treatment (Keith et al., 1996, 1998; Patel et al., 2002; Stafford et al., 2001; Yoburn et al., 2004; however see Haberstock-Debic et al., 2005). Overall, these results are consistent with our suggestion that analgesic efficacy (τ) can predict the magnitude of tolerance and μ-opioid receptor regulation.
Fig. 6.
The effect of chronic (7 day) infusion with hydromorphone, oxycodone and etorphine on morphine analgesic potency. Oxycodone and etorphine data are from Pawar et al., 2007 using a treatment protocol identical to that used for hydromorphone. The shift in ED50 relative to control was calculated as: ED50 treated / ED50 control.
A previous report from our lab raised the possibility that acute or intermittent administration of opioid agonists might have different effects from continuous treatment (Duttaroy and Yoburn, 1995; Yoburn et al., 1993). These studies suggested that intermittent treatment produces minimal tolerance that is generally unrelated to estimated analgesic efficacy. In support of these prior findings, single day and 7 day intermittent treatment (Fig. 4, Table 3) produced no significant tolerance, except for 7 day intermittent treatment with ≈100 times the ED50 hydromorphone dose. In radioligand binding studies, there was no significant regulation of μ-opioid receptors following intermittent treatment (Fig. 5). Overall, the magnitude of tolerance appeared to be dramatically reduced following acute and intermittent treatment compared to infusions. This is particularly striking since the total drug delivered was the same for animals treated intermittently or infused for 7 days with ≈10–100 times the ED50 (Fig. 4). Thus, tolerance was maximal following continuous infusion, an observation that may be important clinically in treating chronic pain and minimizing tolerance.
The ED50 for hydromorphone in the tail-flick assay was estimated using standard and cumulative dosing protocols. The standard dosing protocol is the most common and accurate way to estimate the ED50. Cumulative dosing greatly limits the number of animals required and reduces the need for costly drugs with limited availability (e.g., clocinnamox). On the other hand, the ED50 determined by cumulative dosing is dependent on the dosing schedule (starting dose and increment doses) and can vary if different doses are used (Duttaroy et al., 1997). Therefore, we used the standard ED50 value in this and previous studies (Duttaroy and Yoburn, 1995; Pawar et al., 2007) as the metric for determining equi-effective treatments (multiple of the ED50; see Fig. 4 and 6). This provides a relatively straight-forward comparison of dosing among various opioids. In addition, tolerance following hydromorphone treatment was examined using morphine, which is primarily a μ-opioid receptor agonist that lacks activity in μ-opioid receptor knock out mice (Kieffer, 1999). By using morphine to assess tolerance, tolerance to various opioid agonists can be directly compared.
Overall, the current data indicate that hydromorphone has relatively low analgesic efficacy and produces more tolerance following chronic infusion at equi-effective doses relative to a higher analgesic efficacy opioid such as etorphine (Fig. 6). Interestingly, intermittent hydromorphone treatment produced little tolerance following a single injection or after 7 days treatment. The mechanisms that mediate these effects are not clear, but it is possible that chronic infusion treatment with low efficacy agonists desensitizes more receptors since a low efficacy agonist must occupy more receptors to produce an equivalent effect to that of a higher efficacy agonist (Zimmerman et al., 1987; Adams et al., 1990; Comer et al., 1992; Pawar et al., 2007). Furthermore, it has been proposed that higher efficacy agonists can induce internalization, but lower efficacy agonists are unable to induce internalization, which may reduce the recycling of the resensitized receptors, and this may result in more tolerance (Martini and Whistler, 2007; Koch et al., 1998). The fact that chronic infusion treatment produces more tolerance compared to chronic intermittent treatment may be related to phasic activation of μ-opioid receptors which may less efficiently engage substrates responsible for tolerance. These data also raise the possibility that less tolerance might be anticipated clinically with intermittent administration of opioid analgesics compared to treatment options that rely on continuous release formulations and infusions of opioids for pain.
In summary, hydromorphone is a lower analgesic efficacy agonist as determined using a quantitative model to estimate the efficacy parameter τ. Like other lower analgesic efficacy opioid agonists, hydromorphone does not downregulate μ-opioid receptors in vivo and produces more tolerance compared to higher efficacy opioid agonists. Furthermore, the schedule of drug administration determines the magnitude of tolerance. When hydromorphone was administered intermittently at the same daily infusion dose, substantially reduced tolerance was observed. These data indicate that analgesic efficacy and treatment protocol are important determinants of opioid tolerance.
Acknowledgments
The authors are grateful to Dr. M.T. Turnock who provided continuous encouragement during this study. Quiyu Zhang provided technical support. Priyanka Madia reviewed the manuscript and provided helpful comments. Supported in part by a grant from the National Institute on Drug Abuse (DA 19959, to BCY).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JU, Paronis CA, Holtzman SG. Assessment of relative intrinsic activity of mu–opioid analgesics in vivo by using beta-funaltrexamine. J Pharmacol Exp Ther. 1990;255:1027–1032. [PubMed] [Google Scholar]
- Black JW, Leff P. Operational models of pharmacological agonism. Proc R Soc Lond B Biol Sci. 1983;220:141–162. doi: 10.1098/rspb.1983.0093. [DOI] [PubMed] [Google Scholar]
- Black JW, Leff P, Shankley NP, Wood J. An operational model of pharmacological agonism: the effect of E/[A] curve shape on agonist dissociation constant estimation. Br J Pharmacol. 1985;84:561–571. doi: 10.1111/j.1476-5381.1985.tb12941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A refined and sensitive method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Inciardi JA, Muñoz A. Trends in abuse of Oxycontin and other opioid analgesics in the United States: 2002–2004. J Pain. 2005;6:662–672. doi: 10.1016/j.jpain.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Comer SD, Burke TF, Lewis JW, Woods JH. Clocinnamox: a novel, systemically-active, irreversible opioid antagonist. J Pharmacol Exp Ther. 1992;262:1051–1056. [PubMed] [Google Scholar]
- Duttaroy A, Kirtman R, Farrell F, Phillips M, Philippe J, Monderson T, et al. The effect of cumulative dosing on the analgesic potency of morphine in mice. Pharmacol Biochem Behav. 1997;58:67–71. doi: 10.1016/s0091-3057(96)00463-7. [DOI] [PubMed] [Google Scholar]
- Duttaroy A, Yoburn BC. The effect of intrinsic efficacy on opioid tolerance. Anesthesiology. 1995;82:1226–1236. doi: 10.1097/00000542-199505000-00018. [DOI] [PubMed] [Google Scholar]
- Finney DJ. Probit Analysis. 3. Cambridge University Press; London: 1973. [Google Scholar]
- Galandrin S, Bouvier M. Distinct signaling profiles of beta1 and beta2 adrenergic receptor ligands toward adenylyl cyclase and mitogen-activated protein kinase reveals the pluridimensionality of efficacy. Mol Pharmacol. 2006;70:1575–1584. doi: 10.1124/mol.106.026716. [DOI] [PubMed] [Google Scholar]
- Haberstock-Debic H, Kim KA, Yu YJ, von Zastrow M. Morphine promotes rapid, arrestin-dependent endocytosis of μ-opioid receptors in striatal neurons. J Neurosci. 2005;25:7847–7857. doi: 10.1523/JNEUROSCI.5045-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith DE, Anton B, Murray SR, Zaki PA, Chu PC, Lissin DV, Monteillet-Agius G, Stewart PL, Evans CJ, von Zastrow M. mu-Opioid receptor internalization: opiate drugs have differential effects on a conserved endocytic mechanism in vitro and in the mammalian brain. Mol Pharmacol. 1998;53:377–384. [PubMed] [Google Scholar]
- Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kang L, Evans CJ, von Zastrow M. Morphine activates opioid receptors without causing their rapid internalization. J Biol Chem. 1996;271:19021–19024. doi: 10.1074/jbc.271.32.19021. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Collateral efficacy in drug discovery: taking advantage of the good (allosteric) nature of 7TM receptors. Trends Pharmacol Sci. 2007;28:407–415. doi: 10.1016/j.tips.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Drug efficacy at G protein-coupled receptors. Annu Rev Pharmacol Toxicol. 2002;42:349–379. doi: 10.1146/annurev.pharmtox.42.091401.113012. [DOI] [PubMed] [Google Scholar]
- Kieffer BL. Opioids: first lessons from knockout mice. Trends Pharmacol Sci. 1999;20:19–26. doi: 10.1016/s0165-6147(98)01279-6. [DOI] [PubMed] [Google Scholar]
- Koch T, Schulz S, Schröder H, Wolf R, Raulf E, Höllt V. Carboxyl-terminal splicing of the rat mu opioid receptor modulates agonist-mediated internalization and receptor resensitization. J Biol Chem. 1998;273:13652–13657. doi: 10.1074/jbc.273.22.13652. [DOI] [PubMed] [Google Scholar]
- Koch T, Widera A, Bartzsch K, Schulz S, Brandenburg LO, Wundrack N, Beyer A, Grecksch G, Höllt V. Receptor endocytosis counteracts the development of opioid tolerance. Mol Pharmacol. 2005;67:280–287. doi: 10.1124/mol.104.004994. [DOI] [PubMed] [Google Scholar]
- Kumar MG, Lin S. Hydromorphone in the Management of Cancer-Related Pain: An Update on Routes of Administration and Dosage Forms. J Pharm Pharmaceut Sci. 2007;10:504–518. doi: 10.18433/j3vc75. [DOI] [PubMed] [Google Scholar]
- Leff P, Prentice DJ, Giles H, Martin GR, Wood J. Estimation of agonist affinity and efficacy by direct, operational model-fitting. J Pharmacol Methods. 1990;23:225–237. doi: 10.1016/0160-5402(90)90066-t. [DOI] [PubMed] [Google Scholar]
- Martini L, Whistler JL. The role of mu opioid receptor desensitization and endocytosis in morphine tolerance and dependence. Curr Opin Neurobiol. 2007;17:556–564. doi: 10.1016/j.conb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Murray A, Hagen NA. Hydromorphone. J Pain Sympt Manag. 2005;29:S57–66. doi: 10.1016/j.jpainsymman.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Paronis CA, Holtzman SG. Development of tolerance to the analgesic activity of mu agonists after continuous infusion of morphine, meperidine or fentanyl in rats. J Pharmacol Exp Ther. 1992;262:1–9. [PubMed] [Google Scholar]
- Patel MB, Patel CN, Rajashekara V, Yoburn BC. Opioid agonists differentially regulate mu-opioid receptors and trafficking proteins in vivo. Mol Pharmacol. 2002;62:1464–1470. doi: 10.1124/mol.62.6.1464. [DOI] [PubMed] [Google Scholar]
- Pawar M, Kumar P, Sunkaraneni S, Sirohi S, Walker EA, Yoburn BC. Opioid agonist efficacy predicts the magnitude of tolerance and the regulation of μ-opioid receptors and dynamin-2. Eur J Pharmacol. 2007;563:92–101. doi: 10.1016/j.ejphar.2007.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham EM, Traynor JR. Comparison of the antinociceptive response to morphine and morphine-like compounds in male and female Sprague-Dawley rats. J Pharmacol Exp Ther. 2006;316:1195–1201. doi: 10.1124/jpet.105.094276. [DOI] [PubMed] [Google Scholar]
- Pitts RC, Allen RM, Walker EA, Dykstra LA. Clocinnamox antagonism of the antinociceptive effects of mu opioids in squirrel monkeys. J Pharmacol Exp Ther. 1998;285:1197–1206. [PubMed] [Google Scholar]
- Stafford K, Gomes AB, Shen J, Yoburn BC. mu-opioid receptor downregulation contributes to opioid tolerance in vivo. Pharmacol Biochem Behav. 2001;69:233–237. doi: 10.1016/s0091-3057(01)00525-1. [DOI] [PubMed] [Google Scholar]
- Stevens CW, Yaksh TL. Potency of infused spinal antinociceptive agents is inversely related to magnitude of tolerance after continuous infusion. J Pharmacol Exp Ther. 1989;250:1–8. [PubMed] [Google Scholar]
- Walker EA, Young AM. Differential tolerance to antinociceptive effects of mu opioids during repeated treatment with etonitazene, morphine, or buprenorphine in rats. Psychopharmacology (Berl) 2001;154:131–142. doi: 10.1007/s002130000620. [DOI] [PubMed] [Google Scholar]
- Walker EA, Zernig G, Young AM. In vivo apparent affinity and efficacy estimates for mu opiates in a rat tail-withdrawal assay. Psychopharmacology (Berl) 1998;136:15–23. doi: 10.1007/s002130050534. [DOI] [PubMed] [Google Scholar]
- Whistler JL, Chuang HH, Chu P, Jan LY, von Zastrow M. Functional dissociation of mu opioid receptor signaling and endocytosis: implications for the biology of opiate tolerance and addiction. Neuron. 1999;23:737– 746. doi: 10.1016/s0896-6273(01)80032-5. [DOI] [PubMed] [Google Scholar]
- Wolfram S. Mathematica. Addison-Wesley Publishing Company, Reading; MA: 1991. A system for doing mathematics by computer. [Google Scholar]
- Yoburn BC, Billings B, Duttaroy A. Opioid receptor regulation in mice. J Pharmacol Exp Ther. 1993;265:314–320. [PubMed] [Google Scholar]
- Yoburn BC, Purohit V, Patel K, Zhang Q. Opioid agonist and antagonist treatment differentially regulates immunoreactive u-opioid receptors and dynamin-2 in vivo. Eur J Pharmacol. 2004;498:87–96. doi: 10.1016/j.ejphar.2004.07.052. [DOI] [PubMed] [Google Scholar]
- Zaki PA, Keith DE, Brine GA, Carroll FI. Ligand-induced changes in surface μ-opioid receptor number: relationship to G protein activation? J Pharmacol Exp Ther. 2000;292:1127–1134. [PubMed] [Google Scholar]
- Zernig G, Issaevitch T, Broadbear JH, Burke TF, Lewis JW, Brine GA, Woods JH. Receptor reserve and affinity of mu opioid agonists in mouse antinociception: correlation with receptor binding. Life Sci. 1995;57:2113–2125. doi: 10.1016/0024-3205(95)02204-v. [DOI] [PubMed] [Google Scholar]
- Zernig G, Issaevitch T, Woods JH. Calculation of agonist efficacy, apparent affinity, and receptor population changes after administration of insurmountable antagonists: comparison of different analytical approaches. J Pharmacol Toxicol Methods. 1996;35:223–237. doi: 10.1016/1056-8719(96)00053-6. [DOI] [PubMed] [Google Scholar]
- Zimmerman DM, Leander JD, Reel JK, Hynes MD. Use of beta-funaltrexamine to determine mu opioid receptor involvement in the analgesic activity of various opioid ligands. J Pharmacol Exp Ther. 1987;241:374–378. [PubMed] [Google Scholar]