Abstract
A ‘binge’ is defined by National Institute on Alcohol Abuse and Alcoholism as an excessive pattern of alcohol drinking that produces blood–alcohol levels (BALs) greater than 0.08 g% within a 2-h period and may or may not be associated with dependence. The purpose of this investigation was to explore the effects of several neuropharmacological agents in an animal model in which outbred rats voluntarily and orally self-administer pharmacologically meaningful alcohol doses that produce BALs ≥ 0.08 g% in daily limited access two-bottle choice and operant drinking sessions. Rats were trained to self-administer either 10% (w/v) alcohol solution sweetened with ‘supersac’ (3% glucose + 0.125% saccharin) or supersac alone versus water in a two-bottle choice or operant situation during 30-min daily sessions. Rats were then injected systemically with multiple doses of duloxetine, naltrexone, and the corticotropin-releasing factor antagonist, MPZP, in Latin-square designs. Alcohol binge drinkers reliably consumed amounts of alcohol sufficient to produce BALs ≥ 0.08 g%. Duloxetine dose-dependently suppressed two-bottle choice alcohol binge drinking and operant alcohol responding as well as operant supersac responding, but did not affect two-bottle choice supersac drinking. Naltrexone-suppressed alcohol binge drinking at very low doses and suppressed supersac drinking at moderate-to-high doses. MPZP did not affect alcohol or supersac consumption. Different profiles for drugs that suppress binge-like alcohol drinking compared with dependence-induced drinking provide a heuristic foundation for future medications development.
Keywords: alcoholism, binge, corticotropin-releasing factor antagonist, duloxetine, MPZP, naltrexone, rat
Introduction
Like all patterns of alcohol abuse, binge drinking is problematic for the individual and for society. A substantial portion of the human population drinks alcohol in a binge pattern (Wechsler and Isaac, 1992; Wechsler et al., 2000), and there are several adverse consequences associated with this alcohol abuse pattern, including the increased risk of developing alcohol dependence following binge-like drinking during adolescence (Hingson et al., 2005, 2007; Miller et al., 2007). Billions of dollars in the United States alone are lost annually owing to problems that stem from alcoholism (Harwood et al., 1998).
A ‘binge’ is defined by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) as a pattern of drinking that produces blood–alcohol levels (BALs) greater than 0.08 g% (i.e., 80 mg%; approved by NIAAA National Advisory Council, 2004). The pattern of drinking required to produce these BALs in the average human is generally accepted to be 4–5 drinks in a 2-h period (NIAAA, 2004). It is worth noting that a binge-drinking pattern is fundamentally different from NIAAA definitions of both ‘risky’ drinking (< 0.08 g% BALs) and a ‘bender’ (at least 2 days of sustained heavy drinking).
A procedure that has long been used to promote ethanol drinking by rats is the addition of sweeteners to the ethanol solution. This animal model, unlike many others, contains construct validity for the human condition because humans tend to drink alcohol in a sweetened form (Gilbert, 1978; Samson et al., 1996), especially early in the development of alcohol abuse/alcohol dependence when consumption patterns are reflective of the NIAAA definition of binge drinking (NIAAA, 2004). The addition of sweeteners (usually sucrose or saccharin) to the ethanol solution produces increase in consumption by adult rats relative to ethanol alone in water (Samson, 1986; Rassnick et al., 1992; Doremus et al., 2005; Truxell et al., 2007) and sweeteners (at specific concentrations) alone in water (Samson et al., 1996). Historically, however, studies employing sweetened ethanol have also failed to reliably produce BALs (0.08 g%) determined by NIAAA to be the defining factor in binge alcohol drinking (e.g., Doremus et al., 2005; Truxell et al., 2007) or the studies have not measured BALs (e.g., Samson et al., 1996).
The present investigation sought to test several neuropharmacological compounds, selected for their different mechanisms of action and behavioral profiles, in a rat model of binge-like alcohol drinking. Duloxetine is a mixed selective serotonin/norepineprhine reuptake inhibitor (SSNRI) that has powerful effects in facilitating serotonin neurotransmission (Westanmo et al., 2005) and produces robust reductions in operant alcohol responding by both alcohol-dependent and nondependent rats (unpublished findings). Naltrexone is an opioid antagonist that is prescribed clinically as a treatment for alcoholism (Heilig and Egli, 2006). In rats, naltrexone suppresses alcohol drinking (e.g., Altshuler et al., 1980; Reid and Hunter, 1984; Walker and Koob, 2007), and this effect is exaggerated in rats selectively bred for high alcohol preference [Sardinian alcohol-preferring (sP) rats; Sabino et al., 2006]. Finally, a corticotropin-releasing factor type 1 (CRF1) receptor antagonist, N,N-bis(2-methoxyethyl)-3-(4-methoxy-2-methylphenyl)-2,5-dimethyl-pyrazolo [1,5a]pyrimidin-7-amine (MPZP) was tested. CRF1-receptor antagonists robustly suppress dependence-induced increases in alcohol drinking without affecting alcohol drinking by nondependent animals (Funk et al., 2006, 2007). More specifically, dependence-induced increases in drinking are blocked by the particular antagonist (MPZP) used in this study (Richardson et al., 2007).
The purpose of the present investigation was to explore pharmacological challenges in an animal model of excessive drinking with face validity for binge drinking in humans. Outbred rats achieve binge-like drinking without the use of food or water deprivation, and rats voluntarily and orally self-administer pharmacologically relevant alcohol doses capable of producing BALs that reliably exceed 0.08 g% in daily limited access two-bottle choice and operant drinking sessions. The overall hypothesis was that binge-like alcohol drinking (both operant and two-bottle choice) would be sensitive to compounds that suppress drinking motivated by the positive reinforcing effects of alcohol, but not compounds that suppress drinking motivated by alcohol dependence-related factors. It was hypothesized that (i) duloxetine would suppress binge-like drinking of alcohol but not a sweetened solution, (ii) naltrexone would suppress binge-like drinking of alcohol and, possibly, a sweetened solution, and (iii) MPZP would not affect binge-like drinking of either alcohol or a sweetened solution.
Methods
Subjects
Twelve adult male Wistar rats obtained from Charles River (Kingston, New York, USA) were used in Experiment 1 and 24 adult male Wistar rats were used in Experiment 2. Animals weighed between 250 and 320 g at the start of the experiment and 522 and 567 g at the end of the experiment (body weights were not significantly different between the two groups at any point of the study; data not shown). Animals were single-housed in standard plastic cages with wood chip bedding under a 12 h light/12 h dark cycle (lights off at 10.00 h in Experiment 1; lights off at 08.00 h in Experiment 2). Animals were given free access to food and water throughout except during experimental drinking sessions. All procedures were conducted in the dark cycle and met the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experiment 1
Two-bottle choice oral self-administration
Rats were trained to self-administer ‘supersac’ (3% glucose + 0.125% saccharin; Valenstein et al., 1967) or 10% (w/v) ethanol solution sweetened with supersac, versus water in a two-bottle choice home cage situation during 30-min daily sessions that occurred 4 h into the dark cycle. Food was removed from the lid of the home cage and the rats were weighed immediately prior to drinking sessions. Bottles were attached to cage lids with stainless steel springs to reduce spilling. The positions of the bottles were alternated daily to avoid a possible side preference. All bottles were weighed immediately before and after 30-min drinking sessions. Differences in bottle weights were converted to volume intakes by accounting for solution densities as follows: supersac volume (ml) = weight (g)/1.0118; and 10% ethanol + supersac volume (ml) = weight (g)/0.9868.
Development of binge-like self-administration
Animals were divided into alcohol binge drinkers (n = 6) and supersac controls (n = 6). Prior to two-bottle choice training, all rats were given an initial 2-h training session during which they were allowed to drink supersac in a single-bottle situation. All rats acquired the behavior during this initial training session, and two-bottle choice training started the following day. Prior to pharmacological manipulations, animals in the alcohol binge group were allowed 30-min drinking sessions for 9 consecutive days whereas supersac controls were allowed 30-min drinking sessions for 8 consecutive days.
Duloxetine dose–response test
Duloxetine is highly absorbed and extensively metabolized in rats and it readily crosses the blood–brain barrier (Bymaster et al., 2005). Rats were injected intraperitoneally (i.p.) with duloxetine (0, 2, 4, 8 mg/kg) 40 min before (doses and pretreatment time chosen based on preliminary data in which duloxetine effectively blocked ethanol responding by alcohol-dependent and nondependent rats) two-bottle choice test sessions in a within-subjects (rats) Latin-square design. Rats were allowed to self-administer every day during this time but were injected every other day. Rats were injected in the colony room and returned to the home cage immediately thereafter for the 40-min interim period.
Naltrexone dose–response test
Naltrexone (0, 16, 50, 150, 450 μg/kg), which readily crosses the blood–brain barrier, was injected subcutaneously into rats 30 min before (doses and pretreatment times chosen based on Sabino et al., 2006) two-bottle choice test sessions in a within-subjects Latin-square design. Rats were allowed to self-administer every day during this time but were injected every other day. Rats were injected in the colony room and returned to the home cage immediately thereafter for the 30-min interim period.
MPZP dose–response test
MPZP is a small molecule, non-peptide CRF1-receptor selective antagonist that readily crosses the blood–brain barrier. Rats were injected ssubcutaneously with MPZP (0, 5, 10, 20 mg/kg) 60 min prior to two-bottle choice test sessions in a within-subjects (rats) Latin-square design. These doses and pretreatment times were chosen because these parameters were used in a study that showed the ability of MPZP to suppress anxiety-like behavior and dependence-induced alcohol drinking in rats (Richardson et al., in press). Rats were allowed to self-administer every day during this time but were injected every other day. Rats were injected in the colony room and returned to the home cage immediately thereafter for the 30-min interim period.
Experiment 2
Operant ethanol self-administration
Operant ethanol self-administration was conducted in standard operant chambers (Coulbourn Instruments, Allentown, Pennsylvania, USA) housed in sound-attenuated ventilated cubicles. Animals were trained to orally self-administer ethanol or water in a concurrent, two-lever, free-choice contingency during daily 30-min sessions that occurred at the start of the dark cycle. Syringe pumps (Razel Scientific Instruments, Stamford, Connecticut, USA) dispensed ethanol or water into two stainless steel drinking cups mounted 4.0 cm above the grid floor in the middle of one side panel. Two retractable levers were located 4.5 cm to either side of the drinking cups. Fluid delivery and recording of operant self-administration were controlled by a standard PC computer. Lever presses were not recorded during the 0.5 s in which the pumps were active. A continuous reinforcement (fixed ratio-1) schedule was used such that each response resulted in delivery of 0.1 ml of fluid. Fluid delivery and recording of operant responding were controlled by a microcomputer. All rats were weighed prior to drinking sessions twice per week.
Development of operant binge-like self-administration
On a single initial training day, all rats were trained to press a lever for supersac in a single-lever situation for 2 h. Then all rats underwent 5 days of 30-min training sessions with two levers available (supersac versus water). All rats successfully learned the lever-pressing contingency during these training sessions. Rats were then divided into alcohol binge (n = 12) and supersac control (n = 12) groups, matched for responding during the operant training phase. Rats were then allowed 17 consecutive days of operant self-administration in which alcohol binge rats were allowed to press lever for sweetened ethanol (10% w/v ethanol in supersac) versus water, whereas supersac controls were still allowed to press lever for supersac versus water in a two-lever situation.
Duloxetine dose–response test
Rats were injected intraperitoneally with duloxetine (0, 2, 4, 8 mg/kg) 40 min before (pretreatment time chosen based on preliminary data) operant test sessions in a within-subjects Latin-square design. Rats were allowed to self-administer every day during this time but were injected every 3 to 4 days.
Naltrexone dose–response test
Rats were injected subcutaneously with naltrexone (0, 16, 50, 150, 450 μg/kg) 30 min before operant test sessions in a within-subjects Latin-square design. Rats were allowed to self-administer every day during this time but were injected every 3 to 4 days.
MPZP dose–response test
Rats were injected subcutaneously with MPZP (0, 5, 10, 20 mg/kg) 60 min before operant sessions in a within-subjects Latin-square design. Rats were allowed to self-administer every day during this time but were injected every 3 to 4 days.
Drugs
Duloxetine (LY248686) is a SSNRI. Naltrexone hydrochloride (Sigma-Aldrich, St Louis, Missouri, USA) is a nonselective opioid receptor antagonist. MPZP is an antagonist of CRF1 receptors. All drugs were administered systemically. Duloxetine hydrochloride (Cymbalta, Eli lilly and Co., Indianapolis, Indiana, USA) was acquired in enteric-coated pellets that were crushed using a mortar and pestle, suspended in 1 mol/l HCl (10% of total volume), then diluted with 20% (w/v) 2-hydroxypropyl-β-cyclodextrin (Sigma-Aldrich) in distilled water (90% of total volume). MPZP was dissolved in 1 mol/l HCl (10% of total volume), then diluted with 20% (w/v) 2-hydroxypropyl-β-cyclodextrin in distilled water (90% of total volume). Solutions were then back-titrated with NaOH to pH 4.5 (Sabino et al., 2006). Naltrexone HCl was dissolved in saline. The duloxetine suspension was injected intraperitoneally in a volume of 1 ml/kg body weight. Naltrexone hydrochloride solution was administered subcutaneously in a volume of 1 ml/kg body weight. MPZP solution was administered subcutaneously in a volume of 2 ml/kg body weight.
Blood–alcohol level determinations
Tail blood was sampled at the end of two representative baseline drinking sessions. Rats were gently restrained while the tip of the tail (2 mm) was cut with a clean razor blade. Tail blood (0.2 ml) was collected and centrifuged. Plasma (5μl) was used for the measurement of BALs using an Analox AM 1 analyzer (Analox Instruments Ltd, Lunenberg, Massachusetts, USA). The reaction is based on the oxidation of alcohol by alcohol oxidase in the presence of molecular oxygen (alcohol + O2 → acetaldehyde + H2O2). The rate of oxygen consumption is directly proportional to the alcohol concentration. Single-point calibrations were made for each set of samples with reagents provided by Analox Instruments (0.025– 0.400 g%).
Statistical analysis
Solution consumption is expressed as mean ± SEM and normalized for body weight (i.e., g ethanol/kg body weight; ml, supersac/kg body weight). The effects of duloxetine, naltrexone, and MPZP on alcohol (g/kg) intake and supersac (ml/kg) intake, were separately analyzed using a series of one-way repeated-measures analyses of variance, with dose as a within-subjects factor. Post-hoc comparisons were made using the Student Newman–Keuls test. Statistical significance was set at P < 0.05.
Results
Experiment 1
Development of two-bottle choice binge-like self-administration
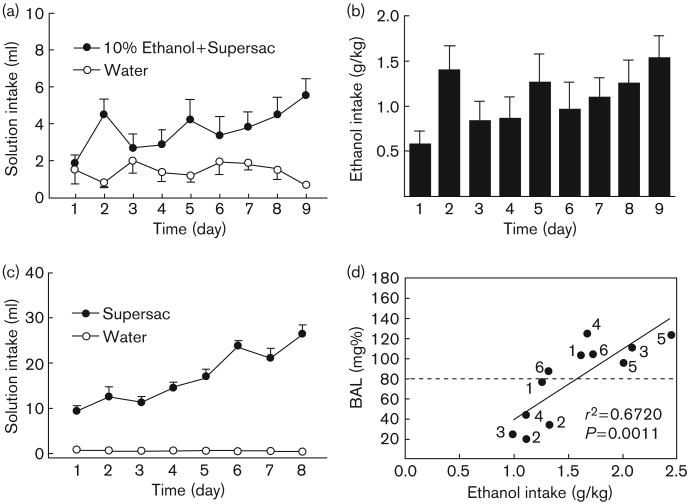
Average intake of sweetened alcohol solution (Fig. 1a) by animals in the alcohol binge group increased from 1.8 ± 0.5 ml (0.58 ± 0.15 g/kg) on day 1 of the baseline period to 5.5 ± 1.0 ml (1.53 ± 0.27 g/kg) on day 9 of the same period (Fig. 1b). Average supersac intake by supersac controls increased from 9.4 ± 1.0 ml on day 1 of the baseline period to 26.6 ± 1.9 ml on the final day of the same period (Fig. 1c). Tail blood samples were collected and BALs determined on two representative drinking days. Five of the six animals in the alcohol binge group achieved BALs of greater than 0.10 gm% on at least one of those 2 days (Fig. 1d). The mean BAL for animals in the alcohol binge group across 2 days was 0.079 g%, the approximate equivalent of the BAL criterion for binge drinking in humans.
Fig. 1.
(a and b) Mean ± SEM intake (ml and g/kg) of sweetened (3% glucose + 0.125% saccharin) 10% (w/v) alcohol solution during the initial 9-day baseline binge period; (c) mean ± SEM intake (ml) of supersac (3% glucose + 0.125% saccharin) by controls during the initial 8-day baseline binge period; and (d) scatter plot of blood–alcohol levels produced by alcohol intake (g/kg) by animals in the alcohol binge group during two representative drinking sessions. Points are individually labeled with rat identification numbers to show that five of the six animals in the alcohol binge group achieved BALs ≥ 0.08 g% during at least one of the two drinking sessions.
Effects of duloxetine on two-bottle choice binge-like drinking
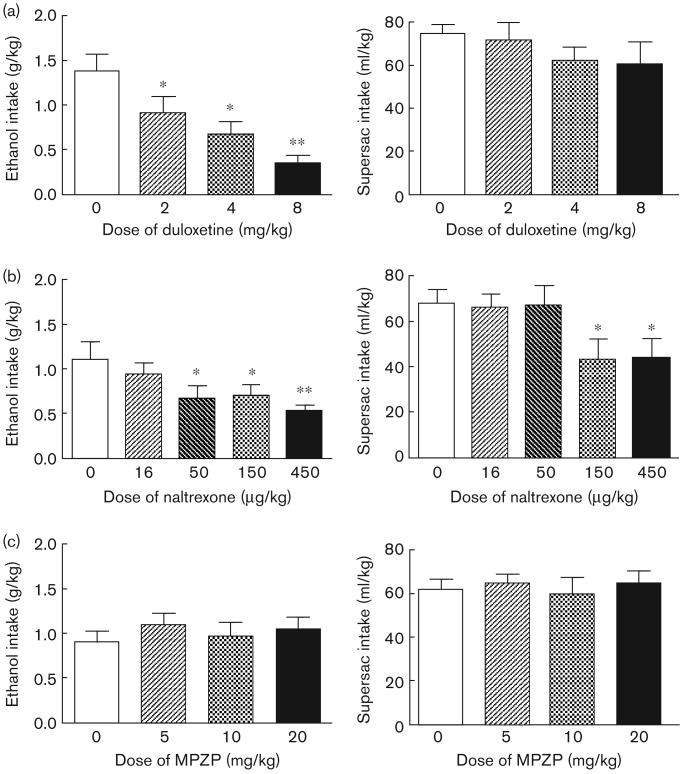
Duloxetine pretreatment dose-dependently reduced intake of sweetened alcohol solution by animals in the alcohol binge group, F(3,15) = 8.48, P < 0.01 (Fig. 2a; left panel). Post-hoc analysis indicated that all doses of duloxetine significantly suppressed binge-like alcohol intake (P < 0.05 in all cases), whereas water intake was not affected (Table 1). Duloxetine administration did not affect supersac intake (Fig. 2a; right panel) or water intake (Table 1) by animals in the supersac group.
Fig. 2.
Mean ± SEM alcohol intake (g/kg) by animals in the alcohol binge group (n = 6; left panel) and supersac intake (ml/kg) by supersac controls (n = 6; right panel) following pretreatment with (a) one of four doses (0, 2, 4, 8 mg/kg) of duloxetine (intraperitoneal injection 40 min prior to drinking session), (b) one of five doses (0, 16, 50, 150, 450 μg/kg) of naltrexone (subcutaneous injection 30 min prior to drinking session), or (c) one of four doses (0, 5, 10, 20 mg/kg) of MPZP (subcutaneous injection 60 min prior to drinking session). *P<0.05, **P<0.001 significant difference from vehicle condition.
Table 1. Effects of duloxetine, naltrexone, and MPZP on water intake, total fluid intake, and preference [ethanol/supersac:total (E/S:T)] for sweetened alcohol solution by animals in the two-bottle choice (experiment 1) alcohol binge group, and of supersac solution by supersac controls.
| Water intake (ml) | Total fluid (ml) | Preference (E/S:T) | |
|---|---|---|---|
| Duloxetine | |||
| Alcohol binge drinkers | |||
| 0 mg/kg | 0.88 ± 0.25 | 6.12 ± 0.98 | 0.85 ± 0.04 |
| 2 mg/kg | 0.72 ± 0.11 | 4.30 ± 0.81 | 0.79 ± 0.05 |
| 4 mg/kg | 0.57 ± 0.05 | 3.22 ± 0.61* | 0.79 ± 0.06 |
| 8 mg/kg | 0.58 ± 0.11 | 2.00 ± 0.38** | 0.67 ± 0.08* |
| Supersac controls | |||
| 0 mg/kg | 0.32 ± 0.06 | 26.77 ± 1.55 | 0.99 ± 0.00 |
| 2 mg/kg | 0.28 ± 0.06 | 26.17 ± 3.92 | 0.99 ± 0.00 |
| 4 mg/kg | 0.22 ± 0.07 | 22.02 ± 2.75 | 0.99 ± 0.00 |
| 8 mg/kg | 0.20 ± 0.04 | 21.02 ± 4.02 | 0.99 ± 0.00 |
| Naltrexone | |||
| Alcohol binge drinkers | |||
| 0 μg/kg | 0.70 ± 0.15 | 5.88 ± 0.92 | 0.86 ± 0.04 |
| 16 μg/kg | 0.87 ± 0.25 | 5.17 ± 0.50 | 0.83 ± 0.05 |
| 50 μg/kg | 1.07 ± 0.55 | 4.13 ± 1.01 | 0.73 ± 0.08 |
| 150 μg/kg | 0.80 ± 0.37 | 4.02 ± 0.60 | 0.80 ± 0.07 |
| 450 μg/kg | 0.87 ± 0.29 | 3.27 ± 0.38* | 0.74 ± 0.09 |
| Supersac controls | |||
| 0 μg/kg | 0.28 ± 0.08 | 32.75 ± 3.56 | 0.99 ± 0.00 |
| 16 μg/kg | 0.33 ± 0.09 | 31.63 ± 3.37 | 0.99 ± 0.00 |
| 50 μg/kg | 0.47 ± 0.21 | 32.10 ± 3.90 | 0.98 ± 0.01 |
| 150 μg/kg | 0.45 ± 0.23 | 21.17 ± 4.71** | 0.95 ± 0.04 |
| 450 μg/kg | 0.23 ± 0.04 | 21.38 ± 4.40** | 0.99 ± 0.00 |
| MPZP | |||
| Alcohol binge drinkers | |||
| 0 mg/kg | 0.45 ± 0.13 | 5.22 ± 0.61 | 0.90 ± 0.04 |
| 5 mg/kg | 0.63 ± 0.24 | 6.30 ± 0.49 | 0.90 ± 0.04 |
| 10 mg/kg | 0.52 ± 0.13 | 5.48 ± 0.84 | 0.89 ± 0.04 |
| 20 mg/kg | 0.58 ± 0.14 | 5.98 ± 0.56 | 0.89 ± 0.03 |
| Supersac controls | |||
| 0 mg/kg | 0.37 ± 0.06 | 35.23 ± 2.39 | 0.99 ± 0.00 |
| 5 mg/kg | 0.33 ± 0.08 | 37.20 ± 3.85 | 0.99 ± 0.00 |
| 10 mg/kg | 0.37 ± 0.07 | 33.68 ± 4.26 | 0.99 ± 0.00 |
| 20 mg/kg | 0.32 ± 0.09 | 36.22 ± 3.20 | 0.99 ± 0.00 |
P<0.05,
P<0.01 relative to vehicle condition.
Effects of naltrexone on two-bottle choice binge-like drinking
Naltrexone pretreatment dose-dependently reduced intake of sweetened alcohol solution by animals in the alcohol binge group, F(4,20) = 4.88, P < 0.01 (Fig. 2b; left panel). Post-hoc analysis revealed that 50, 150, and 450 μg/kg significantly suppressed binge-like alcohol intake (P < 0.05 in all cases). Naltrexone also dose-dependently suppressed supersac intake by supersac controls F(4,20) = 7.20, P < 0.01. Post-hoc analysis revealed that 150 and 450 μg/kg suppressed supersac intake (P < 0.05 in both cases) in those rats (Fig. 2b; right panel). Water intake was not affected in either of the two groups (Table 1).
Effects of MPZP on two-bottle choice binge-like drinking
MPZP pretreatment did not affect consumption of sweetened alcohol solution, supersac, or water by either of the two groups (Fig. 2c and Table 1).
Experiment 2
Development of operant binge-like self-administration
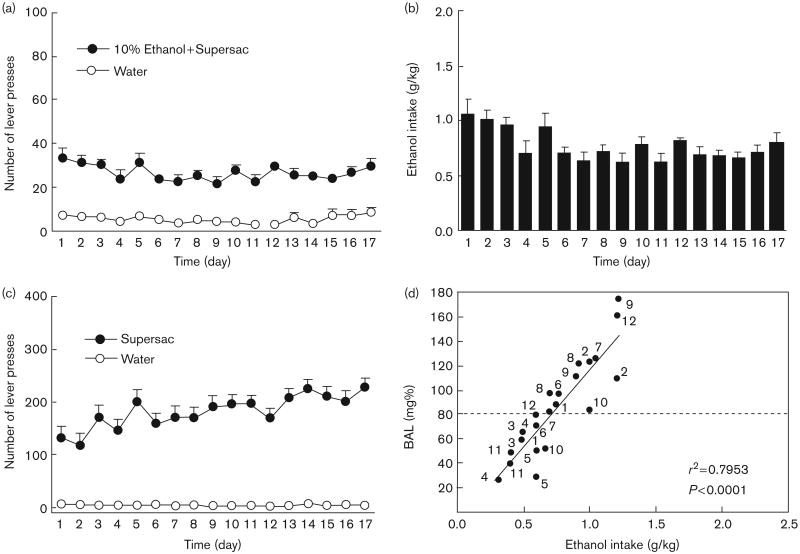
Figure 3 (panels a and b) shows the mean number of daily lever presses and average ethanol intake by animals in the alcohol binge group. Figure 3c shows the average number of lever presses by the supersac control group. Tail blood samples were collected and BALs determined on days 13 and 16 of the baseline period. Eight of the 12 animals in the alcohol binge group achieved BALs of greater than 0.08 g% on at least one of those 2 days (Fig. 3d). The mean BAL for all 12 animals in the alcohol binge group across 2 days was 0.084 g%, which is higher than the BAL criterion for binge drinking in humans.
Fig. 3.
(a) Mean ± SEM number of lever presses for sweetened (3% glucose + 0.125% saccharin) 10% (w/v) alcohol solution, and (b) mean ± SEM intake (g/kg) of sweetened alcohol solution during the initial 17-day baseline binge period; (c) mean ± SEM number of lever presses for supersac (3% glucose + 0.125% saccharin) by controls during the initial 17-day baseline binge period; and (d) scatter plot of blood–alcohol levels produced by alcohol intake (g/kg) by animals in the alcohol binge group during two representative drinking sessions. Points are individually labeled with rat identification numbers to show that 8 of the 12 animals in the alcohol binge group achieved BALs ≥ 0.08 g% during at least one of the two drinking sessions.
Effects of duloxetine on operant binge-like drinking
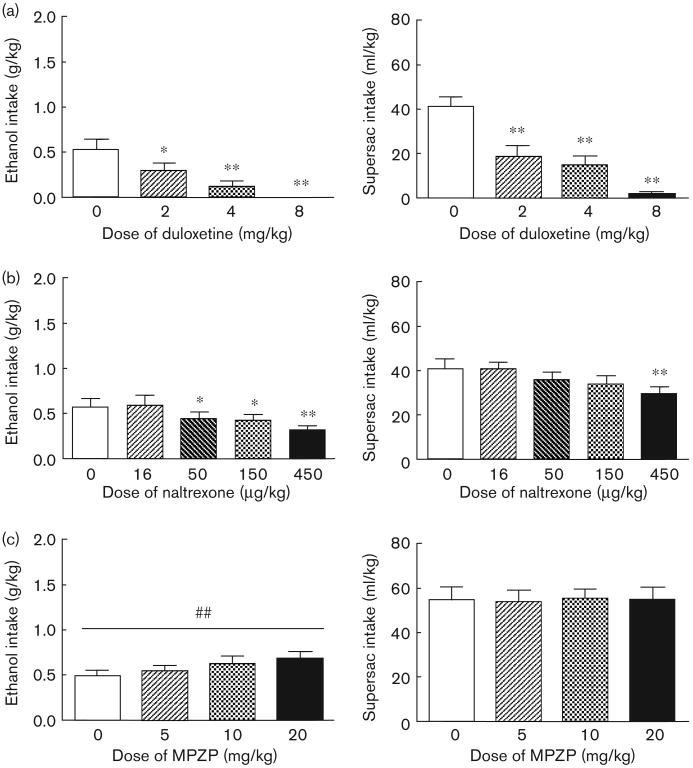
Duloxetine pretreatment dose-dependently reduced intake of sweetened alcohol solution by animals in the alcohol binge group, F(3,33) = 12.62, P < 0.01 (Fig. 4a; left panel). Post-hoc analysis revealed that all doses significantly suppressed binge-like alcohol intake (P < 0.05 in all cases). Duloxetine also suppressed supersac intake by controls F(3,33) = 28.54, P < 0.01. Post-hoc analysis revealed that all doses significantly suppressed supersac intake (P < 0.01 in all cases) in those rats (Fig. 4a; right panel). Water intake was not affected in either of the two groups (Table 2).
Fig. 4.
Mean ± SEM alcohol intake (g/kg) by animals in the alcohol binge group (n = 11; left panel) and supersac intake (ml/kg) by supersac controls (n = 12; right panel) following pretreatment with (a) one of four doses (0, 2, 4, 8 mg/kg) of duloxetine (intraperitoneal injection 40 min prior to drinking session), (b) one of five doses (0, 16, 50, 150, 450 μg/kg) of naltrexone (s.c. injection 30 min prior to drinking session), or (c) one of four doses (0, 5, 10, 20 mg/kg) of MPZP (s.c. injection 60 min prior to drinking session). *P<0.05, **P<0.01 significant difference from vehicle condition.
Table 2. Effects of duloxetine, naltrexone, and MPZP and naltrexone on water intake, total fluid intake, and preference [ethanol/supersac:total (E/S:T)] for sweetened alcohol solution by animals in the operant (experiment 2) alcohol binge group and supersac solution by supersac controls.
| Water intake (ml) | Total fluid (ml) | Preference (E/S:T) | |
|---|---|---|---|
| Duloxetine | |||
| Alcohol binge drinkers | |||
| 0 mg/kg | 0.88 ± 0.30 | 3.68 ± 0.47 | 0.70 ± 0.10 |
| 2 mg/kg | 0.43 ± 0.18 | 1.93 ± 0.42** | 0.72 ± 0.08 |
| 4 mg/kg | 0.33 ± 0.16 | 0.92 ± 0.34** | 0.57 ± 0.14 |
| 8 mg/kg | 0.01 ± 0.01 ** | 0.03 ± 0.03** | N/A |
| Supersac controls | |||
| 0 mg/kg | 0.40 ± 0.10 | 21.30 ± 2.44 | 0.98 ± 0.00 |
| 2 mg/kg | 0.08 ± 0.04** | 9.84 ± 2.55** | 0.99 ± 0.00 |
| 4 mg/kg | 0.07 ± 0.03** | 7.43 ± 2.23** | 0.98 ± 0.01 |
| 8 mg/kg | 0.03 ± 0.02** | 0.74 ± 0.39** | 0.91 ± 0.06 |
| Naltrexone | |||
| Alcohol binge drinkers | |||
| 0 μg/kg | 1.30 ± 0.34 | 3.91 ± 0.36 | 0.68 ± 0.08 |
| 16 μg/kg | 1.29 ± 0.27 | 4.17 ± 0.54 | 0.67 ± 0.06 |
| 50 μg/kg | 1.33 ± 0.38 | 3.45 ± 0.42 | 0.63 ± 0.08 |
| 150 μg/kg | 1.12 ± 0.33 | 3.15 ± 0.41 | 0.66 ± 0.08 |
| 450 μg/kg | 0.93 ± 0.25 | 2.42 ± 0.34** | 0.62 ± 0.07 |
| Supersac controls | |||
| 0 μg/kg | 0.22 ± 0.10 | 19.23 ± 2.30 | 0.99 ± 0.01 |
| 16 μg/kg | 0.23 ± 0.13 | 19.13 ± 1.43 | 0.98 ± 0.01 |
| 50 μg/kg | 0.09 ± 0.04 | 16.87 ± 1.70 | 1.00 ± 0.00 |
| 150 μg/kg | 0.24 ± 0.12 | 16.05 ± 1.93 | 0.98 ± 0.01 |
| 450 μg/kg | 0.14 ± 0.10 | 13.70 ± 1.79** | 0.99 ± 0.01 |
| MPZP | |||
| Alcohol binge drinkers | |||
| 0 mg/kg | 1.04 ± 0.16 | 3.00 ± 0.15 | 0.65 ± 0.05 |
| 5 mg/kg | 1.00 ± 0.43 | 3.18 ± 0.39 | 0.75 ± 0.08 |
| 10 mg/kg | 1.06 ± 0.29 | 3.58 ± 0.42 | 0.71 ± 0.06 |
| 20 mg/kg | 1.09 ± 0.26 | 3.80 ± 0.39 | 0.72 ± 0.07 |
| Supersac controls | |||
| 0 mg/kg | 0.23 ± 0.08 | 22.18 ± 2.26 | 0.99 ± 0.00 |
| 5 mg/kg | 0.19 ± 0.08 | 21.62 ± 2.07 | 0.99 ± 0.01 |
| 10 mg/kg | 0.28 ± 0.06 | 22.29 ± 1.74 | 0.99 ± 0.00 |
| 20 mg/kg | 0.23 ± 0.07 | 21.96 ± 2.25 | 0.98 ± 0.01 |
P<0.01 relative to vehicle condition;
N/A indicates denominator (total fluid intake) was zero for many of the individual rats.
Effects of naltrexone on operant binge-like drinking
Naltrexone pretreatment dose-dependently reduced intake of sweetened alcohol solution by animals in the alcohol binge group, F(4,40) = 7.95, P < 0.01 (Fig. 4b; left panel). Post-hoc analysis revealed that 50, 150, and 450 μg/kg significantly suppressed binge-like alcohol intake (P < 0.05 in all cases). Naltrexone also suppressed supersac intake by controls F(4,44) = 4.43, P < 0.01, though only at the highest dose; post-hoc analysis revealed that only the 450 μg/kg dose suppressed super-sac intake (P < 0.05) in those rats (Fig. 4b; right panel). Water intake was not affected in either of the two groups (Table 2).
Effects of MPZP on operant binge-like drinking
Analyses of variance revealed no effect of MPZP pretreatment on the consumption of sweetened alcohol solution, supersac or water by either of the two groups (Fig. 4c and Table 2). The analysis did, however, reveal a significant upward linear trend of MPZP dose (Fig. 4c) on binge-like alcohol drinking, F(3,43) = 2.69, P < 0.01, but not on supersac drinking (P > 0.05). Water intake was not affected in either of the two groups (Table 2).
Discussion
The present series of experiments was designed to explore a rat model of binge-like alcohol drinking and the neuropharmacological mechanisms involved in this type of alcohol drinking. Animals voluntarily and orally self-administered amounts of alcohol sufficient to reliably produce BALs greater than 0.08 g% following 30-min self-administration sessions (two-bottle choice and operant situations) in the absence of food or water deprivation. The satisfaction of these criteria qualifies this animal model as one with face validity for human binge drinking, as defined by NIAAA. In the present investigation, rats consumed substantial amounts of alcohol and exhibited pharmacologically relevant post-session BALs after a modest period of training, a pragmatic advantage of this model.
Other animal models of binge-like alcohol exposure are limited by a reliance on either nonoral routes of alcohol administration, exposure via forced alcohol administration, or selective breeding for high alcohol preference. Binge alcohol exposure procedures that utilize forced alcohol administration that is either passive (e.g. repeated experimenter-administered intragastric alcohol infusions; Crews et al., 2000; Crews and Braun, 2003) or active (e.g., bout drinking via consumption of alcohol liquid diet as the sole source of nutrition; Fidler et al., 2006; N.W. Gilpin and G.F. Koob, unpublished findings), produce significant neurobiological perturbations that are likely associated with dependence-induced drinking. Other models of binge-like alcohol self-administration have employed water deprivation (e.g., Hubbell et al., 1986; Reid et al., 1996; Gardell et al., 1997) or food deprivation (e.g., MacDonall and Marcucella, 1979; Falk and Tang, 1988) to promote ethanol self-administration during daily limited-access periods. These designs are problematic, however, because animals are primarily motivated by thirst during self-administration sessions and body weight gain is slowed or completely blocked with such procedures. Collectively, these models have weak construct validity for the human condition (i.e., humans do not consume ethanol because they are hungry or thirsty). Other studies have produced voluntary ethanol consumption by rats during limited access sessions without the use of any of these manipulations but those studies either did not produce BALs (0.08 g%) determined by NIAAA to be the defining factor in binge alcohol drinking (e.g., Stewart and Grupp, 1984; Gill et al., 1986; Linseman, 1987) or did not measure BALs (e.g., Macdonall and Marcucella, 1979). Finally, genetic manipulations have been used to produce rats selectively bred for high alcohol preference based on either continuous access ethanol intakes (e.g., alcohol-preferring P rats; Murphy et al., 1986) or limited access ethanol intakes (i.e., high alcohol-consuming HARF rats; Lê et al., 2001). P rats exhibit binge-like patterns of voluntary alcohol drinking behavior and achieve BALs that exceed 0.08 g% under a variety of alcohol access conditions (Murphy et al., 1986) and have been used extensively for the study of genetic and neurobiological mechanisms of alcoholism. HARF rats also appear to achieve ethanol intake levels sufficient to qualify as binge-like drinking (Lê et al., 2001).
In this experiment, intake of both sweetened alcohol and supersac tended to be higher in the two-bottle choice situation than in the operant situation, an effect that might be attributable to the increased work (lever pressing versus drinking from spout) required of rats to attain those solutions during operant sessions. In both experiments, there was some oscillation in alcohol intake over time; however, supersac intake by control rats exhibited the same pattern of change across time. In both experiments, there was also a strong correlation between alcohol intake and BALs but it is curious that this function exhibits a substantial rightward shift in two-bottle choice alcohol binge rats versus operant alcohol binge rats. This discrepancy could be due to different contributions of food intake to alcohol absorption rates in the two experiments. From a circadian perspective, rats typically consume the most food during the first 2–3 h of the dark cycle and then again just before the end of the dark cycle (Whishaw and Kolb, 2005). In Experiments 1 and 2 of the present investigation, self-administration sessions occurred at different time points during the dark cycle. More specifically, self-administration sessions occurred during the middle of the dark cycle in Experiment 1, but at the start of the dark cycle in Experiment 2. Presumably, rats in Experiment 1 (two-bottle choice drinkers) consumed more food during the hours preceding self-administration sessions relative to rats in Experiment 2 (operant responders). Therefore, the rightward shift in the BAL versus intake function of two-bottle choice alcohol drinkers versus operant alcohol responders might be due to the delay in alcohol absorption that occurs in animals with full stomachs (Goldberg, 1943).
This model combines saccharin and low glucose concentrations in a solution shown to have high palatability in rats (Valenstein et al., 1967). Adding sweeteners to ethanol solutions to produce higher ethanol intake is not a new experimental strategy. Some of the disadvantages of earlier procedures are, however, circumvented in the present model, such as the need for food deprivation (e.g., Macdonall and Marcucella, 1979) or water deprivation (e.g., Hubbell et al., 1986; Reid et al., 1996; Gardell et al., 1997), or the lack of a defined BAL criterion (e.g., Stewart and Grupp, 1984; Gill et al., 1986; Linseman, 1987; Sinclair et al., 1992). Although genetic manipulations have been used to produce animals that reliably and voluntarily consume large quantities of ethanol (e.g., HARF animals, Lê et al., 2001; P rats, Murphy et al., 1986), selective breeding is not a practical solution for most laboratories. In addition, existing rat lines are in high demand and can be difficult to acquire. The model presented here combines a previously used strategy for the induction of pharmacologically meaningful ethanol drinking in rats with a sweetening procedure (Valenstein et al., 1967) that appears to optimize ethanol consumption by nonalcohol-dependent rats.
Using a flavored vehicle (i.e., supersaccharin) with positive reinforcing properties as a control procedure is an advantage of the present procedure. The majority of ethanol self-administration studies allows rats a choice between an unsweetened ethanol solution and water, where water is the only alternate reinforcer available for intake comparisons. Such an experimental design has some limitations for testing the behavioral specificity of subsequent pharmacological manipulations because water has little reinforcing value in water-sated rats. That is, low water responding can produce a floor effect and make it difficult to discuss the behavioral specificity of drug effects. Furthermore, analysis of limited-access water response data is complicated by the fact that rats have continuous access to water during the nonexperimental periods, presumably resulting in lower reinforcement value for water during experimental sessions; indeed, water availability has complex effects on operant responding for a variety of reinforcers (Freed and Mendelson, 1977; Johnson et al., 1991). The model used here is more conducive to pharmacological manipulations that suppress ethanol intake because the effects on ethanol intake can be compared with effects on the intake of a highly reinforcing alternate solution (i.e., ethanol vehicle). Thus, effects of pharmacological manipulations on the intake of sweetened ethanol solution in the absence of effects on supersaccharin can be more aptly called ‘behaviorally specific.’ Treatments that produce generalized suppressive effects on consumption lack specificity for ethanol, although such behavioral changes could result from drug effects on a reinforcement pathway common to both ethanol and natural reinforcers.
Another important point to address is the difference in intake levels between sweetened ethanol and supersaccharin in this investigation. Rats consume substantially more supersaccharin alone than they do supersaccharin plus ethanol. This aspect of the procedure, however, also exhibits face validity with the human condition. Rats and humans, given the choice between sweet solutions that do and do not contain ethanol, generally prefer solutions that do not contain ethanol as ethanol has aversive taste properties in both species (Myers and Ewing, 1980; Shoaib and Almeida, 1996). This, however, does not diminish the significance of ethanol consumption by those same humans and rats, especially when ethanol is being consumed in a pathological behavioral pattern (e.g., binge drinking). That is, excessive or detrimental consumption patterns of sweetened ethanol are made no less relevant by the fact that rats and humans consume less total volume of those solutions than they do of sweetened solutions that do not contain ethanol.
Related to this point, P rats exhibit an increased preference for saccharin solutions relative to their nonpreferring counterparts and also relative to outbred Wistar rats, lending support for a genetic correlation between ethanol preference and saccharin preference (Sinclair et al., 1992). Similarly, rats selectively bred for high saccharin consumption consume more unsweetened ethanol than their low-saccharin-consuming counterparts (Dess et al., 1998). It should, however, be noted that outbred rats that show an initial low preference for ethanol (versus both water and saccharin) eventually, given prolonged access, self-administer amounts of ethanol identical to Wistar rats that show an initial high preference for ethanol (Kampov-Polevoy et al., 1990).
Binge alcohol drinking can be considered either as a phase in the development of alcoholism that precedes physical and psychological dependence on alcohol (NIAAA, 2004) or as a separate entity. As a result, binge alcohol drinkers often do not exhibit somatic or motivational signs of alcohol dependence. One approach for differentiating the behaviors and neural mechanisms associated with binge-like alcohol drinking and dependence-induced drinking is to examine a profile of treatment with different pharmacological agents. Following stabilization of drinking behavior by animals in the alcohol binge groups and supersac controls, the groups were tested for the effects of various drugs on drinking behavior.
Duloxetine is a SSNRI used in humans to treat major depression, pain from diabetic peripheral neuropathy, and stress urinary incontinence (Westanmo et al., 2005). Selective serotonin reuptake inhibitors suppress nondependent alcohol drinking in rats (Gill and Amit, 1989) and early stage problem drinking in humans (Naranjo and Sellers, 1989), but not drinking by later-stage human alcoholics (Kabel and Petty, 1996). Furthermore, low serotonin functioning in humans has long been associated with impulsivity and a predisposition to alcohol dependence (Linnoila et al., 1994). In the current investigation, duloxetine exhibited differential effects based on the drinking model: in the two-bottle choice situation, duloxetine dose-dependently suppressed alcohol binge-like drinking, but did not affect supersac drinking, whereas in an operant situation, duloxetine suppressed responding for both alcohol and supersac. As some selective serotonin reuptake inhibitors have been reported to have anorexic actions in rats (Gill and Amit, 1989), it could be argued that duloxetine suppressed intake of alcohol owing to its caloric value. Duloxetine, however, did not affect supersac intake by two-bottle choice drinkers, indicating that its effects are likely not attributable to any potential anorexic actions. It is not clear why duloxetine suppressed supersac drinking in an operant situation, and not in a two-bottle choice situation, but one hypothesis would be the work requirement for operant self-administration. This effect of work requirement might manifest owing to locomotor or motivational properties of the drug, although the locomotor explanation is unlikely as duloxetine does not appear to affect activity in rats (Brocco et al., 2002).
Naltrexone is a nonselective opioid antagonist used clinically in the treatment of alcoholism. Naltrexone has long been known to suppress alcohol drinking by rats (e.g., Altshuler et al., 1980; Reid and Hunter, 1984; Walker and Koob, 2007), and this effect is exaggerated in selectively bred Sardinian alcohol-preferring (sP Sabino et al., 2006 rats. In the current investigation, a very low dose (50 μg/kg) of naltrexone suppressed alcohol binge-like drinking, whereas a three-fold higher dose was required to reduce supersac consumption. These results are consistent with past findings that high doses of naltrexone (5–10 mg/kg; Reid et al., 1996; Gardell et al., 1997) suppress consumption of a sweetened alcohol solution [5% (w/v) sucrose + 6% ethanol (w/v)]. The heightened sensitivity of alcohol binge-like drinking to the suppressive effects of naltrexone is comparable to the ability of a very low naltrexone dose (50 μg/kg) to suppress alcohol drinking by sP rats (Sabino et al., 2006). A low naltrexone dose (100 μg/kg) also suppresses operant responding for unsweetened alcohol by nondependent Wistar rats, but naltrexone is considerably less efficacious in alcohol-dependent rats as a considerably higher dose (500 μg/kg) is required to suppress operant alcohol responding in those rats (Walker and Koob, 2007). Together, these findings indicate that naltrexone is more effective in suppressing excessive binge-like alcohol drinking than excessive drinking related to alcohol dependence.
The ability of naltrexone to suppress supersac consumption in the current investigation is in agreement with other reports in which naltrexone blocked the development of preference for a sucrose diet (Levine et al., 2002) and consumption of saccharin solution (Goodwin et al., 2001). These results are consistent with the hypothesis that blockade of opioid receptors by naltrexone at higher doses blocks the positive reinforcing effects of both natural and drug reinforcers.
Extra-hypothalamic CRF systems are thought to be perturbed during the transition to alcohol dependence, and to be an important factor in subsequent relapse alcohol drinking (Koob, 2003). MPZP is a CRF antagonist that effectively blocks CRF1 receptors (George et al., 2007; Specio et al., 2007). Previous findings from this laboratory indicate that this analog suppresses operant alcohol responding in dependent, but not nondependent, Wistar rats (Richardson et al., 2007). In the current investigation, MPZP did not affect alcohol binge-like drinking or supersac drinking, but there was a significant upward linear trend of dose on alcohol drinking. This result is consistent with the hypothesis that the activation of extra-hypothalamic CRF systems is more involved in alcohol drinking motivated by the negative reinforcing properties of the drug (i.e., dependence-induced drinking), but not drinking motivated by the positive reinforcing effects of the drug (i.e., binge-like alcohol drinking and nondependent alcohol drinking; Koob, 2003). Consistent with this notion, nondependent CRF1-receptor knockout mice have been observed to drink more alcohol than wild-type controls (Sillaber et al., 2002), but CRF1-receptor knockout mice do not exhibit the dependence-induced increases in alcohol drinking observed in wild-type controls (Chu et al., 2007). It remains to be determined if long-term binge-like drinking in a model such as the one used here is capable of eventually producing the motivational symptoms associated with alcohol dependence.
It is unlikely that the drug effects observed in the present investigation are because of nonspecific drug effects (e.g. activity, taste sensitivity, thirst, hunger). Naltrexone suppresses water intake induced by angiotensin II injection at doses similar to those used in the present investigation (Ruegg et al., 1994). The absence of effects of naltrexone on water intake by any group in the present investigation, however, indicates that the observed suppression of experimental solutions was not because of nonspecific thirst effects. Systemically administered naltrexone also suppresses food intake (Hobbs et al., 1994), and opioid antagonists typically suppress locomotor activity (Leventhal et al., 1996), but these effects occur at doses significantly higher than those used in the present investigation. Duloxetine suppresses locomotor activity (Bymaster et al., 2005) and food intake (Jackson et al., 1997) in rodents, and can also affect salivation (Katoh et al., 1995), but these effects occur at doses much higher (30–200 mg/kg) than those used in the present investigation. Relative to naltrexone and duloxetine, less is known about nonspecific behavioral effects of MPZP. MPZP suppresses anxiety-like behavior and dependence-induced alcohol drinking (Richardson et al., 2007). Brain CRF systems are involved in feeding behavior (Zorrilla et al., 2003), but those effects are likely mediated by CRF2 receptors (Ohata et al., 2002; Cottone et al., 2007). CRF1-receptor antagonists may be capable of suppressing locomotor activity (Ohata et al., 2002), but this effect likely did not alter behavior in this study because MPZP produced either increases or no effect on drinking in the various binge groups.
In summary, in the absence of food or water deprivation, rats consumed alcohol voluntarily and orally in amounts sufficient to produce BALs that define alcohol binge drinking in humans. This binge-like model is highly sensitive to compounds that suppress drinking via opioidergic (naltrexone) and serotonergic (duloxetine) mechanisms, but not sensitive to compounds that suppress drinking via decreases in CRF activity. Animal models of binge-like alcohol drinking will be valuable in assessing the motivational aspects and neural consequences of predependence heavy alcohol drinking behavior. The different profiles of compounds that affect alcohol binge-like drinking versus dependence-induced drinking should advance the effort to develop potential pharmacotherapeutics for subpopulations of alcohol abusers and alcoholics (Egli, 2005).
Acknowledgments
The authors thank Mike Arends for his excellent editorial assistance. They also thank Dr Barbara Mason for suggesting duloxetine as a neuropharmacological probe. This is manuscript number 18772 from The Scripps Research Institute. Support: Pearson Center for Alcoholism and Addiction Research and NIAAA grants AA06420, AA08459, and AA12602.
References
- Altshuler HL, Phillips PE, Feinhandler DA. Alteration of ethanol self-administration by naltrexone. Life Sci. 1980;26:679–688. doi: 10.1016/0024-3205(80)90257-x. [DOI] [PubMed] [Google Scholar]
- Brocco M, Dekeyne A, Veiga S, Girardon S, Millan MJ. Induction of hyperlocomotion in mice exposed to a novel environment by inhibition of serotonin reuptake: a pharmacological characterization of diverse classes of antidepressant agents. Pharmacol Biochem Behav. 2002;71:667–680. doi: 10.1016/s0091-3057(01)00701-8. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Lee TC, Knadler MP, Detke MJ, Iyengar S. The dual transporter inhibitor duloxetine: a review of its preclinical pharmacology, pharmacokinetic profile, and clinical results in depression. Curr Pharmaceut Des. 2005;11:1475–1493. doi: 10.2174/1381612053764805. [DOI] [PubMed] [Google Scholar]
- Chu K, Koob GF, Cole M, Zorrilla EP, Roberts AJ. Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF(1) receptor antagonist antalarmin and by CRF(1) receptor knockout. Pharmacol Biochem Behav. 2007;86:813–821. doi: 10.1016/j.pbb.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Nagy TR, Coscina DV, Zorrilla EP. Feeding microstructure in diet-induced obesity susceptible versus resistant rats: central effects of urocortin 2. J Physiol. 2007;583:487–504. doi: 10.1113/jphysiol.2007.138867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Braun CJ. Binge ethanol treatment causes greater brain damage in alcohol-preferring P rats than in alcohol-nonpreferring NP rats. Alcohol Clin Exp Res. 2003;27:1075–1082. doi: 10.1097/01.ALC.0000075826.35688.0D. [DOI] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, III, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–1723. [PubMed] [Google Scholar]
- Dess NK, Badia-Elder NE, Thiele TE, Kiefer SW, Blizard DA. Ethanol consumption in rats selectively bred for differential saccharin intake. Alcohol. 1998;16:275–278. doi: 10.1016/s0741-8329(98)00010-x. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Egli M. Can experimental paradigms and animal models be used to discover clinically effective medications for alcoholism? Addiction Biol. 2005;10:309–319. doi: 10.1080/13556210500314550. [DOI] [PubMed] [Google Scholar]
- Falk JL, Tang M. What schedule-induced polydipsia can tell us about alcoholism. Alcohol Clin Exp Res. 1988;12:577–585. doi: 10.1111/j.1530-0277.1988.tb00246.x. [DOI] [PubMed] [Google Scholar]
- Fidler TL, Clews TW, Cunningham CL. Reestablishing an intragastric ethanol self-infusion model in rats. Alcohol Clin Exp Res. 2006;30:414–428. doi: 10.1111/j.1530-0277.2006.00046.x. [DOI] [PubMed] [Google Scholar]
- Freed WJ, Mendelson J. Water-intake volume regulation in the rat: schedule-induced drinking compared with water-deprivation-induced drinking. J Comp Phsyiol Psychol. 1977;91:564–573. doi: 10.1037/h0077336. [DOI] [PubMed] [Google Scholar]
- Funk CK, O'Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self- administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardell LR, Whalen CA, Chattopadhyay S, Cavallaro CA, Hubbell CL, Reid LD. Combination of naltrexone and fluoxetine on rats' propensity to take alcoholic beverage. Alcohol Clin Exp Res. 1997;21:1435–1439. [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Zorrilla EP, Parsons LH, O'Dell LE, et al. A neurobiological mechanism for the ‘hook’ in nicotine dependence. Proc Nat Acad Sci. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert RM. Schedule-induced self-administration of drugs. In: Blackman DE, Sanger DJ, editors. Contemporary Research in Behavioral Pharmacology. New York: Plenum; 1978. pp. 289–323. [Google Scholar]
- Gill K, Amit Z. Serotonin uptake blockers and voluntary alcohol consumption. A review of recent studies. Recent Dev Alcohol. 1989;7:225–248. doi: 10.1007/978-1-4899-1678-5_12. [DOI] [PubMed] [Google Scholar]
- Gill K, France C, Amit Z. Voluntary ethanol consumption in rats: an examination of blood/brain ethanol levels and behavior. Alcohol Clin Exp Res. 1986;10:457–462. doi: 10.1111/j.1530-0277.1986.tb05124.x. [DOI] [PubMed] [Google Scholar]
- Goldberg L. Quantitative studies on alcohol tolerance in man. Acta Physiologica Scandinavica Supplementum. 1943;5:1–128. [Google Scholar]
- Goodwin FL, Campisi M, Babinska I, Amit Z. Effects of naltrexone on the intake of ethanol and flavored solutions in rats. Alcohol. 2001;25:9–19. doi: 10.1016/s0741-8329(01)00163-x. [DOI] [PubMed] [Google Scholar]
- Harwood HJ, Fountain D, Livermore G. The economic costs of alcohol and drug abuse in the United States, 1992 (NIH pub no 98–4327) Rockville, MD: National Institute on Drug Abuse/National Institute on Alcohol Abuse and Alcoholism; 1998. [Google Scholar]
- Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Therapeutics. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Hingson R, Heeren T, Winter M, Wechsler H. Magnitude of alcohol-related mortality and morbidity among U.S. college students ages 18–24: changes from 1998 to 2001. Ann Rev Public Health. 2005;26:259–279. doi: 10.1146/annurev.publhealth.26.021304.144652. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR. Age of alcohol-dependence onset: associations with severity of dependence and seeking treatment. Pediatrics. 2007;118:e755–e763. doi: 10.1542/peds.2006-0223. [DOI] [PubMed] [Google Scholar]
- Hobbs DJ, Koch JE, Bodnar RJ. Naltrexone, dopamine receptor agonists and antagonists, and food intake in rats: 1. Food deprivation. Pharmacol Biochem Behav. 1994;49:197–204. doi: 10.1016/0091-3057(94)90476-6. [DOI] [PubMed] [Google Scholar]
- Hubbell CL, Czirr SA, Hunter GA, Beaman CM, LeCann NC, Reid LD. Consumption of ethanol solution is potentiated by morphine and attenuated by naloxone persistently across repeated daily administrations. Alcohol. 1986;3:39–54. doi: 10.1016/0741-8329(86)90070-4. [DOI] [PubMed] [Google Scholar]
- Jackson HC, Needham AM, Hutchins LJ, Mazurkiewicz SE, Heal DJ. Comparison of the effects of sibutramine and other monoamine reuptake inhibitors on food intake in the rat. Br J Pharmacol. 1997;121:1758–1762. doi: 10.1038/sj.bjp.0701312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LM, Bickel WK, Higgins ST, Morris EK. The effects of schedule history and the opportunity for adjunctive responding on behavior during a fixed-interval schedule of reinforcement. J Exp Analysis Behav. 1991;55:313–322. doi: 10.1901/jeab.1991.55-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabel DI, Petty F. A placebo-controlled, double-blind study of fluoxetine in severe alcohol dependence: adjunctive pharmacotherapy during and after inpatient treatment. Alcohol Clin Exp Res. 1996;20:780–784. doi: 10.1111/j.1530-0277.1996.tb01686.x. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Kasheffskaya RP, Sinclair JD. Initial acceptance of ethanol: gustatory factors and patterns of ethanol drinking. Alcohol. 1990;7:83–85. doi: 10.1016/0741-8329(90)90065-k. [DOI] [PubMed] [Google Scholar]
- Katoh A, Eigyo M, Ishibashi C, Naitoh Y, Takeuchi M, Ibii N, et al. Behavioral and electroencephalographic properties of duloxetine ( LY248686), a reuptake inhibitor of norepinephrine and serotonin, in mice and rats. J Pharmacol Exp Therapeut. 1995;272:1067–1075. [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Lê AD, Israel Y, Juzytsch W, Quan B, Harding S. Genetic selection for high and low alcohol consumption in a limited-access paradigm. Alcohol Clin Exp Res. 2001;25:1613–1620. doi: 10.1111/j.1530-0277.2001.tb02168.x. [DOI] [PubMed] [Google Scholar]
- Leventhal L, Cole JL, Bodnar RJ. Reductions in locomotor activity following central opioid receptor subtype antagonists in rats. Phsyiol Behav. 1996;60:833–836. doi: 10.1016/0031-9384(96)00103-5. [DOI] [PubMed] [Google Scholar]
- Levine AS, Grace MK, Cleary JP, Billington CJ. Naltrexone infusion inhibits the development of preference for a high-sucrose diet. Am J Physiol: Reg Integ Comp Physiol. 2002;283:R1149–R1154. doi: 10.1152/ajpregu.00040.2002. [DOI] [PubMed] [Google Scholar]
- Linnoila M, Virkkunen M, George T, Eckardt M, Higley JD, Nielsen D, Goldman D. Serotonin, violent behavior and alcohol. EXS. 1994;71:155–163. doi: 10.1007/978-3-0348-7330-7_16. [DOI] [PubMed] [Google Scholar]
- Linseman MA. Alcohol consumption in free-feeding rats: procedural, genetic and pharmacokinetic factors. Psychopharmacology. 1987;92:254–261. doi: 10.1007/BF00177925. [DOI] [PubMed] [Google Scholar]
- Macdonall JS, Marcucella H. Increasing the rate of ethanol consumption in food- and water-satiated rats. Pharmacol Biochem Behav. 1979;10:211–216. doi: 10.1016/0091-3057(79)90089-3. [DOI] [PubMed] [Google Scholar]
- Miller JW, Naimi TS, Brewer RD, Jones SE. Binge drinking and associated health risk behaviors among high school students. Pediatrics. 2007;119:76–85. doi: 10.1542/peds.2006-1517. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Gatto GJ, Waller MB, McBride WJ, Lumeng L, Li TK. Effects of scheduled access on ethanol intake by the alcohol-preferring (P) line of rats. Alcohol. 1986;3:331–336. doi: 10.1016/0741-8329(86)90010-8. [DOI] [PubMed] [Google Scholar]
- Myers RD, Ewing JA. Aversive factors in alcohol drinking in humans and animals. Pharmacol Biochem Behav. 1980;13(Supp 1):269–277. doi: 10.1016/s0091-3057(80)80041-4. [DOI] [PubMed] [Google Scholar]
- Naranjo CA, Sellers EM. Serotonin uptake inhibitors attenuate ethanol intake in problem drinkers. Recent Dev Alcohol. 1989;7:255–266. doi: 10.1007/978-1-4899-1678-5_13. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. NIAAA Council approves definition of binge drinking. NIAAA Newsletter, No 3 (NIH pub no 04–5346) Bethesda MD: National Institute on Alcohol Abuse and Alcoholism; 2004. [Google Scholar]
- Ohata H, Arai K, Shibasaki T. Effect of chronic administration of a CRF(1) receptor antagonist, CRA1000, on locomotor activity and endocrine responses to stress. Eur J Pharmacol. 2002;457:201–206. doi: 10.1016/s0014-2999(02)02663-8. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Pulvirenti L, Koob GF. Oral ethanol self-administration in rats is reduced by the administration of dopamine and glutamate receptor antagonists into the nucleus accumbens. Psychopharmacol. 1992;109:92–98. doi: 10.1007/BF02245485. [DOI] [PubMed] [Google Scholar]
- Reid LD, Hunter GA. Morphine and naloxone modulate intake of ethanol. Alcohol. 1984;1:33–37. doi: 10.1016/0741-8329(84)90033-8. [DOI] [PubMed] [Google Scholar]
- Reid LD, Gardell LR, Chattopadhyay S, Hubbell CL. Periodic naltrexone and propensity to take alcoholic beverage. Alcohol Clin Exp Res. 1996;20:1329–1334. doi: 10.1111/j.1530-0277.1996.tb01130.x. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Fekete EM, Zhao Y, Funk CK, Zorrilla EP, Koob GF. A novel small molecule antagonist of the corticotropin-releasing factor type 1 receptor (CRF1) is a potent anxiolytic and reduces excessive alcohol intake in dependent male rats. Pharmacol Biochem Behav. 2007 doi: 10.1016/j.pbb.2007.10.008. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegg H, Hahn B, Koch JE, Bodnar RJ. Differential modulation of angiotensin II and hypertonic saline-induced drinking by opioid receptor subtype antagonists in rats. Brain Res. 1994;635:203–210. doi: 10.1016/0006-8993(94)91440-0. [DOI] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Koob GF, Steardo L, Lee MJ, Rice KC, Zorrilla EP. Dissociation between opioid and CRF1 antagonist sensitive drinking in Sardinian alcohol-preferring rats. Psychopharmacol. 2006;189:175–186. doi: 10.1007/s00213-006-0546-5. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Samson H, Files F, Brice G. Patterns of ethanol consumption in a continuous access situation: the effect of adding sweetener to the ethanol solution. Alcohol Clin Exp Res. 1996;20:101–109. doi: 10.1111/j.1530-0277.1996.tb01052.x. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Almeida OFX. Absence of tolerance to the aversive stimulus properties of ethanol following oral ethanol self-administration. Alcohol. 1996;13:175–180. doi: 10.1016/0741-8329(95)02039-x. [DOI] [PubMed] [Google Scholar]
- Sillaber I, Rammes G, Zimmermann S, Mahal B, Zieglgansberger W, Wurst W, et al. Enhanced and delayed stress-induced alcohol drinking in mice lacking functional CRH1 receptors. Science. 2002;296:931–933. doi: 10.1126/science.1069836. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Kampov-Polevoy A, Stewart R, Li TK. Taste preferences in rat lines selected for low and high alcohol consumption. Alcohol. 1992;9:155–160. doi: 10.1016/0741-8329(92)90027-8. [DOI] [PubMed] [Google Scholar]
- Specio SE, Wee S, O'Dell LE, Boutrel B, Zorrilla EP, Koob GF. CRF1 receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology. 2007 doi: 10.1007/s00213-007-0983-9. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart RB, Grupp LA. A simplified procedure for producing ethanol self-selection in rats. Pharmacol Biochem Behav. 1984;21:255–258. doi: 10.1016/0091-3057(84)90223-5. [DOI] [PubMed] [Google Scholar]
- Truxell EM, Molina JC, Spear NE. Ethanol intake in the juvenile, adolescent, and adult rat: effects of age and prior exposure to ethanol. Alcohol Clin Exp Res. 2007;31:755–765. doi: 10.1111/j.1530-0277.2007.00358.x. [DOI] [PubMed] [Google Scholar]
- Valenstein ES, Cox VC, Kakolewski JW. Polydipsia elicited by the synergistic action of a saccharin and glucose solution. Science. 1967;157:552–554. doi: 10.1126/science.157.3788.552. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301438. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler H, Isaac N. ‘Binge’ drinkers at Massachusetts colleges. Prevalence, drinking style, time trends, and associated problems. J Am Med Assoc. 1992;267:2929–2931. doi: 10.1001/jama.267.21.2929. [DOI] [PubMed] [Google Scholar]
- Wechsler H, Lee JE, Kuo M, Lee H. College binge drinking in the 1990s: a continuing problem. Results of the Harvard School of Public Health 1999 College Alcohol Study. J Am College Health. 2000;48:199–210. doi: 10.1080/07448480009599305. [DOI] [PubMed] [Google Scholar]
- Westanmo AD, Gayken J, Haight R. Duloxetine: a balanced and selective norepinephrine- and serotonin-reuptake inhibitor. Am J Health-System Pharmacy. 2005;62:2481–2490. doi: 10.2146/ajhp050006. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Kolb B. The behavior of the laboratory rat: a handbook with tests. Oxford, New York: Oxford University Press; 2005. p. 198. [Google Scholar]
- Zorrilla EP, Tache Y, Koob GF. Nibbling at CRF receptor control of feeding and gastrocolonic motility. Trends Pharmacol Sci. 2003;24:421–427. doi: 10.1016/S0165-6147(03)00177-9. [DOI] [PubMed] [Google Scholar]