Abstract
Background
Experience with non-antigenic galactose α1,3 galactose (αGal) polymers and development of αGal deficient pigs has reduced or eliminated the significance of this antigen in xenograft rejection. Despite these advances, delayed xenograft rejection (DXR) continues to occur most likely due to antibody responses to non-Gal endothelial cell (EC) antigens.
Methods
To gauge the diversity of the non-Gal antibody response we used antibody derived from CD46 transgenic heterotopic cardiac xenografts performed without T-cell immunosuppression, Group A (n = 4) and Gal knockout (GT-KO) heart transplants under tacrolimus and sirolimus immunosuppression, Group B (n = 8). Non-Gal antibody was measured by flow cytometry and by Western blots using GT-KO EC membrane antigens. A nanoLC/MS/MS analysis of proteins recovered from 2D gels was used to identify target antigens.
Results
Group A recipients exhibited a mixed cellular and humoral rejection. Group B recipients mainly exhibited classical DXR. Western blot analysis showed a non-Gal antibody response induced by GT+ and GT-KO hearts to an overlapping set of pig aortic EC membrane antigens. Proteomic analysis identified 14 potential target antigens but failed to define several immunodominant targets.
Conclusions
These experiments indicate that the non-Gal antibody response is directed to a number of stress response and inflammation related pig EC antigens and a few undefined targets. Further analysis of these antibody specificities using alternative methods is required to more fully define the repertoire of non-Gal antibody responses.
Keywords: xenotransplantation, endothelial cell, non-Gal antibody, proteomics
Introduction
Xenotransplantation has the potential to resolve the chronic shortage of organs for transplantation if innate and induced immune responses to the graft can be controlled. Cardiac xenograft rejection was initially dominated by hyperacute rejection (HAR) which is dependent on complement and preformed anti-Gal antibody (1–3). When HAR is blocked xenografts succumb to delayed xenograft rejection (DXR) within days to a few months. This rejection is characterized by vascular antibody deposition and microvascular thrombosis and coincides with an induction of anti-Gal antibody (4, 5). Nonantigenic polymers of αGal trisaccharide, such as TPC and GAS, can effectively block anti-Gal antibody in vivo and reduce or eliminate the induction of anti-Gal antibody after transplantation (6–9). When coupled to appropriate immunosuppression TPC can block anti-Gal sensitization and results in prolonged organ survival (10). Under these conditions or when αGal deficient (GT-KO) pig organs are used, xenograft rejection remains associated with antibody deposition, variable complement activation and microvascular thrombosis (9, 11–14). Induction of circulating non-Gal anti-pig antibody has been reported in some recipients (9, 13) and recovery of non-Gal anti-pig antibody is associated with organ rejection (11). These observations suggest that xenograft rejection, in the absence of an anti-Gal response, is limited by an antibody response to non-Gal pig antigens. Alternatively, incompatibilities between pig and primate regulation of coagulation may create an inherently procoagulant xenograft vasculature and thereby contribute to DXR. Although coagulation incompatibilities are well defined in vitro (15–17) we and others (11, 18, 19) have shown that several anticoagulant regimens fail to prolong xenograft survival and do not eliminate microvascular thrombosis. This suggests to us that antibody responses to the xenograft remain the dominant initiating factor in xenograft failure.
There is no direct evidence identifying the specificity of non-Gal antibody in the pig to primate system. Buhler et al using sensitized sera from a variety of GT+ xenograft procedures reported that non-Gal antibody was not directed towards a limited number of carbohydrate antigens and showed only minor anti-SLA specificity (20). Similarly Tseng et al analyzed sera from GT-KO cardiac xenograft recipients and found that non-Gal antibody was directed to shared antigens present in all three swine SLA haplotypes (21). In this report we used IgG purified from sensitized GT+ cardiac xenograft recipient sera and IgG recovered from rejected GT-KO cardiac xenografts for a Western blot and proteomic analysis of the specificity of non-Gal antibody.
Materials and Methods
Animals and transplants
Transgenic donor pigs (Sus scrofa) expressing the human complement regulatory protein CD46 have been previously described (22). GT-KO pigs produced at the Mayo Clinic were derived from pig fetal fibroblasts with a targeted insertion in the GGTA-1 locus (23). Recipient adult olive baboons (Papio anubis) were supplied by the Southwest Regional Primate Research Center, San Antonio, TX. All animals were housed and received humane care in accordance with the standards established by the Institutional Animal Care and Use Committee of the Mayo Clinic and as described in the “Guide for the Care and Use of Laboratory Animals”(NIH publication no. 86-23, revised 1996).
Group A (n = 4) heterotopic transplants using CD46 donors without T-cell immunosuppression have been previously described (24). Recipients were splenectomized prior to transplant and received no T-cell immunosuppression. One transplant was performed without further treatment. Other recipients received TPC (n = 2), a polymer of polyethylene glycol and αGal trisaccharide (6) or received TPC and Rituximab (Genentech Inc., South San Francisco, CA) (n = 1) to modulate anti-Gal antibody. TPC was administered daily until necropsy which was performed 14 days after graft removal.
Group B (n = 8) heterotopic GT-KO cardiac xenograft recipients were splenectomized on postoperative day (POD) -7 and treated with Rituximab at 19mg/kg on POD -7 and weekly for four doses. Immunosuppression with tacrolimus and sirolimus began on POD -6 and was as previously described (11). Induction with rabbit ATG (1.5mg/kg) began on POD 1 for 5 consecutive treatments. Prophylactic antibiotic and anti-viral therapy but no anticoagulation was used.
Cell isolation and culture
Pig aortic endothelial cells (PAEC) were isolated from GT-KO aortas by lightly swabbing the luminal side of the aorta with a cotton-tipped swab soaked in 0.05% trypsin (Invitrogen Corp, Carlsbad, CA). Cells were cultured in EGM media (Cambrex BioScience, Walkersville, MD) and incubated at 37°C, 5% CO2. PAEC identification was based on a cobblestone morphology, expression of CD31 and induction of swine leukocyte antigen class II after stimulation with porcine IFNγ (Biosource, Camarillo, CA.) as detected by the corresponding antibodies (anti-CD31; Serotec, Raleigh, NC. anti-swine DR; BD Biosciences, San Diego, CA).
Antibody recovery and purification
Antibodies were eluted from rejected pig heart tissue as previously described (11). Eluted material typically contained 10 – 50 μg/ml IgG. Recovered antibody was diluted with an equal volume of FACS buffer (1%BSA, PBS, 0.01% sodium azide) for flow cytometry and Western blots were performed using a 1:4 dilution. Group A IgG was purified from necropsy serum using Prosep-G chromatography (Millipore, Billerica, MA).
Flow cytometry
PAECs (50 – 100 × 103 cells) were stained with 50 μl of heat inactivated baboon serum diluted into FACS buffer for 45 minutes at 4°C, washed in 3 ml FACS buffer and labeled for 30 minutes at 4°C with Zymed FITC conjugated goat anti-human IgG or IgM (Invitrogen Corp). Cells were analyzed in the Becton Dickson FACscalibur using CellQuest software. Mean fluorescence was calculated as (specific mean fluorescence -mean fluorescence of the FITC-secondary antibody alone).
Protein antigen preparation, Western blot and proteomic analysis
Endothelial cell membrane proteins were enriched from 0.5 – 1.0 × 108 cultured GT-KO PAECs. Cultured cells were resuspended in hypotonic buffer (10mM Tris (7.5), 0.5mM MgCl2) at 1 × 107 cells/ml, disrupted using a Dounce homogenizer. The tonicity of the buffer was restored by adding 10mM Tris (7.5), 0.5mM MgCl2 and 0.6M NaCl to achieve a NaCl concentration of 0.15mM. Debris was removed by centrifugation at 500 × g, 4°C for 5 minutes. EDTA (pH 7.6) was added to 5mM and the membrane fraction was recovered by ultracentrifugation at 85,000 × g in a Beckman Optima TLX ultracentrifuge (Beckman Coulter Inc, Fullerton, CA). The membrane pellet was resuspended in 50mM Tris (7.5), 150 mM NaCl, 0.5% Triton X-100. All buffers contained a cocktail of protease inhibitors (Roche Diagnostics, Mannheim, Germany).
For standard single dimension Western blot analysis membrane enriched proteins were electrophoresed using a BioRad 10.5–14% Criterion acrylamide gels (BioRad, Hercules, CA). For 2D Westerns PAEC membrane enriched antigens were electrophoresed in IPG strips (pH ranges 3–10, 5–8 and 3–6) using a BioRad PROTEAN IEF Cell and subsequently electrophoresed in the second dimension as above. Proteins were transferred to Immobilon P PVDF membranes for Western blot analysis (Millipore Inc, Boston, MA). Primary antibody diluted into blocking buffer (PBS containing 0.3% Tween-20, 5% milk, 3% BSA) consisted of purified naïve baboon or Group A IgG or IgG recovered from rejected GT-KO tissue. Antibody binding was detected with an HRP conjugated goat anti-human IgG (Invitrogen Corp) and chemiluminescence using Kodak Biomax Light film and SuperSignal West Femto Max. Sensitivity Substrate. (Pierce Biotechnology, Rockford, IL). Protein spots were excised from representative gels and peptides were extracted after trypsin digestion by 2% trifluoroacetic acid and acetonitrile. Peptides were analyzed by nanoflow LC/MS/MS on a LTQ (Thermo Scientific, Waltham, MA) and the results were searched using Mascot 8 and Scaffold (Proteome Software, Portland, OR). Protein identification required a 99% probability with a minimum of 2 peptides per protein (25, 26).
Isolation of GT-KO plasma fibronectin and ELISA analysis
Porcine fibronectin was purified from GT-KO plasma using a Gelatin-Sepharose 4B (GE Healthcare, Piscataway, NJ) column and further purified by size exclusion chromatography using a Superdex 200 16/60 column (GE Healthcare). For ELISA detection of induced anti-fibronectin antibody the purified GT-KO fibronectin was biotinylated using Sulfo NHS-biotin (Pierce Biotechnology) and biotin-fibronectin (5 μg/ml) was bound to streptavidin-coated (10 μg/ml) ELISA plates. Baboon serum was diluted in PBS with 1% BSA, 0.3% Tween-20 and IgG binding to fibronectin was detected using HRP conjugated goat anti-human IgG (Bethyl Labs, Montgomery, TX).
Results
Graft rejection and histology
Group A transplants underwent a vigorous cellular and humoral rejection within 5 – 7 days which was characterized by prominent lymphocytic infiltrates (Figure 1A) with vascular deposition of antibody and complement (data not shown). In Group B graft survival ranged from 0 to 128 days with median survival of 24.5 days. One recipient died with a vigorously contracting xenograft on POD 2 as a result of a vascular hemorrhage. All other xenografts were rejected as evidenced by the complete loss or severe reduction in contractility. In one instance a group B xenograft rejected 90 minutes after reperfusion and exhibited widespread intragraft hemorrhage (Figure 1B) with vascular antibody and complement deposition (data not shown) consistent with hyperacute rejection. The histology, immunopathology and timing of this rejection was consistent with a preformed non-Gal antibody induced hyperacute rejection. All other grafts underwent DXR and showed coagulative necrosis with microvascular thrombosis (Figure 1C). Further details of Group B transplant results will be provided in a separate publication.
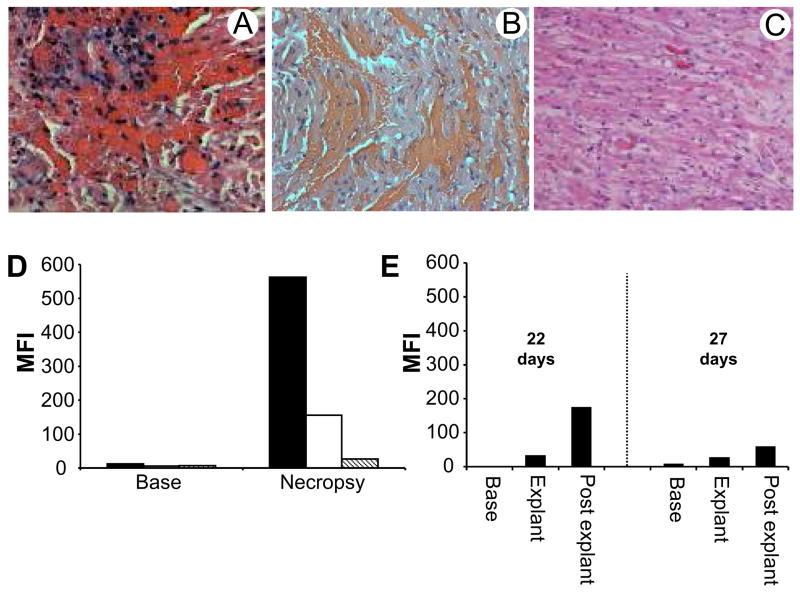
Figure 1.
Histology and antibody responses after cardiac xenotransplantation. A. Representative histology of Group A rejected xenografts exhibiting florid intragraft cellular infiltration. B. Histology showing widespread hemorrhage of a GT-KO Group B xenograft rejected 90 minutes after reperfusion. C. Typical histology of Group B rejected xenografts showing microvascular thrombosis and myocardial ischemic injury. D. The graph shows mean fluorescence intensity of IgG staining on GT-KO PAECs stained with pretransplant (Base) and necropsy serum (Necropsy) from Group A recipients. No treatment (black), treated with TPC (white) and treated with TPC and Rituximab (striped). E. Graph shows mean fluorescence intensity of IgG binding to GT-KO PAEC after staining with pretransplant (base), explant and post explant serum from Group B recipients that rejected their grafts on days 22 and 27.
Non-Gal Antibody Responses
Group A recipients, without immunosuppression, showed a strong induction of non-Gal IgG in necropsy serum that bound to GT-KO PAECs (Figure 1D). The highest induction was evident in the untreated recipient with progressively weaker responses detected in the TPC and TPC plus Rituximab treated recipients. In Group B two recipients rejected their xenografts on day 22 and 27 and survived organ removal. These recipients showed a moderate anti-PAEC non-Gal IgG response that was evident at the time of rejection and in post explant serum (Figure 1E). No induced response was observed in the other Group B recipients prior to explant (data not shown).
Complexity of the induced anti-pig antibody
The highly sensitized necropsy serum from Group A recipients was well suited for a Western blot analysis. Purified IgG from Group A necropsy serum was diluted to 3 – 10 μg/ml for optimal Western blot results. For a negative control IgG (5 μg/ml) purified from a pool of naïve baboon serum was used. At these concentrations the sensitized IgG, but not the naïve IgG, bound to extracellular membrane antigens on GT-KO PAECs (Figure 2A). The IgG from sensitized recipients uniformly bound to a group of high molecular weight bands greater than 150 kDa in 1D Western blots to GT-KO PAEC membrane enriched antigens (Figure 2B). The level of IgG binding to these antigens appeared to parallel the reactivity of serum IgG binding to PAECs with the strongest reactivity present in the untreated recipient and progressively weaker reactivity in the TPC and TPC and Rituximab treated recipients. Additionally a doublet of approximately 130 and 140 kDa was prominently detected with IgG from the untreated recipient. One or both of these bands may also be present in the TPC-treated recipients but they were not detected by IgG from the TPC and Rituximab treated recipient. Three other immunodominant bands at 35, 27 and 19 kDa were observed along with a series of less reactive material between 37 and 100 kDa. Both TPC treated recipients showed a very similar immunoreactivity (data not shown). Under these conditions the naïve IgG (lane 1) did not reproducibly bind to any PAEC GT-KO membrane enriched antigens.
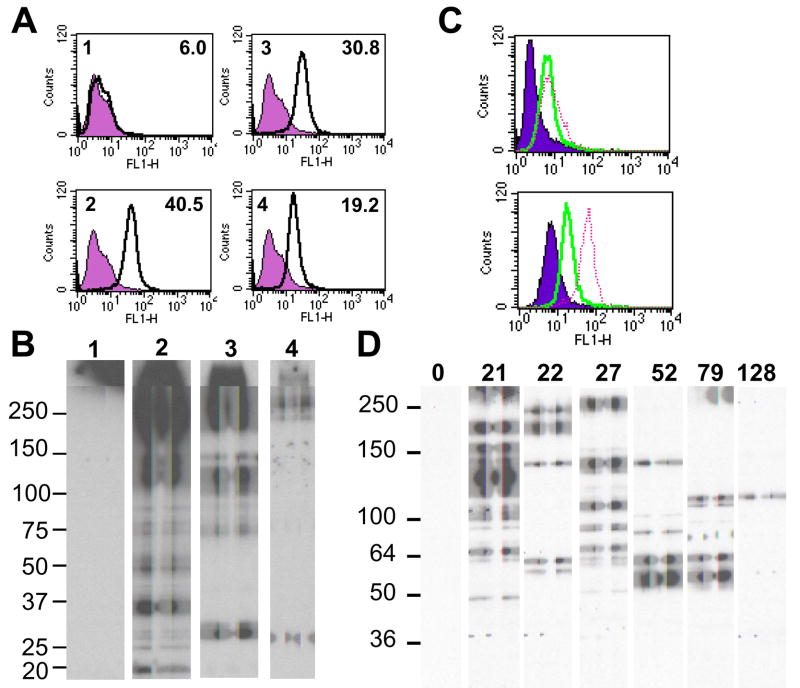
Figure 2.
Specificity of induced anti-pig IgG. A. Flow cytometry measuring the binding of IgG purified from Group A necropsy serum to GT-KO PAECs. Upper left number in each panel indicates the source of the IgG and the number in the upper right is the mean fluorescence intensity. Specific binding is the dark line. Background binding (filled histogram) was determined using only the FITC conjugated secondary antibody. 1; IgG from a pool of naive baboon serum. 2 – 4; IgG purified from necropsy serum of recipients that received no treatment for antibody (2), TPC (3), or TPC and Rituximab (4). B. Western blot analysis of purified Group A IgG binding to GT-KO PAEC membrane antigens. Numbers of each lane indicate the source of IgG and correspond to FACS panels in A. C. Flow cytometry analysis of IgG binding to GT-KO PAECs using antibody recovered from rejected Group B xenografts. Xenograft rejected on day 52 (solid) and day 79 (dashed) are presented in the top panel and xenograft rejected on day 21 (solid) and day 27 (dashed) in the bottom panel. Background binding (filled) was determined using the FITC secondary antibody only. D. Western blot detection of recovered IgG binding to GT-KO PAEC membrane antigens. Duration of graft survival is indicated for each lane.
In Group B only 2 of the 7 recipients that rejected their xenograft (graft survival 22 and 27 days) survived for an appreciable time (>7 days) after organ removal. Since the graft is expected to absorb the majority of induced serum antibody we chose to recover IgG from each of the GT-KO rejected hearts in order to get a broader assessment of non-Gal antibody bound to these xenografts. This recovered antibody exhibited variable levels of IgG binding to GT-KO PAECs as illustrated for antibody recovered from grafts rejected on POD 52, 79, 21 and 27 (Figure 2C). The recovered IgG bound to a variety of PAEC membrane enriched antigens in 1D Western blots, some of which appeared to be detected by antibody recovered from more than one xenograft (Figure 2D). Notably a band of approximately 140kDa appeared to be detected with antibody recovered from grafts rejected on days 21, 22, 27 and 52. Prominent reactivity was also evident to bands of 57 and 62kDa in grafts rejected on days 52 and 79 and to a band of 110 kDa in graft rejected on days 79 and 128. Variable immunoreactivity to antigens greater than 150kDa was also evident with antibodies recovered from grafts rejected on days 21, 22 and 27. The antibody recovered from the group B xenograft lost due to an apparent HAR (day 0) exhibited negligible IgG binding in the Western blot (Figure 2D). This is consistent with the general absence of preformed serum non-Gal IgG in baboons and suggests that this apparent HAR may have resulted form the effects of preformed non-Gal IgM.
Specificity of non-Gal antibody
To determine the specificity of non-Gal antibodies present after cardiac xenotransplantation we used IgG from the untreated and from a TPC-treated recipient in Group A (corresponding to lanes 2 and 3 of Figure 2B) and a pool of IgG recovered from 4 of the rejected GT-KO hearts (rejected on days 79, 52, 27 and 21, Figure 2D) to probe 2D Western blots. These antibody sources appeared to encompass the entire repertoire of IgG specificity as defined by 1D Western blots. A representative 2D Western blot is presented in Figure 3A and a syproRed stained gel showing total protein summarizes all of the spots analyzed in this study (Figure 3B). Many of the most prominent immunodominant antigens detected in 1D Western blots were poorly detected in 2D Westerns. This was particularly true for antigens greater than 150 kDa that were commonly observed in the Group A transplants. An example of this phenomenon is illustrated in Figure 3A which shows IgG binding to a prominent band greater then 150 kDA when the membrane enriched antigens are separated by molecular weight in one dimension and the absence of a corresponding spot when the antigens are separated in two dimensions. To partially address this issue we used a high resolution 1D SDS polyacrylamide gel and the sensitized Group A baboon IgG to resolve the high molecular weight complex into a 250 kDa doublet and a band of 180kDa (Figure 4A). Trypsinized fragments recovered from these bands identified fibronectin as the predominant antigen in the 250kDa doublet and MG-160 (also called Human golgi apparatus protein -1 or E-selectin ligand) in the other band (Table 1). Since fibronectin is a ubiquitous protein and since 1D electrophoretic separation is not an ideal way to isolate proteins for peptide analysis we purified serum fibronectin from a GT-KO pig and used this material to access the anti-fibronectin response. Antibody recovered from Group A xenografts bound to the purified fibronectin in a 1D Western blot (Figure 4B). An ELISA analysis comparing pretransplant and necropsy serum showed that all Group A recipients exhibited an increase in the titer of anti-fibronectin IgG (Figure 4C). A weaker increase in anti-fibronectin antibody was also observed in post explant serum from the two of the Group B transplants which survived organ removal (Figure 4D).
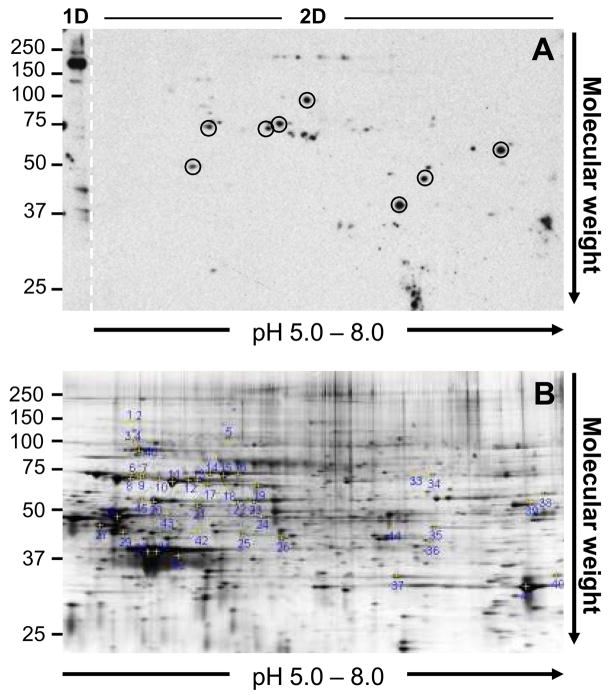
Figure 3.
Proteomic analysis of non-Gal antigens. A. Representative 2D Western blot of Group A IgG binding to membrane enriched GT-KO PAEC antigens. The left edge of the gel (labeled 1D and demarcated with a dashed line) contains an individual lane of GT-KO PAEC membrane enriched antigens which were separated by molecular weight in one dimension only. The remainder of the gel (labeled 2D) is the second dimension separation of membrane enriched antigens after previous isoelectric focusing through a pH 5 – 8 IPG strip. The circled spots in the Western corresponded to immunoreactive spots which aligned with spots in the total protein gel and were excised for nanoLC/MS/MS peptide analysis. Note that the prominent immunoreactive band above 150kDa detected after separating membrane enriched proteins by molecular weight only (see 1D section) lacks a corresponding spot when the membrane enriched antigens are separated by both isoelectric focusing and molecular weight (see 2D section). B. A representative syproRed stained 2D gel showing total protein distribution and indicating the position of each spot recovered and analyzed in this study. The Western blot in A and the total protein gel in B are not matched.
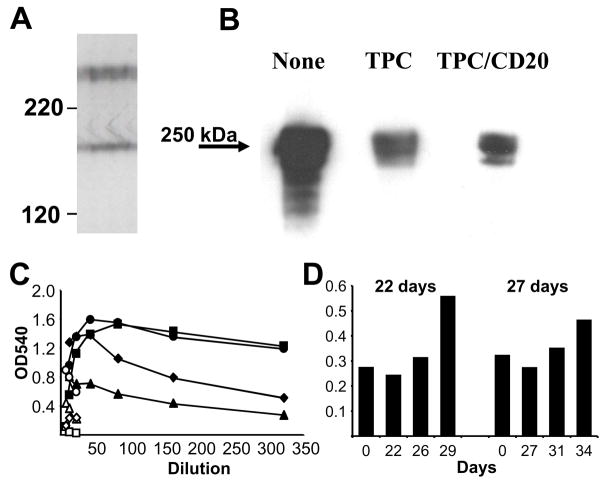
Figure 4.
Characterization of high molecular weight non-Gal antigens detected by Group A antibody. A. High resolution 1D Western blot of high molecular weight antigens detected by IgG purified from a TPC treated recipient. B. Western blot analysis of antibody eluted from Group A rejected xenograft binding to purified pig fibronectin (1 μg/lane) electrophoresed in a 5% denaturing acrylamide gel. The treatment for each recipient is presented above the lane. C. ELISA analysis of pretransplant (open symbols) and necropsy serum (closed symbols) of Group A IgG binding to porcine fibronectin. No treatment (circle), TPC treated (square and triangle), TPC and Rituximab treated (diamond). D. ELISA analysis of anti-fibronectin IgG activity in 2% serum from Group B recipients that survived explant. The x-axis indicates the transplant day for each serum sample and the explant day is indicated above each graph.
Table 1.
| Prospective non-Gal anti-EC targets | ||||
|---|---|---|---|---|
| Protein | Name | MW (kDa) | Function | |
| P07589 | Fibronectin a | 249 | Extracellular matrix | |
| Q92896 | MG-160 a | 180 | Membrane protein | |
| Q9Y4L1 | ORP150 a | 111 | Stress response | |
| P34932 | HSP70RY a,b | 94 | Stress response | |
| P14625 | HSP90B1 a,b | 92 | Stress response | |
| P38646 | GRP75 a | 74 | Stress response | |
| P11021 | GRP78 a | 72 | Stress response | |
| P11142 | HSC70 a,b | 70 | Stress response | |
| P79134 | Annexin A6 a | 69 | Inflammation | |
| P10809 | HSP-60 a,b | 61 | Stress response | |
| P40227 | TCP1a,b,c | 58 | Stress response | |
| P08670 | Vimentin a,b | 53 | Cytoskeleton | |
| P19619 | Annexin A1 a | 38 | Inflammation | |
| P07355 | Annexin A2 b | 38 | Inflammation | |
| Additional non-Gal anti-EC targets | ||||
| Protein | Name | MW (kDa) | Function | |
| Q14764 | Major vault protein a | 99 | Signaling | |
| P55072 | Transitional ER ATPaseb | 89 | Biosynthesis | |
| P16276 | Aconitate hydratasea | 85 | Metabolism | |
| P27594 | Mx1a,b | 75 | Unknown | |
| Q13409 | Dynein intermediate chain a | 71 | Cytoskeletal | |
| P13796 | Plastin-2 b | 70 | Cytoskeletal | |
| P37879 | Lysyl-tRNA synthetase a | 68 | Biosynthesis | |
| P26038 | Moesin a | 68 | Cytoskeletal | |
| Q32LP2 | Radixin b | 68 | Cytoskeletal | |
| P14866 | hnRNP-La | 60 | Biosynthesis | |
| Q5R8T5 | Tyrosyl-tRNA synthase a,b | 59 | Biosynthesis | |
| P14618 | Pyruvate kinasea | 58 | Metabolism | |
| P38657 | Protein disulfide isomerasea | 57 | Biosynthesis | |
| P20000 | aldehyde dehydrogenase b | 56 | Metabolism | |
| P00829 | ATP synthase b-chaina,b | 56 | Metabolism | |
| Q2TBU9 | RuvB-like 2 b | 51 | Biosynthesis | |
| P81948 | Tubulin b | 50 | Cytoskeletal | |
| P49410 | Elongation factor Tua | 49 | Biosynthesis | |
| P17980 | 26S protease regulatory subunit b | 49 | Catabolism | |
| P06733 | Alpha-enolasea,b | 47 | Metabolism | |
| P61158 | actin like protein 3b | 47 | Cytoskeletal | |
| P63261 | actina,b | 42 | Cytoskeletal | |
| P00346 | Malate dehydrogenase a | 35 | Metabolism | |
| P15924 | desmoplakin b | 33 | Cytoskeletal | |
Identified with antibody from sensitized baboon serum.
Identified with antibody recovered from GT-KO heart transplants.
Antibody responses to TCP-1 beta, theta and zeta subunits detected. Molecular weights are based on the values listed in the UniProtKB/Swiss-Prot database.
All other non-Gal antigens in Table 1 were identified by recovering proteins identified in 2D Western blot. In addition to fibronectin and MG-160, 36 proteins were identified from 2D gels and 66% of these (n = 24) were cytoplasmic proteins involved in cellular metabolism or components of the cytoskeleton. These cytoplasmic proteins were identified at similar frequencies using Group A IgG purified from sensitized serum (identified 15 of 24 proteins) or IgG recovered from rejected GT-KO tissue (identified 14 of 24) with 5 of 24 proteins detected by both antibody sources. The remaining proteins (n = 12) included members of the heat shock family of stress response proteins, members of the annexin family and vimentin. Group A IgG detected nearly all of these proteins (11 of 12) and 6 of 12 were detected by both antibody sources.
Discussion
Organs from GT-KO pigs are widely considered to be central for clinical xenotransplantation of solid organs. Thus non-Gal antibody will play an important role in xenograft rejection and identifying these antigens will be essential for monitoring the efficacy of immunosuppression and potentially for developing strategies for establishing antigen specific tolerance (27–31). This study was an initial foray into the identification of non-Gal antibody specificities after pig-to-primate cardiac xenotransplantation. In this report we use a Western blot and proteomic analysis. These techniques have been widely used to identify antibody targets in allotransplantation and in the identification of tumor antigens (32–34). For our study we used two antibody sources; IgG purified from sensitized serum of baboons transplanted with GT+ hearts in the absence of T-cell immunosuppression (Group A) and IgG recovered from GT-KO cardiac xenografts performed under tacrolimus and sirolimus immunosuppression (Group B). Both antibody sources have advantages and disadvantages. Group A antibody had a high titer and was well suited for Western blot procedures, but the antibody was produced in response to GT+ xenografts and was collected 2 weeks after organ explant and may contain antibody not directly related to initiation of xenograft rejection. Group B antibody was produced in the presence of immunosuppression which is likely to be a component of any clinical program but the antibody recovered from rejected GT-KO transplants is a crude mixture of proteins, has limited antibody concentration and may also contain specificities directed to damaged or necrotic tissue. In any case both antibody sources contained IgG that bound to GT-KO PAEC cells (Figure 2A and C) and based on 1D Westerns they seemed to provide a wide array of non-Gal specificities to access the diversity of non-Gal antigens.
Antibody from Group A transplants exhibited strong reactivity to a set of high molecular weight bands (greater than 150kDa) and a few other immunodominant bands in 1D Western blots (Figure 2B). The immunoreactivity to antigens greater than 150 kDa was distinct from the antigens previously detected with anti-Gal antibody (35) and was in part directed towards porcine fibronectin and MG-160. In the case of fibronectin we were able to confirm an induced immune response in the serum of each Group A recipients and also detected increased levels of anti-fibronectin IgG in post explant serum of two of the Group B recipients.
Antibody recovered from rejected Group B xenografts exhibited a much more diverse specificity on 1D Western blots. While antibody from some grafts appeared to detect similar bands, there was no obvious immunodominant target common to all GT-KO transplants. The absence of a common immunoreactivity could be the result of several factors. Antibody recovered from rejected tissue may be biased towards antibodies that bind to proteins present in high concentration. This would over represent antibody binding to common intracellular proteins and protein with abundance in cardiomyocytes and could include many of the proteins listed in Table 1. Our FACS analysis shows that the elution process recovers antibodies that bind to PAEC membranes (Figure 2C) however the concentration of these antibodies may not be sufficient for subsequent detection in Western blots. Alternatively, the presence of immunosuppression may have suppressed antibody class switching such that rejection in this group may result more from preformed non-Gal IgM which was not measured in our analysis. Preformed non-Gal IgM may also have contributed to the apparent HAR rejection in group B. The proteomic techniques we have used in this study are not well suited for an analysis of IgM specificity. Alternative approaches, such as screening a PAEC expression library using flow cytometry, would be well adapted for an analysis of both non-Gal IgM and IgG.
The 2D Western blot and proteomic analysis indicated that antibody from both groups of transplants bound to an array of cytoskeletal and metabolic proteins. Antibody with specificity to these intracellular proteins should not contribute to the induced anti-PAEC response that we observed by flow cytometry (Figure 1D and E) and most likely represent a preformed or secondary response to injured or necrotic tissues. Interestingly both IgG purified from Group A necropsy serum and antibody recovered from Group B rejected tissues showed a similar tendency to detect these proteins. This highlights the problem of selecting an antibody source for this analysis and suggests that most, if not all, antibody sources will have significant background levels of reactivity that may not be related to the initiation of xenograft rejection. Both antibody sources also detected an overlapping set of specificities which included vimentin and members of the annexin and heat shock family of proteins. Some of the heat shock proteins, which are classically considered to be intracellular or even mitochondrial proteins, have been shown to be expressed on the cell surface (HSP60, GRP78, HSP90B1 and HSC70 in Table 1) and antibody responses to many of these proteins have been associated with allograft rejection, inflammation or autoimmunity (36–38). Non-Gal antibody directed to this group of proteins may have a more direct role in initiating xenograft rejection however, elaboration of these antibodies as a result of a secondary antibody response due to ongoing xenograft damage cannot be excluded. Indeed, the prevalence of antibodies with these specificities, as discussed below, may also result from a bias inherent in the use of 2D Western blots.
Non-Gal antibody response(s) that initiate DXR are likely directed to PAEC membrane antigens. For Group A recipients, antibody reactivity of necropsy IgG to PAECs in flow cytometry (Figure 1D) and to PAEC membrane enriched antigens in 1D Western blots (Figure 2B) appeared to parallel each other suggesting that both assays detected IgG with similar or overlapping specificity. This coordination did not appear to extend to our 2D Western blot analysis where most of the highly immunoreactive material detected in the 1D Westerns was not detected in the 2D blots (Figure 3A). This discrepancy has been noted previously in other systems (39) and is likely related to the inefficient solubilization and poor solubility of transmembrane proteins. If this is the case, then our proteomic analysis is biased towards intracellular antigens and the immunodominant bands in the 1D Westerns may represent antibody binding to transmembrane PAEC proteins which could be the most likely candidates for initiating xenograft rejection. Indeed the molecular weight distribution of proteins identified from 2D gels was similar to the distribution of antigens showing modest immunoreactivity in the 1D Western blots. It will be essential then to examine these antibody specificities using methods which do not rely on 2D electrophoresis, such as expression library cloning or protein microarray analysis, to identify these immunodominant non-Gal antigens.
Our analysis suggests that after xenotransplantation non-Gal antibodies are directed towards a series of stress response and inflammation related proteins in addition to a limited number of as yet uncharacterized PAEC antigens. Antibody to many of the proteins we identified have the potential to contribute to xenograft rejection but may also represent preformed or induced responses that are secondary to the rejection process. This underscores the difficulty of identifying the critical non-Gal targets and suggests that multiple analytical techniques, a thorough vetting of potential target antigens and the establishment of new model systems to test non-Gal antibody pathogenicity will be required to identify the important non-Gal target antigens.
Acknowledgments
This project was supported by: NIH Immunobiology of Xenotransplantation grant AI66310 and The William J. von Liebig Foundation, Naples, Florida, USA
Abbreviations
- αGal
α1,3 galactose
- DXR
delayed xenograft rejection
- EC
endothelial cell
- GT-KO
Gal knockout
- TPC
an alpha-galactosyl-polyethylene glycol conjugate
- PAEC
pig aortic endothelial cell
References
- 1.LEVENTHAL JR, SAKIYALAK P, WITSON J, et al. The synergistic effect of combined antibody and complement depletion on discordant cardiac xenograft survival in nonhuman primates. Transplantation. 1993;57:974–978. [PubMed] [Google Scholar]
- 2.LIN SS, KOOYMAN DL, DANIELS LJ, et al. The role of natural anti-Gal-alpha-1-3Gal antibodies in hyperacute rejection of pig-to-baboon cardiac xenotransplants. Transplant Immunol. 1997;5:212–218. doi: 10.1016/s0966-3274(97)80040-8. [DOI] [PubMed] [Google Scholar]
- 3.LOGAN JS. Prospects for xenotransplantation. Curr Opin Immunol. 2000;12:563–568. doi: 10.1016/s0952-7915(00)00139-4. [DOI] [PubMed] [Google Scholar]
- 4.DORLING A. Are anti-endothelial cell antibodies a pre-requisite for the acute vascular rejection of xenografts? Xenotransplantation. 2003;10:16–23. doi: 10.1034/j.1399-3089.2003.01134.x. [DOI] [PubMed] [Google Scholar]
- 5.MCGREGOR CGA, TEOTIA SS, BYRNE GW, et al. Cardiac xenotransplantation: Progress toward the clinic. Transplantation. 2004;78:1569–1575. doi: 10.1097/01.tp.0000147302.64947.43. [DOI] [PubMed] [Google Scholar]
- 6.BYRNE GW, SCHWARZ A, FESI JR, et al. Evaluation of different alpha-Galactosyl glycoconjugates for use in xenotransplantation. Bioconjug Chem. 2002;13:571–581. doi: 10.1021/bc015565e. [DOI] [PubMed] [Google Scholar]
- 7.DIAMOND LE, BYRNE GW, SCHWARZ A, et al. Analysis of the control of the anti-Gal immune response in a non-human primate by galactose alpha-1-3 galactose trisaccharide-polyethylene glycol conjugate. Transplantation. 2002;73:1780–1787. doi: 10.1097/00007890-200206150-00014. [DOI] [PubMed] [Google Scholar]
- 8.KATOPODIS AG, WARNER RG, DUTHALER RO, et al. Removal of anti-Galα1,3Gal xenoantibodies with an injectable polymer. J Clin Invest. 2002;110:1869–1877. doi: 10.1172/JCI200216526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LAM TT, PANIAGUA R, SHIVARAM G, et al. Anti-non-Gal porcine endothelial cell antibodies in acute humoral xenograft rejection of hDAF-transgenic porcine hearts in cynomolgus monkeys. Xenotransplantation. 2004;11:531–535. doi: 10.1111/j.1399-3089.2004.00175.x. [DOI] [PubMed] [Google Scholar]
- 10.MCGREGOR CGA, DAVIES WR, OI K, et al. Cardiac xenotransplantation: Recent preclinical progress with 3-month median survival. J Thorac Cardiovasc Surg. 2005;130:844–851. doi: 10.1016/j.jtcvs.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 11.BYRNE GW, SCHIRMER JM, FASS DN, et al. Warfarin or low-molecular-weight heparin therapy does not prolong pig-to-primate cardiac xenograft function. Am J Transplant. 2005;5:1011–1020. doi: 10.1111/j.1600-6143.2005.00792.x. [DOI] [PubMed] [Google Scholar]
- 12.KUWAKI K, TSENG YL, DOR FJ, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 13.CHEN G, QIAN H, STARZL T, et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat Med. 2005;11:1295–1298. doi: 10.1038/nm1330. Epub 2005 Nov 1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CHEN G, SUN H, YANG H, et al. The role of anti-non-Gal antibodies in the development of acute humoral xenograft rejection of hDAF transgenic porcine kidneys in baboons receiving anti-Gal antibody neutralization therapy. Transplantation. 2006;81:273–283. doi: 10.1097/01.tp.0000188138.53502.de. [DOI] [PubMed] [Google Scholar]
- 15.KOPP CW, SIEGEL JB, HANCOCK WW, et al. Effect of porcine endothelial tissue factor pathway inhibitor on human coagulation factors. Transplantation. 1997;63:749–758. doi: 10.1097/00007890-199703150-00023. [DOI] [PubMed] [Google Scholar]
- 16.SIEGEL JB, GREY ST, LESNIKOSKI BA, et al. Xenogeneic endothelial cells activate human prothrombin. Transplantation. 1997;64:888–896. doi: 10.1097/00007890-199709270-00017. [DOI] [PubMed] [Google Scholar]
- 17.MAZZUCATO M, DEMARCO L, PRADELLA P, et al. Porcine von Willebrand factor binding to human platelet GPIb induces transmembrane calcium influx. Thromb Haemost. 1996;75:655–660. [PubMed] [Google Scholar]
- 18.COZZI E, SIMIONI P, BOLDRIN M, et al. Effects of long-term administration of high-dose recombinant human antithrombin in immunosuppressed primate recipients of porcine xenografts. Transplantation. 2005;80:1501–1510. doi: 10.1097/01.tp.0000178377.55615.8b. [DOI] [PubMed] [Google Scholar]
- 19.SCHIRMER JM, FASS DN, BYRNE GW, et al. Effective antiplatelet therapy does not prolong transgenic pig to baboon cardiac xenograft survival. Xenotransplantation. 2004;11:436–443. doi: 10.1111/j.1399-3089.2004.00159.x. [DOI] [PubMed] [Google Scholar]
- 20.BUHLER L, XU Y, LI W, et al. An investigation of the specificity of induced anti-pig antibodies in baboons. Xenotransplantation. 2003;10:88–93. doi: 10.1034/j.1399-3089.2003.01122.x. [DOI] [PubMed] [Google Scholar]
- 21.TSENG YL, MORAN K, DOR FJ, et al. Elicited antibodies in baboons exposed to tissues from alpha1,3-galactosyltransferase gene-knockout pigs. Transplantation. 2006;81:1058–1062. doi: 10.1097/01.tp.0000197555.16093.98. [DOI] [PubMed] [Google Scholar]
- 22.MCCURRY KR, KOOYMAN DL, ALVARADO CG, et al. Human complement regulatory proteins protect swine-to-primate cardiac xenografts from humoral injury. Nat Med. 1995;1:423–427. doi: 10.1038/nm0595-423. [DOI] [PubMed] [Google Scholar]
- 23.SHARMA A, NAZIRUDDIN B, CUI C, et al. Pig cells that lack the gene for alpha1-3 galactosyltransferase express low levels of the gal antigen. Transplantation. 2003;75:430–436. doi: 10.1097/01.TP.0000053615.98201.77. [DOI] [PubMed] [Google Scholar]
- 24.DAVILA E, BYRNE GW, LABRECHE PT, et al. T-cell responses during pig-to-primate xenotransplantation. Xenotransplantation. 2006;13:31–40. doi: 10.1111/j.1399-3089.2005.00258.x. [DOI] [PubMed] [Google Scholar]
- 25.KELLER A, NESVIZHSKII AI, KOLKER E, AEBERSOLD R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 26.NESVIZHSKII AI, KELLER A, KOLKER E, AEBERSOLD R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 27.MORELLI AE, THOMSON AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 28.YATES SF, PATERSON AM, NOLAN KF, et al. Induction of regulatory T cells and dominant tolerance by dendritic cells incapable of full activation. J Immunol. 2007;179:967–976. doi: 10.4049/jimmunol.179.2.967. [DOI] [PubMed] [Google Scholar]
- 29.SELA M, MOZES E. Therapeutic vaccines in autoimmunity. Proc Natl Acad Sci U S A. 2004;101(Suppl 2):14586–14592. doi: 10.1073/pnas.0404826101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.IERINO FL, GOJO S, BANERJEE PT, et al. Transfer of swine major histocompatibility complex class II genes into autologuous bone marrow cells of baboons for the induction of tolerance across xenogeneic barriers. Transplantation. 1999;67:1119–1128. doi: 10.1097/00007890-199904270-00006. [DOI] [PubMed] [Google Scholar]
- 31.BRACY JL, IACOMINI J. Induction of B-cell tolerance by retroviral gene therapy. Blood. 2000;96:3008–3015. [PubMed] [Google Scholar]
- 32.BOROZDENKOVA S, WESTBROOK JA, PATEL V, et al. Use of proteomics to discover novel markers of cardiac allograft rejection. J Proteome Res. 2004;3:282–288. doi: 10.1021/pr034059r. [DOI] [PubMed] [Google Scholar]
- 33.CARON M, CHOQUET-KASTYLEVSKY G, JOUBERT-CARON R. Cancer immunomics using autoantibody signatures for biomarker discovery. Mol Cell Proteomics. 2007;6:1115–1122. doi: 10.1074/mcp.R600016-MCP200. [DOI] [PubMed] [Google Scholar]
- 34.LATIF N, BAKER CS, DUNN MJ, et al. Frequency and specificity of antiheart antibodies in patients with dilated cardiomyopathy detected using SDS-PAGE and western blotting. J Am Coll Cardiol. 1993;22:1378–1384. doi: 10.1016/0735-1097(93)90546-d. [DOI] [PubMed] [Google Scholar]
- 35.PARKER W, BRUNO D, HOLZKNECHT ZE, PLATT JL. Characterization and affinity isolation of xenoreactive human natural antibodies. J Immunol. 1994;153:3791–3803. [PubMed] [Google Scholar]
- 36.FOTEINOS G, AFZAL AR, MANDAL K, et al. Anti-heat shock protein 60 autoantibodies induce atherosclerosis in apolipoprotein E-deficient mice via endothelial damage. Circulation. 2005;112:1206–1213. doi: 10.1161/CIRCULATIONAHA.105.547414. [DOI] [PubMed] [Google Scholar]
- 37.POCKLEY AG, MUTHANA M. Heat shock proteins and allograft rejection. Contrib Nephrol. 2005;148:122–134. doi: 10.1159/000086057. [DOI] [PubMed] [Google Scholar]
- 38.RESCHER U, GERKE V. Annexins--unique membrane binding proteins with diverse functions. J Cell Sci. 2004;117:2631–2639. doi: 10.1242/jcs.01245. [DOI] [PubMed] [Google Scholar]
- 39.SANTONI V, MOLLOY M, RABILLOUD T. Membrane proteins and proteomics: un amour impossible? Electrophoresis. 2000;21:1054–1070. doi: 10.1002/(SICI)1522-2683(20000401)21:6<1054::AID-ELPS1054>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]