Abstract
Marine and freshwater mussels are notorious foulers of natural and manmade surfaces, secreting specialized protein adhesives for rapid and durable attachment to wet substrates. Given the strong and water-resistant nature of mussel adhesive proteins, significant potential exists for mimicking their adhesive characteristics in bioinspired synthetic polymer materials. An important component of these proteins is L-3,4-dihydroxylphenylalanine (DOPA), an amino acid believed to contribute to mussel glue solidification through oxidation and crosslinking reactions. Synthetic polymers containing DOPA residues have previously been shown to crosslink into hydrogels upon the introduction of oxidizing reagents. Here we introduce a strategy for stimuli responsive gel formation of mussel adhesive protein mimetic polymers. Lipid vesicles with a bilayer melting transition of 37 °C were designed from a mixture of dipalmitoyl and dimyristoyl phosphatidylcholines and exploited for the release of a sequestered oxidizing reagent upon heating from ambient to physiologic temperature. Colorimetric studies indicated that sodium-periodate-loaded liposomes released their cargo at the phase transition temperature, and when used in conjunction with a DOPA-functionalized poly(ethylene glycol) polymer gave rise to rapid solidification of a crosslinked polymer hydrogel. The tissue adhesive properties of this biomimetic system were determined by in situ thermal gelation of liposome/polymer hydrogel between two porcine dermal tissue surfaces. Bond strength measurements showed that the bond formed by the adhesive hydrogel (mean = 35.1 kPa, SD = 12.5 kPa, n = 11) was several times stronger than a fibrin glue control tested under the same conditions. The results suggest a possible use of this biomimetic strategy for repair of soft tissues.
1. Introduction
The human body is able to repair soft and hard tissue wounds in many cases when the injured tissue edges are securely held in close proximity to each other. Often this is achieved by mechanical fastening of tissues by methods such as sutures or staples [1]. In certain surgical situations, suturing and stapling are not ideal approaches for tissue approximation, for example where suturing may cause bleeding or damage to the surrounding tissue or where a tight seal is required to prevent the leakage of body fluids. These shortcomings as well as the desire for convenient and rapid tissue adhesion have led to the development and use of liquid tissue adhesives as alternatives to sutures and staples. Three families of tissue adhesives are most frequently used [2]: cyanoacrylates, gelatin resorcinol and fibrin-based glues. Gelatin resorcinol and cyanoacrylates in particular have favorable mechanical properties, but both are known to have cytotoxic effects due to the breakdown of cyanoacrylates into formaldehyde and the use of formaldehyde as a curing agent in the gelatin-resorcinol system [3, 4]. Several labs have reported on efforts to lower cytotoxicity of these systems [5–7]. The fibrin glues, on the other hand, are generally considered highly biocompatible and adhesive to tissue surfaces but have very low cohesive strength [8, 9]. The use of blood components in the production of fibrin glues remains a concern as it introduces a risk of disease transmission. While autologous fibrin tissue adhesives can be produced, this approach is impractical or prohibitively expensive in many situations.
With these limitations in mind, our group is currently undertaking the development of new biomaterials for use in tissue adhesion, drug delivery and tissue reconstruction. Like others [10], we are inspired by biological adhesives, in particular the adhesive proteins secreted by aquatic organisms for secure attachment to wet substrates [11]. Freshwater and marine mussels, in particular, have long been recognized for their wet adhesive abilities. The amino acid L-3,4-dihydroxylphenylalanine (DOPA) has been found in high concentrations in glue proteins secreted by mussels [12, 13]. The liquid protein glues harden rapidly after secretion due to crosslinking of reactive species formed as a result of oxidation of DOPA residues. The ability of the mussel protein glues to harden rapidly from a liquid precursor and achieve adhesion to both organic and inorganic surfaces in the presence of water [14] has captured the interest of several groups as a useful model system from which to undertake the design of new adhesive polymers [11, 13, 15–17].
Unfortunately, relatively few tissue adhesion studies have been performed using mussel adhesive proteins (MAPs) or polymer mimics of these proteins. In one recent study, a highly concentrated paste of purified MAPs was applied to pig skin tissue surfaces and the bond strength determined mechanically [18]. The results of this study suggest potential use as an adhesive, although the curing time of the MAP glue was too slow for possible clinical use and under most conditions the adhesive strength of MAP-bonded tissue was similar to or lower than fibrin glue. Chemically synthesized polypeptide analogues of MAPs have also been investigated in tissue adhesion experiments, and in one notable case [19], in vitro tissue adhesion to pig skin was clearly enhanced in polypeptides containing tyrosine, which was converted to DOPA and presumably further oxidized during curing of the adhesive. An in vivo pig skin tissue adhesion study was also performed using these polypeptides; however, no mechanical performance data were reported [20].
Our group has focused on the modification of biocompatible polymers such as poly(ethylene glycol) (PEG) with DOPA in an effort to impart adhesive qualities to the polymers [21–24]. Although PEG itself is not adhesive, it represents a good building block for a synthetic tissue adhesive because of its high water solubility, low immunogenicity and toxicity, and availability with endgroup chemistries suitable for facile modification with amino acids and peptides [25]. Previously, we synthesized several linear and branched PEG molecules with endgroups modified by DOPA residues and characterized their oxidation-induced crosslinking to form robust hydrogels [22]. Under optimal conditions, aqueous solutions of these polymers formed rigid hydrogels within 30 s of mixing with an appropriate chemical (periodate) or enzymatic (tyrosinase or peroxidase enzyme) oxidizing reagent [22]. Although rapid solidification is desired for intended use of the material as a surgical adhesive, this property presents new challenges for storage, mixing and handling of the precursor solution.
In this paper we report the use of thermally triggered release of an oxidizing reagent from liposomes to rapidly form adhesive hydrogels from a soluble DOPA-modified PEG polymer. The approach exploits the sequestering ability of liposomes to segregate reactants (NaIO4 and DOPA-modified PEG) during storage and manipulation of the polymer solution at ambient temperature, and allows for the release of entrapped NaIO4 at body temperature to afford rapid hydrogel formation. UV/vis spectroscopy was used to detect the thermal release of NaIO4, dynamic rheology performed to measure gelation and elastic properties of the crosslinked PEG-DOPA hydrogel, and lap shear adhesion experiments were carried out to demonstrate the suitability of the material to function as a tissue adhesive. PEG-DOPA gels achieved shear bond strengths approximately five times greater than commercial fibrin surgical adhesive, suggesting potential use of these polymers as medical adhesives.
2. Materials and methods
2.1. Materials
4-arm DOPA-modified PEG (PEG-DOPA4, figure 1) was synthesized as previously described [22], starting from a branched PEG polymer (10 kDa, SunBioUSA, Orinda, CA). 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC, >99%) and 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC, >99%) were obtained from Avanti Polar Lipids. Phosphate buffered saline (PBS), chloroform, NaIO4, CaCl2, L-DOPA, sodium citrate, Triton 100X, PEG (8 kDa) and NaI were obtained from Sigma. Ethanol was obtained from Pharmco. Petroleum jelly was obtained from VWR. Fresh full thickness porcine skin was obtained from T&J Meat Packing, Chicago Heights, IL, USA.
Figure 1.
Chemical structure of the PEG-DOPA4 polymer used in this study. The average value of n is 55.
2.2. Liposome preparation
Thermally responsive NaIO4-filled liposomes were prepared by a slight modification of a previously described method [26]. Briefly, a dry thin film of phospholipids (90 mol% DPPC and 10 mol% DMPC) was hydrated with aqueous NaIO4 in PBS pH 6 (42 mM NaIO4; 357m osm) in a round bottom flask for 15 min at 50 °C. The resulting vesicle suspension was sonicated at 50 °C until optically clear using a probe-type ultrasonicator to form small unilamellar vesicles. The vesicle suspension was centrifuged to remove metal particles released from the probe tip, and 100% ethanol was added with vortexing to achieve a final ethanol concentration of 4M. After incubation at room temperature for 15 min, the suspension was heated to 50 °C and bubbled with N2 gas for 30 min to remove EtOH, yielding periodate containing vesicles (PCVs). All subsequent manipulations of liposomes were performed at 20 °C to prevent the thermal release of entrapped NaIO4. Unentrapped NaIO4 was removed from the liposome suspension by washing with iso-osmotic PBS (pH 6), centrifuging at 20 000 × g, and decanting the supernatant. This process was repeated a minimum of five times or until NaIO4 could not be detected in the supernatant, using a standard spectrophotometric method [27]. CaCl2-entrapped liposomes (CCVs) used for control experiments were prepared by the reported method [26].
All liposomes were stored at 20 °C until use. NaIO4 concentration was determined spectrophotometrically as follows [27]. Standard solutions containing 0–72 mM NaIO4 were prepared with surfactant and citrate buffer solutions (2 mL 0.1M citrate, pH 6, 2 mL 0.01% Triton) and deionized water added to 9 mL total volume. Sodium iodide (1 mL) was added with vortexing and then incubated for 3 min. The absorbance of the solution was then measured at 352 nm (Hitachi U-2010 UV/VIS spectrophotometer) and a linear standard curve was produced. The concentration of periodate in the liposome suspension was measured by substituting 15 μL PCVs in place of aqueous NaIO4 in the method above. Calculations based on the calibration curve yielded an average concentration of 33.4 mM NaIO4 in the liposome suspension.
2.3. Thermal release of NaIO4
Temperature-dependent release of NaIO4 was observed spectrophotometrically by heating PCVs suspended in an isoosmotic solution containing either L-DOPA or PEG-DOPA4. The release of periodate from the liposomes was detected by rapid oxidation of both L-DOPA and PEG-DOPA4, yielding a color change that is readily detectable by UV/vis spectroscopy [22]. The release of periodate in the presence of L-DOPA was first determined by diluting 15 μL of NaIO4 liposomes into 5 mL osmotically balanced PBS (pH 6) containing 50 μM L-DOPA. A portion of this solution was placed in a cuvette, and a water bath and heat exchanger were used to increase the temperature of the suspension from 25 °C to 50 °C at a rate of 1 °C min− 1. The release of periodate was followed by measuring the absorbance of the liposome suspension at 320 nm corresponding to the oxidation of L-DOPA [22]. The thermal release of periodate and subsequent oxidation of PEG-DOPA4 was detected using the same method by replacing L-DOPA with PEG-DOPA4 (2.75 mg mL− 1).
2.4. Thermally triggered gelation of the PEG-DOPA4 hydrogel
Hydrogels were obtained by heating an equivolume mixture of PEG-DOPA4 (300 mg mL− 1) and PCVs to 37 °C. Rheological measurements of the gelation process were made using a Paar Physica modular compact rheometer 300. Measurements of storage modulus, G′, and loss modulus, G″, versus time and temperature were made using a 50 mm diameter stainless steel cone and plate geometry with a cone angle of 1° respectively. 600 μL of solution was placed on the thermostated plate prior to repositioning of the cone. The hydrogel was maintained in the hydrated state during measurement by placing the rheometer’s cover over the sample and filling an area above the cone with water as intended by design. Approximately, 2 min elapsed between the application of the sample and beginning of data collection. Measurements were taken at 0.1 Hz and 1.0% strain over a 70 min period. During the first 10 min, the temperature was maintained at 25 °C. The temperature was then ramped to 37 °C over the next 10 min and then maintained at 37 °C for the remaining 50 min. At the completion of the gelation experiment, both strain (0.04–1.0% strain at 0.1 Hz) and frequency sweep (1.68–10.0 Hz at 1.0% strain) experiments were performed.
2.5. Shear stress tensile measurements of the PEG-DOPA4 hydrogel on porcine tissue
Lap-shear tensile stress measurements were performed on porcine tissue following the procedures described in ASTM standard F 2255-03 [28]. Slight modifications to the protocol were used when appropriate. Aluminum test fixtures of dimensions 4 cm long, 2.5 cm wide and 2 mm thick were used. Fresh porcine skin was obtained from the slaughterhouse and then frozen for later use. A small piece of thawed skin was cut into rectangular sections slightly larger than 2 cm × 2.5 cm and then placed onto the cryotome stage and allowed to refreeze to − 25 °C. 120 μm thick slices were then cut using the microtome and placed in PBS for immediate use. Porcine tissue slices were adhered to the aluminum fixtures with gel-type cyanoacrylate glue and PBS-soaked gauze placed over the skin to maintain the hydration of the tissue. A mark was placed 1 cm from the edge of the substrate and petroleum jelly spread on the tissue beyond this line to limit the area of adhesive overlap between the two tissue surfaces.
For each tissue adhesion experiment, PEG-DOPA4 was first dissolved in PBS to a concentration of 300 mg mL− 1 by vortexing for 2 min. PEG-DOPA4 adhesive precursor fluid was prepared by mixing equal parts of the PEG-DOPA4 solution with PCVs to a final PEG-DOPA4 concentration of 150 mg mL− 1. 100 μL of adhesive precursor fluid was applied at room temperature to one surface of the aluminum-backed tissue substrate. Two copper wire spacers (250 μm) were placed one at each end of the overlap area to create a space between the two tissue surfaces as described by Yu and Deming [29]. A second aluminum-backed tissue substrate was then placed on top of the first while being careful to ensure that the proper overlap area was maintained. Saturated gauze and a 200 g weight were then placed on top of the test pieces and placed into a container with PBS such that the ends of the gauze strips were immersed into the solution to maintain hydration throughout the duration of the cure time. The entire container was covered to avoid water evaporation and placed into a 37 °C oven for 24 h. The elapsed time between applying adhesive to the first surface and placing the test pieces into the oven was approximately 10 min. For comparison purposes, tissue surfaces adhered together using a commercial fibrin surgical adhesive (Baxter Tisseel) were prepared according to the manufacturer’s directions with a slight modification of the above protocol to account for the absence of liposomes. The tissue surfaces were immediately approximated after the application of adhesive to the tissue surfaces.
Lap-shear tensile strength was measured using an Instron machine. Test pieces were removed from the container after curing for 24 h and allowed to equilibrate to room temperature for 15 min. The bonded fixtures were loaded until failure at a cross-head speed of 5 mm min− 1. A minimum of ten samples per group were tested using this approach.
3. Results
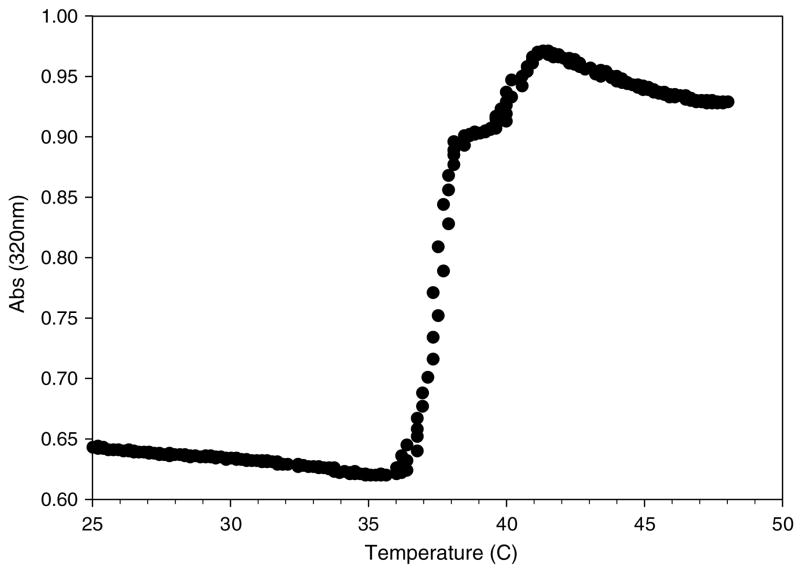
Processing of PCVs yielded an average of 33.3 ± 4.2 mM NaIO4 from a starting concentration of 42 mM, equating to an entrapment efficiency of 78%, which is similar to our previous results for entrapment of aqueous salt solutions [30]. The PCVs were stable for at least 24 h of storage at room temperature without appreciable release of entrapped NaIO4. Liposome suspensions were tested for their ability to release NaIO4 upon heating by colorimetrically (320 nm) monitoring oxidation of either L-DOPA or PEG-DOPA4. Mixtures of PCVs and L-DOPA heated from 25 to 45 °C exhibited a marked increase in absorbance at roughly 37 °C (figure 2), indicating a rapid escape of NaIO4 from the PCVs and oxidation of L-DOPA. Similar results were obtained for mixtures of PCVs and PEG-DOPA4 (figure 3), indicating that both release of NaIO4 from the liposome interior and subsequent oxidation of polymer bound DOPA were unaffected by the presence of PEG. Oxidation of the PEG-DOPA4 solution in this case did not give rise to gelation due to the low polymer concentration used in the UV/vis experiments.
Figure 2.
Thermally triggered release of NaIO4 from PCVs as detected by the oxidation of L-DOPA.
Figure 3.
Thermally triggered oxidation of dilute PEG-DOPA4 by release of NaIO4 from PCVs.
At higher polymer concentration, hydrogel formation was observed upon heating of the PEG-DOPA4/PCV adhesive precursor fluid. This was studied by performing cone and plate rheological measurements in which a solution containing one part PEG-DOPA4 in PBS and one part PCVs was placed in the rheometer at ambient temperature. After an initial equilibration period at 25 °C, the temperature was increased at a constant rate to 37 °C and was then held at this temperature for an extended time. During the ambient temperature equilibration and the initial portion of the heating curve, a slight change in storage modulus (G′) occurred (figure 4), indicating that the material remained fluid during this time. Upon reaching approximately 37 °C, G′ increased to over 1 kPa in less than 5 min, indicating that rapid gelation occurred. Over the final 45 min of the experiment, G′ continued to increase, eventually rising to greater than 6 kPa in the first 50 min after reaching 37 °C. Frequency and strain sweeps were both performed at the completion of the gelation experiment. These experiments revealed <6% change in G′ across the range of frequency and strain tested (data not shown), indicating that an elastic gel had formed.
Figure 4.
Storage modulus and temperature versus time for thermally triggered gelation of the PEG-DOPA4/PCV mixture. (This figure is in colour only in the electronic version)
The tissue adhesive properties of PEG-DOPA4/PCV gels were studied on porcine dermal skin surfaces. After curing the adhesive for 24 h at 37 °C, the PEG-DOPA4/PCV gels exhibited a mean lap shear strength of 35 kPa (SD = 12.5 kPa, n = 11) (figure 5). Inspection of the tissue surfaces after failure indicated a PEG-DOPA4/NaIO4 gel film on both tissue surfaces, which is indicative of cohesive failure as opposed to adhesive failure at the tissue-adhesive surface. Control experiments in which calcium-loaded liposomes [30] were used in place of PCVs, or in which PEG-DOPA4 was replaced with unmodified PEG, showed no signs of adhesion as all specimens failed before or during attempts to load into the mechanical test machine. Fibrin adhesive yielded a mean lap shear strength of 6.9 kPa (SD = 3.2 kPa, n = 11) (figure 5), which is within the range of strengths reported by our group and others for fibrin adhesives [2, 10, 31]. Failure of the fibrin glue bound samples was noted to be of the cohesive type.
Figure 5.
Lap shear strength of porcine dermal tissue surfaces adhered for 24 h at 37 °C with mixtures of PEG-DOPA4/PCVs, PEG-DOPA4/PCVs, PEG/PCVs and commercial fibrin adhesive. The sample size for each group was n = 11.
4. Discussion
The barrier properties of liposome membranes are temperature dependent, having low permeability to ions and small molecules at most temperatures but high permeability to these species at temperatures near the lipid chain melting transition [32]. Thus at most temperatures liposomes function as effective sequestering structures, giving rise to their well-known ability to entrap biologically active molecules and therapeutic agents [33]. The concept of the thermal release of reagents for medical applications was introduced many years ago by Yatvin and coworkers, who sought to exploit intravenously administered liposomes for delivery of therapeutics to tissues under the influence of mild external heating [34]. More recently, several studies in our lab demonstrated the use of stimuli responsive liposomes to trigger the rapid formation of biomaterials by warming from ambient to body temperature [35]. By using a combination of DPPC (90%) and DMPC (10%), a phase transition temperature of approximately 37 °C was achieved such that entrapped Ca2+ could be released through temperature control to activate calcium-dependent reactions leading to polymer hydrogel, mineral and mineral/polymer composite formation [30, 36–40]. In the experiments described here, NaIO4 instead of calcium was entrapped within liposomes and used as an oxidation reagent to polymerize the PEG-DOPA4 solution into a rigid hydrogel suitable for use as a tissue adhesive.
UV/vis experiments were designed to detect the temperature at which entrapped NaIO4 was released during heating, relying upon the redox reaction between the periodate and the catechol side chain of DOPA to form colored products absorbing in the visible range. The 320 nm wavelength used in this experiment was selected based on the results of prior UV/vis studies involving oxidation of DOPA-modified PEG polymers by NaIO4 and other reagents [22]. The NaIO4 release data shown in figure 2 indicate that the PCVs behave as anticipated, exhibiting a sharp increase in absorbance between 36 and 38 °C, reflecting the release of sequestered periodate at the lipid chain melting temperature. Similar results were obtained when DOPA was present in the form of a dilute PEG-DOPA4 solution, indicating that the presence of a PEG polymer does not affect either the temperature of liposome release or the subsequent oxidation reaction. In both cases a slight further increase in absorbance was observed at approximately 41°C, which is likely attributed to NaIO4 release from a small population of lipid vesicles enriched in DPPC [30].
When PCVs were mixed with PEG-DOPA4 at high concentration, heating of the suspension gave rise to rapid solidification into a hydrogel. This process was captured by oscillatory rheology experiments, as is shown in figure 4. A good correlation between the spectroscopic release data (figures 2 and 3) and the onset of gel formation was found, confirming that the gel formation was directly correlated to the thermal release of NaIO4 from lipid vesicles. The crosslinking reactions were studied in detail in a previous publication [22] and involve the formation of quinone species which react via aryl coupling reactions or by a quinone tanning pathway. In this way polymer chains become crosslinked together, with rapid gelation aided by the branched architecture of the PEG-DOPA4 molecule. The maximum storage modulus achieved was lower than reported previously for NaIO4 crosslinked PEG-DOPA4 [22], which may be explained by the presence of liposomes entrapped within the hydrogel matrix.
To measure the tissue adhesive properties of the PEG-DOPA4 hydrogel, we chose a protocol based on an ASTM lap shear method so that the results could be readily compared to the existing and future studies of tissue adhesives. The use of appropriate controls and commercial fibrin adhesive for comparison allows us to make some judgments about the tissue adhesive potential of PEG-DOPA4 using this method. The results shown in figure 5 demonstrate that oxidation by NaIO4 as well as the presence of DOPA on the PEG polymer are necessary requirements for developing a strong adhesive bond. The PEG-DOPA4/PCV hydrogel yielded impressive shear strength results compared to fibrin adhesive, with mean shear strengths of 35 kPa and 6.9 kPa, respectively.
The strength of the existing medical adhesives such as cyanoacrylate, fibrin and gelatin resorcinol is relatively well known and has been reviewed [2, 10]. The loading environment existing across an adhesively bonded soft tissue joint depends on the tissue type and anatomical location, and protocols used for testing tissue adhesion vary widely among research groups. Although it is difficult to directly compare literature values for tissue adhesives due to the variety of methods and tissues used, Chivers and Wolowacz in their review of tissue adhesives [2] stated as a general rule that typical tissue adhesive strengths are on the order of 1000 kPa for cyanoacrylates, 100 kPa for gelatin resorcinol and approximately 10 kPa or less for fibrin glues.
Our experimentally determined value for fibrin adhesive strength to porcine skin is therefore consistent with the literature values described above, supporting our conclusion that the PEG-DOPA4 hydrogel mechanically outperforms fibrin. We speculate that the improved mechanical properties observed with the PEG-DOPA4 hydrogel reflect significant differences in the composition and solidification mechanism compared to the fibrin adhesive. Fibrin adhesive solidifies through noncovalent self-assembly of fibrin molecules into an insoluble fibrillar network [41], which is subsequently rigidified through crosslinking of fibrin α and γ chains by activated factor XIII (FXIIIa) enzyme [42]. The solidification of the PEG-DOPA4 hydrogel, on the other hand, relies on covalent bond formation between soluble polymers to form a crosslinked polymer network with a small mesh size [22]. It is noted that the storage modulus of the PEG-DOPA4 hydrogel (~6000 Pa after 50 min) is considerably greater than that of a fibrin clot (~50–500 Pa after 2 h [43]), which likely impacts the bulk mechanical (cohesive) performance.
As for adhesive interactions that contribute to shear bond strength, both fibrin and PEG-DOPA4 hydrogel are capable of forming covalent bonds at the interface with tissue, albeit via very different mechanisms. In the case of fibrin adhesive, covalent bonds to tissue can arise through FXIIIa catalyzed isopeptide bond formation between fibrin and extracellular matrix proteins [44]. On the other hand, oxidation of PEG-DOPA4 gives rise to reactive quinone species that are reactive toward amines and other residues present in proteins and can yield covalent bonds to tissue surfaces [14].
Although we consider the current results highly favorable for the use of PEG-DOPA4 as a surgical adhesive, additional refinement of the approach described in this study could give rise to further improvements in adhesive strength. In this respect it is interesting to note that the PEG-DOPA4 polymer used in this study has a DOPA content of only 6% by weight, whereas mussel adhesive proteins have DOPA contents as high as 30% [45]. Thus, in the future it may be possible to improve the adhesive performance by increasing the DOPA concentration in these polymers. The observed shear strength is also interesting in view of our estimate that over 30% of the volume of the adhesive hydrogel is occupied by liposomes, which presumably contribute little to force transmission within the gel and may even be considered weakening elements. If it is determined that liposomes weaken the adhesive, an alternative approach involving a double barrel syringe device could be implemented to facilitate the mixing of polymer and NaIO4 reagents just prior to use.
The use of NaIO4 to induce the gelation of PEG-DOPA4 hydrogels is a potential source of concern for eventual clinical use due to its oxidizing properties [46, 47]. Although the local and systemic biological effects of periodate can only be determined through future in vivo studies, we can make a few comments based on what we know about the redox reactions giving rise to gel formation [22]. In the presence of a suitable species, consumption of electrons by an oxidation reaction will be accompanied by the reduction of periodate ion to iodate ion, which in turn can be further reduced to iodide ion as follows [48]:
| (1) |
| (2) |
In the crosslinking system described here, the catechol side chain of DOPA is ultimately oxidized to quinone and then further reacts to give rise to crosslinking of PEG-DOPA4. It is important to note that DOPA exists in greater than threefold excess over periodate in the reactions described here. Therefore, it is possible that most or all periodate is reduced to iodate, a less toxic ion [49], and some iodate may even be further reduced to iodide. Nevertheless, more detailed studies on the fate and biological effects of the oxidizing reagent are needed.
5. Conclusions
In this paper we describe a novel tissue adhesive that with further study could prove to be valuable as an alternative to current solutions for tissue adhesion. By utilizing liposomes to compartmentalize an oxidizing reagent in the same suspension with PEG-DOPA4, a liquid adhesive precursor was created that can be stored at room temperature and then activated at the wound site to form a rapid gel simply through warming to body temperature. The PEG-DOPA4 gel produced lap shear strength to porcine dermal tissue, five times higher than that of fibrin adhesive. With further research into the optimization of the polymer composition and structure, the oxidizing reagent and method of delivery, it may be possible to create a mussel adhesive mimetic polymer tissue adhesive that outperforms the existing surgical adhesives.
Acknowledgments
This work was supported by NIH grants R01 DE 13030, R37 DE 14193 and R01 EB 003806. The authors would like to thank Dr Guillermo Ameer for assistance with mechanical testing experiments, Dr Wesley Burghardt for assistance with rheological measurements and Caren Nguyen for preliminary experiments.
References
- 1.Chu CC, von Fraunhofer JA, Greisler HP. Wound Closure Biomaterials and Devices. Boca Raton, FL: CRC Press; 1997. [Google Scholar]
- 2.Chivers RA, Wolowacz RG. The strength of adhesive-bonded tissue joints. Int J Adhes Adhes. 1997;17:127–32. [Google Scholar]
- 3.Tseng YC, Tabata Y, Hyon SH, Ikada Y. In vitro toxicity test of 2-cyanoacrylate polymers by cell culture method. J Biomed Mater Res. 1990;24:1355–67. doi: 10.1002/jbm.820241007. [DOI] [PubMed] [Google Scholar]
- 4.Papatheofanis FJ. Cytotoxicity of alkyl-2-cyanoacrylate adhesives. J Biomed Mater Res. 1989;23:661–8. doi: 10.1002/jbm.820230609. [DOI] [PubMed] [Google Scholar]
- 5.Levrier O, Mekkaoui C, Rolland PH, Murphy K, Cabrol P, Moulin G, Bartoli JM, Raybaud C. Efficacy and low vascular toxicity of embolization with radical versus anionic polymerization of n-butyl-2-cyanoacrylate (NBCA). An experimental study in the swine. J Neuroradiol. 2003;30:95–102. [PubMed] [Google Scholar]
- 6.Kuijpers AJ, Engbers GHM, Feijen J, De Smedt SC, Meyvis TKL, Demeester J, Krijgsveld J, Zaat SAJ, Dankert J. Characterization of the network structure of carbodiimide crosslinked gelatin gels. Macromolecules. 1999;32:3325–33. [Google Scholar]
- 7.Nakayama Y, Matsuda T. Photocurable surgical tissue adhesive glues composed of photoreactive gelatin and poly(ethylene glycol) diacrylate. J Biomed Mater Res. 1999;48:511–21. doi: 10.1002/(sici)1097-4636(1999)48:4<511::aid-jbm17>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 8.Siedentop KH, Harris DM, Sanchez B. Autologous fibrin tissue adhesive: factors influencing bonding power. Laryngoscope. 1988;98:731–3. doi: 10.1288/00005537-198807000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Sierra DH, Feldman DS, Saltz R, Huang S. A method to determine shear adhesive strength of fibrin sealants. J Appl Biomater. 1992;3:147–51. doi: 10.1002/jab.770030210. [DOI] [PubMed] [Google Scholar]
- 10.Graham LD. Biological adhesives from nature. In: Wnek GE, Bowlin GL, editors. Encyclopedia of Biomaterials and Biomedical Engineering. Boca Raton, FL: Taylor and Francis; 2005. [Google Scholar]
- 11.Lee B, Dalsin JL, Messersmith PB. Biomimetic adhesive polymers based on mussel adhesive proteins. In: Smith AM, Callow JA, editors. Biological Adhesives. Berlin: Springer; 2006. pp. 257–78. [Google Scholar]
- 12.Waite JH. Evidence for a repeating 3,4-dihydroxyphenylalanine- and hydroxyproline-containing decapeptide in the adhesive protein of the mussel. Mytilus edulis J Biol Chem. 1983;258:2911–5. [PubMed] [Google Scholar]
- 13.Waite JH. Reverse engineering of bioadhesion in marine mussels. Ann New York Acad Sci. 1999;875:301–9. doi: 10.1111/j.1749-6632.1999.tb08513.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee H, Scherer NF, Messersmith PB. Single-molecule mechanics of mussel adhesion. Proc Natl Acad Sci. 2006;103:12999–3003. doi: 10.1073/pnas.0605552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deming TJ, Yu M, Hwang J. Mechanical studies of adhesion and crosslinking in marine adhesive protein analogs. Polym Mater: Sci Eng. 1999;80:471–2. [Google Scholar]
- 16.Wang J, Liu C, Lu X, Yin M. Co-polypeptides of 3,4-dihydroxyphenylalanine and l-lysine to mimic marine adhesive protein. Biomaterials. 2007;28:3456. doi: 10.1016/j.biomaterials.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Westwood G, Horton TN, Wilker JJ. Simplified polymer mimics of cross-linking adhesive proteins. Macromolecules. 2007;40:3960–4. [Google Scholar]
- 18.Ninan L, Monahan J, Stroshine RL, Wilker JJ, Shi R. Adhesive strength of marine mussel extracts on porcine skin. Biomaterials. 2003;24:4091–9. doi: 10.1016/s0142-9612(03)00257-6. [DOI] [PubMed] [Google Scholar]
- 19.Tatehata H, Mochizuki A, Kawashima T, Yamashita S, Yamamoto H. Model polypeptide of mussel adhesive protein: I. Synthesis and adhesive studies of sequential polypeptides (X-Tyr-Lys)n and (Y-Lys)n. J Appl Polym Sci. 2000;76:929–37. [Google Scholar]
- 20.Tatehata H, Mochizuki A, Ohkawa K, Yamada M, Yamamoto H. Tissue adhesive using synthetic model adhesive proteins inspired by the marine mussel. J Adhes Sci Technol. 2001;15:1003–13. [Google Scholar]
- 21.Dalsin JL, Hu B-H, Lee BP, Messersmith PB. Mussel adhesive protein mimetic polymers for the preparation of nonfouling surfaces. J Am Chem Soc. 2003;125:4253–8. doi: 10.1021/ja0284963. [DOI] [PubMed] [Google Scholar]
- 22.Lee BP, Dalsin JL, Messersmith PB. Synthesis and gelation of DOPA-modified poly(ethylene glycol) hydrogels. Biomacromolecules. 2002;3:1038–47. doi: 10.1021/bm025546n. [DOI] [PubMed] [Google Scholar]
- 23.Lee BP, Huang K, Nunalee FN, Shull KR, Messersmith PB. Synthesis of 3,4-dihydroxyphenylalanine (DOPA) containing monomers and their copolymerization with PEG-diacrylate to from hydrogels. J Biomater Sci, Polym Ed. 2004;15:449–64. doi: 10.1163/156856204323005307. [DOI] [PubMed] [Google Scholar]
- 24.Lee B, Chao C-Y, Motan E, Nunalee FN, Shull K, Messersmith P. Rapid gel formation and adhesion in photocurable and biodegradable block copolymers with high DOPA content. Macromolecules. 2006;39:1740–8. [Google Scholar]
- 25.Harris JM, Zalipsky S. Poly(ethylene glycol): Chemistry and Biological Applications. Vol. 12. Washington, DC: American Chemical Society; 1997. p. 489. [Google Scholar]
- 26.Messersmith PB, Vallabhaneni S, Nguyen V. Preparation of calcium-loaded liposomes and their use in calcium phosphate formation. Chem Mater. 1998;10:109–16. [Google Scholar]
- 27.Afkhami A, Madrakian T, Zarei AR. Spectrophotometric determination of periodate, iodate and bromate mixtures based on their reaction with iodide. Anal Sci. 2001;17:1199–202. doi: 10.2116/analsci.17.1199. [DOI] [PubMed] [Google Scholar]
- 28.ASTM 2003 F 2255-03 Standard Test Method for Strength Properties of Tissue Adhesives in Lap-Shear by Tension Loading
- 29.Yu M, Deming TJ. Synthetic polypeptide mimics of marine adhesives. Macromolecules. 1998;31:4739–45. doi: 10.1021/ma980268z. [DOI] [PubMed] [Google Scholar]
- 30.Messersmith PB, Starke S. Thermally triggered calcium phosphate formation from calcium-loaded liposomes. Chem Mater. 1998;10:117–24. [Google Scholar]
- 31.Hu B-H, Messersmith PB. Enzymatically cross-linked hydrogels and their adhesive strength to biosurfaces. Orthod Craniofac Res. 2005;8:145–9. doi: 10.1111/j.1601-6343.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- 32.Papahadjopoulos D, Jacobsen K, Nir S, Isac T. Phase transitions in phospholipid vesicles. Fluorescence polarization and permeability measurements concerning the effect of temperature and cholesterol. Biochim Biophys Acta. 1973;311:330–48. doi: 10.1016/0005-2736(73)90314-3. [DOI] [PubMed] [Google Scholar]
- 33.Gregoriadis G. Engineering liposomes for drug delivery: progress and problems. Trends Biotechnol. 1995;13:527–37. doi: 10.1016/S0167-7799(00)89017-4. [DOI] [PubMed] [Google Scholar]
- 34.Yatvin MB, Weinstein JN, Dennis WH, Blumenthal R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science. 1978;202:1290–3. doi: 10.1126/science.364652. [DOI] [PubMed] [Google Scholar]
- 35.Collier JC, Messersmith PB. Phospholipid strategies in biomineralization and biomaterials research. Ann Rev Mater Sci. 2001;31:237–63. [Google Scholar]
- 36.Collier JH, Hu BH, Ruberti JW, Zhang J, Shum P, Thompson DH, Messersmith PB. Thermally and photochemically triggered self-assembly of peptide hydrogels. J Am Chem Soc. 2001;123:9463–4. doi: 10.1021/ja011535a. [DOI] [PubMed] [Google Scholar]
- 37.Sanborn TJ, Messersmith PB, Barron AE. In situ crosslinking of a biomimetic peptide-PEG hydrogel via thermally triggered activation of factor XIII. Biomaterials. 2002;23:2703–10. doi: 10.1016/s0142-9612(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 38.Westhaus E, Messersmith PB. Triggered release of calcium from lipid vesicles: a bioinspired strategy for rapid gelation of polysaccharide and protein hydrogels. Biomaterials. 2001;22:453–62. doi: 10.1016/s0142-9612(00)00200-3. [DOI] [PubMed] [Google Scholar]
- 39.Murphy WL, Messersmith PB. Compartmental control of mineral formation: adaptation of a biomineralization strategy for biomedical use. Polyhedron. 2000;19:357–63. [Google Scholar]
- 40.Pederson A, Ruberti J, Messersmith PB. Thermal assembly of a biomimetic mineral-collagen composite. Biomaterials. 2003;24:4881–90. doi: 10.1016/s0142-9612(03)00369-7. [DOI] [PubMed] [Google Scholar]
- 41.Sierra D, Saltz R. Surgical Adhesives and Sealants: Current Technology and Applications. Lancaster, PA: Technomic; 1996. [Google Scholar]
- 42.Ryan EA, Mockros LF, Stern AM, Lorand L. Influence of a natural and a synthetic inhibitor of factor XIIIa on fibrin clot rheology. Biophys J. 1999;77:2827–36. doi: 10.1016/S0006-3495(99)77114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryan EA, Mockros LF, Weisel JW, Lorand L. Structural origins of fibrin clot rigidity. Biophys J. 1999;77:2813–26. doi: 10.1016/S0006-3495(99)77113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corbett SA, Lee L, Wilson CL, Schwarzbauer JE. Covalent crosslinking of fibronectin to fibrin is required for maximal cell adhesion to a fibronectin-fibrin matrix. J Biol Chem. 1997;272:24999–5005. doi: 10.1074/jbc.272.40.24999. [DOI] [PubMed] [Google Scholar]
- 45.Waite JH, Qin X. Polyphosphoprotein from the adhesive pads of Mytilus edulis. Biochemistry. 2001;40:2887–93. doi: 10.1021/bi002718x. [DOI] [PubMed] [Google Scholar]
- 46.Noda I, Fujieda S, Saito H, Saito T, Otsubo T, Yagita M. Enhancement of cytolytic activity of human peripheral blood lymphocytes by sodium periodate (IO4) possible involvement of protein kinase C. Int J Immunopharmacol. 1998;20:15. doi: 10.1016/s0192-0561(97)00111-2. [DOI] [PubMed] [Google Scholar]
- 47.Novogrodsky A. Induction of lymphocyte cytotoxicity by modification of the effector or target cells with periodate or with neuraminidase and galactose oxidase. J Immunol. 1975;114:1089–93. [PubMed] [Google Scholar]
- 48.Corlett RD, Breck WG, Hay GW. Analysis of periodate oxidation of carbohydrates by polarography. Can J Chem. 1970;48:2474–83. [Google Scholar]
- 49.Webster SH, Rice ME, Highman B, Von Oettingen WF. Toxicology of potassium and sodium iodates; acute toxicity in mice. J Pharmacol Exp Therap. 1957;120:171–8. [PubMed] [Google Scholar]