Abstract
Background
Mammographic density (MD) is a risk factor for breast cancer. MD and breast MRI volume (MRIV) assess the amount of fibroglandular tissue in the breast. MD and MRIV can be modulated with hormonal interventions, suggesting that these imaging modalities may be useful as surrogate endpoint biomarkers for breast cancer chemoprevention trials. We evaluated the effect of raloxifene on MD and MRIV in premenopausal women at increased risk for breast cancer.
Materials and Methods
Mammograms and MRIs were obtained at baseline and after one and two years of raloxifene 60 mg by mouth daily for 27 premenopausal women. Mammographic percent dense area was calculated using a semi-quantitative thresholding technique. T1 weighted spoiled gradient-echo MRI with fat suppression was used to determine breast MRIV using a semiautomatic method. Mean change in MD and median change in MRIV were assessed by the Wilcoxon signed-rank test.
Results
No significant change in MD was seen after treatment with raloxifene. Mean change after one year was 1% (95% CI -3 to +5) and after two years was 1% (95% CI -2 to +5). MRIV decreased on raloxifene. Median relative change in MRIV after one year was -17% (95% CI % -28 to -9; p=0.0017) and after two years was -16% (95% CI -31 to -4; p=0.0004).
Conclusions
In high risk premenopausal women MD did not change on raloxifene, while MRIV significantly declined. Our findings suggest that MRIV is a promising surrogate biomarker in premenopausal women at increased risk for breast cancer and should be investigated further in breast cancer prevention trials.
Keywords: mammographic density, breast MRI, biomarkers, raloxifene, high risk, breast cancer
Introduction
MD is a well recognized risk factor for breast cancer and was first described in 1976 by John Wolfe using qualitative assessments(1). Semi-quantitative methods of measuring MD have since been developed and studies incorporating these techniques have consistently shown a positive association with breast cancer risk. The risk for developing breast cancer for the most dense compared with the least dense breast tissue categories ranges from 1.8-6.0, with most studies yielding an odds ratio of 4.0 or greater.(2) MD is a dynamic value influenced by age, parity(3), menstrual cycle phase(4), menopause(5), insulin-like growth factor pathway(6),body mass index(7) and genetics(8). Endocrine agents that change breast cancer risk also affect MD. Notably, estrogen and progesterone hormone replacement therapy (HRT) increases the risk of breast cancer(9) and likewise increases MD,(10) while tamoxifen, a selective estrogen receptor modulator (SERM), decreases the risk of breast cancer as well as MD in both premenopausal and postmenopausal women.(11-13) Tamoxifen decreases MD 7.9% compared with a 3.5% decrease in the placebo arm (p<0.01).(11) Changes in MD are noted in the first year after starting tamoxifen. These observations suggest that MD may be useful as a surrogate endpoint biomarker for breast cancer chemoprevention trials.
Breast MRI is another imaging modality that can assess the amount of fibroglandular tissue in the breast, i.e. MRI volume (MRIV).(14) This technique is of interest due to the limitations of mammography which presents a two dimensional image of a 3 dimensional object. The tomographic images obtained with MRI allow for a more comprehensive assessment of tissue throughout the breast. While not as extensively studied as MD, MRI also reflects biologic effects, and MRI enhancement decreases with age(14), varies with the menstrual cycle and HRT.(15) It should be recognized that the methodology used for reporting change on MRI is variable and less well established than for MD.(16, 17) Two studies have evaluated the relationship between MD and MRI fibroglandular parameters and report high correlation between MD and MRIV. Correlation coefficients ranged from 0.63 to 0.91, with one study noting a stronger correlation in postmenopausal women indicating that these radiologic studies reflect similar measures of stromal and connective tissue versus fat.(14, 18)
Raloxifene, another SERM, has been recently found to be effective in breast cancer prevention(19). Evaluation of the effect of raloxifene on MD has been conducted in postmenopausal women at risk for osteoporosis and/or cardiovascular disease. In the largest cohort (N=280) Jackson determined that no increase in MD occurred in women on raloxifene as opposed to those on combined hormone replacement therapy, while in smaller cohorts MD declined from baseline on raloxifene or showed no significant change. The methodology to assess change in MD in these studies included semi-quantitative and in some cases purely qualitative means to describe MD.(20-22), Although raloxifene has not been shown to have a large effect on MD in these studies, the effect of raloxifene on mammographic density in premenopausal women is unknown. Based on tamoxifen’s ability to reduce mammographic density to an even greater degree in pre versus postmenopausal women(11), we postulated that raloxifene would reduce MD and MRIV in premenopausal women at increased risk for invasive breast cancer. .
Materials and methods
Trial Design
Participants enrolled in a Phase II trial of raloxifene in premenopausal women at high-risk for developing invasive breast cancer. Details of the study have been previously reported.(23-26) In brief, all participants provided written informed consent. Eligible patients had an increased risk of breast cancer by at least one of the following criteria: Gail model risk assessment of 1.7% or greater over five years, a family history consistent with hereditary breast cancer, or a histologically documented diagnosis of lobular carcinoma in situ, atypical ductal hyperplasia, or locally treated ductal carcinoma in situ. Subjects were required to have regular menstrual cycles (defined as 26-35 days) for the 6 months preceding enrollment in the trial. Premenopausal status was additionally verified by a follicle stimulating hormone level <20 mIU/ml. Raloxifene was administered orally at 60 mg/day with calcium 1250 mg/day, for two years. Because raloxifene is contraindicated in women who are or may become pregnant due to possible teratogenic effects, all women were required to use non-hormonal birth control for the duration of the study and for three months after completion. After stopping raloxifene, subjects were followed off-drug for one year.
Imaging studies
Standard four-view bilateral film-screen mammograms were obtained in the follicular phase of the menstrual cycle at baseline prior to treatment, after one and two years of raloxifene treatment, and one year post-cessation of raloxifene. The craniocaudal view of one breast was selected for digitization. The denser breast was chosen at baseline and this same breast was followed over the course of the study. For patients who had had no prior surgery, the side with greater density was selected. For patients who had undergone breast surgery for non-invasive cancer or other reasons, mammograms of the unaffected side were selected and digitized. Mammograms were scanned with a Better Light (San Carlos, CA) Digital Scan Back through a Nikon 4×5 camera using a resolution of 267 pixels per inch and saved as TIFF files.
Digitized mammograms were analyzed using the scripting language of the MEDx image analysis and visualization software package (Medical Numerics, Stirling, Virginia). Using this program, the skin/air interface was manually outlined. This region was then interactively thresholded by segmenting the image based on gray level and applying a tint over the fibroglandular densities above the specified threshold. The percentage of the breast occupied by fibroglandular tissue (percent MD) was calculated. Percent MD was determined independently by two radiologists (CC and CG) masked to the subject’s duration on raloxifene as well as current use of raloxifene.
Breast MRI was performed on the same day as the mammogram. T1 weighted spoiled gradient-echo (SPGR) fat suppressed MRI using gadolinium contrast with images at 3mm intervals were obtained. The MRIV was determined using an automated classification and calculation method developed by our group. We have previously compared our automated tissue classification method with manually generated tissue classification by two experienced radiologists and found 94.95% agreement. (27) Due to the high concordance of these measurements, the current study did not include further assessment of inter- or intra-observer variability in determining MRIV. The same breast was evaluated for change in MD and MRIV.
Statistics
The change in MD was calculated as the difference in the percent density. The change in MRIV was determined by subtracting the earlier MRIV from the later MRIV and dividing by the earlier MRIV to adjust for large changes in MRIV associated with large baseline MRIV values. Changes over time in paired images were tested for a mean change of zero using the Wilcoxon signed rank test. Correlation between MD at baseline and clinical factors age and body mass index (BMI) was also evaluated. The association between MD and MRIV was determined by Spearman rank correlation. Intra-radiologist reproducibility of the determination of MD for the baseline mammograms was assessed as well as inter-radiologist correlation for change in MD from baseline to one year on raloxifene using Spearman rank correlation.
Results
Thirty-seven women enrolled in the trial and of these 7 did not start drug. Paired mammograms were available for 27 subjects at baseline and 12 months, 25 at baseline and 24 months, and 19 at baseline and 36 months (one year off raloxifene). We evaluated MRIV only for those women who also had paired mammograms. Paired MRIs were available for 16 subjects at baseline and 12 months, 19 at baseline and 24 months, and 17 at baseline and 36 months (one year off raloxifene). Characteristics of the 27 subjects included in the study are shown in Table 1.
Table 1.
Subject Characteristics (N=27)
| Mean age (range) | 43 (35-47) | |
| Race: | ||
| Caucasian | 26 | |
| Hispanic | 1 | |
| Mean BMI (range) | 24 (18-41) | |
| Mean mammographic density (range) | 39% (7-78) | |
| Risk Category: | ||
| Gail Risk >1.7% | 20 (median 2.2%) | |
| Family History | 1 | |
| DCIS | 3 | |
| LCIS | 3 | |
Change in Mammographic Density
Mean percent MD for the 27 eligible patients at baseline was 39% and 37% for radiologists A (CC) and B (CG), respectively. No significant change in percent MD was seen after one or two years of treatment with raloxifene by either reader. The mean change from baseline to one year was 1% (95% CI = -3 to +5) for radiologist A and 2% (95% CI = -4 to +7) for radiologist B. The mean change from baseline to two years on raloxifene was 1% (95% CI = -2 to +5) for radiologist A and 6% (95% CI = -0.2 to +13) for radiologist B. After twelve months post cessation of raloxifene little overall change in MD was recorded (Table 2). No associations between baseline MD and age (r=-0.02, p=0.91) or BMI (r=-0.27, p=0.18) were found. Of the 19 subjects with baseline to year 2 MRIV data, 18 have baseline to year 2 MD changes, with the following summary statistics: for radiologist A, mean change 3%, 95% CI -1% to 8%, p=0.17 for the null hypothesis of zero mean change; for radiologist B, mean change 9%, 95% CI 3% to 15%, p=0.008. These changes are a little higher than those of the other 7 subjects without MRIV data, but not significantly higher (p=0.17 and p=0.30, respectively, by the Wilcoxon rank sum test), thus the results are comparable to the entries in Table 2.
Table 2.
Mean change in mammographic density
| Radiologist A | Radiologist B | ||||||
|---|---|---|---|---|---|---|---|
| Time on study | N | Mean change in % MD | 95% CI | P value | Mean change in % MD | 95% CI | P value |
| 1 year | 27 | 1 | -3 to 5 | 0.93 | 2 | -4 to 7 | 0.86 |
| 2 years | 25 | 1 | -2 to 5 | 0.58 | 6 | -0.2 to 13 | 0.05 |
| 3 years | |||||||
| (from year 2 to year 3) | 19 | -5 | -10 to -0.5 | 0.02 | -6 | -15 to 34 | 0.26 |
To assess the reproducibility and reliability of readers’ measurements, both intra-radiologist and inter-radiologist correlations were determined. Radiologist A recalculated percent MD for 27 baseline mammograms. The difference between the initial and rescored measurements (second set - first set) has a mean of -0.4 +/- 10% (range, -17% to 39.0%). These differences are consistent with a mean of zero (p = 0.56). The 39% at the upper end of the range is an extreme outlier from one subject; the second highest difference is 7%. Inter-radiologist correlation for change in percent mammographic density from baseline to one year was high (r=0.63, p=0.0006). This correlation was also high from baseline to two years and after twelve months post cessation of raloxifene (Table 3).
Table 3.
Inter-radiologist correlation for mammographic density
| Time on study | r coefficient | P value |
|---|---|---|
| 1 year | 0.63 | 0.0006 |
| 2 years | 0.62 | 0.0013 |
| 3 years (one year off raloxifene) | 0.39 | 0.1 |
Change in MRIV
MRIV significantly decreased while on raloxifene for one and two years. The median percent change in MRIV from baseline to one year was -17% (95% CI -28 to -9, p=0.0017). The median percent change from baseline to two years on raloxifene was -16% (95% CI -31 to -14, p=0.0004). The median percent change in MRIV from 2 years to one year later off of raloxifene was -9% (95% CI -18 to 20, p=0.64) and not significantly different from the reading at 2 years (Table 4). BMI had moderate negative correlations with MRIV at all time points, range r=-0.41, (p=0.081) to r= -0.56 (p=0.005) and no correlation with age at baseline r=-0.01, p=0.98.
Table 4.
Median change in breast MRIV
| Time on study | N | Median % change | range | 95% CI | P value |
|---|---|---|---|---|---|
| 1 year | 16 | -17 | -64 to 12 | -28 to -9 | 0.0017 |
| 2 years | 19 | -16 | -57 to 25 | -31 to -14 | 0.0004 |
| 3 years | |||||
| (from year 2 to year 3) | 17 | -9 | -23 to 28 | -18 to 20 | 0.64 |
Correlation between MD and MRIV measurements
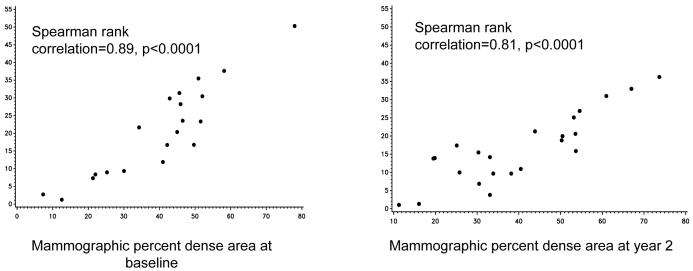
MD and MRIV measurements were well correlated at all time points: baseline r=0.89, p<0.0001, one year on raloxifene r=0.67, p=0.0005, two years on raloxifene r=0.81, P<0.0001, and one year after stopping study drug r=0.80, p=0.0005 (Figure 1). However change in readings over time was not well correlated between MD and MRIV, e.g. baseline to year 2 r=0.33, p=0.18.
Figure 1.
Correlation between MD and MRIV
Discussion
In this trial in premenopausal women at high risk for breast cancer, we found that percent MD did not change on raloxifene, while MRIV significantly decreased. While these results may appear to be discordant with each other, further consideration of each of the imaging modalities and prior intervention studies clarifies these findings and underscores promising aspects of MRIV assessments. Determination of change in MRIV may offer a more reproducible and sensitive measure of breast fibroglandular tissue. While MD has been embraced as a risk marker for breast cancer and incorporated into standard risk assessments(28), its deficiencies must also be recognized. MD readings are based on a single 2-D image subject to technical manipulation.(14) Thus any given instrumentation of mammograms to obtain MD may influence readings, e.g. digitizing the image, the underlying contrast of the image. While MRIs also are mechanically manipulated, the much larger volume of data makes for a more stable assessment. Thus while no changes were seen in MD, relatively little data was available for analysis in comparison with MRIV. The statistically significant correlation between the MD and MRIV at all time points on our study shows that they indeed produce similar quantitative evaluations of fibroglandular tissue. The lack of correlation of change in MRIV and MD is likely due to range of measurements in MD, the standard deviation of the changes in MD and measurement fluctuation which taken together may cloud a potential correlation. The three dimensional nature of MRI technology might overcome some of the limitations of mammography, allowing more accurate and stable assessment of fibroglandular volume. The reduction in variation in MRIV compared with MD supports this imaging modality as a more reliable and reproducible surrogate biomarker, and thus more amenable as an endpoint in phase II prevention trials which employ smaller sample sizes.
Additionally, it is important to interpret these results in the scope of literature on endocrine agents and MD. The Study of Tamoxifen and Raloxifene (STAR)(29) reported that raloxifene is as effective as tamoxifen in reducing the risk of invasive breast cancer in postmenopausal women at increased risk, yet, unlike tamoxifen, raloxifene has not been found to have an effect on MD. Christodoulakos et al reported no statistically significant change in MD in postmenopausal women at risk for osteoporosis or cardiovascular disease after one year of raloxifene (N=48) compared to controls (N=27) using qualitative measures to score MD.(21) Similarly, in a nested pilot study Freedman et al found that the mean MD in a subgroup of postmenopausal women participating in an osteoporosis prevention trial did not significantly change in women receiving raloxifene compared with placebo. MD decreased slightly in both groups over two years:- 1.3% in the 45 women receiving placebo versus -1.5% in the 45 women receiving raloxifene.(20) This study used semi-quantitative methods, similar to ours, to determine MD. Typically the studies with tamoxifen have included larger cohorts of women, both pre and postmenopausal, who are also at increased risk for breast cancer and tamoxifen has repeatedly been shown to decrease MD. These differences in study populations may contribute to the ability to see a change in MD. An examination of baseline MD across these trials underscores this point. For example, in the IBIS-I, a tamoxifen chemoprevention trial, evaluation of MD included 818 pre and postmenopausal women with a baseline MD of approximately 42%, while in the Freedman raloxifene study baseline MD ranged from 8.1 to 13.5%, approximately 4 fold lower than the women at high risk for breast cancer (11, 20). This difference in baseline MD reflects the dissimilar risk profiles of the women enrolled in these trials and raises the question that perhaps the MD in the raloxifene treated women may not be elevated enough to detect a significant change. It is also possible that raloxifene as a chemopreventive works through a mechanism that does not affect mammographic density, although this seems unlikely given the similarity in mechanism of action to tamoxifen.
Our study is the first to report on the effects of raloxifene on MD and MRIV in premenopausal high risk women, and the first to assess change in MRIV in a prospective intervention trial. Additional strengths of our trial include our well defined study population, timing of imaging studies to the menstrual cycle, and same day imaging for mammograms and MRI. However, our study also had several limitations. Based on the number of evaluable subjects in our study, we could only detect a change in mammographic density greater than 7%. Although this is similar to the magnitude of reduction seen in trials of tamoxifen on breast density our trial did not have sufficient power to detect smaller reductions in MD. We had no control group or historical series to suggest the natural history of MRIV in a similar cohort over time without intervention. Based on a study of women participating in the Canadian National Breast Screening Study, the estimated average annual reduction in percent MD for premenopausal women is about 1%(5) while the natural history of MRIV over time is unknown.
Future directions of research should be aimed at further understanding the histologic correlates of MD and MRIV. Boyd et al (30) demonstrated that the risk of hyperplasia (with or without atypia) or carcinoma in situ in biopsies is related to increasing mammographic density, providing the first significant link to histologic data. Further work has evaluated the link between breast tissue/fluid and MD.(31)(32)(33) These results support the hypothesis that mammographically dense breast tissue reflects the degree of stromal and epithelial proliferation and may be closely linked to growth factor activity.
These findings are important because they are the only report on changes in breast MRIV with a prevention agent. Our results raise important considerations for planning prevention intervention trials since this technique may offer a highly sensitive method to identify promising agents which should be moved forward into larger scale testing. Although breast MRI is on average five times more expensive than mammogram, if this technique can be employed thoughtfully in targeted studies it may provide reliable data that ultimately reduces research costs. For example a highly precise test for efficacy will allow design of phase II trials requiring smaller numbers of subjects. MRIV may be a more informative surrogate biomarker for breast cancer risk than MD. Breast MRI will be of increasing relevance since recent ACS guidelines now recommend breast cancer screening with MRI in addition to mammogram in high risk women.(34) These changes will increase the use of MRI and thus the need to better understand how these images can be evaluated prospectively and used clinically.
Acknowledgements
The authors would like to thank Mr. John Ward and Ms. Danielle Deshommes for their technical assistance preparing and scanning the mammograms.
References
- 1.Wolfe JN. Breast patterns as an index of risk for developing breast cancer. Am J Roentgenol. 1976;126:1130–7. doi: 10.2214/ajr.126.6.1130. [DOI] [PubMed] [Google Scholar]
- 2.Boyd NF, Rommens JM, Vogt K, et al. Mammographic breast density as an intermediate phenotype for breast cancer. 2005;6:798–808. doi: 10.1016/S1470-2045(05)70390-9. [DOI] [PubMed] [Google Scholar]
- 3.Boyd NF, Lockwood GA, Byng JW, Tritchler DL, Yaffe MJ. Mammographic densities and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1998;7:1133–44. [PubMed] [Google Scholar]
- 4.Buist DS, Aiello EJ, Miglioretti DL, White E. Mammographic breast density, dense area, and breast area differences by phase in the menstrual cycle. Cancer Epidemiol Biomarkers Prev. 2006;15:2303–6. doi: 10.1158/1055-9965.EPI-06-0475. [DOI] [PubMed] [Google Scholar]
- 5.Boyd N, Martin L, Stone J, et al. A longitudinal study of the effects of menopause on mammographic features. Cancer Epidemiol Biomarkers Prev. 2002;11:1048–53. [PubMed] [Google Scholar]
- 6.Byrne C, Colditz GA, Willett WC, et al. Plasma insulin-like growth factor (IGF) I, IGF-binding protein 3, and mammographic density. Cancer Res. 2000;60:3744–8. [PubMed] [Google Scholar]
- 7.Boyd NF, Lockwood GA, Byng JW, et al. The relationship of anthropometric measures to radiological features of the breast in premenopausal women. Br J Cancer. 1998;78:1233–8. doi: 10.1038/bjc.1998.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyd NF, Dite GS, Stone J, et al. Heritability of mammographic density, a risk factor for breast cancer. N Engl J Med. 2002;347:886–94. doi: 10.1056/NEJMoa013390. [DOI] [PubMed] [Google Scholar]
- 9.Buzdar A, Chlebowski R, Cuzick J, et al. Defining the role of aromatase inhibitors in the adjuvant endocrine treatment of early breast cancer. 2006;22:1575–1585. doi: 10.1185/030079906X120940. [DOI] [PubMed] [Google Scholar]
- 10.Greendale GA, Reboussin BA, Slone S, et al. Postmenopausal hormone therapy and change in mammographic density. J Natl Cancer Inst. 2003;95:30–7. doi: 10.1093/jnci/95.1.30. [DOI] [PubMed] [Google Scholar]
- 11.Cuzick J, Warwick J, Pinney E, Warren RM, Duffy SW. Tamoxifen and breast density in women at increased risk of breast cancer. J Natl Cancer Inst. 2004;96:621–8. doi: 10.1093/jnci/djh106. [DOI] [PubMed] [Google Scholar]
- 12.Chow CK, Venzon D, Jones EC, et al. Effect of tamoxifen on mammographic density. Cancer Epidemiol Biomarkers Prev. 2000;9:917–21. [PubMed] [Google Scholar]
- 13.Brisson J, Brisson B, Cote G, et al. Tamoxifen and mammographic breast densities. Cancer Epidemiol Biomarkers Prev. 2000;9:911–5. [PubMed] [Google Scholar]
- 14.Lee NA, Rusinek H, Weinreb J, et al. Fatty and fibroglandular tissue volumes in the breasts of women 20-83 years old: comparison of X-ray mammography and computer-assisted MR imaging. AJR Am J Roentgenol. 1997;168:501–6. doi: 10.2214/ajr.168.2.9016235. [DOI] [PubMed] [Google Scholar]
- 15.Heywang-Kobrunner SH, Viehweg P, Heinig A, Kuchler C. Contrast-enhanced MRI of the breast: accuracy, value, controversies, solutions. Eur J Radiol. 1997;24:94–108. doi: 10.1016/s0720-048x(96)01142-4. [DOI] [PubMed] [Google Scholar]
- 16.Pfleiderer SO, Sachse S, Sauner D, et al. Changes in magnetic resonance mammography due to hormone replacement therapy. Breast Cancer Res. 2004;6:R232–8. doi: 10.1186/bcr779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delille JP, Slanetz PJ, Yeh ED, et al. Hormone replacement therapy in postmenopausal women: breast tissue perfusion determined with MR imaging--initial observations. Radiology. 2005;235:36–41. doi: 10.1148/radiol.2351040012. [DOI] [PubMed] [Google Scholar]
- 18.Wei J, Chan HP, Helvie MA, et al. Correlation between mammographic density and volumetric fibroglandular tissue estimated on breast MR images. Medical Physics. 2004;31:933–942. doi: 10.1118/1.1668512. [DOI] [PubMed] [Google Scholar]
- 19.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes - The NSABP study of tamoxifen and raloxifene (STAR) P-2 trial. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 20.Freedman M, San Martin J, O’Gorman J, et al. Digitized mammography: a clinical trial of postmenopausal women randomly assigned to receive raloxifene, estrogen, or placebo. J Natl Cancer Inst. 2001;93:51–6. doi: 10.1093/jnci/93.1.51. [DOI] [PubMed] [Google Scholar]
- 21.Christodoulakos GE, Lambrinoudaki IV, Vourtsi AD, et al. Mammographic changes associated with raloxifene and tibolone therapy in postmenopausal women: a prospective study. Menopause-the Journal of the North American Menopause Society. 2002;9:110–116. doi: 10.1097/00042192-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Jackson VP, San Martin JA, Secrest RJ, et al. Comparison of the effect of raloxifene and continuous-combined hormone therapy on mammographic breast density and breast tenderness in postmenopausal women. American Journal of Obstetrics and Gynecology. 2003;188:389–394. doi: 10.1067/mob.2003.21. [DOI] [PubMed] [Google Scholar]
- 23.Eng-Wong J, Reynolds JC, Venzon D, et al. Effect of raloxifene on bone mineral density in premenopausal women at increased risk of breast cancer. 2006;91:3941–3946. doi: 10.1210/jc.2005-2827. [DOI] [PubMed] [Google Scholar]
- 24.Eng-Wong J, Hursting SD, Venzon D, Perkins SN, Zujewski JA. Effect of raloxifene on insulin-like growth factor-I, insulin-like growth factor binding protein-3, and leptin in premenopausal women at high risk for developing breast cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:1468–73. [PubMed] [Google Scholar]
- 25.Chatterton RT, Jr., Zujewski J, Mateo ET, Eng-Wong J, Jordan VC. Effect of raloxifene on salivary sex steroid concentrations in premenopausal women. J Endocrinol. 2006;191:599–604. doi: 10.1677/joe.1.06791. [DOI] [PubMed] [Google Scholar]
- 26.Faupel-Badger JM, Prindiville SA, Venzon D, et al. Effects of raloxifene on circulating prolactin and estradiol levels in premenopausal women at high risk for developing breast cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:1153–8. doi: 10.1158/1055-9965.EPI-05-0898. [DOI] [PubMed] [Google Scholar]
- 27.Yao J, Zujewski JA, Orzano J, Prindiville S, Chow S. Classification and calculation of breast fibroglandular tissue volume on SPGR fat suppressed MRI. Medical Imaging: Image Processing Proceedings of the SPIE. 2005;5747:1942–1949. [Google Scholar]
- 28.Chen JB, Pee D, Ayyagari R, et al. Projecting absolute invasive breast cancer risk in white women with a model that includes mammographic density. JOURNAL OF THE NATIONAL CANCER INSTITUTE. 2006;98:1215–1226. doi: 10.1093/jnci/djj332. [DOI] [PubMed] [Google Scholar]
- 29.Study of Tamoxifen and Raloxifene (STAR) Trial. National Cancer Institute; 2006. 2006. [Google Scholar]
- 30.Boyd NF, Jensen HM, Cooke G, Han HL. Relationship between mammographic and histological risk factors for breast cancer. J Natl Cancer Inst. 1992;84:1170–9. doi: 10.1093/jnci/84.15.1170. [DOI] [PubMed] [Google Scholar]
- 31.Li T, Sun LM, Miller N, et al. The association of measured breast tissue characteristics with mammographic density and other risk factors for breast cancer. Cancer Epidemiology Biomarkers & Prevention. 2005;14:343–349. doi: 10.1158/1055-9965.EPI-04-0490. [DOI] [PubMed] [Google Scholar]
- 32.Guo YP, Martin LJ, Hanna W, et al. Growth factors and stromal matrix proteins associated with mammographic densities. Cancer Epidemiol Biomarkers Prev. 2001;10:243–8. [PubMed] [Google Scholar]
- 33.Lee MM, Petrakis NL, Wrensch MR, et al. Association of abnormal nipple aspirate cytology and mammographic pattern and density. Cancer Epidemiol Biomarkers Prev. 1994;3:33–6. [PubMed] [Google Scholar]
- 34.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]