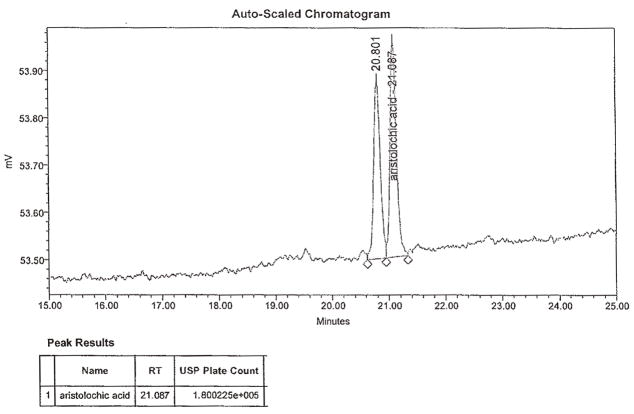
Abstract
The presence of aristolochic acid in some dietary supplements is a concern to regulators and consumers. A method has been developed, by initially using a reference method as a guide, during single laboratory validation (SLV) for the determination of aristolochic acid I, also known as aristolochic acid A, in botanical species and dietary supplements at concentrations of approximately 2 to 32 μg/g. Higher levels were determined by dilution to fit the standard curve. Through the SLV, the method was optimized for quantification by liquid Chromatography with ultraviolet detection (LC-UV) and LC/mass Spectrometry (MS) confirmation. The test samples were extracted with organic solvent and water, then injected on a reverse phase LC column. Quantification was achieved with linear regression using a laboratory automation system. The SLV study included systematically optimizing the LC-UV method with regard to test sample size, fine grinding of solids, and solvent extraction efficiency. These parameters were varied in increments (and in separate optimization studies), in order to ensure that each parameter was individually studied; the test results include corresponding tables of parameter variations. In addition, the chromatographic conditions were optimized with respect to injection volume and detection wavelength. Precision studies produced overall relative standard deviation values from 2.44 up to 8.26% for aristolochic acid I. Mean recoveries were between 100 and 103% at the 2 μg/g level, between 102 and 103% at the 10 μg/g level, and 104% at the 30 μg/g level.
The U.S. Food and Drug Administration (FDA) is concerned about botanicals and botanical containing products that may contain aristolochic acid, either naturally or through adulteration. Some of these botanicals are known to contain, or are suspected of containing, aristolochic acid. These botanicals include Aristolochia species (spp.), Asarum spp., and Bragantia spp. Botanicals that may be adulterated with aristolochic acid include Akebia spp., Clematis spp., Coculus spp., Diploclisia spp., Menispernum spp., Sinomenium spp., and Stephania spp. Additional names associated with these species include Mu tong, Fang ji, Guang fang ji, Fang chi, Kan-Mokotsu, and Mokutsu. The similarity of names, as well as the identification and handling of botanicals in preparation of traditional Chinese medicines, makes it difficult to avoid inadvertent adulteration of both botanicals and dietary supplements. Reported serious adverse effects in humans include nephropathy and urothelial carcinomas. Rodents administered aristolochic acid developed lymphoma and cancers in the kidney, bladder, stomach, and lung, implying a potential increased risk of developing malignancies in humans (1, 2). The test materials used in the validation were Aristolochia spp. root, Aristolochia manschuriensis stem, Akebia trifoliata stem, Clematis armandii stem at 20 ppm high fortification, Stephania tetrandra root at 10 ppm low fortification, tablets containing a 20 ppm high fortification, and tablets with a 10 ppm low fortification. The fortified materials were spiked with ground Aristolochia spp. root. All test materials were authenticated by voucher or analysis for aristolochic acid I (Aristolochia spp. root). Due to the amount of Aristolochia spp. root needed for some experiments in the single laboratory validation (SLV), the ground Aristolochia spp. root was further diluted, as necessary, with ground Stephania tetrandra root (negative control) to produce the necessary amounts of Aristolochia spp. root containing aristolochic acid I.
Experimental
Apparatus
Balances.—Analytical, capable of weighing to 0.00001 g and top loading to 0.001 g.
Mini grinder.—Coffee and spice grinder, Type F408 (KRUPS, Ecully, Cedex, France).
Multi-wrist shaker.—Model 75 (Barrell Corp., Pittsburgh, PA).
Liquid chromatography (LC) column.—Zorbax SB-CIS, 5 μ 3.0 mm id × 25 cm (Agilent, Palo Alto, CA).
Sieve.—400 μm (U.S. standard No. 40; Fisher Scientific, Fair Lawn, NJ).
Mass spectrometer.—Equipped with a diode array detector, 1100 Series (Agilent).
UV-Vis spectrophotometer.—Capable of measuring absorbace at 390 nm (Agilent, Palo Alto, CA).
LC system.—Equipped with a UV-Vis detector, 1100 Series (Agilent).
HPLC column.—Zorbax SB-CIS, 5 μm, 2.1 mm id × 5 cm (for LC/MS triple quad; Agilent).
Syringe filter.—13 mm with 0.45 μm PTFE membrane (Pall Life Sciences, Ann Arbor, MI).
Data system for HPLC.—Empower Version Build 1154 (Waters Corp., Milford, MA).
Data system for Micromass Quattro.—Masslynx Version 4.1 (Waters Corp.).
HPLC system.—Equipped with a mass spectrometer for triple quad, Micromass Quattro LC (Waters Corp.).
(Note: Equivalent equipment may be substituted.)
Reagents
Acetonitrile.—HPLC grade, No. A996-4 (Fisher Scientific).
Water.—Prepared with a Milli-Q purification system (Millipore Corp., Bedford, MA).
Trifluoroacetic acid.—99.8%, No. 3–3076 (Supelco, Bellefonte, PA).
Ethanol.—200 proof, HPLC/spectrophotometric grade, No. 459828-4L (Sigma-Aldrich, St. Louis, MO).
Methanol.—HPLC grade, No. A454-4 (Fisher Scientific).
Formic acid.—ACS 88+%, No. 36504 (Alfa Aesar, Ward Hill, PA).
Ammonium acetate.—HPLC grade, No. A639-500 (Fisher Scientific).
(Note: Equivalent reagents may be substituted.)
Reference Standard
Aristolochic acid I (also known as aristolochic acidA).—94.03% pure, No. 011002–10 (ChromaDex, Santa Ana, CA).
Preparation of Standard Solutions
(a) Stock solution (ca 200 μg/mL)
Prepared by weighing ca 0.002 g aristolochic acid to 0.00001 g and transferring into a 10 mL volumetric flask. The aristolochic acid was dissolved and diluted to volume with acetonitrile. One milliliter was diluted to 5 mL with ethanol and read on a spectrophotometer at 390 nm. The molar extinction coefficient (∈) for aristolochic acid from the Merck Index is 6500 (concentration in g-mole/L).
where Abs = absorbance at 390 nm. The solution is stable for 30 days when stored protected from light in a refrigerator set to maintain 5 ± 3°C.
(Note: The concentration was verified on the spectrophotometer prior to making working standards.)
(b) Working standard solutions
Prepared by diluting the standards at 6 concentration levels from 0.040 to 0.64 μg/mL in a solution of acetonitrile and purified water (50 + 50, v/v). The solutions are stable for 14 days when stored protected from light in a refrigerator set to maintain 5 ± 3°C.
Preparation of Reagents
Mobile phase A was prepared by adding 0.1% (v/v) trifluoroacetic acid to purified water. Mobile phase B consisted of 0.1% (v/v) trifluoroacetic acid in acetonitrile. The extraction solvent was acetonitrile and purified water (50 + 50, v/v).
Procedure
The material was ground to pass through a 400 μm sieve and mixed well. Approximately 2 g of test sample was weighed into an appropriate flask with a cap, and 100 mL of extraction solvent was added. The solution was shaken for a minimum of 30 min on a wrist shaker set at full stroke. The test samples greater than 32.0 μg/g in concentration were diluted to fit on the standard curve. The extracts were passed through a 0.45 μm filter and were then ready for LC analysis and quantification. The LC parameters are shown in Table 1. Each concentration standard was injected at least twice. Standards were injected after every 4 test samples and also at the end of the series.
Table 1.
HPLC-UV parameters
| Detection | Ultraviolet detector (390 nm) | ||
| Column temperature | 40°C | ||
| Flow rate | Approximately 0.5 mL/min. Flow rate should be adjusted so that desired retention time will be obtained | ||
| Injected volume | 25 μL | ||
| Injection time | 40 min | ||
| Pump program
| |||
| Time, min | %Aa | %Ba | Gradient curve |
|
| |||
| 0 | 80 | 20 | NAb |
| 25 | 30 | 70 | 1 |
| 30 | 0 | 100 | 1 |
| 31 | 80 | 20 | 1 |
| 40 | 80 | 20 | NA |
Mobile phase A = 0.1% (v/v) trifluoroacetic acid and purified water; Mobile phase B = 0.1% (v/v) trifluoroacetic acid and acetonitrile.
NA = Not applicable.
Quantification and Confirmation
The standard curve was obtained by a linear regression of all of the peak areas and the concentrations of the working standards. Determination of the aristolochic acid concentration of the injected test sample was calculated from the regression analysis. The concentration was calculated using the following equation:
where C = concentration from regression analysis (μg/mL); V = extraction solvent volume (mL); D = dilution factor, if any; and W = test sample weight (g).
When aristolochic acid I was detected at greater than or equal to the 2 μg/g by LC-UV, confirmation was done using LC with tandem mass spectrometry (LC/MS/MS). The spectrum of standard was used for the confirmation of presence of aristolochic acid I with comparison to the spectrum of the test sample of like retention time. The peak area detected in the standard for 3 product ions was divided by the peak area of 1 of 3 product ions and averaged for all standards when multiple injections were made. The test sample ratio of confirmation ions to the chosen product ion should be ±10% (arithmetic difference, not relative difference) of the averaged standard ratio. For example, if an average ratio for the standards is 50%, the window for the test sample ratio is 40 to 60%. The chromatographic conditions are shown in Table 2 and the ions monitored are shown in Table 3.
Table 2.
Chromatographic conditions for LC/triple quad-MS
| HPLC column | Zorbax SB-C18, 5 μm, 2.1 mm id × 50 mm, Agilent | ||
| Mobile phase A | 5.0% methanol, 0.1% formic acid, and 10 mM ammonium acetate in purified water | ||
| Mobile phase B | 1:1 acetonitrile-methanol containing 0.1% (v/v) formic acid and 1 0 mM ammonium acetate in purified water | ||
| Pump program
| |||
| Time, min | %A | %B | Gradient curve |
|
| |||
| 0 | 70 | 30 | NAa |
| 13 | 30 | 70 | 1 |
| 15 | 0 | 100 | 1 |
| 16 | 70 | 30 | 1 |
| 20 | 70 | 30 | NA |
| Column temperature | 40°C | ||
| Flow rate | 0.2 mL/min | ||
| Injection volume | 25 μL | ||
| Sample introduction | Electrospray positive (ES+) | ||
| Mode
| |||
| Mass range | Daughter scan for specific ion, dwell = 0.25 s | ||
| Source temperature | 150°C | ||
| Desolvation temperature | 350°C | ||
| Desolvation gas flow | 600 L/h | ||
| Cone gas flow | 60L/h | ||
NA=Not applicable.
Table 3.
Ions to be monitored for LC/MS confirmation
| Compound | Parent ion, m/z | Daughter ions, m/z | Cone, V | Collision, eV |
|---|---|---|---|---|
| Aristolochic acid | 359.1 | 265, 281, 296 | 18 | 40,40,30 |
Results and Discussion
Several tests were done to evaluate the optimum extraction parameters. The publication by Kite et al. (3) showed 70% aqueous methanol, withoutpH adjustment, to be optimum. An 18 h agitation time was also used. Using the Aristolochia root material, the Kite et al. method (3), and the reference method (4), several extraction efficiency parameters were evaluated. These included the test sample grind size, test sample size, extraction solvent volume and composition, and extraction time. In addition, the effects of repetitive dilution and ASE were measured.
To determine the impact of the test sample grind size, 3 preparations of Aristolochia spp. root were ground: the first to an average size of about 600 μm [size range of approximately 150 μm (U.S. Standard Sieve #100) to approximately 1500 μm (approximately U.S. Standard #14)]: the second to pass through a 600 μm sieve (U. S. Standard #30); and the third to pass through a 400 μm sieve (U.S. Standard #40). The optimum grind size was found to be approximately 400 μm (Table 4).
Table 4.
Test sample grind size verification using Aristolochia spp. root
| Grind size, μm | Replicate | Aristolochic acid concn, μg/g |
|---|---|---|
| 150 to 1500 | 1 | 903 |
| 2 | 860 | |
| 3 | 862 | |
| 4 | 904 | |
| 5 | 887 | |
| Mean | 883 | |
| SDa | 21.4 | |
| RSDrb | 2.42 | |
| 600 | 1 | 969 |
| 2 | 960 | |
| 3 | 942 | |
| 4 | 983 | |
| 5 | 1010 | |
| Mean | 973 | |
| SD | 25.6 | |
| RSDr | 2.63 | |
| 400 | 1 | 1030 |
| 2 | 1060 | |
| 3 | 1040 | |
| 4 | 1060 | |
| 5 | 1040 | |
| Mean | 1050 | |
| SD | 13.4 | |
| RSDr | 1.28 |
SD = Standard deviation.
RSDr = % Relative standard deviation, intralaboratory precision.
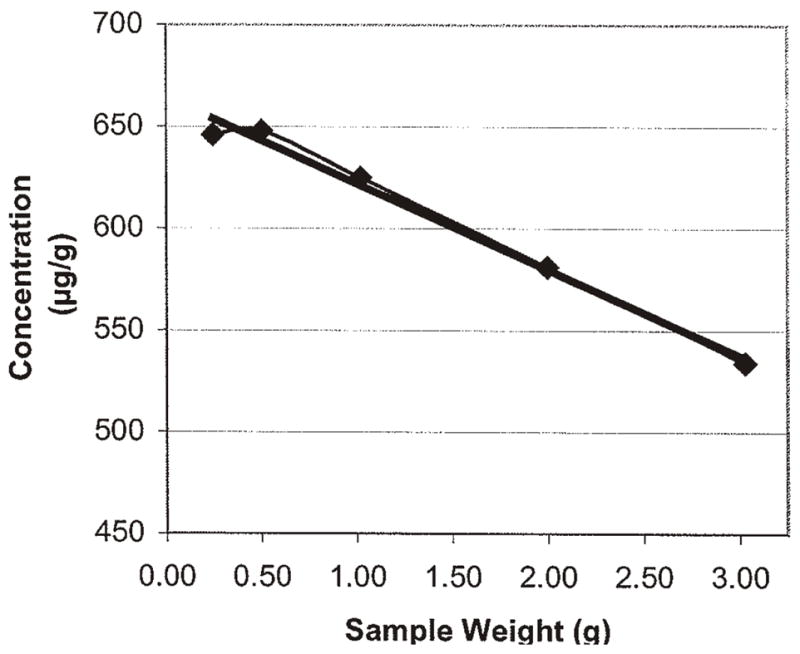
The test sample size parameter was evaluated using portions of 0.25, 0.5, 1, 2, and 3 g that were tested using the various experimental extraction solvents. The optimum test sample size was found to be approximately 2 g using the optimum extracted solvent composition of acetonitrile and Milli-Q water (50 + 50, v/v). The use of 2 g allowed for achieving the desired 2 μg/g limit of quantification (LOQ). Although not optimum, the use of 3 μg/g only slightly decreased the extraction efficiency, thereby allowing for a possible lower LOQ of 1.3 μg/g. These data are presented in Tables 5 and 6 and Figures 1–10.
Table 5.
Aristolochic acid concentration in Aristolochia spp. root diluted with 5. tetrandra root using acetonitrile–water as the extraction solvent (results by test sample weight)
| Test sample weight, g | % Acetonitrile | Aristolochic acid concn, μg/g |
|---|---|---|
| 0.25 | 25 | 646 |
| 50 | 623 | |
| 60 | 604 | |
| 70 | 596 | |
| 80 | 619 | |
| 100 | 224 | |
| 0.50 | 25 | 648 |
| 50 | 621 | |
| 60 | 585 | |
| 70 | 595 | |
| 80 | 629 | |
| 100 | 195 | |
| 1.00 | 25 | 625 |
| 50 | 611 | |
| 60 | 605 | |
| 70 | 608 | |
| 80 | 593 | |
| 100 | 171 | |
| 2.00 | 25 | 581 |
| 50 | 613 | |
| 60 | 611 | |
| 70 | 610 | |
| 80 | 596 | |
| 100 | 164 | |
| 3.00 | 25 | 534 |
| 50 | 594 | |
| 60 | 601 | |
| 70 | 605 | |
| 80 | 569 | |
| 100 | 139 |
Table 6.
Aristolochic acid concentration in Aristolochia spp. root diluted with S. tetrandra root using methanol-water as the extraction solvent (results by test sample weight)
| Test sample weight, g | % Methanol | Aristolochic acid concn, μg/g |
|---|---|---|
| 0.25 | 60 | 627 |
| 70 | 594 | |
| 75 | 588 | |
| 80 | 610 | |
| 85 | 576 | |
| 0.50 | 60 | 550 |
| 70 | 553 | |
| 75 | 588 | |
| 80 | 578 | |
| 85 | 541 | |
| 1.00 | 60 | 571 |
| 70 | 569 | |
| 75 | 576 | |
| 80 | 584 | |
| 85 | 541 | |
| 2.00 | 60 | 610 |
| 70 | 578 | |
| 75 | 584 | |
| 80 | 553 | |
| 85 | 530 | |
| 3.00 | 60 | 576 |
| 70 | 541 | |
| 75 | 526 | |
| 80 | 520 | |
| 85 | 489 |
Figure 1.

0.25 g test sample of Aristolochia spp. root diluted with S. tetrandra root with varying percentages of acetonitrile–water as the extraction solvent.
Figure 10.

3.00 g test sample of Aristolochia spp. root diluted with S. tetrandra root with varying percentages of methanol–water as the extraction solvent.
The extraction solvent volume used was 100 mL. The reference method extraction solvent, methanol and 10% formic acid in water (80 + 20, v/v), was thoroughly evaluated. Aqueous methanol solvents consisting of 60, 70, 75, 80, and 85% methanol were tested to better determine if the methanol level suggested by Kite et al. (3) was adequate. Aqueous acetonitrile solvents consisting of 25, 50, 60, 70, 80, and 100% acetonitrile were also tested. When optimum aqueous methanol and acetonitrile solutions were determined, the use of 10% formic acid in water was evaluated at the optimum conditions found. The presence of formic acid had no effect on the results; therefore, its use was not further considered (Tables 7–9 and Figures 11–21). The optimum extraction solvent composition was found to be a solution of acetonitrile and Milli-Q water (50 + 50, v/v). Acetonitrile and purified water combinations gave higher extraction efficiency results compared to the methanol and purified water. The results varied little from 25 to 70% acetonitrile, but 50% acetonitrile gave slightly higher results. The column mobile phase composition and the use of isocratic versus gradient elution, relative to the reference method, were considered after optimizing the extraction solvent composition. The effect on the LOQ was also considered.
Table 7.
Aristolochic acid concentration in Aristolochia spp. root diluted with S. tetrandra root using acetonitrile-water as the extraction solvent (results by percent acetonitrile)
| % Acetonitrile | Test sample weight, g | Aristolochic acid concn, μg/g |
|---|---|---|
| 25 | 0.24690 | 646 |
| 0.50260 | 648 | |
| 1.0224 | 625 | |
| 1.9969 | 581 | |
| 3.0289 | 534 | |
| 50 | 0.25730 | 623 |
| 0.51120 | 621 | |
| 1.0871 | 611 | |
| 2.0253 | 613 | |
| 3.0004 | 594 | |
| 60 | 0.25350 | 604 |
| 0.50390 | 585 | |
| 1.0615 | 605 | |
| 2.0270 | 611 | |
| 3.0651 | 601 | |
| 70 | 0.25060 | 596 |
| 0.25760 | 595 | |
| 1.0692 | 608 | |
| 2.0106 | 610 | |
| 2.9987 | 605 | |
| 80 | 0.25850 | 619 |
| 0.50500 | 629 | |
| 1.0219 | 593 | |
| 2.0515 | 596 | |
| 2.9842 | 569 | |
| 100 | 0.2504 | 224 |
| 0.5107 | 195 | |
| 0.9947 | 171 | |
| 2.0486 | 164 | |
| 3.0359 | 139 |
Table 9.
Extraction solvent evaluation (10% formic acid versus Milli-Q water) in Aristolochia spp. root diluted with S. tetrandra root
| Aristolochic acid concn, μg/g
|
||
|---|---|---|
| Replicate | Acetonitrile–Milli-Q water (50 + 50, v/v) | Acetonitrile–10% formic acid (50 + 50, v/v) |
| 1 | 589 | 585 |
| 2 | 627 | 634 |
| 3 | 642 | 637 |
| 4 | 646 | 615 |
| 5 | 664 | 636 |
| Meana | 634 | 621 |
| SDb | 28.2 | 22.3 |
| RSDr | 4.45 | 3.59 |
| Methanol-Milli-Q water (75 + 25, v/v) | Methanol-1 0% formic acid (75 + 25, v/v) | |
| 1 | 575 | 576 |
| 2 | 608 | 594 |
| 3 | 586 | 596 |
| 4 | 591 | 609 |
| 5 | 600 | 600 |
| Mean | 592 | 595 |
| SD | 12.7 | 12.1 |
| RSDr | 2.15 | 2.03 |
SD = Standard deviation.
RSDr = % Relative standard deviation, intralaboratory precision.
Figure 11.
Acetonitrile–water (25 + 75, v/v) as the extraction solvent with varying test sample weights of Aristolochia spp. root diluted with S. tetrandra root.
Figure 21.

Methanol–water (85 + 15, v/v) as the extraction solvent with varying test sample weights of Aristolochia spp. root diluted with S. tetrandra root.
With optimized parameters, test samples were analyzed with sonication of 30, 40, 50, and 60 min and compared to a wrist action shaker for the same time periods to evaluate the extraction time. These were compared to an 18 h agitation. There was no advantage seen for more than 30 min extraction with a wrist action shaker or sonication. All results were within the acceptable range, and the wrist action shaker results had a slightly smaller standard deviation. The optimum extraction method was, therefore, determined to be a 30 min time period using a wrist action shaker having an agitation speed of approximately 400 cycles/min with a travel distance of 2.5 cm (Table 10 and Figures 22 and 23).
Table 10.
Extraction time comparison between wrist shaker and sonication for Aristolochia spp. root diluted with S. tetrandra root
| Aristolochic acid concn, μg/g
|
||||
|---|---|---|---|---|
| Extraction time | Replicate 1 | Replicate 2 | Replicate 3 | Mean |
| Wrist shaker
| ||||
| 30 min | 637 | 647 | 655 | 646 |
| 40 min | 623 | 645 | 650 | 639 |
| 50 min | 618 | 642 | 645 | 635 |
| 60 min | 617 | 636 | 637 | 630 |
| Overall mean | 638 | |||
| Overall SDa | 6.76 | |||
| Overall RSDrb | 1.06 | |||
| 18h | 633 | 647 | N/Ac | 640 |
|
| ||||
| Sonication
| ||||
| 30 min | 580 | 671 | 654 | 635 |
| 40 min | 572 | 662 | 649 | 628 |
| 50 min | 574 | 592 | 618 | 595 |
| 60 min | 600 | 644 | 643 | 629 |
| Overall mean | 622 | |||
| Overall SD | 18.1 | |||
| Overall RSDr | 2.91 | |||
SD = Standard deviation.
RSDr = % Relative standard deviation, intralaboratory precision.
N/A = Not applicable.
Figure 22.
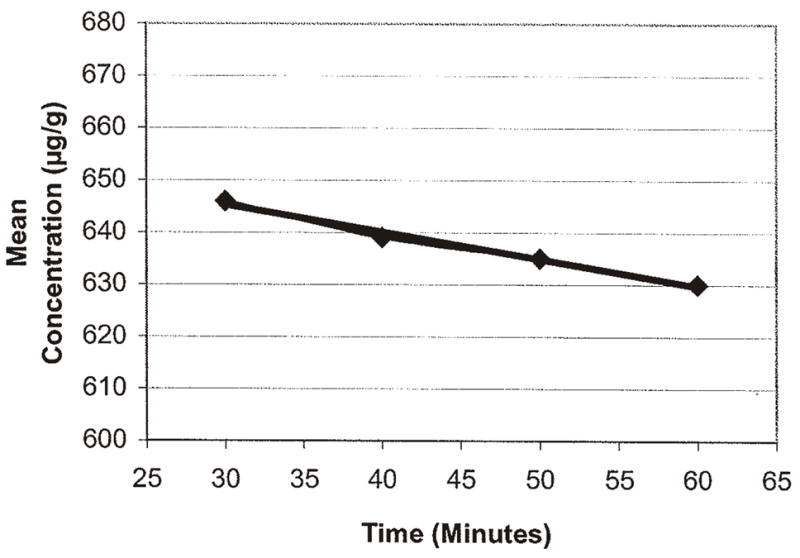
Wrist shaker extraction time for Aristolochia spp. root diluted with S. tetrandra root.
Figure 23.
Sonication extraction time for Aristolochia spp. root diluted with S. tetrandra root.
Using repetitive dilutions, the optimum extraction solvent and time were verified. After the initial 30 min extraction, half of the extract was analyzed and the remaining half was brought to volume and extracted for an additional 30 min. Half of the extract from the diluted solution was then analyzed and the remaining half was brought to volume, extracted for30 min, and analyzed again. The results from the 3 extractions for both wrist action shaking and sonication had minimal variability and were within the target relative standard deviation (RSDr) values, showing that the repetitive dilution did not extract any more aristolochic acid. However, the wrist action shaker extraction overall mean result was slightly higher than the overall mean result for sonication, further verifying that the wrist action shaker extraction method was optimal (Table 11).
Table 11.
Repetitive dilution verification comparison between wrist shaker and sonication for Aristolochia spp. root diluted with S. tetrandra root
| Aristolochic acid concentration, μg/g
|
|||||
|---|---|---|---|---|---|
| Extraction interval | Extraction time, min | Replicate 1 | Replicate 2 | Replicate 3 | Mean |
| Wrist shaker
| |||||
| Initial | 30 | 610 | 626 | 635 | 624 |
| After 1st dilution | 30 | 619 | 617 | 642 | 626 |
| After 2nd dilution | 30 | 611 | 664 | 679 | 651 |
| Overall mean | 634 | ||||
| Overall SDa | 15.0 | ||||
| Overall RSDrb | 2.37 | ||||
| Sonication
| |||||
| Initial | 30 | 599 | 599 | 610 | 603 |
| After 1st dilution | 30 | 583 | 610 | 589 | 594 |
| After 2nd dilution | 30 | 586 | 641 | 649 | 625 |
| Overall mean | 607 | ||||
| Overall SD | 15.9 | ||||
| Overall RSDr | 2.62 | ||||
SD = Standard deviation.
RSDr = % Relative standard deviation, intralaboratory precision.
Using the ASE instrument with the appropriate optimum parameters, the botanical residue (Table 12) from the repetitive dilution section and a fresh test sample (Table 13) were separately but exhaustively extracted. The ASE consisted of 3 consecutive extractions of the same test sample using fresh solvent for each extraction. The levels of aristolochic acid I were determined in both the botanical residue and the fresh test sample extracts to verify that the extraction efficiency was indeed optimized. The amount of aristolochic acid found from the botanical residue extracts was less than 1% of the total amount from the repetitive dilution experiment. The mean of the 3 fresh test samples that were treated by ASE, compared to the overall mean wrist action shaker result, were approximately 1 standard deviation different, 653 μg/g (Table 13) versus 634 μg/g (Table 11), further substantiating good extraction optimization.
Table 12.
Accelerated solvent extraction of repetitive dilution residue of Aristolochia spp. root diluted with S. tetrandra root
| Aristolochic acid concn, μg/g
|
||||
|---|---|---|---|---|
| Extraction type | Replicate | ASEa cut 1 | ASE cut 2 | ASE cut 3 |
| Wristb | 1 | 3.56 | 0.527c | 0.291c |
| 2 | 5.16 | 0.0554c | 0.0259c | |
| 3 | 4.48 | 0.0948c | 0.0111c | |
| Sonicateb | 1 | 8.23 | 0.0997c | NDd |
| 2 | 4.26 | 0.783c | 0.0803c | |
| 3 | 5.28 | 0.274c | ND | |
ASE =Accelerated solvent extraction.
After repetitive dilution, residue was extracted with ASE.
Extrapolated values.
ND = Not detected.
Table 13.
Accelerated solvent extraction of Aristolochia spp. root diluted with S. tetrandra root
| Aristolochic acid concn, μg/g
|
|||||
|---|---|---|---|---|---|
| Extraction type | Replicate | ASEa cut 1 | ASE cut 2 | ASE cut 3 | Total ASE cuts |
| ASE | 1 | 555 | 88.7 | 17.0 | 661 |
| 2 | 582 | 45.1 | 12.6 | 640 | |
| 3 | 490 | 140 | 28.6 | 659 | |
| Mean | 653 | ||||
| SDb | 11.6 | ||||
| RSDrc | 1.78 | ||||
ASE = Accelerated solvent extraction.
SD = Standard deviation.
RSDr = % Relative standard deviation, intralaboratory precision.
Limit of Quantification
After optimization, the LOQ was tested to meet a target value of 2 μg/g (ppm) by LC-UV. The LOQ was further tested by setting the UV detector at several wavelengths, to include at least 390, 322, and 313 nm. The injection volume was also considered. The optimum wavelength was found to be 390 nm (Figures 24–26). Varying the wavelength had no effect on the theoretical plate count, but at 322 and 313 nm a second peak appeared with a peak resolution of approximately 1.3, that could interfere with the aristolochic acid determination. A gradient should be used for the best signal-to-noise ratio at the LOQ (Figures 27–30). The gradient mobile phase gave a plate count of 187 000, while the isocratic mobile phase gave plate counts a factor of 10 lower. The injection volumes tested were 10, 25, 50, and 100 μL. The plate counts were 164 000, 182 000, 176 000, and 146 000, respectively. The best injection volume based on plate count was determined to be 25 μL (Figures 31–34).
Figure 24.
LC-UV chromatogram of a 0.040 μg/mL standard at 390 nm.
Figure 26.
LC-UV chromatogram of a 0.040 μg/mL standard at 313 nm.
Figure 27.
LC-UV chromatogram at 390 nm with isocratic mobile phases A and B (55 + 45, v/v).
Figure 30.
Liquid chromatogram at 390 nm using the gradient elution program in Table 1.
Figure 31.
0.040 μg/mL standard, 10 μL injection using the gradient elution program in Table 1.
Figure 34.
0.040 mg/mL standard, 100 μL injection using the gradient elution program in Table 1.
Calibration Curve
At least 1 calibration curve was generated on 3 separate days. Two different analysts conducted this validation process. The calibration range encompassed approximately the expected concentration of each extracted and diluted test material range of 2.00 to 32.0 μg/g. A calibration standard curve (6 concentration levels ranging from 0.040 to 0.640 μg/mL) was injected twice with each analytical run. Example calibration curve data from each of the 3 days during the precision determination are shown in Table 14. Linearity, calibration curve precision, and tailing factor were all evaluated.
Table 14.
Example calibration curve data from precision determination
| Theoretical standard concn, μg/mL | Standard concn, μg/mL, calculated from linear regression | % Deviation from theoretical |
|---|---|---|
| Day 1
| ||
| 0.03895 | 0.03429 | −12.0 |
| 0.03895 | 0.03482 | −10.6 |
| 0.03902 | 0.03980 | 1.99 |
| 0.03926 | 0.03977 | 1.29 |
| Mean | −4.83 | |
| 0.1169 | 0.1106 | −5.40 |
| 0.1169 | 0.1134 | −3.02 |
| 0.1171 | 0.1135 | −3.09 |
| 0.1171 | 0.1201 | 2.57 |
| Mean | −2.24 | |
| 0.1948 | 0.1951 | 0.156 |
| 0.1948 | 0.1974 | 1.32 |
| 0.1951 | 0.1942 | −0.458 |
| 0.1951 | 0.1933 | −0.902 |
| Mean | 0.0290 | |
| 0.3246 | 0.3341 | 2.92 |
| 0.3246 | 0.3339 | 2.87 |
| 0.3252 | 0.3261 | 0.276 |
| 0.3252 | 0.3254 | 0.060 |
| Mean | 1.53 | |
| 0.4859 | 0.4966 | 2.21 |
| 0.4869 | 0.4958 | 1.84 |
| 0.4878 | 0.4908 | 0.623 |
| 0.4878 | 0.4872 | −0.124 |
| Mean | 1.14 | |
| 0.6492 | 0.6413 | −1.22 |
| 0.6492 | 0.6345 | −2.27 |
| 0.6504 | 0.6511 | 0.108 |
| 0.6504 | 0.6482 | −0.345 |
| Mean | −0.932 | |
|
| ||
| Day 2
| ||
| 0.03895 | 0.03483 | −10.6 |
| 0.03895 | 0.03502 | −10.1 |
| 0.03902 | 0.03490 | 11.8 |
| 0.03902 | 0.03559 | 9.65 |
| Mean | 0.188 | |
| 0.1169 | 0.1128 | −3.52 |
| 0.1169 | 0.1126 | −3.69 |
| 0.1171 | 0.1150 | 1.86 |
| 0.1171 | 0.1163 | 0.645 |
| Mean | −1.18 | |
| 0.1948 | 0.1975 | 1.37 |
| 0.1948 | 0.1928 | −1.01 |
| 0.1951 | 0.1966 | −0.775 |
| 0.1951 | 0.1944 | 0.368 |
| Mean | −0.0118 | |
| 0.3246 | 0.3286 | 1.23 |
| 0.3246 | 0.3329 | 2.57 |
| 0.3252 | 0.3305 | −1.61 |
| 0.3252 | 0.3301 | −1.49 |
| Mean | 0.175 | |
| 0.4859 | 0.5054 | 4.00 |
| 0.4869 | 0.4986 | 2.41 |
| 0.4878 | 0.4965 | −1.74 |
| 0.4878 | 0.4943 | −1.31 |
| Mean | 0.840 | |
| 0.6492 | 0.6350 | −2.19 |
| 0.6492 | 0.6357 | −2.08 |
| 0.6504 | 0.6426 | 1.21 |
| 0.6504 | 0.6424 | 1.24 |
| Mean | −0.455 | |
|
| ||
| Day 3
| ||
| 0.03902 | 0.03664 | −6.09 |
| 0.03902 | 0.03871 | −0.786 |
| 0.03902 | 0.03610 | 8.09 |
| 0.03902 | 0.03625 | 7.65 |
| Mean | 2.22 | |
| 0.1171 | 0.1179 | 0.670 |
| 0.1171 | 0.1212 | 3.51 |
| 0.1171 | 0.1161 | 0.862 |
| 0.1171 | 0.1185 | −1.15 |
| Mean | 0.973 | |
| 0.1951 | 0.1925 | −1.34 |
| 0.1951 | 0.1901 | −2.54 |
| 0.1951 | 0.1958 | −0.373 |
| 0.1951 | 0.1942 | 0.474 |
| Mean | −0.945 | |
| 0.3252 | 0.3255 | 0.101 |
| 0.3252 | 0.3297 | 1.38 |
| 0.3252 | 0.3255 | −0.0827 |
| 0.3252 | 0.3297 | −1.36 |
| Mean | 0.00957 | |
| 0.4878 | 0.4870 | −0.172 |
| 0.4878 | 0.4943 | 1.33 |
| 0.4878 | 0.4941 | −1.28 |
| 0.4878 | 0.4927 | −1.00 |
| Mean | −0.281 | |
| 0.6504 | 0.6467 | −0.565 |
| 0.6504 | 0.6490 | −0.216 |
| 0.6504 | 0.6459 | 0.704 |
| 0.6504 | 0.6445 | 0.922 |
| Mean | 0.211 | |
The relative response (peak area) of the analyte versus concentration was used to construct the calibration curve using a least-squares linear regression method. Calibration curves had a correlation coefficient (r) ranging from 0.99902 to 0.99996.
The back-calculated values for each concentration were within or equal to the targets of ±9% of theoretical (within or equal to ±10% at the LOQ) for within- and between-day precision, except within-day precision ranged from 10.6 to 12.0% for Day 1 LOQ and 10.1 to 11.8% at Day 2 LOQ (Table 11).
The U.S. Pharmacopeia tailing factor (Tf) was used.
where ac = peak width at 5% of the peak height and ab = front half-width measured from the leading edge to a perpendicular dropped from the peak apex. The tailing factor was no more than 1.5 (1.14 to 1.17) for the 6 aristolochic I standard peak concentrations (Figure 35).
Figure 35.
Tailing factors for standards using the gradient elution program in Table 1.
Test Material Precision
At least 3 replicates of each of the 7 test materials were analyzed on each of 3 separate days (Table 15). Using a minigrinder, 20 tablets were homogenized for each determination. Two different analysts were used during this validation process. The precision fell within or equal to the target RSDr for intraassay replicates, with the exception of Day 3 A. manschuriensis stem, Day 1 Stephania tetrandra root (10 μg/g fortification), Day 2 and 3 tablets (10 μg/g fortification), and Day 2 and 3 tablets (20 μg/g fortification). Overall combined (interassay) replicates met RSDr targets, with the exception of the 20 ppm high fortification tablets.
Table 15.
Test material precision
| Aristolochic acid concn, μg/g
|
Days 1–3 combined
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Replicate | Day 1 | Day 2 | Day 3 | Mean, μg/g | SDa | RSDrb | PRSDRc | HorRatd | Target RSDre |
|
Aristolochia spp. root
| |||||||||
| 1 | 1410 | 1410 | 1410 | 1450 | 47.3 | 3.26 | 5.33 | 0.6 | 2.67–3.56 |
| 2 | 1490 | 1490 | 1420 | ||||||
| 3 | 1490 | 1520 | 1400 | ||||||
| Mean | 1460 | 1470 | 1410 | ||||||
| SD | 46.2 | 56.9 | 10.0 | ||||||
| RSDr | 3.16 | 3.87 | 0.709 | ||||||
|
| |||||||||
|
Aritolochia manschuriensis stem
| |||||||||
| 1 | 2890 | 2790 | 2860 | 2830 | 85.3 | 3.01 | 4.82 | 0.6 | 2.41–3.21 |
| 2 | 2900 | 2940 | 2680 | ||||||
| 3 | 2820 | 2850 | 2720 | ||||||
| Mean | 2870 | 2860 | 2750 | ||||||
| SD | 43.6 | 75.5 | 94.5 | ||||||
| RSDr | 1.52 | 2.64 | 3.44 | ||||||
|
| |||||||||
|
Akebia trifoliate stem
| |||||||||
| 1 | <2.00 | <2.00 | <2.00 | <2.00 | NAf | NA | NA | NA | NA |
| 2 | <2.00 | <2.00 | <2.00 | ||||||
| 3 | <2.00 | <2.00 | <2.00 | ||||||
| Mean | <2.00 | <2.00 | <2.00 | ||||||
| SD | NA | NA | NA | ||||||
| RSDr | NA | NA | NA | ||||||
|
| |||||||||
|
Clematis armandii stem, 20 μg/g fortification with, Aristolochia spp.
| |||||||||
| 1 | 23.4 | 22.4 | 22.6 | 22.5 | 0.550 | 2.44 | 9.96 | 0.2 | 4.98–6.64 |
| 2 | 23.2 | 22.0 | 22.1 | ||||||
| 3 | 22.4 | 21.7 | 22.7 | ||||||
| Mean | 23.0 | 22.0 | 22.5 | ||||||
| SD | 0.529 | 0.351 | 0.321 | ||||||
| RSDr | 2.30 | 1.60 | 1.43 | ||||||
|
| |||||||||
|
S. tetrandra root:, 10 μg/g fortification with Aristolochia spp.
| |||||||||
| 1 | 10.0 | 11.6 | 10.1 | 10.5 | 0.776 | 7.39 | 11.2 | 0.7 | 5.60–7.47 |
| 1 | 10.5 | 10.0 | 11.0 | ||||||
| 3 | 11.8 | 9.82 | 9.76 | ||||||
| Mean | 10.8 | 10.5 | 10.3 | ||||||
| SD | 0.929 | 0.980 | 0.641 | ||||||
| RSDr | 8.60 | 9.33 | 6.22 | ||||||
|
| |||||||||
| Tablets, 10 μg/g fortification with Aristolochia spp.
| |||||||||
| 1 | 6.55 | 6.83 | 6.09 | 6.50 | 0.450 | 6.92 | 12.0 | 0.6 | 6.00–8.00 |
| 2 | 6.93 | 5.87 | 5.93 | ||||||
| 3 | 6.43 | 6.76 | 7.10 | ||||||
| Mean | 6.64 | 6.49 | 6.37 | ||||||
| SD | 0.261 | 0.535 | 0.634 | ||||||
| RSDr | 3.93 | 8.24 | 9.95 | ||||||
|
| |||||||||
| Tablets, 20 μg/g fortification with Aristolochia spp.
| |||||||||
| 1 | 16.7 | 18.8 | 16.6 | 17.2 | 1.42 | 8.26 | 10.4 | 0.8 | 5.20–6.94 |
| 2 | 15.0 | 15.7 | 19.1 | ||||||
| 3 | 17.0 | 17.7 | 18.6 | ||||||
| Mean | 16.2 | 17.4 | 18.1 | ||||||
| SD | 1.08 | 1.57 | 1.32 | ||||||
| RSDr | 6.67 | 9.02 | 7.29 | ||||||
SD = Standard deviation.
RSDr = % Relative standard deviation, intralaboratory precision.
PRSDR = 2 C−0.05, % relative standard deviation, predicted interlaboratory precision, C = mean concentration expressed as a mass fraction.
HorRat = RSDr/PRSDR.
Target RSDr = % Acceptable intralaboratory precision (0.500 to 0.667) PRSDR.
NA = Not applicable.
When the initial test sample extract fell above the highest standard of the curve, the extract was diluted to fit on the curve. The target RSDr was calculated and reported as follows:
where C = mean concentration expressed as a mass fraction.
The HorRat values fell within or equal to 0.3 to 1.3 and were calculated and reported as follows:
Although the 20 ppm high fortification tablets did not meet the overall target RSDr range, they did meet the HorRat value acceptance criteria. There may have been a lack of homogeneity for this material, and this will be investigated prior to a collaborative study.
Test samples of the negative control material, S. tetrandra root, were fortified in triplicate with the reference compound mixture at 2, 10, and 30 μg/g. Triplicate unfortified controls were analyzed concurrently. This was repeated for 2 additional days (Table 16).
Table 16.
Negative control recovery
| Test sample identification | Replicate | Spike concn, μg/g | Amt of aristolochic acid found, μg/g | Recovery, % | Mean recovery, % | SDa | RSDrb |
|---|---|---|---|---|---|---|---|
| Day 1
| |||||||
| S. tetrandra root spiked 2 μg/g | 1 | 2.020 | 2.032 | 101 | 100 | 0.854 | 0.854 |
| 2 | 2.020 | 2.006 | 99.3 | ||||
| 3 | 2.020 | 2.025 | 100 | ||||
| S. tetrandra root spiked 1 0 μg/g | 1 | 10.02 | 10.27 | 102 | 102 | 0.577 | 0.566 |
| 2 | 10.02 | 10.35 | 103 | ||||
| 3 | 10.02 | 10.19 | 102 | ||||
| S. tetrandra root spiked 30 μg/g | 1 | 29.96 | 31.36 | 105 | 104 | 1.00 | 0.962 |
| 2 | 29.96 | 31.01 | 104 | ||||
| 3 | 29.96 | 30.71 | 103 | ||||
|
| |||||||
| Day 2
| |||||||
| S. tetrandra root spiked 2 μg/g | 1 | 2.020 | 2.065 | 102 | 103 | 1.53 | 1.49 |
| 2 | 2.020 | 2.072 | 103 | ||||
| 3 | 2.020 | 2.118 | 105 | ||||
| S. tetrandra root spiked 10 μg/g | 1 | 10.02 | 10.57 | 105 | 103 | 2.00 | 1.94 |
| 2 | 10.02 | 10.17 | 101 | ||||
| 3 | 10.02 | 10.37 | 103 | ||||
| S. tetrandra root spiked 30 μg/g | 1 | 29.96 | 31.24 | 104 | 104 | 1.00 | 0.962 |
| 2 | 29.96 | 31.44 | 105 | ||||
| 3 | 29.96 | 30.77 | 103 | ||||
|
| |||||||
| Day 3
| |||||||
| S. tetrandra root spiked 2 μg/g | 1 | 2.024 | 1.991 | 98.4 | 101 | 5.92 | 5.86 |
| 2 | 2.024 | 1.968 | 97.2 | ||||
| 3 | 2.024 | 2.190 | 108 | ||||
| S. tetrandra root spiked 10 μg/g | 1 | 10.07 | 10.51 | 104 | 103 | 0.577 | 0.560 |
| 2 | 10.07 | 10.33 | 103 | ||||
| 3 | 10.07 | 10.34 | 103 | ||||
| S. tetrandra root spiked 30 μg/g | 1 | 30.00 | 31.34 | 104 | 1 04 | NAc | NA |
| 2 | 30.00 | 31.11 | 104 | ||||
| 3 | 30.00 | 31.19 | 104 | ||||
SD = Standard deviation.
RSDr = % Relative standard deviation, intralaboratory precision.
NA= Not applicable.
The mean recovery was found to be between 100 and 103% at the 2 μg/g level, between the 102 and 103% at the 10 μg/g level, and 104% at the 30 μg/g level. The column and UV detector selectivity of the reference method was investigated by injecting mixed standards of aristolochic acid I, II, IIIa, and VII at the highest standard level used for aristolochic acid I. The resolution was calculated as follows:
where t1 and t2 = retention times of peaks; w1 and w2 = peak widths measured at the baseline between tangents drawn to the peak sides. Selectivity was checked prior to starting the extraction efficiency experiment. There was no coelution or resolution of less than 1.3 of any reference material with aristolochic acid I (Figure 30). The LC/MS column and confirmation selectivity was investigated at the lowest standard level to ensure that confirmation by LC/MS was not compromised.
To determine ruggedness, extraction efficiency, LOQ, linearity, precision, accuracy, UV detector wavelength, injection volume, stability of the calibration curve, mobile phase composition, isocratic versus gradient, elution, and the use of more than 1 analyst were monitored. The retention time and column stability were also determined, with the average retention time over 12 months ranging from 20.8 to 21.1 min and the column plate count over the same time period declining less than 5%. In addition, grind size, test sample size in relationship to extraction volume, extraction solvent composition, extraction method, and extraction times were determined. Based on these parameters, the method was found to be rugged.
Confirmation data were determined when aristolochic acid I was detected at greater than or equal to the LOQ (i.e., in the test samples) or fortified negative control. The lowest level standard was used for confirmation comparison to the detected peak in the test sample using LC/MS. No low level test material was found to contain aristolochic acid I near the LOQ; therefore, A. trifoliata stem was fortified at the 2 μg/g level to accomplish the confirmation experiments. Standard instrument operating procedures were used to optimize the LC/MS for this purpose and to ensure system suitability. Quality assurance checks were also made. At least 3 product ions were chosen for confirmation, and 2 confirmation methods were performed (Table 17).
Table 17.
Confirmation by LC/triple quad-MS
| Response, area
|
Retention time. min
|
Confirmation method 1 ion/ion ratio, %
|
Confirmation method 2 ion/total ion area ratio, %
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identification | Replicate | Ion 296 | Ion 281 | Ion 265 | Ion 296 | Ion 281 | Ion 265 | Ion 281/296 | Ion 265/296 | Ion 296 | Ion 281 | Ion 265 |
| 0. 03878 μg/mL standard | 1 | 539 | 214 | 123 | 11.4 | 11.5 | 11.4 | 40 | 23 | 62 | 24 | 14 |
| 2 | 871 | 337 | 283 | 11.6 | 11.6 | 11.6 | 39 | 32 | 58 | 23 | 19 | |
| Mean | 40 | 28 | 60 | 24 | 17 | |||||||
| Akebia trifolata stem spiked at 2 μg/g | 1 | 1048 | 279 | 228 | 11.6 | 11.6 | 11.4 | 27 | 22 | 67 | 18 | 15 |
| 2 | 957 | 332 | 296 | 11.5 | 11.4 | 11.5 | 35 | 31 | 60 | 21 | 19 | |
| 3 | 1079 | 328 | 271 | 11.4 | 11.5 | 11.6 | 30 | 25 | 64 | 20 | 16 | |
| Mean | 31 | 26 | 64 | 20 | 17 | |||||||
In the first confirmation method, the spectrum of standard was used for confirmation of presence of aristolochic acid I with comparison to the spectrum of the test sample of like retention time. The peak area detected in the standard for 3 product ions was divided by the peak area of 1 of 3 product ions and averaged for all standards when multiple injections were made. The test sample ratio of confirmation ions to the chosen product ion were to be within ±10% (arithmetic difference, not relative difference) of the averaged standard ratio. For example, if an average ratio for the standards was 50%, the window for test sample ratio was 40 to 60%. The ratios agreed within 10%.
In the second confirmation method, the spectrum of the standard was also used for the confirmation of presence of aristolochic acid I with comparison to the spectrum of the test sample of like retention time. The standard area ratio of at least 3 product ions versus the total area of the 3 was compared to the same area ratio of the test sample. The ratios were to agree within 10% for confirmation; however, only 2 of the 3 ratios fell within this range.
The first method is the preferred confirmation procedure. Similar confirmation may also be accomplished using LC/ion trap MS.
Conclusions
A method, further validated amongst several laboratories, will facilitate the accurate determination of the quality of botanicals and dietary supplements with respect to aristolochic acid. In addition, the use of the method may allow assessment of the relationship between levels of aristolochic acid in botanicals and dietary supplements and any alleged or reported adverse effects. Based on the results of this validation, the method for the determination of aristolochic acid I in botanicals and tablets is deemed acceptable for use for quantification by LC-UV with confirmation by LC/MS. It is also recommended that a collaborative study be initiated.
Figure 2.

0.50 g test sample of Aristolochia spp. root diluted with S. tetrandra root with varying percentages of acetonitrile–water as the extraction solvent.
Figure 3.

1.00 g test sample of Aristolochia spp. root diluted with S. tetrandra root with varying percentages of acetonitrile–water as the extraction solvent.
Figure 4.

2.00 g test sample of Aristolochia spp. root diluted with S. tetrandra root with varying percentages of acetonitrile–water as the extraction solvent.
Figure 5.

3.00 g test sample of Aristolochia spp. root diluted with S. tetrandra root with varying percentages of acetonitrile–water as the extraction solvent.
Figure 6.
0.25 g test sample of Aristolochia spp. root diluted with S. tetrandra root with varying percentages of methanol–water as the extraction solvent.
Figure 7.

0.50 g test sample of Aristolochia spp. root diluted with S. tetrandra root with varying percentages of methanol–water as the extraction solvent.
Figure 8.

1.00 g test sample of Aristolochia spp. root diluted with S. tetrandra root with varying percentages of methanol–water as the extraction solvent.
Figure 9.

2.00 g test sample of Aristolochia spp. root diluted with S. tetrandra root with varying percentages of methanol–water as the extraction solvent.
Figure 12.
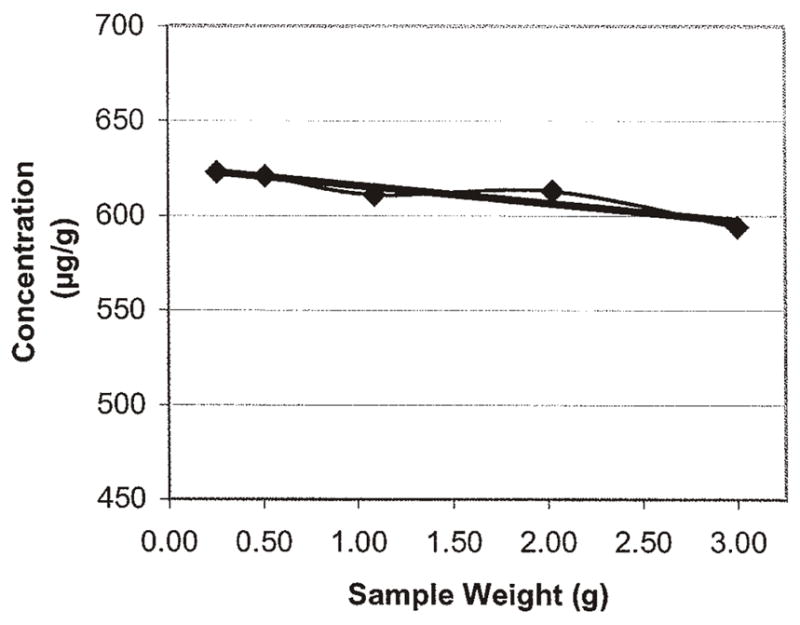
Acetonitrile–water (50 + 50, v/v) as the extraction solvent with varying test sample weights of Aristolochia spp. root diluted with S. tetrandra root.
Figure 13.
Acetonitrile–water (60 + 40, v/v) as the extraction solvent with varying test sample weights of Aristolochia spp. root diluted with S. tetrandra root.
Figure 14.

Acetonitrile–water (70 + 30, v/v) as the extraction solvent with varying test sample weights of Aristolochia spp. root diluted with S. tetrandra root.
Figure 15.

Acetonitrile–water (80 + 20, v/v) as the extraction solvent with varying test sample weights of Aristolochia spp. root diluted with S. tetrandra root.
Figure 16.

Acetonitrile (100%) as the extraction solvent with varying test sample weights of Aristolochia spp. root diluted with S. tetrandra root.
Figure 17.

Methanol–water (60 + 40, v/v) as the extraction solvent with varying test sample weights of Aristolochia spp. root diluted with S. tetrandra root.
Figure 18.

Methanol–water (70 + 30, v/v) as the extraction solvent with varying test sample weights of Aristolochia spp. root diluted with S. tetrandra root.
Figure 19.

Methanol–water (75 + 25, v/v) as the extraction solvent with varying test sample weights of Aristolochia spp. root diluted with S. tetrandra root.
Figure 20.
Methanol–water (80 + 20, v/v) as the extraction solvent with varying test sample weights of Aristolochia spp. root diluted with S. tetrandra root.
Figure 25.
LC-UV chromatogram of a 0.040 μg/mL standard at 322 nm.
Figure 28.
LC-UV chromatogram at 390 nm with isocratic mobile phases A and B (60 + 40, v/v).
Figure 29.
LC-UV chromatogram at 390 nm with isocratic mobile phases A and B (65 + 35, v/v).
Figure 32.
0.040 μg/mL standard, 25 μL injection using the gradient elution program in Table 1.
Figure 33.
0.040 mg/mL standard, 50 μL injection using the gradient elution program in Table 1.
Table 8.
Aristolochic acid concentration in Aristolochia spp. root diluted with S. tetrandra root using methanol–water as the extraction solvent (results by percent methanol)
| % Methanol | Test sample weight, g | Aristolochic acid concn, μg/g |
|---|---|---|
| 60 | 0.25570 | 627 |
| 0.49410 | 550 | |
| 1.0627 | 571 | |
| 2.0393 | 474 | |
| 2.9946 | 553 | |
| 70 | 0.25910 | 594 |
| 0.49900 | 553 | |
| 1.0067 | 569 | |
| 2.0604 | 544 | |
| 3.0549 | 572 | |
| 75 | 0.24900 | 588 |
| 0.50540 | 588 | |
| 1.0470 | 576 | |
| 2.0367 | 569 | |
| 2.9824 | 559 | |
| 80 | 0.24850 | 610 |
| 0.50050 | 578 | |
| 1.0436 | 584 | |
| 2.0494 | 553 | |
| 3.0190 | 530 | |
| 85 | 0.25610 | 576 |
| 0.50990 | 541 | |
| 1.0589 | 526 | |
| 2.0695 | 520 | |
| 3.0381 | 489 |
Acknowledgments
We thank Richard Crowley (Covance Laboratories) for reviewing this manuscript.
References
- 1.U.S. Food and Drug Administration. Letter to Health Care Professionals–FDA Concerned About Botanical Products, Including Dietary Supplements, Containing Aristolochic Acid. College Park, MD: 2000. May 31, [Google Scholar]
- 2.The European Agency for the Evaluation of Medicinal Products. Aristolochia species. London, UK: October 31, Evaluation of Medicines for Human Use (2000) Position Paper on the Risks Associated with the Use of Herbal Products Containing. [Google Scholar]
- 3.Kite GC, Yule MA, Leon C, Simmonds MSJ. Rapid Commun Mass Spectrom. 2002;16:585–590. doi: 10.1002/rcm.611. [DOI] [PubMed] [Google Scholar]
- 4.Flurer RA, Jones MB, Vela N, Ciolino LA, Wolnick KA. Determination of Aristolochic Acid in Traditional Chinese Medicines and Dietary Supplements, marked DRAFT, No. 4212. U.S. Food and Drug Administration, Forensic Chemistry Center; Cincinnati, OH: [Google Scholar]