Abstract
Background
A massive destruction of transplanted tissue occurs immediately following transplantation of pancreatic islets from pig to non-human primates. The detrimental instant blood-mediated inflammatory reaction (IBMIR), triggered by the porcine islets, is a likely explanation for this tissue loss; this reaction may also be responsible for mediating an adaptive immune response in the recipient that requires a heavy immunosuppressive regimen.
Materials and methods
Low molecular weight dextran sulfate (LMW-DS) and the complement inhibitor Compstatin were used in a combination of in vitro and in vivo studies designed to dissect the xenogeneic IBMIR in a non-human primate model of pancreatic islet transplantation. Adult porcine islets (10,000 IEQs/kg) were transplanted intraportally into three pairs of cynomolgus monkeys that had been treated with LMW-DS or heparin (control), and the effects on the IBMIR were characterized. Porcine islets were also incubated in human blood plasma in vitro to assess complement inhibition by LMW-DS and Compstatin.
Results
Morphological scoring and immunohistochemical staining revealed that the severe islet destruction and platelet, macrophage, neutrophilic granulocyte, and T-cell infiltration observed in the control (heparin-treated) animals were abrogated in the LMW-DS-treated monkeys. Both coagulation and complement activation were significantly reduced in monkeys treated with LMW-DS, but IgM and complement fragments were still found on the islet surface. This residual complement activation could be inhibited by Compstatin in vitro.
Conclusions
The xenogeneic IBMIR in this non-human primate model is characterized by an immediate binding of antibodies that triggers deleterious complement activation and a subsequent clotting reaction that leads to further complement activation. The effectiveness of LMW-DS (in vivo and in vitro) and Compstatin (in vitro) in inhibiting this IBMIR provides the basis for a protocol that can be used to abrogate the IBMIR in pig-human clinical islet transplantation.
Introduction
Clinical islet transplantation is a promising treatment for type I diabetic patients. The improved protocol introduced by Shapiro et al. in 2000 has greatly improved the results of this approach, but despite these advances, islets derived from more than one donor pancreas are still generally required to cure an individual diabetic patient (1). This requirement has drawn attention to the limited availability of human islets for transplantation and sparked interest in the use of islets from alternative sources, particularly the pig (2).
One obstacle to be surmounted before porcine islets can be used in clinical islet xenotransplantation is the injurious instant blood-mediated inflammatory reaction (IBMIR) that elicits massive cell destruction when porcine islets are exposed to fresh human blood (3). The xenogeneic IBMIR is characterized by activation of platelets and the coagulation and complement systems. This activation is accompanied by infiltration of the islets by polymorphonuclear lymphocytes (PMNs) (3).
The occurrence of this deleterious IBMIR is supported by studies demonstrating that porcine islets are immediately destroyed when transplanted intraportally into the liver of non-human primates (4, 5). Kirschof et al. reported that most of their porcine islet xenografts (22–73%) were substantially damaged after 24 h when transplanted into non-immunosuppressed monkeys (6). The grafts exhibited cell destruction, with deposition of coagulation and complement components and platelets, supporting the contention that the IBMIR contributes to the islet damage in this model. Further support for the importance of the IBMIR comes from the observation that although porcine islets can successfully survive in the liver of diabetic monkeys for more than 100 days (7, 8), very high quantities of islets (25,000 and 50,000 IEQs/kg BW, respectively) are needed to produce normoglycemia in the monkeys, indicating that there is a substantial loss of transplanted tissue.
Using in vitro and small-animal models, we have previously demonstrated that low molecular weight dextran sulfate (LMW-DS) effectively inhibits the activation of the coagulation and complement systems and the infiltration of leukocytes into the islets during xenogeneic islet transplantation (9). In the present study, we have used LMW-DS together with Compstatin, a new peptide complement inhibitor that is suitable for use in clinical islet xenotransplantation (10), to dissect the IBMIR in in vivo (LMW-DS) and in in vitro (LMW-DS and Compstatin) xenotransplantation models. The results of these studies have broadened our understanding of the innate immune events that might be expected to occur in clinical islet xenotransplantation and have provided the basis for a protocol for abrogating the IBMIR during clinical transplantation with porcine pancreatic islets.
Materials and methods
Animals
Retired breeder pigs, weighing approximately 200 kg, were used as donors for all experiments. Cynomolgus monkeys (Macaca fascicularis; 3–6 years old; 4–6 kg) were used as recipients. All procedures using pigs were approved by the Swedish Council on Medical Ethics. Cynomolgus monkeys were housed according to the guidelines of the Belgian Ministry of Agriculture and Animal Care. All procedures using monkeys were approved by the local Ethical Committee for Animal Care of the Université Catholique de Louvain.
Islet isolation
Isolation of porcine islets was performed as previously described (11), with minimal modifications. Purified islet fractions were pooled and cultured at 37°C in a humidified atmosphere with 5% CO2 in CMRL 1066 medium (Biochrom, Berlin, Germany) supplemented with 20% porcine serum, 2 mM N-acetyl-L-alanyl-L-glutamine, 10 mM HEPES, 100 IU/ml penicillin, 100 μg/ml streptomycin (Biochrom), and 20 μg/ml ciprofloxacin (Bayer, Leverkusen, Germany).
Evaluation of porcine islet quality
The in vitro function and viability of the porcine islets were assessed after overnight culture as described above. Islet viability determined by trypan blue exclusion assay and insulin release defined as the ratio of stimulated (16.5 mM glucose) to basal (1.65 mM glucose) insulin release, were performed as previously described (11). For assays of islet insulin content, 1-mL samples were washed with distilled water, then sonicated (Labsonic, Braun, Melsungen, Germany) for 30 sec. A 200-μL aliquot of each sample was subjected to acid-ethanol extraction (0.18 M HCl) and used for insulin measurement. Another 100-μL aliquot was dried at 60°C overnight for consecutive fluorometric DNA assays (12), using calf thymus DNA type I (Sigma, Deisenhofen, Germany) as a standard. 24-h insulin secretion: Immediately after a medium change, 500-μL samples of the medium were taken in duplicate from the remaining Petri dishes for determination of insulin accumulation in the medium, in order to calculate the 24-h insulin secretion by the islets. Transplantation islets into nude mice was performed as previously described (11).
Islet transplantation
Before each experiment, the monkeys were sedated with 6 mg/kg Zoletil® 100 (Virbac S.A., Carros, France) intramuscularly, and general anesthesia was maintained with inhalation of 1–3% enflurane. During the experiment, electrocardiogram, blood pressure, and pulse were continuously monitored. The pig islets were suspended in 10 mL of transplant medium (Ringer acetate; Braun, Melsungen, Germany) with 25% (w/v) human albumin and 5 mM glucose and injected slowly into the portal vein over the course of 5 min. The animals were treated in pairs, with each pair being given porcine islets from the same donor. One recipient in each pair received LMW-DS (monkeys M-5, M-7, M-9) and the other heparin as a control (monkeys M-6, M-8, M-10):
Intravenous infusion of LMW-DS (MW 5000; Sigma Chemicals, St. Louis, MO, USA) was performed via an indwelling catheter placed in the jugular vein or via a catheter in the portal vein. In the LMW-DS-treated groups, dextran with a molecular weight of 1kDa (Promiten, Pharmalink AB, Upplands Väsby, Sweden) was injected i.v. just before islet transplantations to avoid the risk of anaphylactoid reactions triggered by LMW-DS. After the injection of Promiten, the monkey received a bolus dose of LMW-DS (1.5 mg/kg) i.v. prior to islet infusion, followed by 3.0 mg/kg LMW-DS given together with the porcine islets (10,000 IEQs/kg of recipient BW). The transplantation was followed by a continuous i.v. infusion of LMW-DS (1.0–1.5 mg/kg/h) for up to 24 h.
In the heparin-treated groups, the monkeys received a continuous i.v. infusion of heparin (35U/kg of BW, heparin LEO, 5000 U/mL; Lowen, Sweden) for 24 h, beginning immediately prior to islet infusion.
Blood samples
All blood samples from the monkeys were drawn from a femoral vein catheter at 0, 20, and 40 min and at 1, 3 and 24 h after transplantation. Blood was also drawn from healthy human blood donors into 7-mL tubes containing citrate, EDTA or 500 μg of hirudin, a specific inhibitor of thrombin (Refludan; Pharmion Ltd, Cambridge, UK). To obtain plasma, the samples were centrifuged at 4500 × g for 5 min. If not immediately analyzed for activated partial thromboplastin time (APTT), the samples were stored at −70°C.
Analyses of blood and plasma samples
APTT measurements were performed as previously described (13). Platelet counts and differential leukocyte counts were obtained using a Coulter ACT-diff analyzer (Beckman Coulter, Miami, FL) and EDTA-treated blood. Plasma levels of thrombin-antithrombin (TAT) were quantified using commercially available EIA kits (TAT, Behringswerke, Marburg, Germany). C3a generation was measured in plasma according to the method of Nilsson Ekdahl et al. (14), and sC5b-9 was analyzed using a modification of the enzyme immunoassay described by Nilsson Ekdahl et al. (14) and Mollnes et al. (15).
Plasma IL-6, TNFα, IL-1β, and CRP were measured using a commercial ELISA kit (Immulite IL-6, Immulite TNFα, Immulite IL-1β, and Immulite High Sensitivity CRP, respectively; Diagnostic Products Corporation, Los Angeles, CA, USA).
Histological and immunohistochemical staining
The monkey livers bearing transplanted adult porcine islet grafts were retrieved 24 h after transplantation, at a time when the major part of the IBMIR has generally occurred (3). Some tissue samples were snap-frozen in isopentane and stored at −70°C. Other samples were fixed with 4% paraformaldehyde overnight, then embedded in paraffin. The samples were sectioned and subsequently used for morphological scoring after hematoxylin eosin staining.
Immunohistochemical staining was carried out using guinea pig anti-insulin (DAKO, Carpenteria, CA, USA), mouse anti-human neutrophil elastase (DAKO), mouse anti-human CD68 (DAKO), mouse anti-human MAC387 (Abcam, Cambridge, UK), mouse anti-human CD56 (Monosan, Stockholm, Sweden), rabbit anti-human CD3 (DAKO), mouse anti-human CD20 (DAKO), rabbit anti-human IgG and IgM (DAKO), mouse anti-human CD41 (DAKO), mouse anti-human C3c (QUIDEL, San Diego, CA, USA), or goat anti-human C9 (Serotec Ltd Scandinavia, Oslo, Norway).
Treatment of porcine islets with human plasma
Approximately 1000 pig islets/40μL of plasma (typically 5000 islets in 200 μL) were incubated in human hirudin-treated plasma in heparinized test tubes. Five different islet preparations and five different plasma preparations were used in these experiments. In some experiments, hirudin-treated plasma was preincubated with 20 μM (final concentration) of the potent Compstatin analog, Ac-ICV(1MeW)QDWGAHRCT-NH2 (16), for 15 min at 37°C before the islets were added. The mixture of islets and plasma was then incubated, with gentle shaking, at 37°C for up to 30 min. After centrifugation, the islets were immediately prepared for COPAS analysis and confocal microscopy.
Preparation of islets for flow cytometry and confocal microscopy
Ten microliters of FITC-labeled antibody recognizing one of the following proteins was added to 5000 islets (corresponding to approximately 10 × 106 cells) in 100 μL of PBS according to the manufacturer’s recommendations for single cells: C1q (1.0 g/L; AbCam, Cambridge, UK), C3c (3.2 g/L, for detection of C3b and iC3b; DakoCytomation, Glostrup, Denmark), C4 (1.3 g/L; Dako), C9 (2.6 g/L; Dako), mannose-binding lectin (MBL) (0.7 g/L; Dako), IgG (2.6 g/L; Dako), or IgM (4.0 g/L; Dako). Irrelevant mouse IgG1 (0.1 g/L; Dako) was used as a negative control. For all immunostaining experiments, the islets were incubated, while gently rotating on ice, for 30 min in the presence of an individual antibody. After being washed with PBS, the islets were treated with 1% formaldehyde (Apoteket, Gothenburg, Sweden) and kept on ice until analyzed.
Complex object parametric analyzer and sorter (COPAS) analysis
The fluorescence-stained islets were analyzed using a complex object parametric analyzer and sorter (COPAS; Union Biometrica, Somerville, MA), which is a large particle-based flow cytometry instrument previously used for islet analysis (17). For each experiment, 1000 islets were collected using a 488/514 multi-line laser, and positive cells were sorted out for further analysis by confocal microscopy. The COPAS flow cytometry data were analyzed using CellQuest Pro software (BD Biosciences Immunocytometry Systems). Data were reported as mean fluorescent intensity (MFI).
Confocal microscopy
One to two hundred hand-picked, stained islets were contained in a drop of PBS in a small Petri dish and protected from light before examination in the confocal microscope (Zeiss 510 Meta confocal, Carl Zeiss, Jena, Germany). Examination of the stained islets was performed using the 488-nm laser at 10 times magnification. Counter staining with 4′, 6-diamidino-2-phenylindole (DAPI) was used to visualize the nuclei of living islet cells.
Complement inhibition assay
One hundred microliters of 10% human serum (v/v), diluted in veronal buffer with 1 mM Ca2+, 0.3 mM Mg2+, 1% (w/v) BSA, and 0.05% (v/v) Tween 20, was incubated in the presence of serially diluted LMW-DS and/or Compstatin in wells of microtiter plates for 30 min at 37°C. The wells were then washed with PBS containing 0.05 % (v/v) Tween 20, and the bound C3 fragments were detected using 100μL of HRP-conjugated anti-C3c (Dako AS, Glostrup, Denmark).
Statistical analysis
All values are expressed as means ± standard error of mean and were compared using Student’s unpaired t-test or using the Mann-Whitney test for unpaired samples. P values less than 0.05 were considered statistically significant.
Results
Islet quality
The viability of the APIs used in this study was 96, 100, and 97%, respectively. The stimulation index in the SGS test was 1.29, 1.84, and 1.40, and the mean insulin content was 613, 149, and 685 μU/IEQs, respectively. Adult porcine islets used in each experiment cured diabetic athymic mice. When we assessed the possible detrimental effect of LMW-DS by incubating APIs from three different pancreata in the presence (100, 1000, or 2500 mg/L) or absence of LMW-DS, we found no adverse effect of LMW-DS on insulin release at any of the concentrations tested (data not shown).
Influence of LMW-DS on blood cell counts, liver and renal function, and cytokine induction in the transplanted monkeys
One of the transplanted monkeys (M6) treated with heparin died 2 hours after transplantation due to severe hypoglycemia. The platelet and leukocyte counts and the creatinine levels were kept within normal ranges throughout the experiments with one exception: The granulocyte count increased 2 hours after transplantation in the heparin-treated group (3.9±0.5 vs 9.6±1.6) compared to that of the LMW-DS-treated group (6.0±0.9 vs 7.3±1.4). There was also a tendency towards an increase in the liver enzymes at 24 hours after islet transplantation in the heparin-treated monkeys (heparin vs LMW-DS: AST, 434.7±126.4 vs. 288.0±130.4; ALT, 207.7±68.7 vs. 116.8±47.7). No bleedings or other adverse reactions were observed.
Influence of LMW-DS on cytokine induction was examined using three healthy monkeys. Only a slight increase in the IL-6 levels was seen 24 hours after administration of LMW-DS in two out of three healthy monkeys (maximum 27μg/L). However, LMW-DS did not trigger an increase of plasma IL-1β, TNFα, or CRP (not shown).
LMW-DS concentrations in transplanted monkeys
Previous studies showed a strong correlation between APTT and the concentration of LMW-DS (13). Plasma APTT was therefore used to follow the blood concentration of LMW-DS in the transplanted monkeys (Fig. 1). The APTT in monkeys treated with heparin at concentrations routinely used in clinical islet transplantation (i.e. 500–1000 IU/L) was kept constant at 25–40s throughout the whole study period. The APTT in monkeys treated with LMW-DS reached around 100s at 15 minutes after islet infusion, but gradually decreased during 2 hours after islet transplantation. After 24 hours, the APTTs in monkeys M-5, M-7, and M-9 were 101s, 66s, and 107s, respectively. Thus, both M-5 and M-9 had approximately double the concentration of LMW-DS compared to M-7.
Fig. 1.
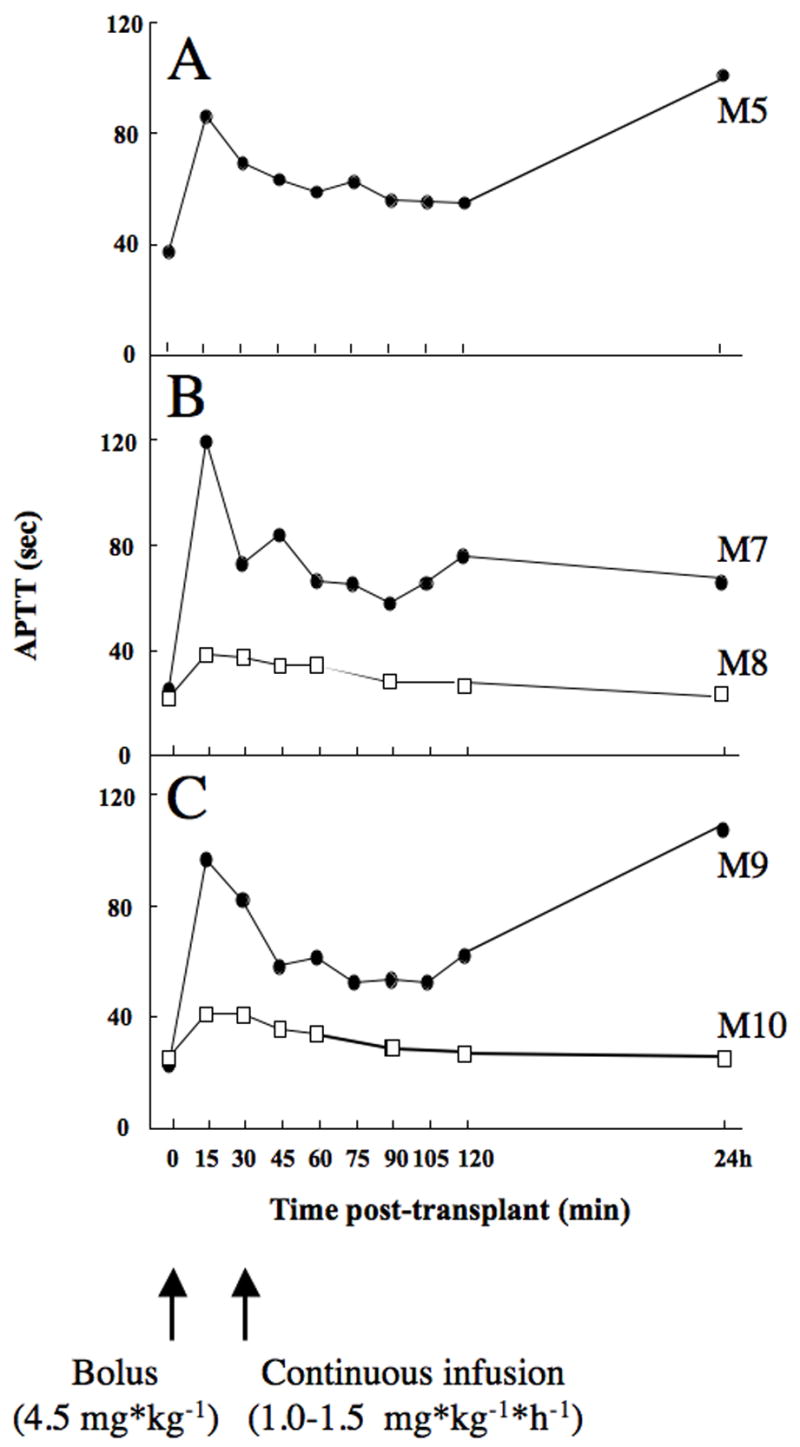
Plasma APTT values in transplanted diabetic monkeys (M5, M7-M10) treated with heparin (squares) or LMW-DS (circles).
Inhibition of the IBMIR by LMW-DS during pig islet xenotransplantation
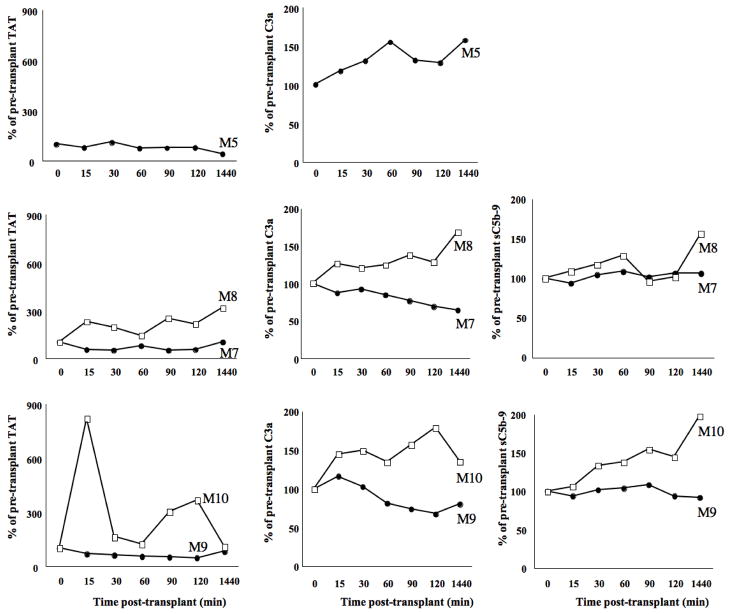
LMW-DS, unlike heparin, diminished both the coagulation and the complement cascade activation in two sets of monkeys. The increase of coagulation marker TAT was effectively inhibited by LMW-DS (Fig. 2). The complement activation parameters C3a and sC5b-9 were also suppressed by LMW-DS in both treated monkeys compared to the controls during the study period (Fig. 2). In M-5, TAT was totally suppressed while C3a was more difficult to evaluate without the corresponding control (M6). In this animal sC5b-9 was not assessed due to an insufficient amount of plasma samples.
Fig. 2.
EDTA blood was drawn from a femoral vein catheter of the transplanted monkeys treated with heparin (squares) or LMW-DS (circles) at varying time points after porcine islet xenotransplantation. TAT, C3a, and sC5b-9 levels were assessed and expressed as percentage of the pre-transplant values.
Histological evaluation of grafted pig islets after intraportal transplantation into the monkeys treated with LMW-DS or heparin
Morphological aspects of islet grafts were scored semiquantitatively according to the representative examples shown in Fig. 3. As summarized in Table 1, histology of the transplanted grafts were well kept in the monkeys treated with LMW-DS in both settings of experiments. However, the beneficial effects of LMW-DS were more pronounced in M-5 and M-9 compared to M-7. Indeed, the completely preserved islets (score 0 in all categories) were encountered in 37.2% and 44% of the LMW-DS treated animals M-5 and M-9 (LMW-DS treated monkeys), respectively, but in only 22% of the control M-10.
Fig. 3.
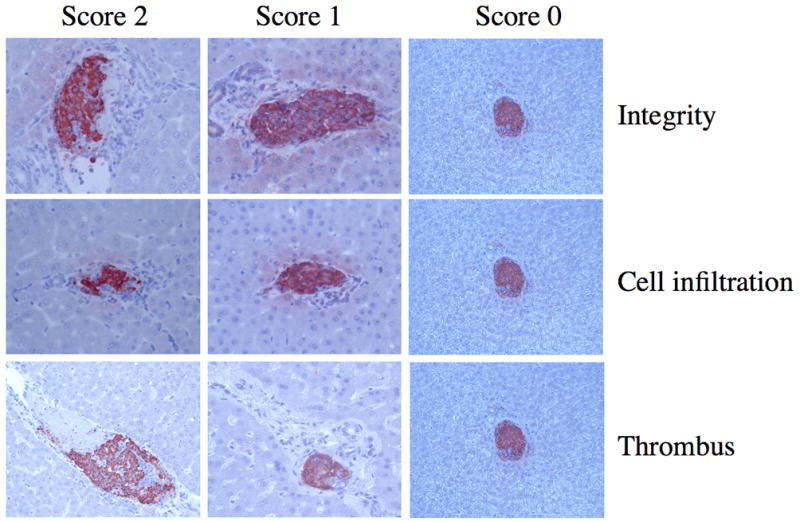
Visual examples of the morphological scoring system used to quantify different aspects of the IBMIR. Hematoxylin eosin-stained porcine islet grafts retrieved 24 hours after intraportal xenotransplantation from diabetic monkeys treated with LMW-DS or heparin were used. A summary of all transplanted monkeys is presented in Table 1.
Table 1.
Summary of the morphological score (as depicted in Fig. 3) of the islets grafts in recipient monkeys M5, M7-M10
| Monkey No | Treatment | Integrity | Thrombus | Cell infiltration | % of Score 0 2 | APTT at 24 hours after transplantation (s) |
|---|---|---|---|---|---|---|
| M5 | LMW-DS, n=113 | 0.66±0.04 1 | 0.26±0.03 | 0.90±0.07 | 37.2 | 101 |
|
| ||||||
| M7 | LMW-DS, n=134 | 0.93±0.06 | 0.52±0.06 | 1.08±0.06 | 26.1 | 66 |
| M8 | Heparin, n=149 | 1.05±0.05 | 0.62±0.06 | 1.17±0.06 | 20.1 | 24 |
| p=0.13 | p=0.28 | p=0.32 | p=0.23 | |||
|
| ||||||
| M9 | LMW-DS, n=134 | 0.63±0.05 | 0.37±0.05 | 0.85±0.06 | 44.0 | 107 |
| M10 | Heparin, n=125 | 0.95±0.06 | 0.54±0.06 | 1.25±0.07 | 22.4 | 25 |
| p<0.0001 | p<0.05 | p<0.0001 | p<0.001 | |||
Values are expressed as means±SEM.
Percentage islets with no signs of IBMIR (score 0).
Immunohistochemical staining of grafted pig islets after intraportal transplantation into the monkeys treated with LMW-DS or heparin
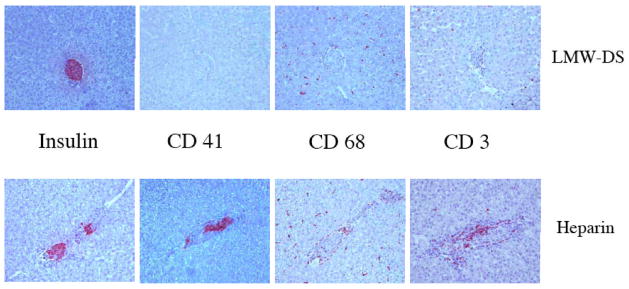
The immunohistochemical findings from the grafts were summarized in Fig. 4 and Table 2. As expected, most parameters involved in innate immune responses were active after 24 hours post islet transplantation in the controls M8 and M10. In particular, CD41+ platelets, CD68+ macrophages, and neutrophil elastase positive PMNs were abrogated in the monkeys treated with LMW-DS compared to the controls given heparin (p=0.056, 0.002, and 0.04, respectively). CD56+ NK cells were found only occasionally. Unlike the soluble complement markers there was no clear inhibition of complement activation as reflected in deposition of C3 fragment and C9 on the surface of the islets. Furthermore, IgM antibodies were found on islet both in LMW-DS and heparin-treated animals. Most of parameters reflecting specific immune responses were yet silent. However, CD3+ T cell infiltration was already seen in the islet grafts of the controls M8 and M10. Notably this infiltration was effectively suppressed by LMW-DS.
Fig. 4.
Immunohistochemical staining of porcine islet grafts retrieved 24 hours after intraportal xenotransplantation from diabetic monkeys treated with LMW-DS or heparin. The figure shows representative expression of insulin and of CD41 (platelets), CD68 (macrophages), and CD3 (T-cells) in the grafts. A summary of all transplanted monkeys is presented in Table 1. Magnification x200.
Table 2.
Summary of the immunohistochemical staining (as depicted in Fig. 4) of the islet grafts in recipient monkeys receiving LMW-DS or heparin
| Treatment | CD41 | C3c | C9 | Neutrophil elastase | CD68 | MAC 387 | CD56 | CD3 | CD20 | IgG | IgM |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LMW-DS (n=21) | (−)~(++) | (−)~(++) | (−)~(+++) | (−)~(++) | (−)~(++) | (−)~(++) | (−)~(+) | (−)~(++) | (−)~(+) | (−) | (−)~(+) |
| 0.59±0.191 | 0.80±0.37 | 1.50±0.31 | 0.42±0.23 | 1.31±0.21 | 0.90±0.28 | 0.10±0.10 | 0.63±0.20 | 0.20±0.13 | 0 | 0.25±0.25 | |
|
| |||||||||||
| Heparin (n=18) | (−)~(+++) | (−)~(++) | (−)~(+++) | (−)~(++) | (++)~(+++) | (+)~(+++) | (−)~(+) | (−)~(+++) | (−)~(++) | (−) | (−)~(++) |
| 1.60±0.51 | 0.63±0.26 | 1.67±0.33 | 1.08±0.23 | 2.17±0.11 | 2.11±0.26 | 0.22±0.15 | 1.90±0.35 | 0.40±0.27 | 0 | 0.60±0.24 | |
|
| |||||||||||
| P value | 0.056 | 0.69 | 0.71 | 0.04 | 0.002 | 0.01 | 0.48 | 0.006 | 0.83 | - | 0.36 |
Values are expressed as means±SEM.
Binding of complement components to porcine islets after incubation in human plasma
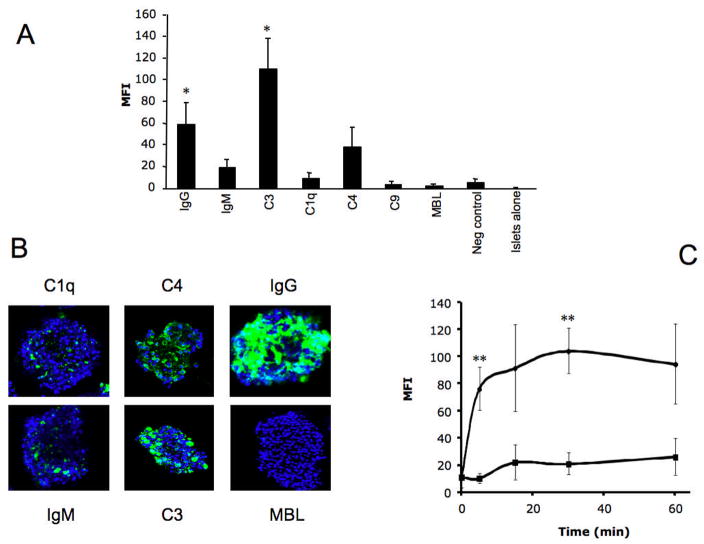
After incubation in hirudin-treated plasma, the porcine islets were stained with FITC-conjugated antibodies recognizing IgG, IgM, C1q, C3b/iC3b, C4 fragments, C9, and MBL. Large particle flow cytometry and confocal microscopy demonstrated that antibodies against IgG, IgM, C1q, C4, and C3 bound strongly to the islets, but the binding of MBL and C9 was less prominent (Fig. 5 A, B). C3b/iC3b fragments were detected on the islets after only 5 min, and the binding of C3b/iC3b continued to increase over time. Addition of Compstatin significantly reduced the binding of C3b/iC3b to the islets (Fig. 5C). Confocal microscopy analyses confirmed these results (not shown).
Fig. 5.
Porcine islets incubated in hirudin-treated plasma for 30 min. The islets were stained for IgG (n=5), IgM (n=5), C3b/iC3b (n=5), C1q (n=3), C4 (n=5), C9 (n=3), and MBL (n=3). As negative control, an antibody recognizing mouse IgG was used (n=5). The islets were analyzed by (A) large particle flow cytometry and (B) confocal microscopy. In C the deposition of C3b/iC3b on the islet in the absence and presence of Compstatin is presented after analysis by large particle flow cytometry (n=5; statistical evaluation was performed at 5 and 30 min where n=7) (*=p<0.05; **=p<0.01).
Inhibition of complement activation by LMW-DS and Compstatin
Ten % (v/v) human serum was incubated in wells of microtiter plates in the presence of LMW-DS and/or Compstatin for 30 min at 37°C (Fig. 6). In the presence of Compstatin there was no effect below 0.5 μM of the compound, but at higher concentrations Compstatin gradually inhibited complement activation. At 5 μM total inhibition was achieved. LMW-DS inhibited complement activation only marginally between 10 and 100 mg/L, but the effect was more pronounced at concentrations above this level. There was no indication of interaction between the drugs regarding this effect on complement activation in serum.
Fig. 6.
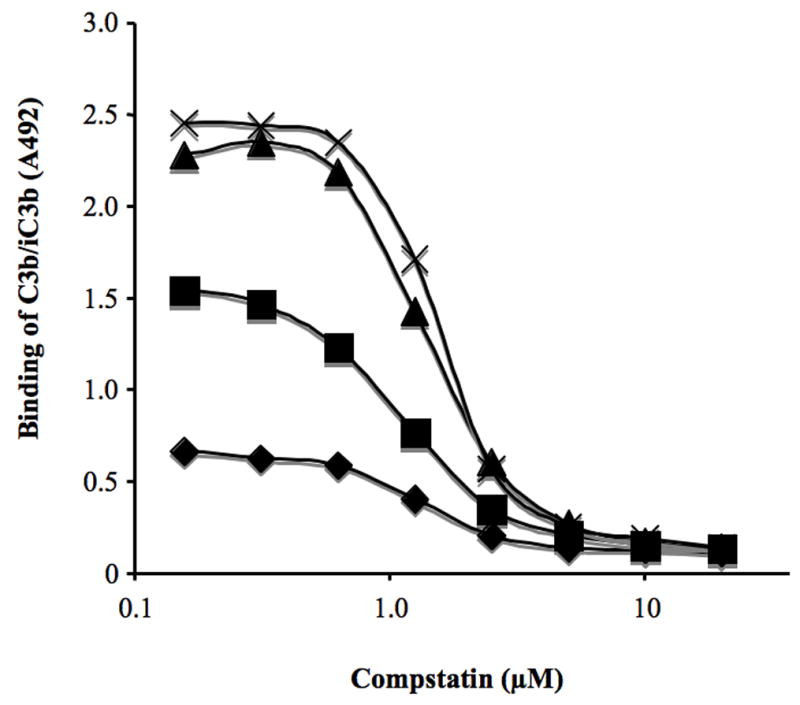
Binding of C3b/iC3b to the surface of microtiter wells after incubation with 10% serum in the presence of increasing doses of Compstatin for 30 min at 37°C. 0 (cross), 10 (triangle), 100 (squares), 1000 (diamond) mg/L of LMW-DS was present in the wells.
Discussion
We have previously shown that LMW-DS efficiently prevents clotting that occurs in both allogeneic and xenogeneic IBMIR triggered by APIs both in vitro and in vivo in a small animal model (9, 18). Here we confirm that LMW-DS is efficient also in a primate model mimicking the clinical setting. The effect of LMW-DS was compared to that of heparin, which is routinely used in clinical islet transplantation. LMW-DS was proved to be far more efficient in inhibiting the IBMIR than heparin. These data confirm those of Rood et al who recently demonstrated longer porcine islet survival in non-human primates treated with LMW-DS (19).
In the present study, both the morphological findings and the measurements in the plasma, were similar to those in our previous studies, in which APIs were surrounded by clots and infiltrated by numerous leukocytes immediately after contact with fresh blood seen in the tubing loop model and our small animal model (9). Most parameters reflecting the IBMIR, i.e. both the coagulation and complement cascades, platelet deposition, and infiltration of macrophages and neutrophils, were attenuated in the monkeys treated with LMW-DS compared to the controls. There was also a tendency that increases in granulocyte count and liver enzymes were attenuated. Notably T cell infiltration observed in some of the transplanted islet grafts was also effectively suppressed, demonstrating that also the adaptive immune responses are attenuated by LMW-DS.
The effects of LMW-DS on the adaptive immune system may be explained by the effects on complement activation since complement is also of great importance in bridging innate immunity and specific immune responses. In allogeneic whole organ transplantation, C3 is one of the essential factors that trigger rejection in mice (20–22) and humans (23). It is therefore reasonable to expect that complement activation will trigger a profound adaptive immune response raised against the graft, necessitating an unwarrantedly heavy immunosuppressive regimen. Previous studies support such a hypothesis (4, 5).
As shown in Table 1, the islet grafts in M-9 that reached an APTT of 107s 24 hours after transplantation, demonstrated well-preserved morphology suggesting that this dose of LMW-DS would be preferable. In a recently performed phase I study in normal individuals, we have shown that this concentration can be reached without an increased risk of bleeding or side effects (manuscript under preparation). This makes treatment with LMW-DS during xenogeneic islet transplantation an attractive alternative. It should be noted that a specific concentration of LMW-DS gives different APTT in blood from different individuals both in vitro and in vivo, probably due to that different allotypes of certain coagulation factors interact with LMW-DS differently.
In our previous studies, we showed that complement activation induced in xenogeneic IBMIR occurs secondarily to coagulation activation; a reaction which is also seen in allogeneic IBMIR (9, 24) and which is elicited by chondroitin sulfate released by activated platelets (25). This explains why complement activation in the present study was suppressed in parallel with the reduction of coagulation activation at substantially lower concentrations (15–35 mg/L) of LMW-DS than used in other studies aiming for a inhibition of hyper acute rejection (26, 27). However, unlike complement activation in the fluid phase, immunohistochemical analyses showed that complement deposition was still seen on the islet grafts in the monkeys treated with LMW-DS. These reactions were analyzed in detail in vitro using large particle flow cytometry and confocal microscopy in order to be able clarify the mechanism of activation. The experiments were performed using human plasma in order to directly translate the findings to clinical islet xenotransplantation. Pig islets incubated in human plasma revealed an almost instantaneous binding of IgM and IgG antibodies and complement components already after 5 min. This rapid activation was completely inhibited by Compstatin. It is possible that the instantaneous insulin dumping in a non-human primate model previously reported by Bennet et al. is explained by this antibody-mediated reaction (3). The severity of this reaction, which was totally abrogated by the recombinant complement inhibitor CR1, is reflected in the fact that the release of insulin corresponded to about 40% of the insulin in the transplanted islets.
One way to fully inhibit complement activation is to increase the dose of LMW-DS, but as shown in our in vitro experiments, doses between 10 and 100 mg/L have only minor effects on complement activation alone. Moreover, higher doses of LMW-DS are likely to give side effects. It is therefore obvious that LMW-DS must be combined with a specific complement inhibitor such as Compstatin in order to block the immediate destructive immunoglobulin-triggered complement activation found both in vitro and in vivo (10, 28). The in vitro studies show that LMW-DS and Compstatin do not interact in human serum.
Taken together, it possible to propose a model of how the different components of IBMIR interact in xenogeneic combinations: 1) Immediately when porcine islets come in contact with human blood there is an instantaneous binding of IgG and IgM antibodies to the islet surface which triggers a deleterious complement activation; 2) This is followed by a clotting reaction with accompanying complement activation. Based upon the experimental data presented, LMW-DS combined with a specific complement inhibitor is an attractive alternative to control the detrimental innate immune responses that are postulated to occur in forthcoming intraportal preclinical and clinical islet xenotransplantation trials. We are at the moment in progress to produce sufficient amounts of Compstatin in order to perform studies in the NHP model with Compstatin combined with LMW-DS.
References
- 1.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Rood PP, Buhler LH, Bottino R, Trucco M, Cooper DK. Pig-to-nonhuman primate islet xenotransplantation: a review of current problems. Cell Transplant. 2006;15(2):89. doi: 10.3727/000000006783982052. [DOI] [PubMed] [Google Scholar]
- 3.Bennet W, Sundberg B, Lundgren T, et al. Damage to porcine islets of Langerhans after exposure to human blood in vitro, or after intraportal transplantation to cynomologus monkeys: protective effects of sCR1 and heparin. Transplantation. 2000;69(5):711. doi: 10.1097/00007890-200003150-00007. [DOI] [PubMed] [Google Scholar]
- 4.Buhler L, Deng S, O’Neil J, et al. Adult porcine islet transplantation in baboons treated with conventional immunosuppression or a non-myeloablative regimen and CD154 blockade. Xenotransplantation. 2002;9(1):3. doi: 10.1034/j.1399-3089.2002.1o044.x. [DOI] [PubMed] [Google Scholar]
- 5.Cantarovich D, Blancho G, Potiron N, et al. Rapid failure of pig islet transplantation in non human primates. Xenotransplantation. 2002;9(1):25. doi: 10.1034/j.1399-3089.2002.0o144.x. [DOI] [PubMed] [Google Scholar]
- 6.Kirchhof N, Shibata S, Wijkstrom M, et al. Reversal of diabetes in non-immunosuppressed rhesus macaques by intraportal porcine islet xenografts precedes acute cellular rejection. Xenotransplantation. 2004;11(5):396. doi: 10.1111/j.1399-3089.2004.00157.x. [DOI] [PubMed] [Google Scholar]
- 7.Cardona K, Korbutt GS, Milas Z, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12(3):304. doi: 10.1038/nm1375. [DOI] [PubMed] [Google Scholar]
- 8.Hering BJ, Wijkstrom M, Graham ML, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006;12(3):301. doi: 10.1038/nm1369. [DOI] [PubMed] [Google Scholar]
- 9.Goto M, Johansson H, Maeda A, Elgue G, Korsgren O, Nilsson B. Low molecular weight dextran sulfate prevents the instant blood-mediated inflammatory reaction induced by adult porcine islets. Transplantation. 2004;77(5):741. doi: 10.1097/01.tp.0000114872.26990.4f. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson B, Larsson R, Hong J, et al. Compstatin inhibits complement and cellular activation in whole blood in two models of extracorporeal circulation. Blood. 1998;92(5):1661. [PubMed] [Google Scholar]
- 11.Brandhorst H, Brandhorst D, Hering BJ, Bretzel RG. Significant progress in porcine islet mass isolation utilizing liberase HI for enzymatic low-temperature pancreas digestion. Transplantation. 1999;68(3):355. doi: 10.1097/00007890-199908150-00006. [DOI] [PubMed] [Google Scholar]
- 12.Kissane JM, Robins E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958;233(1):184. [PubMed] [Google Scholar]
- 13.Johansson H, Goto M, Dufrane D, et al. Low molecular weight dextran sulfate: a strong candidate drug to block IBMIR in clinical islet transplantation. Am J Transplant. 2006;6(2):305. doi: 10.1111/j.1600-6143.2005.01186.x. [DOI] [PubMed] [Google Scholar]
- 14.Nilsson Ekdahl K, Nilsson B, Pekna M, Nilsson UR. Generation of iC3 at the interface between blood and gas. Scand J Immunol. 1992;35(1):85. doi: 10.1111/j.1365-3083.1992.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 15.Mollnes TE, Riesenfeld J, Garred P, et al. A new model for evaluation of biocompatibility: combined determination of neoepitopes in blood and on artificial surfaces demonstrates reduced complement activation by immobilization of heparin. Artif Organs. 1995;19(9):909. doi: 10.1111/j.1525-1594.1995.tb02450.x. [DOI] [PubMed] [Google Scholar]
- 16.Katragadda M, Magotti P, Sfyroera G, Lambris JD. Hydrophobic effect and hydrogen bonds account for the improved activity of a complement inhibitor, compstatin. J Med Chem. 2006;49(15):4616. doi: 10.1021/jm0603419. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez LA, Hatch EW, Armann B, et al. Validation of large particle flow cytometry for the analysis and sorting of intact pancreatic islets. Transplantation. 2005;80(6):729. doi: 10.1097/01.tp.0000179105.95770.cd. [DOI] [PubMed] [Google Scholar]
- 18.Goto M, Groth CG, Nilsson B, Korsgren O. Intraportal pig islet xenotransplantation into athymic mice as an in vivo model for the study of the instant blood-mediated inflammatory reaction. Xenotransplantation. 2004;11(2):195. doi: 10.1046/j.1399-3089.2003.00107.x. [DOI] [PubMed] [Google Scholar]
- 19.Rood PP, Bottino R, Balamurugan AN, et al. Reduction of early graft loss after intraportal porcine islet transplantation in monkeys. Transplantation. 2007;83(2):202. doi: 10.1097/01.tp.0000250680.36942.c6. [DOI] [PubMed] [Google Scholar]
- 20.Pratt JR, Basheer SA, Sacks SH. Local synthesis of complement component C3 regulates acute renal transplant rejection. Nat Med. 2002;8(6):582. doi: 10.1038/nm0602-582. [DOI] [PubMed] [Google Scholar]
- 21.Chowdhury P, Zhou W, Sacks SH. Complement in renal transplantation. Nephron Clin Pract. 2003;95(1):c3. doi: 10.1159/000073012. [DOI] [PubMed] [Google Scholar]
- 22.Peng Q, Li K, Patel H, Sacks SH, Zhou W. Dendritic cell synthesis of C3 is required for full T cell activation and development of a Th1 phenotype. J Immunol. 2006;176(6):3330. doi: 10.4049/jimmunol.176.6.3330. [DOI] [PubMed] [Google Scholar]
- 23.Brown KM, Kondeatis E, Vaughan RW, et al. Influence of donor C3 allotype on late renal-transplantation outcome. N Engl J Med. 2006;354(19):2014. doi: 10.1056/NEJMoa052825. [DOI] [PubMed] [Google Scholar]
- 24.Ozmen L, Ekdahl KN, Elgue G, Larsson R, Korsgren O, Nilsson B. Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes. 2002;51(6):1779. doi: 10.2337/diabetes.51.6.1779. [DOI] [PubMed] [Google Scholar]
- 25.Hamad O, Nilsson Ekdahl K, Nilsson P, et al. Complement activation is triggered by chondroitin sulfate released by thrombin receptoractivatedplatelets. J Thrombosis Haemostasis. 2007 doi: 10.1111/j.1538-7836.2008.03034.x. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiorante P, Banz Y, Mohacsi PJ, et al. Low molecular weight dextran sulfate prevents complement activation and delays hyperacute rejection in pig-to-human xenotransplantation models. Xenotransplantation. 2001;8(1):24. doi: 10.1046/j.0908-665x.2000.00088.x. [DOI] [PubMed] [Google Scholar]
- 27.Thomas H, Maillet F, Letourneur D, Jozefonvicz J, Fischer E, Kazatchkine MD. Sulfonated dextran inhibits complement activation and complement-dependent cytotoxicity in an in vitro model of hyperacute xenograft rejection. Mol Immunol. 1996;33(7–8):643. doi: 10.1016/0161-5890(96)00028-4. [DOI] [PubMed] [Google Scholar]
- 28.Soulika AM, Khan MM, Hattori T, et al. Inhibition of heparin/protamine complex-induced complement activation by Compstatin in baboons. Clin Immunol. 2000;96(3):212. doi: 10.1006/clim.2000.4903. [DOI] [PubMed] [Google Scholar]