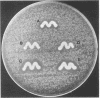
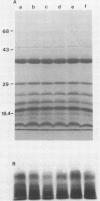
Abstract
Four hundred thirty-eight strains of Haemophilus influenzae were examined for production of and sensitivity to haemocin, a bacteriocin produced by some members of this species. Whereas 199 of 212 (94%) type b isolates produced haemocin, 131 of 134 (98%) nontypeable and 91 of 92 (99%) encapsulated non-type b isolates were sensitive to haemocin. Among strains previously genetically characterized by multilocus enzyme electrophoresis, haemocin production was detected in type b isolates belonging to 25 of 29 (86%) clonally distinct electrophoretic types. None of 60 clonally distinct nontypeable strains produced this substance, and all were sensitive to it in vitro. The genes encoding haemocin production were transformed independently of the genes necessary for capsule expression from a prototypic type b strain to a nontypeable strain. After intranasal inoculation of infant rats with an equal mixture of a non-haemocin-producing strain and its haemocin-producing transformant, organisms capable of haemocin production predominated in both nasopharyngeal and blood cultures. These data demonstrate that haemocin production is strongly associated with type b encapsulated members of this species and suggest a mechanism by which haemocin might play a role in host nasopharyngeal colonization by this pathogen.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. W., Pichichero M. E., Connor E. M. Enhanced nasopharyngeal colonization of rats by piliated Haemophilus influenzae type b. Infect Immun. 1985 May;48(2):565–568. doi: 10.1128/iai.48.2.565-568.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braude A. I., Siemienski J. S. The influence of bacteriocins on resistance to infection by gram-negative bacteria. II. Colicin action, transfer of colicinogeny, and transfer of antibiotic resistance in urinary infections. J Clin Invest. 1968 Aug;47(8):1763–1773. doi: 10.1172/JCI105866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin B. W., Bendler J. W., 3rd, Goodgal S. H. The type b capsulation locus of Haemophilus influenzae: map location and size. J Gen Microbiol. 1972 May;70(3):411–422. doi: 10.1099/00221287-70-3-411. [DOI] [PubMed] [Google Scholar]
- Chapin K. C., Doern G. V. Selective media for recovery of Haemophilus influenzae from specimens contaminated with upper respiratory tract microbial flora. J Clin Microbiol. 1983 Jun;17(6):1163–1165. doi: 10.1128/jcm.17.6.1163-1165.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajani A. S., Asmar B. I., Thirumoorthi M. C. Systemic Haemophilus influenzae disease: an overview. J Pediatr. 1979 Mar;94(3):355–364. doi: 10.1016/s0022-3476(79)80571-5. [DOI] [PubMed] [Google Scholar]
- Farkas-Himsley H., Yu H. Purified colicin as cytotoxic agent of neoplasia: comparative study with crude colicin. Cytobios. 1985;42(167-168):193–207. [PubMed] [Google Scholar]
- Fuska J., Fusková A., Smarda J., Mach J. Effect of colicin E3 on leukemia cells P388 in vitro. Experientia. 1979 Mar 15;35(3):406–407. doi: 10.1007/BF01964380. [DOI] [PubMed] [Google Scholar]
- Gilsdorf J. R., Ferrieri P. Role of nasopharyngeal colonization with and without bacteremia in the protection of infant rats against Haemophilus influenzae type b challenge. Infect Immun. 1985 Mar;47(3):648–653. doi: 10.1128/iai.47.3.648-653.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy K. G. Colicinogeny and related phenomena. Bacteriol Rev. 1975 Dec;39(4):464–515. doi: 10.1128/br.39.4.464-515.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herriott R. M., Meyer E. M., Vogt M. Defined nongrowth media for stage II development of competence in Haemophilus influenzae. J Bacteriol. 1970 Feb;101(2):517–524. doi: 10.1128/jb.101.2.517-524.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiseth S. K., Gilsdorf J. R. The relationship between type b and nontypable Haemophilus influenzae isolated from the same patient. J Infect Dis. 1988 Sep;158(3):643–645. doi: 10.1093/infdis/158.3.643. [DOI] [PubMed] [Google Scholar]
- Kilian M., Mestecky J., Schrohenloher R. E. Pathogenic species of the genus Haemophilus and Streptococcus pneumoniae produce immunoglobulin A1 protease. Infect Immun. 1979 Oct;26(1):143–149. doi: 10.1128/iai.26.1.143-149.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Hansen E. J. Antigenic and phenotypic variations of Haemophilus influenzae type b lipopolysaccharide and their relationship to virulence. Infect Immun. 1986 Jan;51(1):69–79. doi: 10.1128/iai.51.1.69-79.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konisky J. Colicins and other bacteriocins with established modes of action. Annu Rev Microbiol. 1982;36:125–144. doi: 10.1146/annurev.mi.36.100182.001013. [DOI] [PubMed] [Google Scholar]
- Michaels R. H., Stonebraker F. E., Robbins J. B. Use of antiserum agar for detection of Haemophilus influenzae type b in the pharynx. Pediatr Res. 1975 May;9(5):513–516. doi: 10.1203/00006450-197505000-00010. [DOI] [PubMed] [Google Scholar]
- Moxon E. R., Murphy P. A. Haemophilus influenzae bacteremia and meningitis resulting from survival of a single organism. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1534–1536. doi: 10.1073/pnas.75.3.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxon E. R., Smith A. L., Averill D. R., Smith D. H. Haemophilus influenzae meningitis in infant rats after intranasal inoculation. J Infect Dis. 1974 Feb;129(2):154–162. doi: 10.1093/infdis/129.2.154. [DOI] [PubMed] [Google Scholar]
- Moxon E. R., Vaughn K. A. The type b capsular polysaccharide as a virulence determinant of Haemophilus influenzae: studies using clinical isolates and laboratory transformants. J Infect Dis. 1981 Apr;143(4):517–524. doi: 10.1093/infdis/143.4.517. [DOI] [PubMed] [Google Scholar]
- Musser J. M., Barenkamp S. J., Granoff D. M., Selander R. K. Genetic relationships of serologically nontypable and serotype b strains of Haemophilus influenzae. Infect Immun. 1986 Apr;52(1):183–191. doi: 10.1128/iai.52.1.183-191.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser J. M., Kroll J. S., Moxon E. R., Selander R. K. Evolutionary genetics of the encapsulated strains of Haemophilus influenzae. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7758–7762. doi: 10.1073/pnas.85.20.7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Rubin L. G., Moxon E. R. Haemophilus influenzae type b colonization resulting from survival of a single organism. J Infect Dis. 1984 Feb;149(2):278–278. doi: 10.1093/infdis/149.2.278. [DOI] [PubMed] [Google Scholar]
- Senior B. W. The purification, structure and synthesis of proticine 3. J Med Microbiol. 1983 Aug;16(3):323–331. doi: 10.1099/00222615-16-3-323. [DOI] [PubMed] [Google Scholar]
- Smith A. L., Smith D. H., Averill D. R., Jr, Marino J., Moxon E. R. Production of Haemophilus influenzae b meningitis in infant rats by intraperitoneal inoculation. Infect Immun. 1973 Aug;8(2):278–290. doi: 10.1128/iai.8.2.278-290.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs D. R. Bacteriocins and antagonism: the killing fields. J Infect Dis. 1986 Apr;153(4):809–810. [PubMed] [Google Scholar]
- Streelman A. J., Snyder I. S., Six E. W. Modifying effect of colicin on experimental Shigella keratoconjunctivitis. Infect Immun. 1970 Jul;2(1):15–23. doi: 10.1128/iai.2.1.15-23.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stull T. L., LiPuma J. J., Edlind T. D. A broad-spectrum probe for molecular epidemiology of bacteria: ribosomal RNA. J Infect Dis. 1988 Feb;157(2):280–286. doi: 10.1093/infdis/157.2.280. [DOI] [PubMed] [Google Scholar]
- Stull T. L., Mack K., Haas J. E., Smit J., Smith A. L. A comparison of techniques for isolation of the outer membrane proteins of Haemophilus influenzae type b. Anal Biochem. 1985 Nov 1;150(2):471–480. doi: 10.1016/0003-2697(85)90537-8. [DOI] [PubMed] [Google Scholar]
- Stull T. L., Mendelman P. M., Haas J. E., Schoenborn M. A., Mack K. D., Smith A. L. Characterization of Haemophilus influenzae type b fimbriae. Infect Immun. 1984 Dec;46(3):787–796. doi: 10.1128/iai.46.3.787-796.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuy J. H. On the nature of nontypable Haemophilus influenzae. Antonie Van Leeuwenhoek. 1978;44(3-4):367–376. doi: 10.1007/BF00394313. [DOI] [PubMed] [Google Scholar]
- Tagg J. R., Dajani A. S., Wannamaker L. W. Bacteriocins of gram-positive bacteria. Bacteriol Rev. 1976 Sep;40(3):722–756. doi: 10.1128/br.40.3.722-756.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takala A. K., van Alphen L., Eskola J., Palmgren J., Bol P., Mäkelä P. H. Haemophilus influenzae type b strains of outer membrane subtypes 1 and 1c cause different types of invasive disease. Lancet. 1987 Sep 19;2(8560):647–650. doi: 10.1016/s0140-6736(87)92440-8. [DOI] [PubMed] [Google Scholar]
- Turk D. C. The pathogenicity of Haemophilus influenzae. J Med Microbiol. 1984 Aug;18(1):1–16. doi: 10.1099/00222615-18-1-1. [DOI] [PubMed] [Google Scholar]
- Venezia R. A., Matusiak P. M., Robertson R. G. Bactericidal factor produced by Haemophilus influenzae b: partial purification of the factor and transfer of its genetic determinant. Antimicrob Agents Chemother. 1977 Apr;11(4):735–742. doi: 10.1128/aac.11.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venezia R. A., Robertson R. G. Bactericidal substance produced by Haemophilus influenzae b. Can J Microbiol. 1975 Oct;21(10):1587–1594. doi: 10.1139/m75-232. [DOI] [PubMed] [Google Scholar]