Summary
Polymorphisms of IL-1β are associated with an increased risk of solid malignancies. Here, we show that stomach-specific expression of human IL-1β in transgenic mice leads to spontaneous gastric inflammation and cancer that correlates with early recruitment of myeloid-derived suppressor cells (MDSCs) to the stomach. IL-1β activates MDSC in vitro and in vivo through an IL-1RI/NF-κB pathway. IL-1β transgenic mice deficient in T and B lymphocytes develop gastric dysplasia accompanied by a marked increase in MDSCs in the stomach. Antagonism of IL-1 receptor signaling inhibited the development of gastric preneoplasia and suppressed MDSC mobilization. These results demonstrate that pathologic elevation of a single proinflammatory cytokine may be sufficient to induce neoplasia and provide a direct link between IL-1β, MDSCs and carcinogenesis.
INTRODUCTION
Many solid malignancies appear to be initiated by tissue injury or chronic inflammation (Coussens and Werb, 2002). Long-term use of non-steroidal anti-inflammatory drugs (NSAIDs) reduces the risk of many cancers (Baron and Sandler, 2000). Gastric adenocarcinoma is the 2nd most common cancer in the world and is strongly linked to chronic inflammation (Fox and Wang, 2007). It is now well accepted that infection with a bacterium, Helicobacter pylori (H. pylori), plays a pivotal role in triggering the chronic inflammation (gastritis) leading to malignancy (Fox and Wang, 2007). Chronic inflammation of the stomach initiates histopathologic progression of chronic gastritis to gastric atrophy, intestinal metaplasia, dysplasia and finally gastric cancer (Fox and Wang, 2001). While H. pylori infection is extremely prevalent, only a small minority (e.g. 1%) of infected individuals will, after many years, develop gastric cancer. The variable response to this common pathogen appears to be governed by a genetic predisposition for high expression levels of pro-inflammatory cytokines (El-Omar et al., 2001).
A number of clinical studies have suggested that polymorphisms in pro-inflammatory cytokine genes such as IL-1β, TNF-α and IL-6, are associated with diverse diseases, including cancer (Bidwell et al., 1999; Howell et al., 2002). The strongest association with cancer has been reported for the IL-1β gene cluster, where polymorphisms of IL-1β have been shown to increase the risk of a number of human tumors (Barber et al., 2000; Howell et al., 2003; Wang et al., 2003), particularly gastric cancer (El-Omar et al., 2001; Figueiredo et al., 2002). IL-1β is a pleiotropic proinflammatory cytokine that has profound effects on inflammation and immunity, and has been shown to be induced by H. pylori infection (El-Omar 2001). Carriers of IL-1B polymorphisms (IL-1B-511T and IL-1B-31C), which have been linked to enhanced IL-1β production and increased circulating levels of the cytokine in humans, showed an increased risk of gastric cancer (El-Omar 2001; Fox and Wang, 2007).
While genetic studies in humans have suggested an important role for IL-1β in cancer, direct evidence that IL-1β contributes to the pathogenesis of cancer has been lacking. In addition, the primary cellular targets of IL-1β ’s effects have not been defined. Studies in mice have suggested that gastric carcinogenesis is a Th1 mediated disease, and that CD4+ T cells are a necessary component for the induction of atrophic gastritis and preneoplasia of the stomach (Roth et al., 1999). Mice deficient in T and B, or only T lymphocytes, are resistant to Helicobacter-induced preneoplasia; however infusion of CD4+ T cells is able to reproduce atrophic gastritis in immunodeficient mice (Eaton et al., 2001). While IL-1β has direct effects on T lymphocyte function, recent studies have pointed to myeloid cells as a critical downstream target of IL-1β ’s actions. IL-1β is known to activate the NF-κB pathway in myeloid cells through binding to its receptor (IL-1RI) (Dinarello, 1996). A number of reports have demonstrated that the transcription factor NF-κB is a key player linking inflammation and cancer (Karin and Greten, 2005).
Recent studies have indicated a possible role for IL-1β in the activation of myeloid-derived suppressor cells (MDSCs), also Gr-1+CD11b+ immature myeloid cells, a heterogeneous cellular population believed to have immunosuppressive effects (Dolcetti et al., 2008). While MDSCs are increased in a number of pathologic conditions (Serafini et al., 2006), they are significantly overproduced in the bone marrow and spleens of tumor-bearing mice (Melani et al., 2003; Serafini et al., 2006) and are elevated in the peripheral blood of cancer patients (Almand et al., 2001; Young and Lathers, 1999). Accumulating data have shown that MDSCs infiltrate into tumors and promote tumor angiogenesis by producing high levels of MMP9 and by directly incorporating into tumor endothelium (Ahn and Brown, 2008; Du et al., 2008). MDSCs have been implicated in tumor refractoriness to anti-VEGF treatment and likely contribute to TGF-α-mediated metastasis (Shojaei et al., 2007; Yang et al., 2008). MDSCs can be mobilized by a variety of tumor-derived factors, including IL-1β and can promote tumor progression (Bunt et al., 2006; Bunt et al., 2007). Xenograft tumors with IL-1β overexpression show greater accumulation of MDSCs and more rapid tumor progression (Song XP et al., 2005), while 4T1 mammary carcinoma tumors implanted into IL-1R-deficient mice exhibit delayed accumulation of MDSCs and slower growing tumors (Bunt et al., 2007).
Thus, while studies in patients and mice have shown a strong correlation between MDSC infiltration and tumor progression (Serafini et al., 2006), these models have all been based on MDSCs activation in response to tumor-derived signals. A possible role for MDSCs in initiating carcinogenesis has not been studied, and a possible link between IL-1β and MDSCs in models of chronic inflammation has not been investigated. Thus, we generated a transgenic mouse model of gastric-specific overexpression of human IL-1β (hIL-1β ) and investigated the role of IL-1β in gastric carcinogenesis.
RESULTS
IL-1β transgenic mice develop spontaneous gastric inflammation and dysplasia
To investigate a direct pathogenic role of IL-1β in gastric carcinogenesis in vivo, we generated an H/K-ATPase/hIL-1β transgene (Figure 1A) that targets constitutively secreted hIL-1β specifically to parietal cells of the stomach. One high-expressing hIL-1β transgenic line (Line 19) and one low-expressing line (Line 42) in the stomach were identified by hIL-1β ELISA (Figure 1B and Figure S1A) and mRNA measurements (Figure 1C and Figure S1B). The levels of IL-6 and TNF-α mRNAs in the stomachs were significantly increased in 4-month old transgenic mice compared to wild type mice. Furthermore, serum from transgenic mice, in a human IL-1β bioassay, could stimulate (3-fold) secretion of IL-8 from IL-1R receptor-expressing HEK-293 cells, an effect that could be blocked by IL-1RA or an antibody to human IL-1β but not by an antibody to murine IL-1β (data not shown). Taken together, these data confirm that the IL-1β transgenic mice express bioactive human IL-1β.
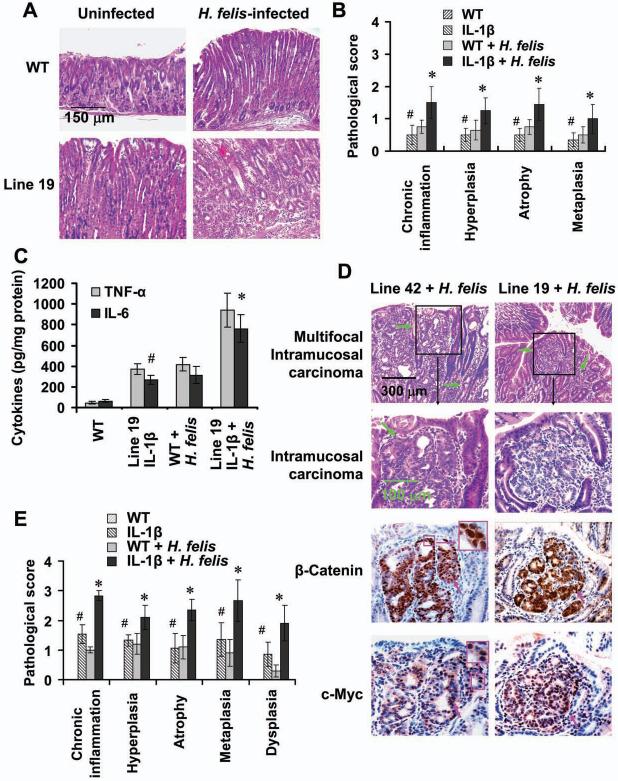
Figure 1. IL-1β transgenic mice develop gastric inflammation and dysplasia/carcinoma.
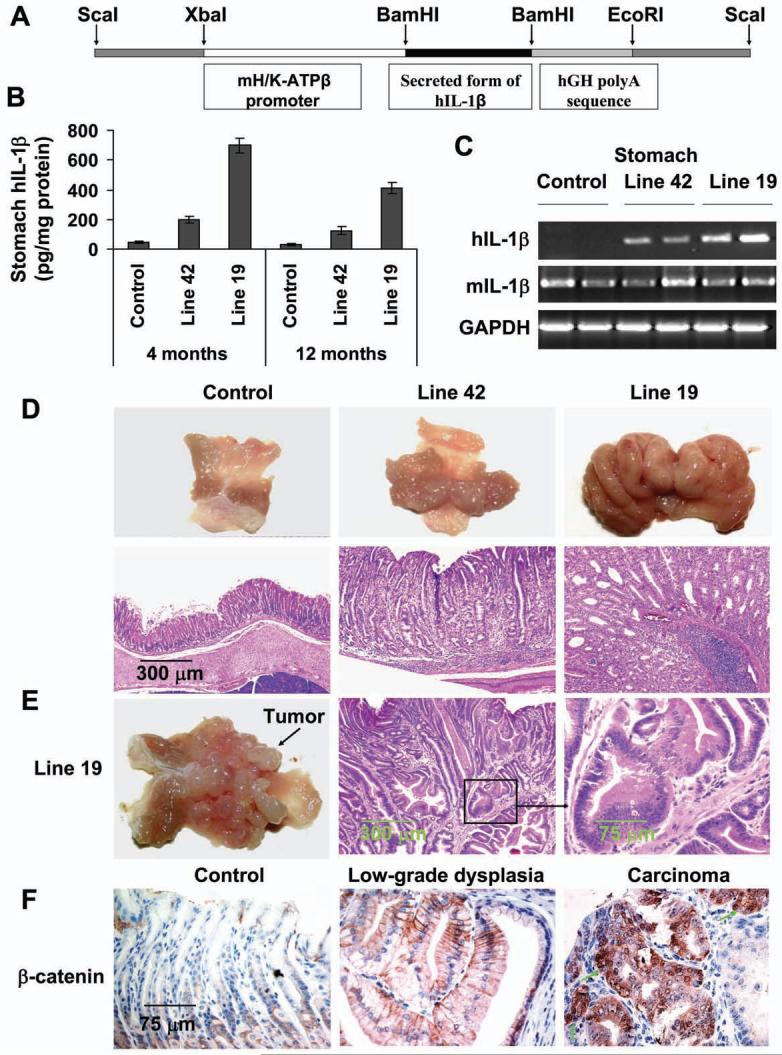
(A) The construct pBS/HKATPase/ß globin/IL-1β, which contains the mouse H+/K+-ATPase ß subunit gene and secreted form of hIL-1β cDNA, was microinjected into fertilized mouse oocytes. (B) Expression of hIL-1β in the stomachs of 4 and 12 month old IL-1β transgenic mice and control mice was determined using a human-specific IL-1β ELISA kit. Data are the mean ± SD of 10 mice. (C) Expression of human (transgenic) and mouse (endogenous) IL-1β mRNA in stomach as assessed by RT-PCR. (D) IL-1β transgenic mice develop gastric hyperplasia (upper lane) and gastritis (H&E staining, lower lane). (E) Male Line 19 IL-1β transgenic mice develop stomach cancer. (F) Activation and relocalization of β-catenin in gastric cancer of IL-1β transgenic mice. The stomach sections were stained with an anti-β-catenin antibody. Arrows show nuclear β-catenin+ cells (brown).
IL-1β transgenic mice on a C57BL/6J background (5 generations) older than 1 year of age exhibited marked gastric hyperplasia (Figure 1D, upper lane) and gastritis with inflammatory cell infiltration, compared to age-matched wild type (WT) control mice (Figure 1D, lower lane). More than 70% of older Line 19 mice developed severe hyperplasia, chronic inflammation, parietal cell loss (atrophy), metaplasia and dysplasia. Fewer Line 42 mice developed atrophy and metaplasia and none developed dysplasia (Table S1), consistent with the lower levels of IL-1β expression in Line 42. Overall, inflammation and histopathologic alteration scores were significantly higher in IL-1β transgenic mice than in control mice, and were significantly higher in Line 19 mice than in Line 42 mice (Table S2). Mucus metaplasia with expansion of TFF2/SP-expressing mucus cells, a characteristic premalignant change, was frequently observed in IL-1β transgenic but not in control mice (Figure S1C). Importantly, 30% (6/20) of male Line 19 mice developed high-grade dysplasia or adenocarcinoma localized to the body of the stomach (Figure 1E and Table S1). The adenocarcinomas were well-differentiated and did not invade into the submucosa (intramucosal). Immunohistochemical staining for β-catenin showed nuclear and cytoplasmic localization of β-catenin in all cases of high grade gastrointestinal epithelial neoplasia (GIN)/adenocarcinoma (6/6), while cytoplasmic and membranous β-catenin staining was observed in other preneoplastic lesions and only weak membranous β-catenin staining was observed in normal stomach (Figure 1F). In addition, nuclear c-Myc staining was also observed in all cases of GIN/adenocarcinoma, while only rare scattered nuclear c-Myc staining was noted in other preneoplastic lesions. No nuclear c-Myc staining was observed in the foveolar epithelium of normal stomach (Figure S1D). Interestingly, we found that male IL-1β mice showed higher levels of IL-6 in both serum and stomach tissues compared to female mice (Figure S1E and S1F), while there were no differences in the level of TNF-α between male and female mice (data not shown). These data indicate that the higher frequency of dysplasia and cancer in male animals may be associated with a higher level of IL-6, as previously reported (Naugler et al., 2007). There were no histological alterations in other organs examined, including liver, kidney, lung, heart (data not shown). These results clearly show that, in the absence of gastric Helicobacter infection, overexpression of IL-1β can lead to chronic gastritis, metaplasia, and high-grade dysplasia/carcinoma, suggesting that chronic inflammation may be sufficient for cancer initiation and progression.
Overexpression of IL-1β accelerates the development of gastric inflammation and carcinoma in the setting of H. felis infection
Individuals with high-expressing IL-1β genetic polymorphisms have an increased risk for gastric cancer, but only in the context of H. pylori infection (El-Omar et al., 2001). Consistent with these clinical observations, IL-1β mice infected with H. felis developed more severe gastric inflammation and histologic alterations 5-months post-infection (Figure 2A). Scores for acute and chronic inflammation, hyperplasia, atrophy and metaplasia were significantly higher in H. felis-infected IL-1β transgenic mice compared to infected control mice and uninfected IL-1β mice (Figure 2B). Levels of TNF-α, IL-6 and SDF-1 protein were significantly increased in the stomachs of H. felis-infected IL-1β mice compared to H. felis-infected control mice and uninfected IL-1β mice (Figure 2C and data not shown). Furthermore, all IL-1β mice (Line 42 and Line 19) developed varying grades of dysplasia 12-months post-infection, while such lesions were not seen in H. felis-infected control mice or in uninfected Line 42 mice (Figure 2D and 2E and Table S3). Notably, 8.4% (1/12) of Line 19 and 10% (1/10) of Line 42 IL-1β mice with H. felis infection developed invasive carcinoma. Immunohistochemical staining showed strong nuclear and cytoplasmic β-catenin and c-Myc expression in the majority of cells of the invasive gastric cancers (Figure 2D). Inflammation, atrophy and dysplasia scores were significantly higher in H. felis-infected IL-1β transgenic mice compared to infected control mice and uninfected IL-1β mice (Figure 2E and Table S3). These results confirm, in an in vivo murine model system, earlier clinical observations that high levels of IL-1β expression increase the risk for gastric cancer in the setting of Helicobacter infection (El-Omar et al., 2001; Figueiredo et al., 2002).
Figure 2. Overexpression of IL-1β accelerates the development of gastric inflammation and carcinoma in the setting of H. felis infection.
(A) Stomach sections from Line 19 IL-1β transgenic and control mice infected with H. felis for 5 months were stained with H&E. (B) Pathological scores from the stomach of above mice were graded according to the diagnostic criterion described in Methods. The data represent the mean ± SD of 16 mice (#p < 0.05, vs WT mice; *p < 0.01, vs uninfected WT mice). (C) Expression of mouse TNF-α and IL-6 in stomach tissue from mice was determined by ELISA. The data represent the mean ± SD of 6 mice (#p < 0.05, vs WT mice; *p < 0.01, vs uninfected WT mice). (D) IL-1β transgenic mice infected with H. felis for 12 months developed dysplasia and carcinoma with activation of β-catenin. The sections from intramucosal/invasive gastric cancer were stained with anti-β-catenin and anti-c-Myc antibodies. Green arrows indicate invasive gastric cancer and pink arrows show nuclear β-catenin- or c-Myc-positive cells (brown), respectively. (E) Gastric inflammation and pathology scores were graded in Line 19 IL-1β transgenic and control mice infected with H. felis for 12 months. The data represent the mean ± SD of 10 animals (#p < 0.05, vs control mice; *p < 0.01, vs uninfected WT mice).
Overexpression of IL-1β in the stomach leads to mobilization and recruitment of MDSCs
Next, we investigated the mechanisms involved in IL-1β-induced inflammation and carcinogenesis. To study a possible role for inflammatory cells in this model, we examined the distribution of lymphoid and myeloid cells in the peripheral blood, spleen and stomach at various time points in IL-1β transgenic mice. In very young (2 month old) mice, which only developed mild gastritis without gastric atrophy (Figure S2A), there was no change in the frequencies of CD4+, CD8+ T cells or CD11b+/F4/80+monocyte/macrophage in IL-1β transgenic mice compared with age- and gender-matched wild type mice; however, there was a significant increase in the frequencies of MDSCs in the peripheral blood (Figure 3A and Figure S2B), spleen (Figure 3B and Figure S2C) and stomach (Figure 3C and 3D) in 2 month old IL-1β transgenic mice compared to control mice. The serum and stomach tissue levels of TNF-α, IL-6 and SDF-1α were significantly increased in IL-1β transgenic mice compared to controls (Figure 3E and Figure S2D). Real time PCR confirmed these findings (data not shown). The expression of IL-4 and IL-10 in the stomach was not significantly altered in IL-1β transgenic mice (data not shown), consistent with a specific effect on pro-inflammatory cytokines. Thus, transgenic overexpression of IL-1β in the stomach mobilizes MDSC recruitment at the earliest stages of the histolopathologic progression of gastric inflammation to cancer.
Figure 3. Overexpression of IL-1β in the stomach leads to mobilization and recruitment of MDSCs.
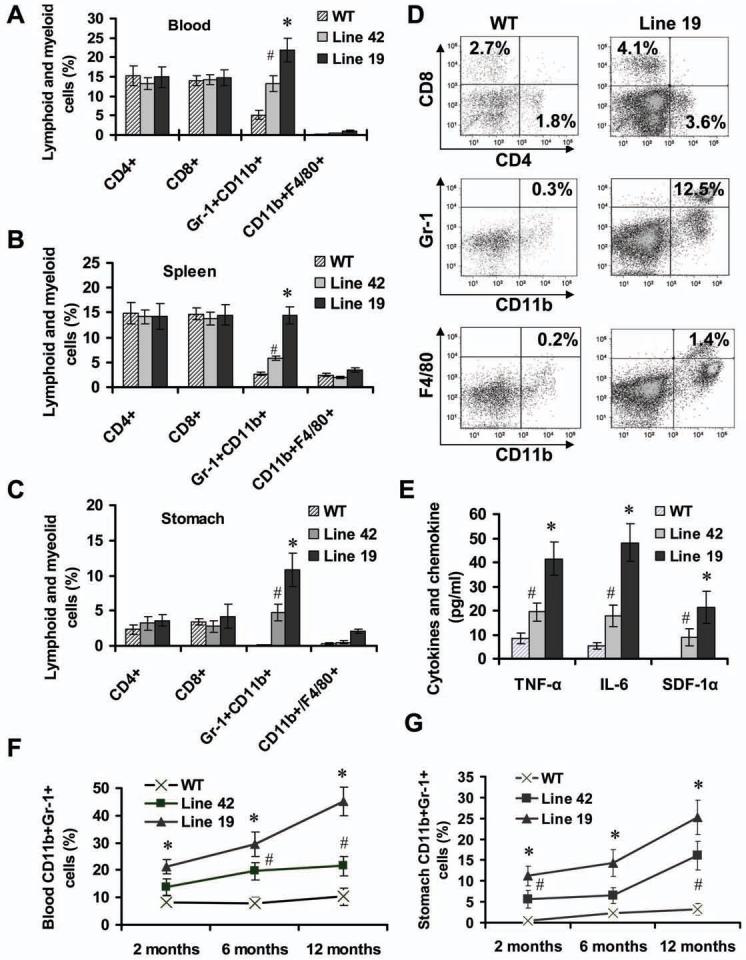
The frequencies of lymphoid and myeloid cells in peripheral blood (A), spleen (B) and stomach (C) from 2 month old IL-1β mice and age-matched WT mice were measured by FACS. The data are the mean ± SD of 6 mice (#p < 0.05, p < 0.01, vs WT mice). (D) Representative FACS blots for detecting lymphoid and myeloid cells in the stomach from WT and Line 19 IL-1β mice. (E) Expression of mouse TNF-α, IL-6 and SDF-1α in the gastric tissue from 2 month old mice was determined by ELISA. The data represent the mean ± SD of 6 animals (*p < 0.01, vs WT mice). (F-G) The kinetics of MDSCs was determined by FACS in peripheral blood (F) and stomach (G) from IL-1β mice and WT mice at different time points. The data represent the mean ± SD of 6 animals (#p < 0.05; *p < 0.01, vs WT mice).
In 6 and 12 month old IL-1β transgenic mice, the accumulation of MDSCs in the blood (Figure 3F), stomach (Figure 3G) and spleen (data not shown) steadily increased and was associated with development of progressive chronic atrophic gastritis, a preneoplastic lesion. Consistent with this histopathologic progression, the numbers of T cells, F4/80+ macrophages and p40-Phox+ neutrophils (Figure S3A and S3B) were significantly increased in the stomachs of older IL-1β transgenic mice, although the increases were less than those observed for MDSCs. There was also a gradual increase in the expression of inflammatory cytokines, chemokines and growth factors (Figure S3C). Furthermore, older IL-1β mice developed splenomegaly (Figure S3D) due largely to the accumulation of large numbers of splenic MDSCs (Figure S3E). Moreover, we found that male IL-1β transgenic mice exhibited higher levels of MDSCs in the blood and stomach than did female mice (Figure S3F), which correlated with the higher circulating levels of IL-6 in male mice compared to female mice (Figure S1E and S1F). These data suggest that in older IL-1β transgenic mice, MDSCs continue to accumulate in addition to recruitment of other immune cells and amplification of the inflammatory response, resulting in the development of gastric neoplasia.
IL-1β activates MDSCs through an NF-κB signal pathway
A number of previous studies have suggested that MDSCs are able to suppress the proliferative response of lymphocytes in tumor bearing animals (Song et al., 2005). Our results confirmed that splenic MDSC from IL-1β transgenic mice exhibited strong suppressive effect on CD4+ T cell proliferation and IFN-γ secretion (Figure S4A and S4B). Next, we investigated whether IL-1β can directly activate MDSCs. Treatment of bone marrow and splenic MDSCs from wild type mice with human IL-1β resulted in a 3-4-fold increase in IL-6 and TNF-α mRNA compared to control MDSCs (Figure S4C). IL-6 protein levels were also significantly increased in IL-1β treated MDSCs (Figure S4D). These data indicate that IL-1β may directly induce IL-6 expression in MDSCs.
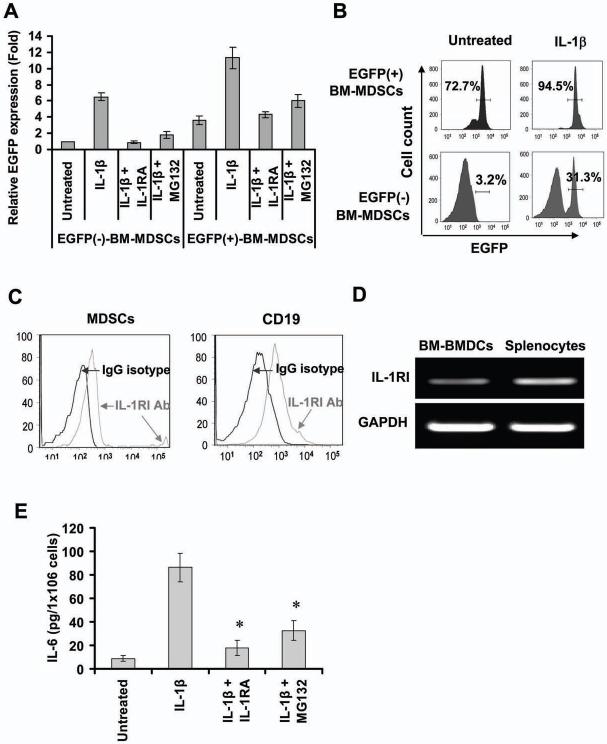
IL-1β has a strong link to NF-κB, a key regulator of cytokine-dependent inflammatory gene expression (Dinarello, 1996; Karin and Greten, 2005). To investigate whether IL-1β induces gene expression in MDSCs by activating the NF-κB signaling pathway, we sorted bone marrow MDSCs from cis-NF-κBEGFP transgenic mice, in which the enhanced GFP (EGFP) is under the transcriptional control of NF-κB cis-elements. In these mice, EGFP expression of the cis-NF-κBEGFP transgene reflects the level of NF-κB activation (Karrasch et al., 2007; Magness et al., 2004). The purity of sorted MDSCs was more than 98% (Figure S4E). Semi-quantitative RT-PCR and Real-time PCR showed that IL-1β treatment significantly upregulated mRNA expression of EGFP in both EGFP- and EGFP+ bone marrow-derived MDSCs from cis-NF-κBEGFP mice (Figure S4F and Figure 4A), indicating that IL-1β specifically activates NF-κB in MDSCs. FACS analysis of IL-1β-stimulated MDSCs from cis-NF-κBEGFP mice further confirmed the upregulation of EGFP protein expression in these cells (Figure 4B), as did antagonism with the NF-κB inhibitor, MG-132, which inhibited IL-1β-induced EGFP expression in MDSCs (Figure 4A and Figure S4F). This suggests that IL-1β can activate the NF-κB pathways in MDSCs.
Figure 4. IL-1β activates MDSCs through an NF-κB signal pathway.
(A) IL-1β upregulates EGFP mRNA expression by activates NF-κB in MDSCs. EGFP+ and EGFP- MDSCs from NF-κBEGFP mice were treated with IL-1β in absence or presence of 50 ng/ml IL-1RA or 1 μM MG132 for 3 hours. mRNA expression was determined by real time-PCR. The data are normalized to untreated EGFP- MDSCs and represent the mean ± SD of four independent experiments. (B) IL-1β upregulates expression of EGFP protein in MDSCs. EGFP+ and EGFP-MDSCs were treated with IL-1β for 24 hours. The EGFP fluorescence intensities were detected by FACS. (C) Expression of IL-1RI protein in MDSCs was determined by FACS using PE-IL-1RI antibody. (D) IL-1RI mRNA expression in MDSCs measured by RT-PCR. (E) Blocking IL-1β /NF-κB signal pathway inhibits IL-1β-stimulates secretion of IL-6 in MDSCs. EGFP+ MDSCs were treated with IL-1β in absence or presence of IL-1RA or MG132 for 36 hours. The level of IL-6 were measured by ELISA. The data represent the mean ± SD of four independent experiments (*p < 0.01, vs IL-1β treated group).
Although studies have been contradictory as to whether the IL-1 receptor I (IL-1RI) is expressed in MDSCs (Bunt et al., 2006), we were able to detect expression of IL-1RI in MDSCs and CD19+ cells (IL-1RI positive cells) by FACS using a PE tagged IL-1RI antibody (Figure 4C), as well as by RT-PCR (Figure 4D). Although the density of IL-1RI is known to be quite low in most cell types, we were able to detect around 2%-6% IL-1RI+ MDSCs in whole bone marrow MDSCs (data not shown). Furthermore, we found that IL-1RA, a natural antagonist of IL-1 receptor that binds to IL-1R and thereby inhibits IL-1β activity (Arend, 2002), blocked IL-1β-induced EGFP expression (Figure 4A and Figure S4F) and IL-6 production (Figure 4E) in MDSCs from cis-NF-κBEGFP transgenic mice. These data confirm that IL-1β can directly activate MDSCs through an IL-1β/IL-1RI/NF-κB pathway.
Overexpression of IL-1β activates NF-κB in MDSCs in vivo
To investigate effects of IL-1β overexpression on NF-κB in vivo, we examined the expression of gastric NF-κB RelA (p65). Strong NF-κB RelA (p65) staining could be observed in inflammatory cells (in young mice) and in occasional dysplastic gastric glands in (older) IL-1β mice, compared to rare p65+ cells in the stomachs of control mice (Figure 5A), suggesting that overexpression of IL-1β may result in the activation of NF-κB in both inflammatory and epithelial cells.
Figure 5. Overexpression of IL-1β activates NF-κB in MDSCs in vivo.
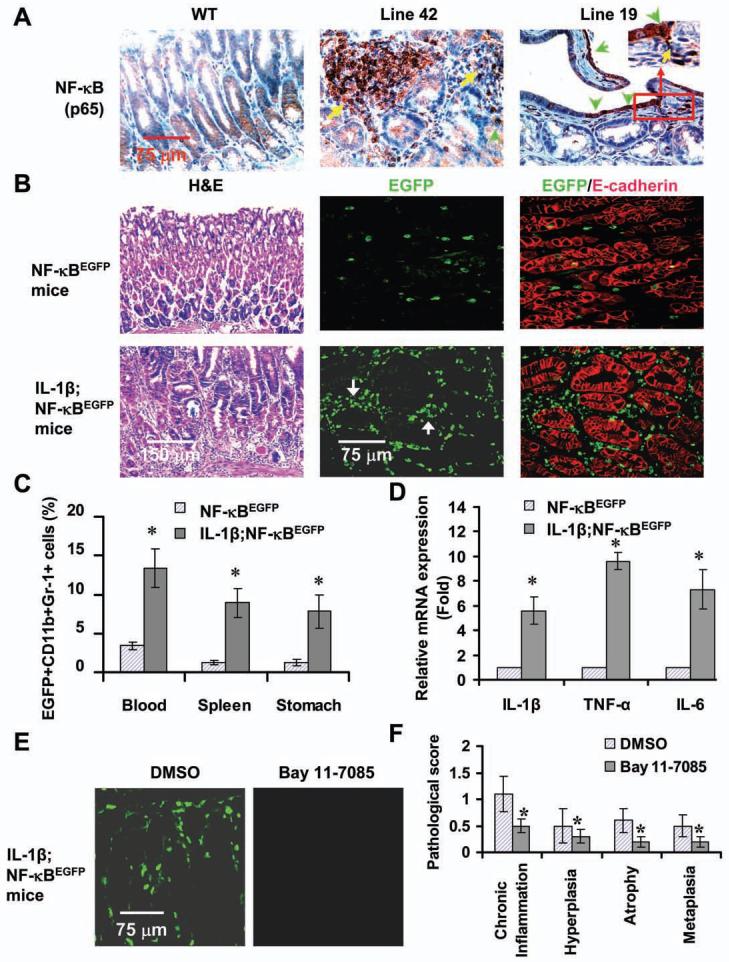
(A) NF-κB activation in the stomachs of IL-1β mice. Sections were stained a NF-κB p65 antibody. Arrows indicate p65+ cells. (B) Enhanced EGFP expression in IL-1β;NF-κBEGFP mice. Frozen gastric sections from 6 month old indicated mice were subjected to H&E staining and double staining with anti-EGFP (green) and E-cadherin (red) antibodies. The red staining indicates epithelial cells. Localization of EGFP+ cells is mostly confined to stromal region. (C) Increased frequencies of EGFP+ MDSCs in peripheral blood, spleen and stomach tissues in IL-1β;NF-κBEGFP mice as analyzed by FACS. The data represent the mean ± SD of 6 animals (*p < 0.05, vs NF-κBEGFP mice). (D) Increased expression of cytokines in stomach MDSCs in IL-1β;NF-κBEGFP mice. Stomach MDSCs sorted from 6 month old NF-κBEGFP and IL-1β;NF-κBEGFP mice were restimulated with PMA for 4 hours. mRNA expression was determined by real-time PCR. The data are normalized to MDSCs of NF-κBEGFP mice and represent the means ± SD of three independent experiments (*p < 0.01, vs NF-κBEGFP mice). (E) Blocking NF-κB activity by injecting i.p. Bay 11-7085 prevents EGFP expression in 3 month old IL-1β;NF-κBEGFP mice. Representative photos were taken from frozen gastric sections under fluorescence microscope. (F) Blocking NF-κB activity inhibits the development of gastritis. The pathological scores were graded in Bay 11-7085 or DMSO-treated IL-1β;NF-κBEGFP mice (*p < 0.05, vs DMSO treated mice, n = 8).
To better define the cellular target of IL-1β, as well as the effects on MDSCs, we next crossed Line 19 IL-1β mice to NF-κBEGFP transgenic mice. Older (> 6 month old) IL-1β;NF-κBEGFP double transgenic mice developed spontaneous gastritis and dysplasia (Figure 5B, left panel). The number of MDSCs were significantly increased in the blood, spleen and stomach in 2 month old IL-1β;NF-κBEGFP transgenic mice compared with NF-κBEGFP transgenic mice (data not shown), consistent with previous results (Figure 3A-3C). Furthermore, the number of EGFP+ MDSCs were also significantly increased in the blood (Figure 5C and Figure S5A), spleen and stomach (Figure 5C) in IL-1β;NF-κBEGFP transgenic mice compared with NF-κBEGFP transgenic mice. Immuofluorescence staining confirmed that EGFP+ cells in the gastric mucosa were mainly CD11b+ cells (Figure S5B) and Gr-1+ cells (data not shown). In older IL-1β;NF-κBEGFPmice, many EGFP+ cells were observed in the gastric mucosa, whereas fewer EGFP+ cells were observed in the stomachs of control NF-κBEGFP mice (Figure 5B). The EGFP+ signal in older mice (>6 months) was localized primarily to immune cells (Figure 5B, middle and right panel), including not only MDSCs but also F4/80+ macrophages (Figure S5C), consistent with late stage activation of the latter immune populations.
To determine whether IL-1β modulates NF-κB-dependent gene expression in MDSCs, we isolated EGFP+ MDSCs from IL-1β;NF-κBEGFP and NF-κBEGFP mice and assessed gene expression by RT-PCR. We found that the mRNA expression of several NF-κB target genes was significantly increased in EGFP+ MDSCs isolated from the stomachs of IL-1β mice compared to control mice (Figure 5D). These data strongly suggest that overexpression of IL-1β activates NF-κB in MDSCs cells in vivo, resulting in amplification of the pro-inflammatory response in a manner that could contribute to the development of dysplasia.
To further investigate the role of NF-κB signaling in IL-1β-induced gastric preneoplasia, we treated 3 month old IL-1β;NF-κBEGFP mice with the specific NF-kB inhibitor, Bay11-7085 or DMSO (solvent control) for 5 weeks. The number of EGFP+ cells in the stomach were strongly reduced in Bay 11-7085-treated IL-1β;NF-κBEGFP mice (Figure 5E). FACS analysis showed that the number of EGFP+ cells in the blood were also significantly decreased in Bay 11-7085-treated IL-1β;NF-κBEGFP mice (Figure S5D). The expression of gastric mouse IL-6, IL-β and TNF-α was significantly decreased in Bay 11-7085-treated IL-1β;NF-κBEGFP mice (Figure S5E). Moreover, the histopathologic scores were significantly lower in Bay 11-7085-treated mice than in DMSO-treated mice (Figure 6F). These data demonstrate that pharmacologic NF-κB inhibition ameliorates IL-1β-induced gastric inflammation. This suggests the possibility that MDSCs could be a relevant target, but NF-κB inhibition likely also affects other cells types, including macrophages, granulocytes and dendritic cells.
Figure 6. MDSCs are implicated in IL-1β-induced chronic gastritis and dysplasia.
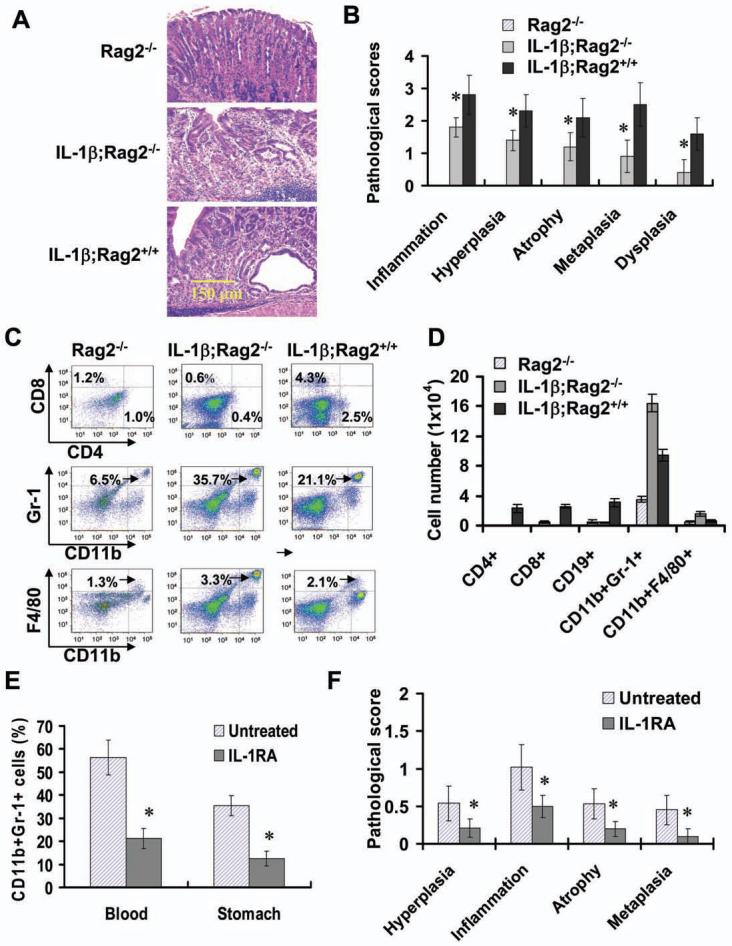
(A) IL-1β;Rag2-/- mice develop spontaneous gastritis and dysplasia (H&E staining). (B) The pathological scores were graded in old Rag2-/-, IL-1β;Rag2-/- and IL-1β;Rag2+/+ mice (>12 months). The data represent the mean ± SD of 10 animals (*p <0.01, vs Rag2-/- mice). (C-D) Increased the number of myeloid cells in the stomach of 6 month old IL-1 β;Rag2-/- mice. Representative FACS blots (C) and the data shown are the mean ± SD of cell number per stomach derived from 6 animals (D). (E) IL-1RA treatment inhibits the mobilization and recruitment of MDSCs in IL-1β;Rag2-/- mice. Three month old IL-1β;Rag2-/- mice were treated with IL-1RA for 6 weeks. Single nucleated cells isolated were stained with APC-CD11b and PerCP-Gr-1 antibodies and analyzed by FACS. (F) IL-1RA treatment inhibits the development of gastritis in IL-1β;Rag2-/- mice. The pathological scores were graded in above indicated mice The data represent the mean ± SD of 6 animals (*p < 0.05, vs untreated mice).
IL-1β induces gastric preneoplasia and mobilizes MDSCs in the absence of lymphocytes
Previous studies have shown that adaptive immune responses, particularly CD4+ T cells, are required for the development of Helicobacter-dependent atrophic gastritis and preneoplasia. Both Rag2-/- and T cell deficient TCRβ δ-/- mice show no detectable epithelial changes or parietal cell loss when infected with H. felis or H. pylori (Lee et al., 2007; Roth et al., 1999). We confirmed that Rag2-/- mice did not develop atrophic gastritis after H. felis infection alone, but did develop atrophy after H. felis infection in combination with a transfer of splenocytes or CD4+T cells from wild type or IL-1β transgenic mice (data not shown).
Rag2-/- mice are deficient in B cells, T cells and NK cells, but have abundant normal maturation of the myeloid lineages including MDSCs (Figure S6A and S6B). Despite the absence of atrophic gastritis in H. felis-infected Rag2-/- mice, there was a notable increase in the number of myeloid cells (both MDSCs and monocytes/macrophages) in the stomachs and spleens of infected mice (data not shown).
To investigate the role of MDSCs, independent of lymphocytes, in gastric preneoplasia, we crossed Line 19 IL-1β mice into a C57BL Rag2-/- genetic background to generate lymphocyte deficient IL-1β transgenic mice. Unexpectedly, IL-1β;Rag2-/- mice showed the spontaneous development of atrophic gastritis, metaplasia and dysplasia (Figure 6A and 6B), accompanied by a marked increase in the number of MDSCs in the stomach (Figure 6C and 6D), blood (Figure S6A) and spleen (Figure S6B). These results strongly suggest that atrophic gastritis/dysplasia can occur in absence of T cells, and that MDCSs may be a critical mediator of early stages of gastric carcinogenesis.
IL-1RA inhibits the development of gastric carcinoma and suppresses MDSC mobilization in H. felis -infected IL-1β mice
Next, we sought to investigate whether deficiency of MDSCs or inhibition of MDSC mobilization and recruitment can suppress the development of gastritis. Since at present no MDSC-specific knockout mice are available, we treated 3 month old IL-1β;Rag2-/- mice with IL-1RA for 6 weeks. Notably, IL-1RA treatment significantly reduced mobilization and recruitment of MDSCs in the circulation and stomach (Figure 6E), and significantly inhibited the development of gastric inflammation and preneoplasia in IL-1β;Rag2-/- mice (Figure 6F).
Since overexpression of IL-1β accelerates the development of gastric carcinoma in the setting of H. felis infection, we investigated whether suppression of MDSC mobilization and recruitment inhibits the development of gastric inflammation and carcinoma in H. felis- infected IL-1β mice using IL-1RA. Prophylactic administration of IL-1RA increased the concentration of IL-1RA in gastric mucosa (Figure S7A) and reduced the mobilization of circulating MDSCs (Figure 7A) and recruitment of MDSCs in the stomach of H. felis-infected transgenic and control mice (Figure S7B), and significantly prevented the development of inflammation in both transgenic and WT mice infected with H. felis for 5 months (Figure 7B). IL-1RA treatment significantly reduced the levels of TNF-α, IL-6 and SDF1 in the gastric mucosa (Figure 7C and 7D). Furthermore, IL-1RA treatment significantly inhibited the development of gastric inflammation and histologic scores of other alterations in both transgenic and WT mice that were infected H. felis for 12 months (Figure 7E). More importantly, none of the H. felis-infected IL-1β mice developed dysplasia in the IL-1RA treated group compared to untreated H. felis-infected IL-1β mice (Figure 7E). These findings demonstrate that IL-1 receptor blockade can inhibit the development of gastric inflammation and carcinogenesis, which may be due in part to a reduction of MDSCs in Helicobacter-infected IL-1β mice. Taken together, the data demonstrate a clear role for IL-1β in the induction of gastric cancer, and implicate MDSCs in promoting carcinogenesis. However, since neither IL-1RA nor Bay 11-7085 target MDSCs specifically, other downstream targets of IL-1β might also contribute to gastric neoplasia.
Figure 7. IL-1RA treatment improves gastric pathology and blocks MDSC recruitment in H. felis infected IL-1β mice.
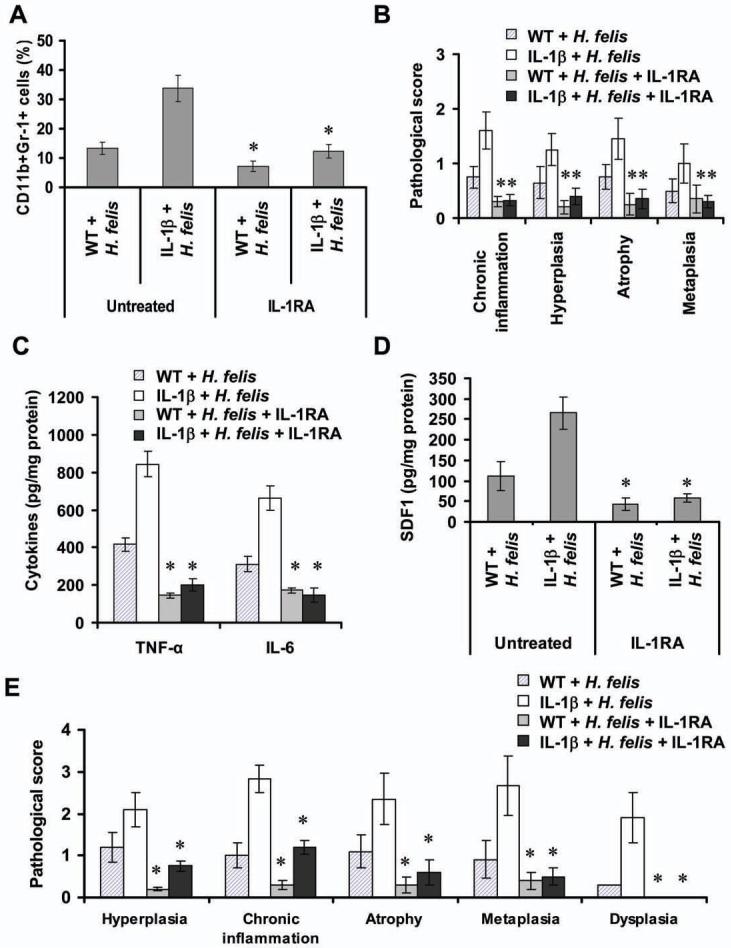
(A) IL-1RA treatment inhibits the mobilization of MDSCs. Single nucleated cells in peripheral blood were isolated from Line 19 IL-1β transgenic and control mice infected with H. felis for 5 months with or without preventive administration of IL-1RA. Cells were stained with fluorescence-labeled CD11b and Gr-1 antibodies and analyzed by FACS (*p < 0.05, vs untreated mice, n = 6). (B) IL-1RA treatment inhibits the development of gastric inflammation and preneoplasia in H. felis-infected mice. Histopathological scores assessed in mice (*p < 0.05, vs untreated mice, n = 6). Gastric expression of mouse TNF-α and IL-6 (C) and SDF-1α (D) in indicated mice were determined by ELISA. (E) IL-1RA treatment inhibits the development of gastric dysplasia in H. felis-infected IL-1β mice. Stomach sections were from Line 19 IL-1β transgenic and WT mice infected with H. felis for 12 months with or without IL-1RA treatment for 3 months. The histopathologic scores were graded (*p < 0.01, vs untreated mice, n = 10). Error bar in (A)-(E) indicate ± SD.
DISCUSSION
In this study, we have established human IL-1β transgenic mice by targeting expression of hIL-1β to the stomach using the H/K-ATPase promoter. The IL-1β transgenic mice provide an in vivo model of inflammation-related cancer that closely mimics development of human cancer. Our results indicate that these IL-1β transgenic mice develop step-wise spontaneous inflammation, metaplasia, dysplasia and carcinoma of the stomach, and that IL-1β activation of NF-κB in MDSCs contribute to the development of gastric inflammation and initiation of carcinogenesis. H. felis infection resulted in more rapid progression to gastric atrophy and cancer in IL-1β transgenic mice. Our current results provide support and validation for clinical studies that suggested a strong link between SNPs in the IL-1β gene and the risk of gastric cancer in the setting of H. pylori infection (El-Omar et al., 2001). In addition, the data offer direct evidence that elevation of a single proinflammatory cytokines, IL-1β, is sufficient for the induction of gastric dysplasia/carcinoma, and thus establish a crucial etiological role for IL-1β in gastric carcinogenesis.
Previous studies have pointed to roles for MDSCs in cancer. Accumulating data have shown that MDSCs can contribute to suppression of tumor immunity, resistance to immunotherapy, tumor angiogenesis and metastasis (Shojaei et al., 2007; Yang et al., 2008). In addition, it has been demonstrated that overexpression of IL-1β by cancer cells themselves can accelerate tumor progression and its spread in part by mobilization and recruitment of MDSCs in tumor tissues (Bunt et al., 2006; Song et al., 2005). Our study suggests for a link between IL-1β and MDSCs in carcinogenesis. Several findings in our study suggest a direct contribution of MDSCs to IL-1β induced gastric inflammation and carcinoma. (1) The mobilization of MDSCs into the blood, and the recruitment of MDSCs to the stomach, occurred at the very earliest stage of gastric inflammation in IL-1β transgenic mice. (2) IL-1β directly activated MDSCs in vivo and in vitro to induce secretion of pro-inflammatory cytokines (IL-6 and TNF-α) and chemokines (SDF1) in IL-1β mice at this early stage. (3) The IL-1β;Rag2-/- mice developed spontaneous gastritis and dysplasia in the absence of T cells. (4) Antagonism of IL-1 receptor signaling by IL-1RA inhibited gastric inflammation and carcinoma and also suppressed MDSC mobilization and recruitment. Interestingly, inhibitory effects of IL-1RA were also observed in H. felis-infected wild type mice, suggesting that the effects were not limited to the transgenic mice. However, given that MDSCs were not specifically ablated in our model, a possible role for other cell types (including neutrophils, macrophages, dendritic cells, myofibroblasts, endothelial cells, etc.) cannot be excluded.
One important finding in this study is that IL-1β can directly activate MSDCs through an IL-1β/ IL-1RI / NF-κB pathway. In prior studies, such a direct link was not well established and other possibilities were considered, including a role for IL-6 in MDSC activation (Bunt et al., 2006). IL-1R-deficient mice have a delayed accumulation of MDSC, which was partially restored by IL-6, indicating that IL-6 is a downstream mediator of the IL-1β-induced expansion of MDSC (Bunt et al., 2007). However, we found that while the levels of expression were low in MDSCs, IL-1RI is clearly expressed in these cells, and IL-1β could directly activate MDSCs. IL-1β stimulation of MDSCs led to increased NF-κB activity, both in vitro and in vivo, and increased secretion of IL-6 and TNF-α. Using an IL-1β;NF-κBEGFP mouse model, we found that overexpression of IL-1β directly activated NF-κB in both epithelial cells and immune cells, but the greatest activation occurred in MDSCs. We thus have linked IL-1β directly to NF-κB activation in MDSCs, and to the NF-κB downstream targets genes, IL-6 and TNF-α.
Activation of the transcription factor, NF-κB, is a key molecular link between inflammation and cancer (Karin and Greten, 2005). Activation of NF-κB occurs downstream of both the TLR and IL-1RI pathways through a complex involving IKK-beta. We have previously shown that Helicobacter infection activates the innate immune system through a TLR pathway (Mandell et al., 2004) leading to induction of NF-κB signaling and cytokine production (such as IL-1β, TNF-α and IL-6) by macrophages. Knockout of IKK-β in myeloid cells has been shown to downregulate the innate immune system and suppress both murine hepatocellular and colon carcinoma (Greten et al., 2004; Maeda et al., 2005; Pikarsky et al., 2004). However, in these conditional knockouts of IKK-β in myeloid cells, the relevant cell population targeted has not been completely defined, although IL-6 levels are consistently downregulated in the knockout animals. In our IL-1β transgenic mice, the levels of IL-6 and TNF-α were significantly increased in both the stomach and the serum, and correlated well with MDSC mobilization and recruitment. IL-6 in particular, which is frequently elevated in patients with cancer, is an important inducer of tumor promotion and progression, and may account for gender differences in cancer susceptibility (Heikkila et al., 2008; Naugler et al., 2007). Thus, IL-6 and TNF-α may be major secreted products of MDSCs and as such serve as direct downstream target of IL-1β and NF-κB that amplify the inflammatory immune response and promote carcinogenesis (Balkwill, 2006; Naugler et al., 2007).
Clinical and epidemiologic studies have suggested a strong association between chronic inflammation and cancer (Coussens and Werb, 2002). Specific polymorphisms in proinflammatory cytokine genes, such as IL-1β, can now be linked to MDSCs. Previous studies have demonstrated that MDCSs have immunosuppressive properties, and we have confirmed that MDSCs from our IL-1β mice can inhibit T and B cell proliferation. However, the development of gastric preneoplasia has in the past been shown to be related to the host immune response to infection, and largely dependent on CD4+ T cells, which we have again confirmed in this study. Rag2-/- were resistant to Helicobacter-induced gastric atrophy, while Rag2-/- mice reconstituted with whole splenocytes or CD4+ T cells developed severe atrophic gastritis after H. felis infection. However, IL-1β transgenic mice showed an early and marked infiltration of the gastric mucosa with MDSCs at a stage when very few T cells were present. More importantly, IL-1β mice crossed into a Rag2-/- background (i.e. IL-1β;Rag2-/- mice) still developed spontaneously gastritis and dysplasia, in association with a marked infiltrate of MDSCs, indicating that IL-1β-induced gastric inflammation may be mediated by MDSCs independent of a T cell-mediated Th1 immune response. IL-1β is able to induce gastric atrophy and mobilize MDSCs in lymphocyte deficient animals. While the data fall short of demonstrating that MDSCs are the primary mediator of carcinogenesis in our model, they do suggest an early role for MDSCs in cancer that does not dependent on an immunosuppressive role, but is instead more consistent with a pro-inflammatory role.
In conclusion, our study strengthens the link between IL-1β and gastric cancer, and implicates MDSCs as being important in the early stages of gastric carcinogenesis. This observation could lead to a more general understanding of the role of inflammation in carcinogenesis and provide a model for test the efficacy of anti-IL-1β therapies in cancer prevention.
EXPERIMENTAL PROCEDURES
Generation of HK-ATPase hIL-1β transgenic mice and double transgenic mice
The 1,060bp fragments of mouse HK-ATPase β subunit promoter (Lorenz and Gordon, 1993) and the 550bp fragments of mature secreted form human IL-1β cDNA fused with the signal sequence human IL-1RA (Bjorkdahl et al., 1999) were subcloned together with human growth hormone polyadenylation sequence into pBluescript vector (Stratagene) (Figure 1A). The transgenic construct was used for pronuclear injection of C57BL/6J x SJL F2 hybrid zygotes. Potential founders were screened using both PCR and southern blot analysis. A high IL-1β-expressing line (Line 19) and a lower IL-1β-expressing line (Line 42) were selected and backcrossed to C57BL/6J mice. The NF-κBEGFP knockin mice (C57BL/6J) were described previously (Magness et al., 2004). Line 19 IL-1β mice (C57BL/6J) were crossed to NF-κBEGFP mice to generate IL-1β;NF-κBEGFP (C57BL/6J background). To generate IL-1β;Rag2-/- double transgenic mice, Line 19 IL-1β mice (C57BL/6J) were crossed to Rag2-/- mice (C57BL6J) (Taconic, New York). All animal studies were performed in AAAIAC approved facilities at Columbia University under approval of the Columbia University Institutional Animal Care and Use Committee.
Helicobacter felis infection and drug treatment
Eight week old IL-1β male mice and age-matched C57BL/6J male mice were infected with H. felis (ATCC strain 49179) by oral gavage with 1 × 108 colony forming units every other day for 3 times as previously described. Human IL-1RA or saline began to be given by intraperitoneal injection (i.p.) at a dose of 100 mg/kg/day per mouse at 3 days after H. felis infection for prevention and at 5 months after H. felis infection for treatment for 90 days. In a separate experiment, three month old IL-1β;Rag2-/- mice were given IL-1RA intraperitoneal injection at a dose of 100 mg/kg/day for 6 weeks. Three month old IL-1β;NF-κBEGFP mice were injected i.p. three times weekly with the NF-κB inhibitor Bay 11-7085 (5 mg/kg) (Calbiochem, Gibbstown, NJ) or vehicle control (DMSO) for 5 weeks. Animals were euthanatized with CO2, and serum stomach and spleen tissues were collected for further analysis.
Single cell preparation and FACS analysis
Bone marrow-derived cells (BMDC) from the femur and tibia of euthanatized mice were flushed and depleted of RBC using RBC lysing buffer (Sigma-Aldrich). Total nucleated cells in peripheral blood were isolated after erythrocyte lysis. Single splenic cells were obtained by disaggregating spleen. For single cell suspension preparation from stomach tissues, the mucosa of whole stomach was gently scraped free from the serosa, minced and digested for 1 hour in 1 mM DTT, 1 mM EDTA, 5% FBS in PBS at 37° and filtered through a 40μm nylon mesh strainer and then resuspended in Dulbecco’s PBS (D-PBS) (Houghton et al., 2004). For FACS analysis, single cell suspensions were stained with fluorence labeled FITC-CD45, PE-CD3, APC-CD19, PE-Cy7-CD8, Alex-700-CD4, APC-CD11b, PerCP-Ly-6G antibodies (BD Pharmingen) and detected using a LSRII flow cytometer (BD biosciences, San Diego, CA). Data were analyzed by FlowJo7 software (Tree Star, Inc, Ashland, OR).
MDSC isolation and treatment
BMDCs from WT, IL-1β and IL-1β;NF-κBEGFP mice were stained with APC-CD11b and PerCP-Gr-1 antibodies, and sorted by FACS BD Aria (BD biosciences) to get CD11b+Gr-1+ MDSCs. MDSCs were cultured in 12-well plates (Costar) in complete medium (RPMI 1640) supplemented with 10% FCS. MDSCs were treated with IL-1β in presence or absence of IL-1RA or NF-κB inhibitor MG 132 (Calbiochem, La Jolla, CA) for 3 hours. Cells mRNA was extracted for RT-PCR. In another experiment, MDSCs were treated with IL-1β in presence or absence of IL-1RA or NF-κB inhibitor MG 132 for 36 hours. Supernatant (culture medium) were harvested and the level of protein in supernatant was measured by ELISA.
IL-1RI assay
The IL-1RI expression was detected by FACS. Briefly, 1 × 105 purified MDSCs from WT mice bone marrow were incubated with 1 μg Fcγ III/Receptor (BD Pharmigen, San Diego, CA) for 30 minutes at room temperature prior to staining. MDSCs were incubated with 2 μl PE-labeled IL-IRI antibody or PE-IgG isotype control (BD Pharmigen) for 45 minutes at 4°C in the dark. Cells were washed twice with PBS buffer and analyzed by flow cytometry for IL-1RI expression. mRNA expression of IL-1RI in MDSCs was determined by RT-PCR. Primers used are listed in supplementary Table S4.
Histopathologic analysis
The stomach and other tissues from transgenic and control mice were fixed in 10% formalin imbedded in paraffin, cut into 5 μm sections, and with hematoxylin/eosin (H&E). Histopathologic scores in the stomach tissues were graded according to previously described criteria (Fox et al., 2000) by two pathologists blinded to treatment groups.
Immunohistochemical staining
Paraffin sections fixed in 10% formalin were incubated with primary antibodies: rabbit polyclonal TFF2 (Tu et al., 2007), NF-κB p65, F4/80, and CD11b (Abcam, Cambridge, MA), β-catenin (BD Bioscience Pharmingen, San Diego, CA), c-Myc (Santa Cruz, San Diego, CA) and control IgG2a. Biotinylated secondary antibodies (Jackson Immunoresearch Laboratories Inc.,West Grove, PA) and ABC avidin-biotin-DAB detection kit (Vector Labs) were used for detection and visualization according to supplied protocol.
Immunofluorescence staining
Frozen stomach sections (5 μm) were subjected to double-immunofluorescence staining by simultaneous incubation of sections with anti-EGFP antibody and anti-E-cadherin (Invitrogen, Carlsbad, CA), or F4/80 and anti-CD11b antibodies (eBiosciences, San Diego, CA) overnight, then incubated with FITC-conjugated anti-rabbit secondary antibody and Texas red-conjugated anti-rat secondary antibody (Vector Laboratories) for 1 hour at room temperature. Slides were counterstained with 2 μg/ml DAPI Vector Laboratories). Specimens were observed with Olympus Fluoview Confocal Microscope and images were analyzed with Adobe Photoshop (Adobe System Incorporated, San Jose, CA).
Measurement of cytokine levels by ELISA
The levels of human IL-1β, IL-8, mouse TNF-α, IL-6, IL-1β, SDF-1, IFN-γ and IL-1RA in supernatant of cells cultured, or serum or gastric tissues of the transgenic mice were determined using ELISA kit (BD Company, San Diego, CA). Absorbance was measured at 450 nm by a Multiscan MC reader, and the samples were analyzed by DELTA SOFT II software (BioMetallics, Inc., Princeton, NJ).
Quantitative and semi-quantitative polymerase chain reaction
Total RNA was isolated from the stomachs and spleen of IL-1β mice and control mice. Reverse transcription was performed using SuperScript III First-Strand Synthesis System and semi quantitative PCR reactions were performed using a PCR Core Kit (Roche, Nutley, NJ). The resulting PCR products were analyzed by agarose gel electrophoresis. Quantitative real-time PCR was performed with a 3-step method using the ABI General System 7300 (ABI Applied System, University Park, IL) and QuantiTect SYBR Green PCR (Qiagen, Valencia, CA). The PCR conditions were as follows: 95°C for 3 minutes, followed by 40 cycles of 95°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds. The sequences of primers were listed in Table S4.
Statistics
Data are represented as the mean ± SD from at least three independent experiments or 6 mice. The significance of the difference between groups was evaluated with the Student’s t-test or χ2 test. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Dr. Jeffrey I. Gordon for providing the mouse H/K-ATPase promoter plasmid and Dr. David A. Brenner for providing the NF-KBEGFP transgenic mice. We would like to thank Shengwen Wang and Guangchun Jin for assistance with FACS analysis, Zina Dubeykovskaya for providing the TFF2 antibody; Vigneshwaran Ramanathan and Shanisha AK Gordon for breeding and genotyping the transgenic mice. This work was supported by grants from the National Institutes of Health: 1U54CA126513 (to T.C.W.), RO1CA093405 and R01CA120979 (to T.C.W and J.G.F.) and RO1AI51415 (to E.K.J.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Significance
Polymorphisms of proinflammatory cytokines have been associated with increased risk for malignancies. Development of appropriate pro-inflammatory cytokine-based cancer models could facilitate the elucidation of molecular mechanisms and development of therapies. We demonstrate that gastric specific overexpression of human IL-1β in transgenic is sufficient for step-wise progression of gastric dysplasia and cancer, and that activation of NF-κB myeloid-derived suppressor cells (MDSCs) is strongly associated with cancer. Blockade by IL-1RA significantly inhibits histologic progression and reduces MDSCs recruitment. Our results suggest that targeted inhibition of IL-1 receptor signalling may be a potential strategy for prevention and treatment of inflammation-dependent cancer. Our data also suggests that MDSCs contribute not only to cancer progression but also to the earlier stages of carcinogenesis.
REFERENCES
- Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell. 2008;13:193–205. doi: 10.1016/j.ccr.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13:323–340. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006;25:409–416. doi: 10.1007/s10555-006-9005-3. [DOI] [PubMed] [Google Scholar]
- Barber MD, Powell JJ, Lynch SF, Fearon KC, Ross JA. A polymorphism of the interleukin-1 beta gene influences survival in pancreatic cancer. Br J Cancer. 2000;83:1443–1447. doi: 10.1054/bjoc.2000.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron JA, Sandler RS. Nonsteroidal anti-inflammatory drugs and cancer prevention. Annu Rev Med. 2000;51:511–523. doi: 10.1146/annurev.med.51.1.511. [DOI] [PubMed] [Google Scholar]
- Bidwell J, Keen L, Gallagher G, Kimberly R, Huizinga T, McDermott MF, Oksenberg J, McNicholl J, Pociot F, Hardt C, D’Alfonso S. Cytokine gene polymorphism in human disease: on-line databases. Genes Immun. 1999;1:3–19. doi: 10.1038/sj.gene.6363645. [DOI] [PubMed] [Google Scholar]
- Bjorkdahl O, Akerblad P, Gjorloff-Wingren A, Leanderson T, Dohlsten M. Lymphoid hyperplasia in transgenic mice over-expressing a secreted form of the human interleukin-1beta gene product. Immunology. 1999;96:128–137. doi: 10.1046/j.1365-2567.1999.00655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- Dolcetti L, Marigo I, Mantelli B, Peranzoni E, Zanovello P, Bronte V. Myeloid-derived suppressor cell role in tumor-related inflammation. Cancer Lett. 2008 doi: 10.1016/j.canlet.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, Song H, Vandenberg S, Johnson RS, Werb Z, Bergers G. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton KA, Mefford M, Thevenot T. The role of T cell subsets and cytokines in the pathogenesis of Helicobacter pylori gastritis in mice. J Immunol. 2001;166:7456–7461. doi: 10.4049/jimmunol.166.12.7456. [DOI] [PubMed] [Google Scholar]
- El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, et al. The role of interleukin-1 polymorphisms in the pathogenesis of gastric cancer. Nature. 2001;412:99. doi: 10.1038/35083631. [DOI] [PubMed] [Google Scholar]
- Figueiredo C, Machado JC, Pharoah P, Seruca R, Sousa S, Carvalho R, Capelinha AF, Quint W, Caldas C, van Doorn LJ, et al. Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J Natl Cancer Inst. 2002;94:1680–1687. doi: 10.1093/jnci/94.22.1680. [DOI] [PubMed] [Google Scholar]
- Fox JG, Beck P, Dangler CA, Whary MT, Wang TC, Shi HN, Nagler-Anderson C. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat Med. 2000;6:536–542. doi: 10.1038/75015. [DOI] [PubMed] [Google Scholar]
- Fox JG, Wang TC. Helicobacter pylori--not a good bug after all! N Engl J Med. 2001;345:829–832. doi: 10.1056/NEJM200109133451111. [DOI] [PubMed] [Google Scholar]
- Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117:60–69. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Heikkila K, Ebrahim S, Lawlor DA. Systematic review of the association between circulating interleukin-6 (IL-6) and cancer. Eur J Cancer. 2008;44:937–945. doi: 10.1016/j.ejca.2008.02.047. [DOI] [PubMed] [Google Scholar]
- Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–1571. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- Howell WM, Calder PC, Grimble RF. Gene polymorphisms, inflammatory diseases and cancer. Proc Nutr Soc. 2002;61:447–456. doi: 10.1079/pns2002186. [DOI] [PubMed] [Google Scholar]
- Howell WM, Turner SJ, Theaker JM, Bateman AC. Cytokine gene single nucleotide polymorphisms and susceptibility to and prognosis in cutaneous malignant melanoma. Eur J Immunogenet. 2003;30:409–414. doi: 10.1111/j.1365-2370.2003.00425.x. [DOI] [PubMed] [Google Scholar]
- Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- Karrasch T, Kim JS, Muhlbauer M, Magness ST, Jobin C. Gnotobiotic IL-10-/-;NF-kappa B(EGFP) mice reveal the critical role of TLR/NF-kappa B signaling in commensal bacteria-induced colitis. J Immunol. 2007;178:6522–6532. doi: 10.4049/jimmunol.178.10.6522. [DOI] [PubMed] [Google Scholar]
- Lee CW, Rao VP, Rogers AB, Ge Z, Erdman SE, Whary MT, Fox JG. Wild-type and interleukin-10-deficient regulatory T cells reduce effector T-cell-mediated gastroduodenitis in Rag2-/- mice, but only wild-type regulatory T cells suppress Helicobacter pylori gastritis. Infect Immun. 2007;75:2699–2707. doi: 10.1128/IAI.01788-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz RG, Gordon JI. Use of transgenic mice to study regulation of gene expression in the parietal cell lineage of gastric units. J Biol Chem. 1993;268:26559–26570. [PubMed] [Google Scholar]
- Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Magness ST, Jijon H, Van Houten Fisher N, Sharpless NE, Brenner DA, Jobin C. In vivo pattern of lipopolysaccharide and anti-CD3-induced NF-kappa B activation using a novel gene-targeted enhanced GFP reporter gene mouse. J Immunol. 2004;173:1561–1570. doi: 10.4049/jimmunol.173.3.1561. [DOI] [PubMed] [Google Scholar]
- Mandell L, Moran AP, Cocchiarella A, Houghton J, Taylor N, Fox JG, Wang TC, Kurt-Jones EA. Intact gram-negative Helicobacter pylori, Helicobacter felis, and Helicobacter hepaticus bacteria activate innate immunity via toll-like receptor 2 but not toll-like receptor 4. Infect Immun. 2004;72:6446–6454. doi: 10.1128/IAI.72.11.6446-6454.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melani C, Chiodoni C, Forni G, Colombo MP. Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/c mice suppresses immune reactivity. Blood. 2003;102:2138–2145. doi: 10.1182/blood-2003-01-0190. [DOI] [PubMed] [Google Scholar]
- Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- Roth KA, Kapadia SB, Martin SM, Lorenz RG. Cellular immune responses are essential for the development of Helicobacter felis-associated gastric pathology. J Immunol. 1999;163:1490–1497. [PubMed] [Google Scholar]
- Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S, Fuh G, Gerber HP, Ferrara N. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- Shojaei F, Wu X, Zhong C, Yu L, Liang XH, Yao J, Blanchard D, Bais C, Peale FV, van Bruggen N, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450:825–831. doi: 10.1038/nature06348. [DOI] [PubMed] [Google Scholar]
- Song X, Krelin Y, Dvorkin T, Bjorkdahl O, Segal S, Dinarello CA, Voronov E, Apte RN. CD11b+/Gr-1+ immature myeloid cells mediate suppression of T cells in mice bearing tumors of IL-1beta-secreting cells. J Immunol. 2005;175:8200–8208. doi: 10.4049/jimmunol.175.12.8200. [DOI] [PubMed] [Google Scholar]
- Tu S, Chi AL, Lim S, Cui G, Dubeykovskaya Z, Ai W, Fleming JV, Takaishi S, Wang TC. Gastrin regulates the TFF2 promoter through gastrin-responsive cis-acting elements and multiple signaling pathways. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1726–1737. doi: 10.1152/ajpgi.00348.2006. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kato N, Hoshida Y, Yoshida H, Taniguchi H, Goto T, Moriyama M, Otsuka M, Shiina S, Shiratori Y, et al. Interleukin-1beta gene polymorphisms associated with hepatocellular carcinoma in hepatitis C virus infection. Hepatology. 2003;37:65–71. doi: 10.1053/jhep.2003.50017. [DOI] [PubMed] [Google Scholar]
- Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, Moses HL. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MR, Lathers DM. Myeloid progenitor cells mediate immune suppression in patients with head and neck cancers. Int J Immunopharmacol. 1999;21:241–252. doi: 10.1016/s0192-0561(99)00008-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.