Abstract
Background
Trimethoprim-sulfamethoxazole (SXT) reduces morbidity and mortality among HIV-infected persons in Africa, but its impact on antimicrobial resistance is of concern.
Methods
HIV-uninfected (group A), HIV-infected but not requiring SXT (group B), and HIV-infected and eligible for SXT (group C) adults were recruited into a prospective observational cohort study in Moshi, Tanzania. Stool was examined for Escherichia coli nonsusceptible to SXT at baseline and at weeks 1, 2, 4, and 24. General estimating equation models were used to assess differences in susceptibility over time and cross-resistance to other antimicrobials.
Results
Of 181 subjects, 118 (65.1%) were female and the median (range) age was 36 (20 to 72) years. At baseline, E. coli nonsusceptible to SXT was isolated from 23 (53.5%) of 43 patients in group A, 25 (67.6%) of 37 patients in group B, and 37 (64.9%) of 57 patients in group C. The odds ratios (P value) for SXT nonsusceptibility in group C at weeks 1, 2, 4, and 24 compared with baseline were 3.4 (0.013), 3.0 (0.019), 2.9 (0.030), and 1.5 (0.515), respectively. SXT nonsusceptibility was associated with nonsusceptibility to ampicillin, chloramphenicol, ciprofloxacin, and nalidixic acid (P ≤ 0.006).
Conclusion
In Tanzania, carriage of fecal E. coli nonsusceptible to SXT is common before SXT prophylaxis. Initiation of SXT leads to further loss of susceptibility to SXT and to other antimicrobials.
Keywords: antibiotic prophylaxis, Escherichia coli, feces, HIV, Tanzania, trimethoprim-sulfamethoxazole combination
Trimethoprim-sulfamethoxazole (SXT) has been shown to reduce morbidity and mortality among persons living with HIV in Africa.1-4 Based on the results of clinical trials from the West African country of Côte d’Ivoire,1,4 in 2000, the World Health Organization (WHO) and the Joint United Nations Program on AIDS (UNAIDS) recommended the use of SXT prophylaxis for persons with symptomatic HIV disease or with CD4 T-lymphocyte counts (CD4 counts) <500 cells/mm3 in Africa.5 At the time, uncertainty was expressed about whether clinical benefits seen in Côte d’Ivoire, where the prevalence of resistance to SXT is relatively low,6 would also be seen in East Africa and southern Africa, where the prevalence of resistance is higher.7,8 In addition, concern was raised that the widespread use of SXT may substantially increase the prevalence of antimicrobial resistance in common community-acquired pathogens.
Inexpensive and relatively safe, SXT and the related compound sulfadoxine-pyrimethamine play central roles in the management of common clinical syndromes in Africa. These drugs are frequently used to treat dysentery, lower respiratory tract infection, and fever in which Shigella spp., non-Typhi serotypes of Salmonella enterica, Streptococcus pneumoniae, and Plasmodium spp., respectively, play major roles.9,10 It follows that increases in resistance to SXT among these pathogens could reduce the effectiveness of empiric treatment strategies, leading to more illness and death. Large, randomized, community-based cohort studies would be required to investigate the role that SXT prophylaxis plays in the emergence of antimicrobial resistance among isolates from patients with these specific infections at the community level. Randomized studies of SXT prophylaxis are no longer acceptable, however, because of the established benefit of SXT on morbidity and mortality.
To understand the role that SXT might play in driving antimicrobial resistance, we selected fecal Escherichia coli as an indicator organism for enteric pathogens. We then examined the hypothesis that initiation of SXT prophylaxis in HIV-infected individuals would lead to rapid and widespread resistance of fecal E. coli to SXT compared with HIV-infected and HIV-uninfected persons not receiving SXT.
METHODS
Study Design and Participants
We designed a 3-group prospective observational cohort study of persons aged ≥18 years who had recently received HIV voluntary counseling and testing (VCT) at VCT centers in Moshi, Tanzania, between August 2004 and December 2005. Some patients were coenrolled in another study that evaluated the role of simple clinical and laboratory evaluations to identify HIV-infected patients with CD4 counts <200 cells/mm3.11
Clinical Procedures
VCT centers referred HIV-infected and HIV-uninfected subjects to the Infectious Diseases Clinic (IDC) at Kilimanjaro Christian Medical Centre (KCMC) for management of HIV infection and for study enrollment. After providing written informed consent, a standardized clinical history and physical examination were done. HIV-infected patients not yet on SXT were staged according to the WHO system. In accordance with WHO/UNAIDS recommendations, HIV-uninfected patients (group A) and those with asymptomatic HIV infection (WHO stage 1; group B) were not offered SXT. Patients with symptomatic HIV infection (WHO stage 2, 3, or 4; group C) were offered free SXT prophylaxis in the form of 2 single-strength tablets, each containing 80 mg of trimethoprim and 400 mg of sulfamethoxazole, daily. Pregnancy in women of reproductive age was excluded at each visit using a menstrual history and urine pregnancy test. Although women in the first trimester of pregnancy were not included in the study, those in the second or third trimester were included.12 Whole stool was collected at the baseline visit and before the first dose of SXT for all subjects. Subjects then returned to the KCMC IDC 1, 2, 4, and 24 weeks after the baseline visit. At each visit, whole stool was collected and the standardized clinical history and physical examination were repeated. Adherence to SXT prophylaxis was assessed at each follow-up visit by patient self-report using a standardized questionnaire. Patients who entered the study in WHO stage 1 and progressed to WHO stages 2 through 4 or those who were found to have CD4 counts <500 cells/mm3 were allowed to move between study groups. This study spanned a period of transition from the availability of antiretroviral therapy (ART) to patients in Tanzania who could afford it to the provision of free therapy.13
Laboratory Procedures
Whole stool was inoculated to MacConkey agar with an SXT disk and incubated for 24 hours at 37°C. Plates were examined for the presence of flat, dry, lactose-utilizing colonies consistent with E. coli. The presence or absence of presumptive E. coli was recorded. If colonies consistent with E. coli were not seen within <16 mm of the SXT disk, the stool was classified as having susceptible E. coli.14,15 If colonies consistent with E. coli were seen within <16 mm of the SXT disk, the stool was classified as having nonsusceptible E. coli and the colony nearest to the disk was picked and subcultured to sheep blood agar. The inoculated sheep blood agar plate was then incubated for 24 hours at 37°C, and the spot indole test was performed on the resulting growth. Indole-positive isolates were stored on nutrient agar at room temperature. All SXT-nonsusceptible E. coli isolates and a sample of susceptible isolates were shipped to the Duke University Medical Center Clinical Microbiology Laboratory (DUMC CMB) for further evaluation.
At the DUMC CMB, isolates were subcultured to sheep blood agar and MacConkey agar and were confirmed as E. coli using oxidase and indole tests. Isolates without classic E. coli colony morphology were identified using the MicroScan Walk-away system panel NEG Combo type 32 (Dade MicroScan, West Sacramento, CA). Susceptibility testing for ceftriaxone, nalidixic acid, ampicillin, ciprofloxacin, chloramphenicol, and azithromycin was done using the Kirby-Bauer disk diffusion method to Clinical Laboratory Standards Institute (CLSI) standards. Staphylococcus aureus interpretive criteria were used to evaluate zone sizes for azithromycin.14 Minimum inhibitory concentration (MIC) to SXT was determined using the E-test (AB BIODISK, Solna, Sweden).14
Statistical Analysis
Prespecified analyses included descriptive analyses of the cohort by study group, comparison of changes in the proportion of E. coli nonsusceptible to SXT by study arm over time, and assessment of the effect of SXT use on coselection of nonsusceptibility to other antimicrobial agents. The characteristics of study subjects and E. coli antimicrobial susceptibility testing results were calculated as medians, ranges, and proportions. Antimicrobial susceptibility patterns were modeled using general estimating equations to account for repeated measures on individuals. Within-group differences over time were assessed in a pooled model with interactions between study group and visit type. All analyses were done using STATA, version 9.2 (Stata Corporation, College Station, TX).
Research Ethics
The protocol for this study was approved by the KCMC Research Ethics Committee, the Tanzania National Institute for Medical Research National Research Ethics Coordinating Committee, and an Institutional Review Board of Duke University Medical Center.
RESULTS
Baseline Characteristics
One hundred eighty-one subjects were seen at baseline. Of these, 118 (65.1%) were female and the median (range) age was 36 (20 to 72) years. Of the 181 subjects, 54 (29.8%) were in group A, 53 (29.3%) were in group B, and 74 (40.9%) were in group C. A greater proportion of subjects in groups B and C had primary education or less than subjects in group A. All patients in group B were in WHO stage 1. All patients in group C were in WHO stage 2, 3, or 4, but 58 (78.4%) of 74 patients in group C were in WHO stage 3 or 4. The median (range) CD4 count at baseline was 297 (56 to 1200) cells/mm3 in group B compared with 187 (2 to 1322) cells/mm3 in group C. Other baseline characteristics of subjects are shown in Table 1.
TABLE 1.
Sociodemographic and Clinical Characteristics of Study Subjects at Baseline Visit, KCMC, 2004 to 2005
| Characteristic | HIV Uninfected (Group A) |
HIV Infected, No SXT (Group B) |
HIV Infected, SXT (Group C) |
All |
||||
|---|---|---|---|---|---|---|---|---|
| n/n | (% or min, max) | n/n | (% or min, max) | n/n | (% or min, max) | n | (% or min, max) | |
| Female, n (%) | 29/54 | (53.7) | 34/53 | (64.2) | 55/74 | (74.3) | 118/181 | (65.2) |
| Median age, y (min, max) | 36 | (20, 72) | 34 | (21, 63) | 39 | (20, 65) | 36 | (20, 72) |
| Primary education or less, n (%) | 7/54 | (12.9) | 13/53 | (24.5) | 16/74 | (21.6) | 36/181 | (19.9) |
| Urban, n (%) | 26/54 | (48.1) | 21/53 | (39.6) | 24/74 | (32.4) | 71/181 | (39.2) |
| WHO stage, n (%) | ||||||||
| 1 | NA | NA | 53/53 | (100.0) | 0/74 | (0.0) | 53/127 | (41.7) |
| 2 | NA | NA | 0/53 | (0.0) | 16/74 | (21.6) | 16/127 | (12.6) |
| 3 | NA | NA | 0/53 | (0.0) | 28/74 | (37.8) | 28/127 | (22.0) |
| 4 | NA | NA | 0/53 | (0.0) | 30/74 | (40.5) | 30/127 | (23.6) |
| Median CD4 count, cells/mm3, (min, max) | NA | NA | 297 | (56, 1200) | 187 | (2, 1322) | 211 | (2, 1322) |
| Median body mass index (range) | 21.8 | (15.8, 39.5) | 22.0 | (17.8, 35.3) | 19.4 | (11.7, 40.5) | 21.2 | (11.7, 40.5) |
max indicates maximum; min, minimum; NA, not applicable.
Use of SXT and Related Antimicrobials
The proportions of subjects in group C reporting 100% adherence to SXT at study weeks 1, 2, 4, and 24 were 79.0%, 80.0%, 73.1%, and 75.0%, respectively, in group C. Two subjects in group A and 7 subjects in group B took short courses of SXT during the study. Three subjects in group A and 1 subject in group B took short courses of sulfadoxine-pyrimethamine during the study. SXT was discontinued by 1 (1.4%) subject in group C because of rash. No patient developed Stevens-Johnson syndrome.
Susceptibility of Fecal Escherichia coli to SXT at Follow-Up
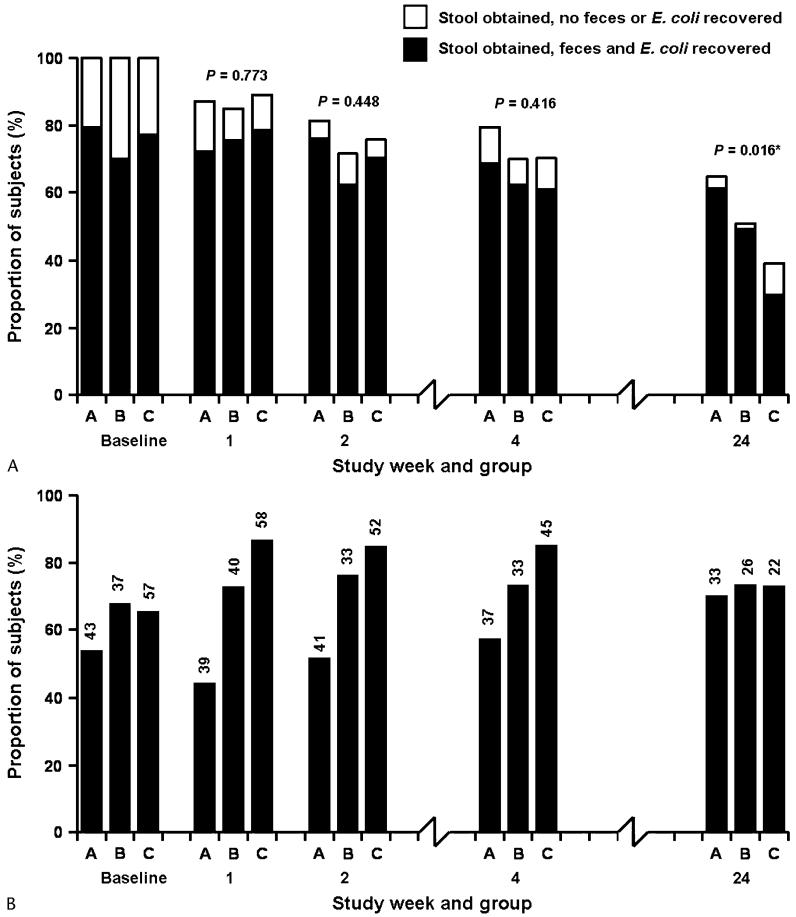
Of 181 study subjects, 158 (87.3%) were retained in follow-up for the week 1 visit, 138 (76.2%) at week 2, 132 (72.9%) at week 4, and 91 (50.3%) at week 24. Subject retention by study group is shown in Figure 1A. There was no difference in subject retention between the 3 groups by study week, except at week 24, when more subjects were retained in group A compared with group C (P = 0.004). Of persons retained to follow-up, E. coli was isolated from 137 (75.7%) persons at baseline, 137 (86.7%) at week 1, 126 (91.3%) at week 2, 115 (87.1%) at week 4, and 81 (89.0%) at week 24. Of baseline stool samples, SXT-nonsusceptible E. coli was isolated from 23 (53.5%) of 43 group A patients, 25 (67.6%) of 37 group B patients, and 37 (64.9%) of 57 group C patients. Baseline proportions of E. coli nonsusceptible to SXT were not significantly different between the 3 groups (P = 0.365). By week 1, SXT nonsusceptibility was present in E. coli from 17 (43.6%) of 39 subjects in group A, 29 (72.5%) of 40 subjects in group B, and 50 (86.2%) of 58 subjects in group C. Changes in the proportion of E. coli isolates in these and all subsequent study groups and study weeks are illustrated in Figure 1B. A comparison of the proportions of E. coli isolates nonsusceptible to SXT across study groups by study week yielded significantly higher proportions nonsusceptible in group C relative to group A at weeks 1, 2, and 4 (P < 0.001, P < 0.001, and P = 0.006, respectively) and in group B relative to group A at weeks 1 and 2 (P = 0.009 and P = 0.031, respectively). The differences between group C and group B were not statistically significant at conventional levels (P > 0.092). In a generalized estimating equation model, the odds ratios (ORs) for resistance in group C at study weeks 1, 2, 4, and 24 compared with baseline were 3.4 (P = 0.013), 3.0 (P = 0.019), 2.9 (P = 0.030), and 1.5 (P = 0.515), respectively. No significant differences in the odds of SXT resistance were seen in group A or B compared with baseline (Table 2).
FIGURE 1.
A, Retention to follow-up of study subjects and recovery of feces and fecal E. coli by study group and week, KCMC, 2004 to 2005. Group A: HIV-uninfected; group B: HIV-infected, no SXT; and group C: HIV-infected, SXT. P values are for the comparison of retention rates across groups by week. The difference in retention between group A and group C at week 24 was statistically significant (P = 0.004). B, Proportion of fecal E. coli isolated from study subjects nonsusceptible to SXT by study group and week, KCMC, 2004 to 2005. Group A: HIV uninfected; group B: HIV infected, no SXT; and group C: HIV infected, SXT. Numbers above bars are the denominators used to calculate proportions.
TABLE 2.
Changes in the Risk of E. coli Nonsusceptibilty to SXT by Study Week Relative to Baseline, KCMC, 2004 to 2005
| Follow-Up Week | HIV Uninfected (Group A) |
HIV Infected, No SXT (Group B) |
HIV Infected, SXT (Group C) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | (95% CI) | P | OR | (95% CI) | P | OR | (95% CI) | P | |
| 1 | 0.67 | (0.30, 1.5) | 0.324 | 1.3 | (0.49, 3.2) | 0.645 | 3.4 | (1.3, 8.9) | 0.013 |
| 2 | 0.92 | (0.40, 2.1) | 0.846 | 1.4 | (0.54, 3.8) | 0.465 | 3.0 | (1.2, 7.3) | 0.018 |
| 4 | 1.14 | (0.44, 2.9) | 0.779 | 1.3 | (0.43, 3.6) | 0.680 | 2.9 | (1.1, 7.7) | 0.030 |
| 24 | 2.04 | (0.75, 5.5) | 0.161 | 1.3 | (0.44, 3.8) | 0.637 | 1.5 | (0.46, 4.6) | 0.515 |
ORs and 95% confidence intervals (CIs) were calculated on the basis of parameter estimates and standard errors from a general estimating equation model with interactions between study group and visit type.
Escherichia coli SXT Nonsusceptibility and Other Antimicrobials
Coselection of antimicrobial nonsusceptibility was assessed among 419 fecal E. coli isolates. SXT nonsusceptibility was associated with nonsusceptibility to ampicillin (OR = 10.2; P < 0.001), chloramphenicol (OR = 7.8; P < 0.001), ciprofloxacin (OR = 17.1; P = 0.006), and nalidixic acid (OR = 26.4; P = 0.001) but not with nonsusceptibility to azithromycin (OR = 1.2; P = 0.545) (Table 3). All fecal E. coli isolates were susceptible to ceftriaxone.
TABLE 3.
Antimicrobial Susceptibility of SXT-Susceptible and Nonsusceptible Fecal E. coli, KCMC, 2004 to 2005
| SXT Susceptibility | Proportion Nonsusceptible to Other Antimicrobials n (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ampicillin |
Azithromycin |
Chloramphenicol |
Ciprofloxacin |
Nalidixic Acid |
||||||
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| SXT susceptible (n = 180) | 25 | (13.9) | 146 | (80.7) | 5 | (2.8) | 1 | (0.6) | 1 | (0.6) |
| SXT nonsusceptible (n = 239) | 153 | (64.6) | 202 | (84.5) | 44 | (18.4) | 20 | (8.4) | 26 | (10.9) |
| OR | 10.2 | 1.2 | 7.8 | 17.1 | 26.4 | |||||
| 95% CI | 5.9 to 17.8 | 0.71 to 1.9 | 3.0 to 20.2 | 2.3 to 127.7 | 3.6 to 194.5 | |||||
| P | <0.001 | 0.545 | <0.001 | 0.006 | 0.001 | |||||
ORs and 95% CIs were calculated using general estimating equation models predicting nonsusceptibility to each antimicrobial.
DISCUSSION
We demonstrate that in northern Tanzania, carriage of fecal E. coli nonsusceptible to SXT is common among HIV-uninfected persons and among HIV-infected patients before the commencement of SXT prophylaxis. Furthermore, the initiation of SXT prophylaxis rapidly leads to further loss of susceptibility not only to SXT but to other important antimicrobial agents. These findings provide valuable insights into the possible negative consequences of widespread use of life-extending SXT for HIV-infected individuals in Africa.
The large proportion of subjects found to be carrying SXT-nonsusceptible E. coli before commencement of SXT prophylaxis was consistent with reports showing SXT nonsusceptibility to be common among other Enterobacteriaceae from patients in East Africa and southern Africa.8,16,17 Concern about the impact of SXT nonsusceptibility among key HIV bacterial copathogens on the efficacy of SXT prophylaxis has been raised.18 Although large observational studies done in East Africa and southern Africa have shown that SXT prophylaxis significantly reduces morbidity and mortality, even in settings where SXT nonsusceptibility is more common,2,19-21 we are aware of no study that has investigated whether the magnitude of the effect of SXT prophylaxis is reduced in populations in which SXT nonsusceptibility is more common.
Most subjects initiating SXT prophylaxis with SXT-susceptible E. coli at baseline were carrying SXT-nonsusceptible E. coli within 1 week. The magnitude of this effect is consistent with that seen in fecal E. coli isolates from tuberculosis and HIV-coinfected patients in Malawi after initiation of SXT.17 Furthermore, in our study, most of those patients assigned to receive SXT continued to carry SXT nonsusceptible E. coli at a proportion higher than baseline. This finding suggests that the impact of SXT prophylaxis on antimicrobial resistance of bacterial flora occurs rapidly and that it is sustained as long as SXT prophylaxis is continued. Although we studied the fecal indicator organism E. coli, there also is evidence from East Africa that antimicrobial use is associated with the frequency of resistance among bacterial enteric pathogens. In Kenya, resistance to antimicrobials among diarrheal non-Typhi Salmonella and Shigella spp. was inversely proportional to the frequency with which the antimicrobials were prescribed, with SXT being the most common treatment prescription and the least effective agent.16
We demonstrated that the initiation of SXT prophylaxis not only selects for SXT-nonsusceptible E. coli but also seems to select for ampicillin, chloramphenicol, and ciprofloxacin nonsusceptibility. It is likely that a mechanism for the coselection of resistance is by means of mobile genetic elements such as integrons in plasmids and transposons. Research on enteroinvasive or enteroaggregative E. coli in Senegal showed that trimethoprim and other antimicrobial resistance was common and that the mechanism was likely within the class 1 integron-containing plasmids that may be horizontally transferred from gut commensal organisms.22 In Tanzania, a study examining prevalence and mechanisms of antimicrobial resistance among Shigella spp. from pediatric stool cultures found that resistance to ampicillin, chloramphenicol, tetracycline, and SXT was common. Ampicillin resistance was most frequently related to an integron-borne OXA-1-type β-lactamase, and resistance to SXT was attributable to the presence of an integron-borne dhfr Ia gene.23 A study of uropathogenic E. coli from Europe and Canada that was resistant to trimethoprim, sulfamethoxazole, or both found dfr or sul gene-containing integrons present in 59% of isolates. Analysis of the regional distribution of these integrons indicated that horizontal gene transfer was the main mechanism of resistance spread rather than clonal expansion.24 These studies suggest that coselection of resistance by means of mobile genetic elements in fecal E. coli attributable to SXT use is likely to occur and that these genetic elements can disseminate from fecal flora to bacterial enteric pathogens.
Azithromycin has been proposed as a possible alternative to SXT for prophylaxis among HIV-infected persons in Africa.8 Azithromycin might provide a replacement antimicrobial for patients with sulfa drug sensitivity or in populations in which SXT nonsusceptibility among important human pathogens becomes sufficiently common so as to impair its efficacy for prophylaxis or treatment. In addition, its spectrum of in vitro activity includes a number of important HIV copathogens such as Streptococcus pneumoniae, Pneumocystis jirovecii, Toxoplasma gondii, and Plasmodium spp. Azithromycin has also been demonstrated to be useful in the treatment of typhoid fever and shigellosis.25,26 Its efficacy in the treatment of typhoid fever suggests that it may also be active against non-Typhi Salmonella. Although we found that the development of SXT-associated rash was uncommon and was consistent with study findings from elsewhere in Africa, suggesting that SXT is well tolerated,1,4,27,28 SXT nonsusceptibility was common among E. coli isolates in our study. Unlike other antimicrobials studied, we found that azithromycin nonsusceptibility did not seem to be coselected by SXT use. In contrast to other studies that have found azithromycin resistance to be uncommon among gram-negative organisms,8,25 however, the proportion of E. coli isolates that were not susceptible to azithromycin in our study exceeded 80%. Comparing azithromycin antimicrobial susceptibility testing results for gram-negative organisms across studies is hampered by the lack of established interpretive criteria for zone sizes for the Kirby-Bauer disk diffusion method and by the occurrence of the dual-zone phenomenon.29 Although we arbitrarily used interpretive criteria for S. aureus14 and read the zone of complete inhibition rather than the zone of partial inhibition on the disk diffusion test, other investigators may have selected different interpretive criteria leading to quite different reported rates of resistance. Nonetheless, the high proportion of E. coli isolates that were not susceptible to azithromycin in our area casts doubt on whether it would be useful to prevent or treat infections caused by gram-negative organisms in our setting.
Our study has a number of limitations. Because of the established efficacy of SXT in preventing morbidity and mortality in HIV-infected patients, our study was of an observational rather than randomized design. This limitation was addressed to some extent by obtaining baseline stool samples from patients in each study group to establish differences in SXT nonsusceptibility before SXT use. The high baseline proportion of E. coli isolates nonsusceptible to SXT limited the number of individuals who could switch from carrying SXT-susceptible E. coli to carrying SXT nonsusceptible E. coli. Despite this limitation, we were able to demonstrate rapid and statistically significant changes in antimicrobial resistance of the fecal indicator organism. Although our loss to follow-up rate was consistent with projections, loss to follow-up may have introduced bias into our results if there were differences in rates of SXT nonsusceptibility between retained and lost patients. Two factors may have diluted the observed effect of SXT on E. coli antimicrobial susceptibility: reported adherence <100% occurred in a quarter of patients in group C, and group A and B subjects were contaminated by the use of short courses of SXT and sulfadoxine-pyrimethamine for intercurrent illness. Finally, our interpretation of the impact of the effect of SXT prophylaxis on antimicrobial resistance in key human pathogens is, by necessity, an extrapolation from observations made on the indicator organism, fecal E. coli. Fecal E. coli has a long track record of use as an indicator organism for resistance among enteric pathogens,30,31 and there is ample evidence that resistance genes are freely shared between fecal flora such as E. coli and clinically important enteric pathogens.22
Our study demonstrates that fecal E. coli, an indicator organism for enteric pathogens, rapidly develops resistance to SXT and a number of other clinically important antimicrobials after initiation of SXT prophylaxis. Furthermore, it is likely that mobile genetic elements would facilitate the movement of the selected resistance genes between fecal flora and enteric pathogens. These data suggest that while the substantial benefits of SXT prophylaxis are realized in Africa, surveillance for its ongoing efficacy for prophylaxis against HIV coinfections and for the empiric management of dysentery, fever, and pneumonia syndromes should be established and maintained. Larger long-term studies are needed to evaluate the impact of widespread use of SXT prophylaxis on these clinical outcomes. In addition, efforts to monitor the prevalence of resistance to other antimicrobials that are coselected by SXT use among important pathogens and research to evaluate alternative effective and inexpensive antimicrobial agents are warranted.
ACKNOWLEDGMENTS
The authors are grateful to the staff of Kikundi cha Wanawake Kilimanjaro Kupambana na UKIMWI (KIWAKKUKI; Women Against AIDS in Kilimanjaro), Angaza, and the Rainbow Centre for referring recent VCT clients and to Dr. Mark. E. Swai, Director of Hospital Services, KCMC, for making space available for patient follow-up for the study. Anna Mchaki, Praxed Moshi, Rhoda Mremi, Robert Shughulu, Helen Y. Chu, L. Brett Caram, Cynthia A. Moylan, Susanna Naggie, and Keren Z. Landman assisted with operations of the follow-up clinic. The authors thank Richard Tarimo and Aloyce Ole Sululu for performing stool screening and storing E. coli isolates at the KCMC Clinical Laboratory; Anne B. Morrissey for assistance with shipping of isolates; Dolores Calley and Hina Patel at the DUMC CMB for assisting with confirmation of organism identification and antimicrobial susceptibility testing; and Francis P. Karia and Stanley Mirrett for administrative support for the study.
The infrastructure to support this study was supplemented by a Comprehensive International Program of Research on AIDS award (R03 AI-01-018), and the study itself was funded by a charitable donation. Additional investigator support was obtained from the Fogarty International Center (D43 PA-03-018; N. M. Thielman, H. O. Ramadhani, J. D. Hamilton, H. J. Shao, J. F. Shao, J. A. Bartlett, and J. A. Crump), the Duke University Center for AIDS Research (P30 AI 64518; J. A. Bartlett), the Duke Clinical Trials Unit and Clinical Research Sites (U01 AI069484-01; N. M. Thielman, J. A. Bartlett, and J. A. Crump), the International Studies on AIDS Associated Co-Infections award (U01 AI-03-036; N. M. Thielman, H. O. Ramadhani, H. J. Shao, J. A. Bartlett, J. F. Shao, and J. A. Crump) of the US National Institutes of Health, and the Hubert-Yeargan Center for Global Health, Duke University Medical Center (S. C. Morpeth).
REFERENCES
- 1.Anglaret X, Chene G, Attia A, et al. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Cote d’Ivoire: a randomised trial. Lancet. 1999;353:1463–1468. doi: 10.1016/s0140-6736(98)07399-1. [DOI] [PubMed] [Google Scholar]
- 2.Chintu C, Bhat GJ, Walker AS, et al. Cotrimoxazole prophylaxis against opportunistic infections in HIV-infected Zambian children (CHAP): a double-blind randomised placebo-controlled trial. Lancet. 2004;364:1865–1871. doi: 10.1016/S0140-6736(04)17442-4. [DOI] [PubMed] [Google Scholar]
- 3.Mermin J, Lule J, Ekwaru JP, et al. Cotrimoxazole prophylaxis by HIV-infected persons in Uganda reduces morbidity and mortality among HIV-uninfected family members. AIDS. 2005;19:1035–1042. doi: 10.1097/01.aids.0000174449.32756.c7. [DOI] [PubMed] [Google Scholar]
- 4.Wiktor SZ, Sassan-Moroko M, Grant AD, et al. Efficacy of trimethoprim-sulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV-1-infected patients with tuberculosis in Abidjan, Cote d’Ivoire: a randomised controlled trial. Lancet. 1999;353:1469–1475. doi: 10.1016/s0140-6736(99)03465-0. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. UNAIDS Provisional WHO/UNAIDS recommendations on the use of cotrimoxazole prophylaxis in adults and children living with HIV/AIDS in Africa. Afr Health Sci. 2001;1:30–31. [PMC free article] [PubMed] [Google Scholar]
- 6.Anglaret X, Sylla-Koko F, Bonard D, et al. Susceptibilities to cotrimoxazole of pathogens isolated from blood and stool specimens in Abidjan, Ivory Coast, 1994 to 1996. J Clin Microbiol. 1997;35:1915. doi: 10.1128/jcm.35.7.1915-1915.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mwansa J, Mutela K, Zulu I, et al. Antimicrobial sensitivity in Enterobacteria from AIDS patients, Zambia. Emerg Infect Dis. 2002;8:92–93. doi: 10.3201/eid0801.010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Oosterhout JJG, Laufer MK, Graham SM, et al. A community-based study of the incidence of trimethoprim-sulfamethoxazole-preventable infections in Malawian adults living with HIV. J Acquir Immune Defic Syndr. 2005;39:626–631. [PubMed] [Google Scholar]
- 9.World Health Organisation Division of Diarrhoea and Acute Respiratory Disease Control. United Nations Children’s Fund Integrated management of the sick child. Bull World Health Organ. 1995;73:735–740. [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organisation. United Nations Children’s Fund . IMCI Chart Booklet. World Health Organization; Geneva, Switzerland: [Google Scholar]
- 11.Morpeth SC, Crump JA, Shao HJ, et al. Predicting CD4 lymphocyte count <200 cells/mm3 in an HIV-1-infected African population. AIDS Res Hum Retroviruses. 2007;23:1230–1236. doi: 10.1089/aid.2007.0053. [DOI] [PubMed] [Google Scholar]
- 12.Forna F, McConnell M, Kitabire FN, et al. Systematic review of the safety of trimethoprim-sulfamethoxazole for prophylaxis in HIV-infected pregnant women: implications for resource-limited settings. AIDS Rev. 2006;8:24–36. [PubMed] [Google Scholar]
- 13.World Health Organisation . Progress on Global Access to HIV Antiretroviral Therapy: A Report on ‘3 by 5’ and Beyond. World Health Organization; Geneva, Switzerland: 2006. [Google Scholar]
- 14.Clinical Laboratories Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing; Sixteenth Informational Supplement. CLSI; Wayne, PA: 2006. [Google Scholar]
- 15.National Committee on Clinical Laboratory Standards . Performance Standards for Antimicrobial Susceptibility Testing; Thirteenth Informational Supplement. NCCLS; Wayne, PA: 2003. [Google Scholar]
- 16.Brooks JT, Ochieng JB, Kumar L, et al. Surveillance for bacterial diarrhea and antimicrobial resistance in rural western Kenya, 1997-2003. Clin Infect Dis. 2006;43:393–401. doi: 10.1086/505866. [DOI] [PubMed] [Google Scholar]
- 17.Zachariah R, Harries AD, Spielman MP, et al. Changes in Escherichia coli resistance to co-trimoxazole in tuberculosis patients and in relation to co-trimoxazole prophylaxis in Thyolo, Malawi. Trans R Soc Trop Med Hyg. 2002;96:202–204. doi: 10.1016/s0035-9203(02)90306-8. [DOI] [PubMed] [Google Scholar]
- 18.Anglaret X. Trimethoprim-sulfamethoxazole prophylaxis in sub-Saharan Africa. Lancet. 2001;358:1027–1028. doi: 10.1016/S0140-6736(01)06227-4. [DOI] [PubMed] [Google Scholar]
- 19.Mermin J, Lule J, Ekwaru JP, et al. Effect of cotrimoxazole prophylaxis on morbidity, mortality, CD4-count, and viral load in HIV infection in rural Uganda. Lancet. 2004;364:1428–1434. doi: 10.1016/S0140-6736(04)17225-5. [DOI] [PubMed] [Google Scholar]
- 20.Zachariah R, Spielman M-PL, Chinji C, et al. Voluntary counselling, HIV testing and adjunctive cotrimoxazole reduces mortality in tuberculosis patients in Thyolo, Malawi. AIDS. 2003;17:1053–1061. doi: 10.1097/00002030-200305020-00015. [DOI] [PubMed] [Google Scholar]
- 21.Mwaungulu FBD, Floyd S, Crampin AC, et al. Cotrimoxazole prophylaxis reduces mortality in human immunodeficiency virus-positive tuberculosis patients in Karonga District, Malawi. Bull World Health Organ. 2004;82:354–363. [PMC free article] [PubMed] [Google Scholar]
- 22.Gassama A, Aidara-Kane A, Chainer D, et al. Integron-associated antibiotic resistance in enteroaggregative and enteroinvasive Escherichia coli. Microb Drug Resist. 2004;10:27–30. doi: 10.1089/107662904323047763. [DOI] [PubMed] [Google Scholar]
- 23.Navia MM, Capitano L, Ruiz J, et al. Typing and characterization of mechanisms of resistance of Shigella spp. isolated from feces of children under 5 years of age from Ifakara, Tanzania. J Clin Microbiol. 1999;37:3113–3117. doi: 10.1128/jcm.37.10.3113-3117.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blahna MT, Zalewski CA, Reuer J, et al. The role of horizontal gene transfer in the spread of trimethoprim-sulfamethoxazole resistance among uropathogenic Escherichia coli in Europe and Canada. J Antimicrob Chemother. 2006;57:666–672. doi: 10.1093/jac/dkl020. [DOI] [PubMed] [Google Scholar]
- 25.Frenck RW, Nakhla IA, Sultan Y, et al. Azithromycin versus ceftriaxone for the treatment of uncomplicated typhoid fever in children. Clin Infect Dis. 2000;31:1134–1138. doi: 10.1086/317450. [DOI] [PubMed] [Google Scholar]
- 26.Khan WA, Seas C, Dhar U, et al. Treatment of shigellosis: V. Comparison of azithromycin and ciprofloxacin. Ann Intern Med. 1997;126:697–703. doi: 10.7326/0003-4819-126-9-199705010-00004. [DOI] [PubMed] [Google Scholar]
- 27.Colebunders R, Izaley L, Bila K, et al. Cutaneous reactions to trimethoprim-sulfamethoxazole in African patients with acquired immunodeficiency syndrome. Ann Intern Med. 1987;107:599–600. doi: 10.7326/0003-4819-107-4-599_2. [DOI] [PubMed] [Google Scholar]
- 28.Maynart M, Lievre L, Sow PS, et al. Primary prevention with cotrimoxazole for HIV-1-infected adults: results of the pilot study in Dakar, Senegal. J Acquir Immune Defic Syndr. 2001;26:130–136. doi: 10.1097/00042560-200102010-00004. [DOI] [PubMed] [Google Scholar]
- 29.Jain SK, Gupta A, Glanz B, et al. Antimicrobial-resistant Shigella sonnei: limited antimicrobial treatment options for children and challenges of interpreting in vitro azithromycin susceptibility. Pediatr Infect Dis J. 2005;24:494–497. doi: 10.1097/01.inf.0000164707.13624.a7. [DOI] [PubMed] [Google Scholar]
- 30.Lederberg J, Lederberg EM. Replica plating and indirect selection of bacterial mutants. J Bacteriol. 1952;63:399–406. doi: 10.1128/jb.63.3.399-406.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walson JL, Marshall B, Pokhrel BM, et al. Carriage of antibiotic-resistant fecal bacteria in Nepal reflects proximity to Kathmandu. J Infect Dis. 2001;184:1163–1169. doi: 10.1086/323647. [DOI] [PubMed] [Google Scholar]