Abstract
The purpose of this investigation was to examine the clinical and microbiologic effects of apically repositioned flap surgery. Eighteen patients with chronic periodontitis received initial preparation (IP) including scaling and root planing, followed at 3 months by apically repositioned flap surgery at sites with pocket depth > 4 mm. Subjects were monitored clinically and microbiologically at baseline, 3 months after IP, and at 3, 6, 9, and 12 months postsurgery. Clinical assessments of plaque accumulation, gingival redness, suppuration, bleeding on probing, pocket depth, and attachment level were made at six sites per tooth. Subgingival plaque samples were taken from the mesial aspect of each tooth, and the presence and levels of 40 subgingival taxa were determined using checkerboard DNA-DNA hybridization. Significant reductions were seen in mean pocket depth and percentage of sites exhibiting gingival redness and bleeding on probing in both sites that received IP only and in sites receiving IP followed by surgery. Mean attachment level increased significantly for both sets of sites, but the increase was greater at the surgically treated sites. The total DNA probe counts were significantly reduced at sites in both treatment groups. At surgically treated sites, 19 of 40 taxa were significantly reduced posttherapy. At sites receiving IP only, 16 species were significantly reduced over time. While there were some reductions in mean counts after IP in this site group, the major reductions occurred after the surgical phase in these patients, even though these particular sites did not receive surgical therapy. The reduction in pocket depth by surgical means and the associated decrease in reservoirs of periodontal pathogens may be important in achieving sustained periodontal stability.
Levy et al1 examined the effect of apically repositioned flap surgery on the composition of the subgingival microbiota as well as clinical parameters. In that study of 11 subjects, there was a significant decrease in mean pocket depth and the percentage of sites exhibiting gingival redness 3 months after scaling and root planing (SRP) and apically repositioned flap surgery at sites > 4 mm. Further, there was a significant decrease in the percentage of sites with attachment level < 4 mm and a significant increase in the percentage of sites with attachment level between 4 and 6 mm after therapy. There was also a significant decrease in the percentage of sites with periodontal pockets > 6 mm, as well as an increase in the percentage of sites with periodontal pockets < 4 mm after surgery. These clinical changes were accompanied by significant reductions in segments of the microbiota associated with periodontal disease initiation and progression. Some of these pathogenic organisms appeared to occur in microbial complexes.2 In the authors’ previous study,1 the counts of the periodontal disease–associated red complex species Bacteroides forsythus and Porphyromonas gingivalis, as well as the orange complex species Campylobacter gracilis, Campylobacter rectus, and Prevotella nigrescens were significantly reduced.
The purpose of the present investigation was to extend these findings in terms of numbers of subjects examined and postsurgery follow-up. In addition, clinical and microbiologic changes were examined at sites receiving initial preparation (IP) followed by surgery as well as sites in the same subject receiving IP only.
Method and materials
Subject population
Eighteen chronic periodontitis subjects ranging in age from 25 to 71 years were selected from those seen in the Department of Periodontology at the Forsyth Institute, Boston. All subjects had at least 20 teeth and at least eight sites with pockets > 4 mm and eight sites with attachment loss > 3 mm. Exclusion criteria included pregnancy, nursing, previous periodontal therapy or antibiotics in the previous 3 months, any systemic condition that might affect the progression or treatment of periodontitis, and the need for antibiotic prophylaxis prior to monitoring or therapy. The mean baseline clinical parameters for the 18 subjects are presented in Table 1.
Table 1.
Mean ± standard deviation (SD) baseline clinical characteristics of the 18 subjects
| Mean ± SD | Range | |
|---|---|---|
| Age (y) | 50 ± 12 | 25–71 |
| No.of missing teeth | 2.2 ± 1.8 | 0–6 |
| % men | 72 | |
| Mean pocket depth (mm) | 3.3 ± 0.5 | 2.7–4.4 |
| Mean attachment level (mm) | 3.2 ± 0.7 | 2.4–4.6 |
| % of sites with | ||
| Plaque accumulation | 62 ± 26 | 1–92 |
| Gingival redness | 66 ± 23 | 25–100 |
| Bleeding on probing | 27 ± 14 | 8–57 |
| Suppuration | 2 ± 1 | 0–21 |
| No.of surgically treated teeth | 12.2 ± 3.3 | 6–17 |
| No.of current smokers | 7 | |
| No.of past smokers | 5 | |
| No.of never smokers | 6 |
Experimental design
The experimental design, monitoring protocol, treatment procedures, and microbial sample assessments were previously described in detail.1 At baseline, subjects received clinical and microbiologic assessment, followed by complete-mouth SRP and instruction in proper home care techniques. This comprised the IP phase. Patients were clinically and microbiologically monitored again 3 months after completion of this phase and received apically repositioned flap surgery at sites with pocket depth > 4 mm. The surgical procedures were evaluated at 7 and 14 days. Postsurgical clinical and microbiologic monitoring was repeated at 3, 6, 9, and 12 months after completion of the last surgical procedure. In addition, subjects received maintenance supra- and subgingival scaling and reinforcement of home care procedures at these time points.
Clinical meaurements
Plaque accumulation (0, 1), gingival erythema (0, 1), bleeding on probing (0, 1), suppuration (0, 1), probing pocket depth, and probing attachment level were measured at six sites per tooth (mesiobuccal, buccal, distobuccal, distolingual, lingual, and mesiolingual) at all teeth, excluding third molars, at each visit. Each clinical measurement was taken at up to 168 sites in each subject. The pocket depth and attachment level measurements were repeated at each visit, and the means of the pairs of measurements were used to determine changes in attachment level.3
The same clinician performed all measurements at all visits for a given subject. The clinician making the clinical measurements did not perform the therapy for that subject. The examiners were calibrated at 3-month intervals by comparing measurements on selected patients. In addition, duplicate measurements of pocket depth and attachment level made at each visit permitted intraexaminer calibration.
Apically repositioned flap surgery
Prior to the surgical procedure, complete medical and dental histories and vital signs were taken to determine the patient’s health and general well-being. The surgical procedure required that the gingival margin be placed at the crest of the alveolar bone, although in some situations (eg, furcations), this was not possible. In certain cases, root resections, tunnel preparations, or extractions were used to modify intrabony defects and allow for minimum pocket depth. Osteoplasty was performed to recontour bone for better flap adaptation. Ostectomy was performed to remove intraosseous defects, bony ledges, exostoses, and tori. An attempt to recreate positive bony architecture was made, but on occasion flat or negative architecture was left as a compromise. The surgical sites were sutured with 4-0 silk and covered with a periodontal dressing. Subjects were given 0.12% chlorhexidine mouth-rinse to be used twice daily for 14 days. Subjects were seen at 7 and 14 days postoperative, with suture removal generally at 7 days. Acetaminophen or acetaminophen/opiod-containing analgesics were prescribed as necessary. The number of surgically treated teeth in each subject averaged 12.2 (range six to 17). A total of 220 teeth were treated surgically; 49% were multi-rooted.
Microbial assessment
Subgingival plaque samples were taken from the mesiobuccal aspects of all teeth, excluding third molars, at baseline, after IP, and 3, 6, 9, and 12 months after apically repositioned flap surgery. Counts of 40 subgingival species were determined in each plaque sample using a modification4 of the checkerboard DNA-DNA hybridization technique.5 In brief, after the removal of supragingival plaque, subgingival plaque samples were taken with individual sterile Gracey curettes from the mesial aspect of each tooth. The samples were placed in separate Eppendorf tubes containing 0.15 mL TE (10 mM tris-HCl, 1 mM ethylenediaminetetraacetic acid [EDTA], pH 7.6), and 0.15 mL of 0.5 M NaOH was added. The samples were lysed, and the DNA was placed in lanes on a nylon membrane using a Minislot device (Immunetics). After fixation of the DNA to the membrane, the membrane was placed in a Miniblotter 45 (Immunetics) with the lanes of DNA at 90 degrees to the lanes of the device. Digoxigenin-labeled whole genomic DNA probes to 40 subgingival species were hybridized in individual lanes of the Miniblotter. After hybridization, the membranes were washed at high stringency, and the DNA probes were detected using antibody to digoxigenin conjugated with alkaline phosphatase and chemifluorescence detection. Signals were detected using AttoPhos substrate (Amersham Life Science) and a Storm Fluorimager (Molecular Dynamics). Two lanes in each run contained standards at concentrations of 105 and 106 cells of each species. The sensitivity of the assay was adjusted to permit detection of 104 cells of a given species by adjusting the concentration of each DNA probe. Signals were evaluated using the Storm Fluorimager and converted to absolute counts by comparison with the standards on the same membrane. Failure to detect a signal was recorded as zero. A total of 2,749 samples were examined, for a mean of 25.5 samples in each subject per visit.
Statistical analysis
The outcome variables evaluated in this study were the mean levels of clinical and microbiologic parameters. All clinical parameters, including pairs of pocket depth and attachment level measurements, were available from six sites per tooth for up to 28 teeth at each monitoring visit. Values for each parameter were averaged within a subject for the sites that received IP only and separately for the sites that received IP followed by surgery. The values were then averaged separately for the surgically and nonsurgically treated sites across subjects for each visit. The significance of differences over time was assessed using the Quade test individually for the surgically and nonsurgically treated sites.6
In a similar fashion, microbiologic data were analyzed separately for sites receiving IP only and sites that received IP followed by surgery. Pre- and posttherapy microbiologic data were available from 18 subjects. Samples were taken from the mesiobuccal aspect of all teeth present and individually processed for their content of 40 taxa using the checkerboard technique. Mean levels (counts × 105) of each species were computed for each subject in each treatment category at each visit. Significance of differences over time in mean levels of subgingival species was determined separately for each treatment category using the Quade test. All analyses were performed using the subject as the unit of analysis.
Results
Clinical changes resulting from therapy
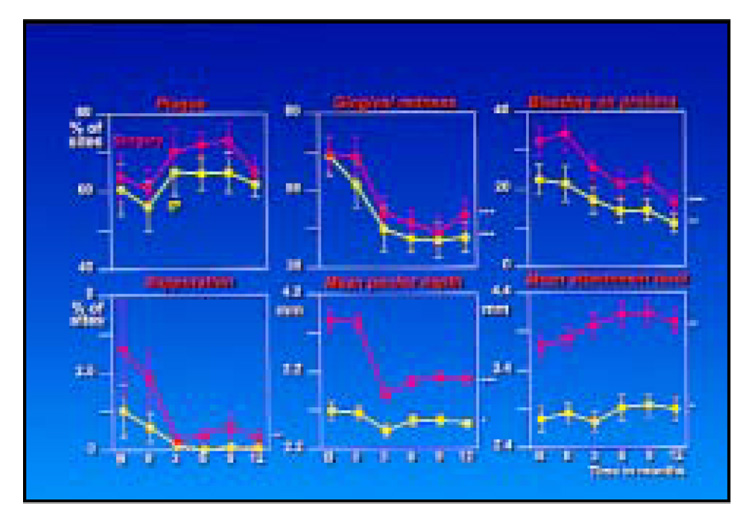
The mean clinical parameters for the sites receiving IP only or IP followed by apically repositioned flap surgery at each time point are presented in Fig 1. Significant reductions were seen for gingival redness, bleeding on probing, and mean pocket depth for both categories of sites and for suppuration at the surgically treated sites. Mean attachment level increased significantly for both sets of sites, but the increase was greater at the surgically treated sites. The greatest decrease in gingival redness, bleeding, suppuration, and probing pocket depth occurred after the surgical phase not only at the surgically treated sites, but also at those sites receiving IP only. Oddly, the percentage of sites exhibiting visible plaque increased after the surgical treatment at sites receiving IP with and without periodontal surgery, although the increase was not statistically significant.
Fig 1.
Mean clinical parameters. The yellow lines represent the sites that received initial preparation only, while the red lines represent sites receiving initial preparation followed by apically repositioned flap surgery (*P < .05, **P < .01, ***P < .001). It should be noted that the mean baseline pocket depth for the sites receiving initial preparation followed by surgery was < 4.0 mm because all sites within a surgical field did not have pocket depth > 4 mm.
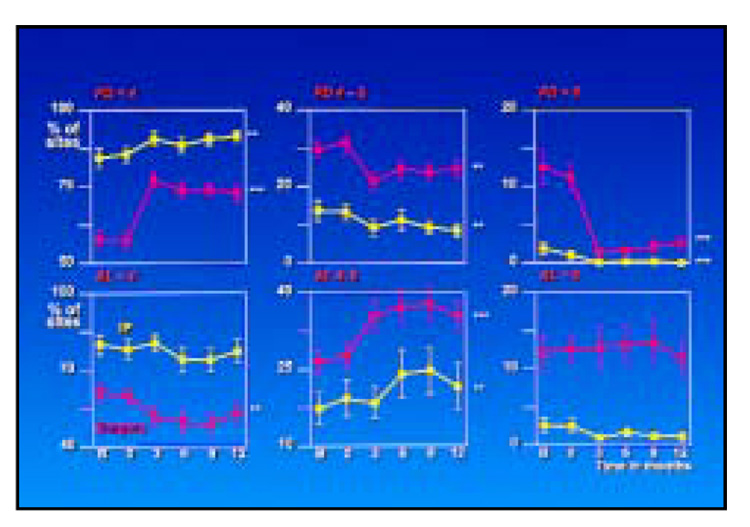
The mean percentage of sites in different pocket depth and attachment level categories at each time point for both site treatment categories is presented in Fig 2. The proportion of shallow pockets (< 4 mm) increased significantly after therapy at both surgically and nonsurgically treated sites. The increase, however, was much more dramatic for the surgically treated areas. The increase in the percentage of shallow sites was accompanied by significant decreases in pockets in the 4 to 6 mm, and in particular in the > 6 mm, category at the surgically treated sites. There was a significant increase in the proportion of sites with attachment level measurements of 4 to 6 mm whether treated by IP alone or followed by surgery. This occurred primarily at the expense of sites with attachment levels < 4 mm for the surgically treated sites.
Fig 2.
Percentage of sites in the three pocket depth and attachment level categories. The yellow lines represent the sites that received initial preparation only, while the red lines represent sites receiving initial preparation followed by apically repositioned flap surgery (**P < .01, ***P < .001).
Microbiologic changes resulting from therapy
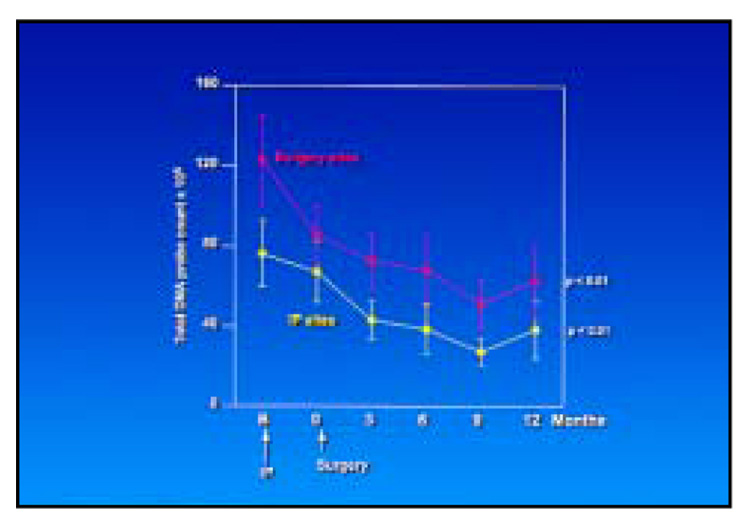
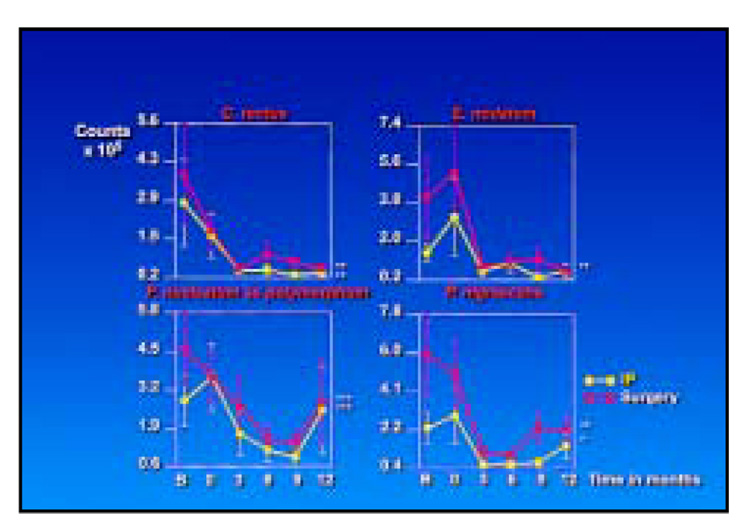
The total DNA probe counts were significantly reduced at sites that received IP only or IP followed by surgery (Fig 3). Not surprisingly, the initial counts were higher at the surgically treated sites, although the slope of the mean reduction at surgically and nonsurgically treated sites was similar. Figure 4 presents the mean counts of the 40 subgingival species evaluated before and after therapy at sites receiving IP followed by apically repositioned flap surgery; 19 of the 40 test taxa were significantly reduced posttherapy. In particular, members of the red complex—B. forsythus, P. gingivalis, and Treponema denticola—and certain species in the orange complex, such as C. rectus and C. gracilis, were reduced after IP and showed further reductions after the surgical phase of therapy. Other members of the orange complex, such as Eubacterium nodatum, Fusobacterium nucleatum ss polymorphum, P. nigrescens, and Streptococcus constellatus, were less affected by IP but showed reductions in mean counts after surgery. The reductions achieved 3 months postsurgery were in general maintained to the 12-month monitoring visit. Figure 5 presents mean counts of the 40 test taxa pre- and posttherapy at sites receiving IP only; 16 species were significantly reduced over time. While there were some reductions in mean counts after IP, the major reductions occurred after the surgical phase, even though these sites did not receive surgical therapy.
Fig 3.
Mean total DNA probe count (× 105, ± standard error of the means [SEM]) for sites receiving initial preparation only (yellow lines) or initial preparation followed by surgery (red lines).
Fig 4.
Mean counts (× 105, ± SEM) of the 40 test species at sites receiving initial preparation followed by surgery. The species are ordered in complexes as described by Socransky et al.2 Nineteen of 40 species were significantly reduced.
Fig 5.
Mean counts (× 105, ± SEM) of the 40 test species at sites receiving initial preparation only. Sixteen of 40 species were significantly reduced.
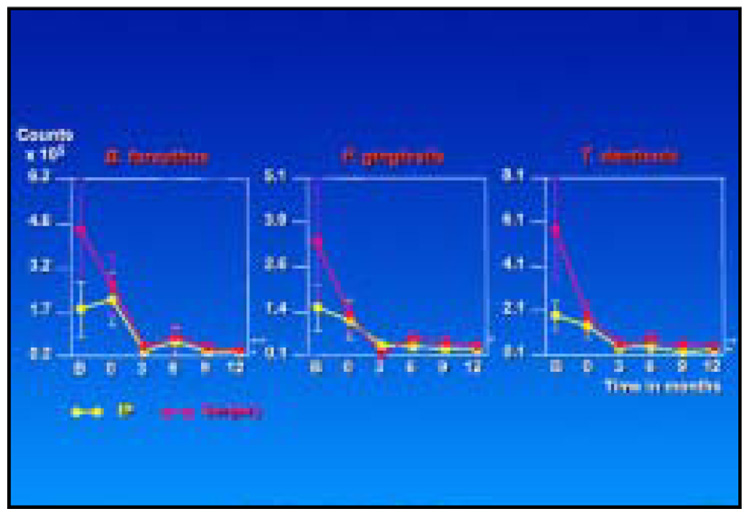
Figure 6 and Figure 7 highlight the changes in mean species counts from pre- to posttherapy at surgically and nonsurgically treated sites for red complex and selected orange complex species, respectively. Not unexpectedly, mean baseline counts for these species were higher at the surgically treated sites. Mean counts of red complex species were reduced markedly by IP at sites that would later receive surgery but less dramatically reduced at sites that received IP only between the baseline visit and 3 months after SRP. After surgery, sites in both treatment categories showed a further reduction in counts of these species that was maintained to 12 months. The mean counts were comparable at all sites from 3 to 12 months posttherapy. Other than for C. rectus, IP had little effect on the mean counts of orange complex species. The major decrease in these species occurred after surgery. Suggestions of an increase of some orange complex species at 12 months were observed.
Fig 6.
Mean counts (× 105, ± SEM) of the red complex species for sampled sites receiving initial preparation only (yellow lines) or initial preparation followed by surgery (red lines) (*P < .05, **P < .01, ***P < .001).
Fig 7.
Mean counts (× 105, ± SEM) of selected orange complex species for sampled sites receiving initial preparation only (yellow lines) or initial preparation followed by surgery (red lines) (*P < .05, **P < .01, ***P < .001).
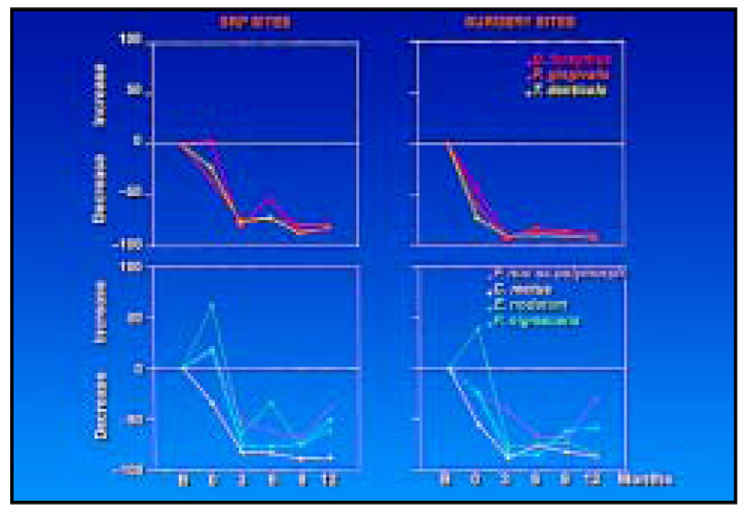
While mean reduction in counts gives an indication that a therapeutic effect is taking place, it does not provide an indication of the magnitude of this effect. Figure 8 presents the proportional change from baseline to each time point for species of the red complex and selected members of the orange complex. The mean proportional reduction of red complex species at 12 months posttherapy was between 90% and 93% and between 78% and 83% for the surgically and nonsurgically treated sites, respectively. The proportional change in orange complex species from baseline to 12 months was less consistent. C. rectus mimicked the pattern of change shown by the red complex species at surgically and nonsurgically treated sites. The other three species either decreased modestly or increased immediately after the IP phase but decreased proportionally after surgery, even in the nonsurgically treated sites. However, the proportional reduction of these three species tended to increase at nonsurgically treated sites, and at 12 months the reduction ranged from 40% to 62%. At the surgically treated sites, the proportional reductions had begun to reverse by 12 months for two of the species, ranging from 31% for F. nucleatum ss polymorphum to 59% for P. nigrescens.
Fig 8.
Proportional change in counts of the red complex species (top) and selected orange complex species (bottom) at sites receiving initial preparation only (left) or initial preparation (0) followed by surgery (right). The horizontal line in each plot represents no change. Points below this line represent a proportional decrease in mean counts, while points above the line represent a proportional increase in mean counts.
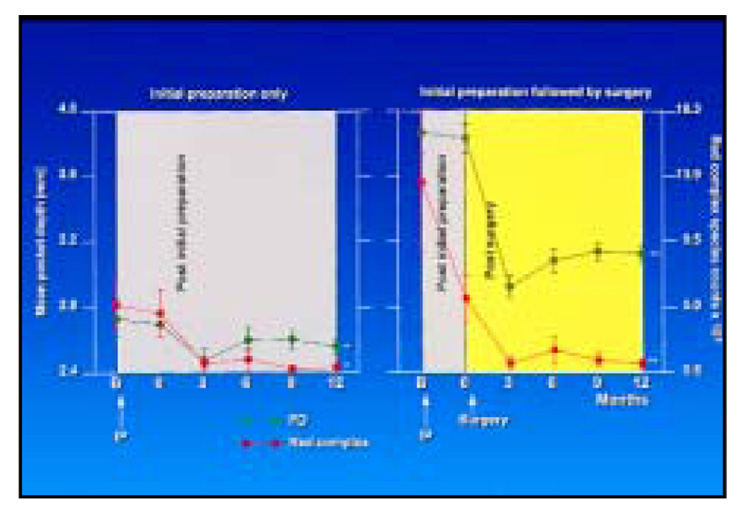
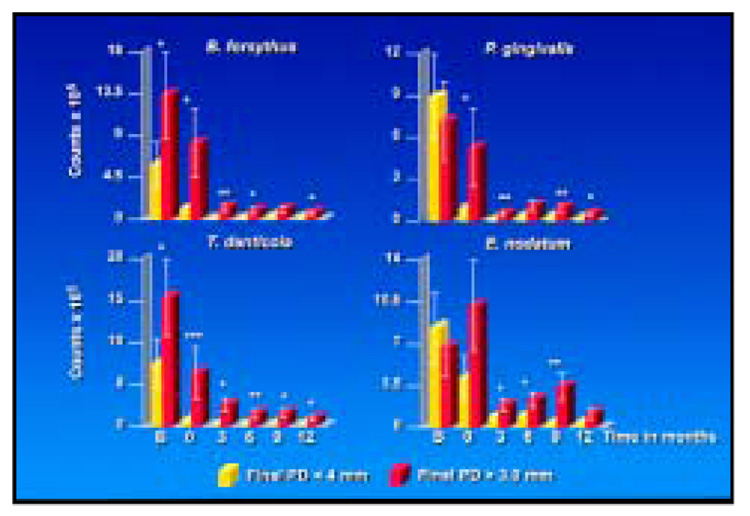
The relationship between mean pocket depth and mean total red complex species counts was examined (Fig 9). At both types of sites, the decrease in mean pocket depth was accompanied by a parallel decrease in mean counts of the red complex species. Further, mean counts of red and orange complex species at periodontal pockets > 4 mm at baseline were reduced markedly at sites with a final pocket depth < 4 mm and somewhat less reduced at sites whose final probing pocket depth at 12 months was ≥ 4 mm (Fig 10).
Fig 9.
Mean pocket depth (± SEM, green lines) and total mean red complex species counts (× 105, ± SEM, red lines) at sampled sites that received initial preparation only (left) or initial preparation (0) followed by surgery (right) (*P < .05, **P < .01). The gray area represents the phase after initial preparation, while the yellow area represents the postsurgery phase. It should be noted that the mean baseline pocket depth for the sites receiving initial preparation followed by surgery was < 4.0 mm because all sites within a surgical field did not have pocket depth > 4 mm.
Fig 10.
Effect of pocket depth reduction (ie, final pocket depth < 4 mm) on mean counts (× 105, ± SEM) of selected species at sites with initial pocket depth > 4 mm. Sites with a final pocket depth < 4 mm are shown in yellow, while those > 4 mm are in red. Significant differences between sites with final pocket depth < 4 mm and ≥ 4 mm at each time point were sought using the Mann-Whitney test (*P < .05, **P < .01).
Discussion
The purpose of the present investigation was to extend the findings of a study that evaluated the effect of apically repositioned flap surgery on clinical parameters and the composition of the subgingival microbiota. That study of 11 chronic periodontitis patients evaluated 3 months posttherapy found that treatment improved clinical parameters and decreased segments of the subgingival microbiota. The current investigation supported and extended these findings and described changes not only at sites receiving apically repositioned flap surgery, but also at sites in the same mouth that received IP only. Clearly, there was a beneficial effect on clinical and microbiologic parameters at the sites that received either form of therapy, and these effects were sustained for 1 year. Mean pocket depth and percentage of sites that exhibited bleeding and gingival redness were reduced at sites that received either form of therapy. There was a significant increase in mean probing attachment level measurements at surgically treated sites, a finding in accord with those of other investigators.7–9
The mean counts of the species of the red complex, which have been strongly associated with periodontal disease,2,10 were markedly reduced, and this reduction was sustained for 1 year at both surgically and nonsurgically treated sites. The species of the red complex have also been shown to be reduced by SRP alone,4 and the organisms tend to remain at lowered levels for 1 year.11 The surgically treated subjects in the current investigation showed a microbiologic effect beyond that of subjects receiving SRP only in the earlier studies, in that members of the orange complex such as C. rectus, E. nodatum, F. nucleatum ss polymorphum, and P. nigrescens were also reduced in mean counts after surgical therapy. This reduction may be of value if orange complex species contribute to the pathogenesis of disease or if they set the environment to one conducive to the proliferation of the pathogenic red complex species. These findings are in accord with data from other laboratories that found reductions in the levels, proportions, or prevalence of gram-negative species of the red and orange complexes following various forms of periodontal surgery.12–15
One of the most intriguing findings of the present investigation was the further decrease in subgingival species at sites that received IP only after the remainder of the mouth had received periodontal surgery. This finding was unexpected, although several hypotheses could be suggested to explain the data. The first is that elimination of pockets by surgery might decrease reservoirs of periodontal pathogens, lowering pathogen load and leading to diminished spread throughout the mouth.16 The second is that the manipulation of tissues during the surgical procedures may have led to an altered host immunologic response to pathogenic species, which then may have manifested beneficial clinical effects even at sites not receiving periodontal surgery.17–22 It should also be pointed out that the patients rinsed with chlorhexidine during the surgical phase and then were repeatedly monitored for plaque control in the postsurgical phase. These procedures may have affected the supragingival plaque, which in turn may have contributed to the observed decrease in subgingival plaque species throughout the mouth.23
The major beneficial clinical and microbiologic effect was observed at 3 months after surgery. However, these beneficial effects were sustained for at least 1 year and conceivably longer. Recently, Serino et al24 compared the clinical effects of modified Widman flap surgery with those of SRP in 64 adult subjects with advanced periodontal disease. They found that surgical therapy is more effective than SRP in reducing mean probing pocket depth and eliminating deep periodontal pockets. The differences between treatment groups in pocket depth were sustained up to 13 years. In addition, more SRP-treated subjects exhibited signs of advanced disease progression in the 1- to 3-year period following active therapy than the surgically treated subjects. Indeed, 29% of the SRP-treated group and 14% of the surgically treated group were exited from the study because of multiple sites exhibiting disease progression in the 1 to 3 years following active therapy. Such data suggest that the rather profound reduction of the periodontal pathogens of the red complex and some of the potential pathogens of the orange complex at surgically treated sites may lead to sustained pocket depth reduction and lower the risk for periodontal disease progression.
Subjects with different smoking histories were included in the present investigation, although the number of subjects in each category was small (Table 1). It has been suggested that subjects who are current cigarette smokers respond less well to mechanical forms of periodontal therapy such as SRP and periodontal surgery than nonsmokers.25–30 In accord with these studies, the data from the present investigation indicated that the mean complete-mouth attachment loss from baseline to 12 months in never, past, and current smokers was 0.05 ± 0.13 mm, 0.04 ± 0.34 mm, and 0.27 ± 0.23 mm, respectively, although mean attachment level in the three smoking groups was comparable at baseline. However, mean pocket depth reductions were comparable among groups and were associated with decreases in mean counts of bacterial species in each of the smoking groups. While these data are intriguing, more subjects are needed to confirm the association between cigarette smoking and clinical and microbiologic therapeutic outcomes in subjects with periodontal disease.
The present investigation was a straightforward study in which the effects of apically repositioned flap surgery on clinical and microbiologic parameters in subjects with chronic periodontitis were examined. The study indicated that there were beneficial changes in most clinical parameters accompanied by clear reductions in the red complex species associated with periodontal disease as well as other potential contributors to disease such as the orange complex taxa. One of the most important aspects of this study was the further improvement at sites that received IP only, once periodontal surgery had been completed at the deeper periodontal pockets. The reduction in pocket depth by surgical means and the associated decrease in reservoirs of periodontal pathogens may be important in achieving sustained periodontal stability. Thus, periodontal surgery appears to be an important part of the armamentarium to control periodontal infections.
Acknowledgments
This work was supported in part by research grants DE-10977 and DE-12108 from the National Institute of Dental and Craniofacial Research and CNPq, Brazil.
References
- 1.Levy RM, Giannobile WV, Feres M, Haffajee AD, Smith C, Socransky SS. The short-term effect of apically repositioned flap surgery on the composition of the subgingival microbiota. Int J Periodontics Restorative Dent. 1999;19:555–567. [PubMed] [Google Scholar]
- 2.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 3.Haffajee AD, Socransky SS, Goodson JM. Comparison of different data analyses for detecting changes in attachment level. J Clin Periodontol. 1983;10:298–310. doi: 10.1111/j.1600-051x.1983.tb01278.x. [DOI] [PubMed] [Google Scholar]
- 4.Haffajee AD, Cugini MA, Dibart S, Smith C, Kent RL, Jr, Socransky SS. The effect of SRP on the clinical and microbiological parameters of periodontal diseases. J Clin Periodontol. 1997;24:324–334. doi: 10.1111/j.1600-051x.1997.tb00765.x. [DOI] [PubMed] [Google Scholar]
- 5.Socransky SS, Smith C, Martin L, Paster BJ, Dewhirst FE, Levin AK. Checkerboard DNA-DNA hybridization. Biotechnology. 1994;17:788–792. [PubMed] [Google Scholar]
- 6.Conover WJ. Practical Nonparametric Statistics. New York: John Wiley; 1980. [Google Scholar]
- 7.Zamet JS. A comparative clinical study of three periodontal surgical techniques. J Clin Periodontol. 1975;2:87–97. doi: 10.1111/j.1600-051x.1975.tb01729.x. [DOI] [PubMed] [Google Scholar]
- 8.Rosling B, Nyman S, Lindhe J, Jern B. The healing potential of the periodontal tissues following different techniques of periodontal surgery in plaque-free dentitions. A 2-year clinical study. J Clin Periodontol. 1976;3:233–250. doi: 10.1111/j.1600-051x.1976.tb00042.x. [DOI] [PubMed] [Google Scholar]
- 9.Nyman S, Lindhe J, Rosling B. Periodontal surgery in plaque-infected dentitions. J Clin Periodontol. 1977;4:240–249. doi: 10.1111/j.1600-051x.1977.tb01896.x. [DOI] [PubMed] [Google Scholar]
- 10.Consensus report for periodontal diseases: Pathogenesis and microbial factors. Ann Periodontol. 1996;1:926–932. doi: 10.1902/annals.1996.1.1.926. [DOI] [PubMed] [Google Scholar]
- 11.Cugini MA, Haffajee AD, Smith C, Kent RL, Jr, Socransky S. The effect of SRP on the clinical and microbiological parameters of periodontal diseases. 12 month results. J Clin Periodontol. 2000;27:30–36. doi: 10.1034/j.1600-051x.2000.027001030.x. [DOI] [PubMed] [Google Scholar]
- 12.Mombelli A, Nyman S, Brägger U, Wennström J, Lang NP. Clinical and microbiologic changes associated with an altered subgingival environment induced by periodontal pocket reduction. J Clin Periodontol. 1995;22:780–787. doi: 10.1111/j.1600-051x.1995.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 13.Rawlinson A, Duerden BI, Goodwin L. Effects of surgical treatment on the microbial flora in residual periodontal pockets. Eur J Prosthet Restorative Dent. 1995;3:155–161. [PubMed] [Google Scholar]
- 14.Danser MM, Timmerman MF, van Winkelhoff AJ, van der Velden U. The effect of periodontal treatment on periodontal bacteria on the oral mucous membranes. J Periodontol. 1996;67:478–485. doi: 10.1902/jop.1996.67.5.478. [DOI] [PubMed] [Google Scholar]
- 15.Tuan MC, Nowzari H, Slots J. Clinical and microbiologic study of periodontal surgery by means of apically positioned flaps with and without osseous recontouring. Int J Periodontics Restorative Dent. 2000;20:468–475. [PubMed] [Google Scholar]
- 16.Riviere GR, Smith KS, Tzagaroulaki E, et al. Periodontal status and detection frequency of bacteria at sites with periodontal health and gingivitis. J Periodontol. 1996;67:109–115. doi: 10.1902/jop.1996.67.2.109. [DOI] [PubMed] [Google Scholar]
- 17.Ebersole JL, Taubman MA, Smith DJ, Haffajee AD. Effect of subgingival scaling on systemic antibody responses to oral microorganisms. Infect Immunol. 1985;48:534–539. doi: 10.1128/iai.48.2.534-539.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aukhil I, Lopatin DE, Syed SA, Morrison EC, Knowles CJ. The effects of periodontal therapy on serum antibody (IgG) levels to plaque microorganisms. J Clin Periodontol. 1988;15:544–550. doi: 10.1111/j.1600-051x.1988.tb02127.x. [DOI] [PubMed] [Google Scholar]
- 19.Chen HA, Johnson BD, Sims TJ, et al. Humoral immune responses to Porphyromonas gingivalis before and following therapy in rapidly progressive periodontitis patients. J Periodontol. 1991;62:781–791. doi: 10.1902/jop.1991.62.12.781. [DOI] [PubMed] [Google Scholar]
- 20.Johnson V, Johnson BD, Sims TJ, et al. Effects of treatment on antibody titer to Porphyromonas gingivalis in gingival crevicular fluid of patients with rapidly progressive periodontitis. J Periodontol. 1993;64:559–565. doi: 10.1902/jop.1993.64.6.559. [DOI] [PubMed] [Google Scholar]
- 21.Sjostrom K, Ou J, Whitney C, et al. Effect of treatment on titer, function, and antigen recognition of serum antibodies to Actinobacillus actinomycetemcomitans in patients with rapidly progressive periodontitis. Infect Immunol. 1994;62:145–151. doi: 10.1128/iai.62.1.145-151.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horibe M, Watanabe H, Ishikawa I. Effect of periodontal treatment on serum IgG antibody titers against periodontopathic bacteria. J Clin Microbiol. 1995;22:510–515. doi: 10.1111/j.1600-051x.1995.tb00798.x. [DOI] [PubMed] [Google Scholar]
- 23.Ximenez-Fyvie LA, Haffajee AD, Som S, Thompson M, Torresyap G, Socransky SS. The effect of repeated professional supragingival plaque removal on the composition of the supra- and subgingival microbiota. J Clin Periodontol. 2000;27:637–647. doi: 10.1034/j.1600-051x.2000.027009637.x. [DOI] [PubMed] [Google Scholar]
- 24.Serino G, Rosling B, Ramberg P, Socransky SS, Lindhe J. Initial outcome and long-term effect of surgical and non-surgical treatment of advanced periodontal disease. J Clin Periodontol. 2001;28:910–916. doi: 10.1034/j.1600-051x.2001.028010910.x. [DOI] [PubMed] [Google Scholar]
- 25.Preber H, Bergström J. Cigarette smoking in patients referred for periodontal treatment. Scand J Dent Res. 1986;94:102–108. doi: 10.1111/j.1600-0722.1986.tb01372.x. [DOI] [PubMed] [Google Scholar]
- 26.Preber H, Bergström J. Effect of cigarette smoking on periodontal healing following surgical therapy. J Clin Periodontol. 1990;17:324–328. doi: 10.1111/j.1600-051x.1990.tb01098.x. [DOI] [PubMed] [Google Scholar]
- 27.Ah MKB, Johnson GK, Kaldahl WB, Patil KD, Kalkwarf KL. The effect of smoking on the response to periodontal therapy. J Clin Periodontol. 1994;21:91–97. doi: 10.1111/j.1600-051x.1994.tb00285.x. [DOI] [PubMed] [Google Scholar]
- 28.Grossi SG, Zambon J, Machtei EE, et al. Effects of smoking and smoking cessation on healing after mechanical periodontal therapy. J Am Dent Assoc. 1997;128:599–607. doi: 10.14219/jada.archive.1997.0259. [DOI] [PubMed] [Google Scholar]
- 29.Kinane DF, Radvar M. The effect of smoking on mechanical and antimicrobial periodontal therapy. J Periodontol. 1997;68:467–472. doi: 10.1902/jop.1997.68.5.467. [DOI] [PubMed] [Google Scholar]
- 30.Renvert S, Dahlen G, Wikstrom M. The clinical and microbiological effects of nonsurgical periodontal therapy in smokers and nonsmokers. J Clin Periodontol. 1998;25:153–157. doi: 10.1111/j.1600-051x.1998.tb02421.x. [DOI] [PubMed] [Google Scholar]