Abstract
Interleukin-23 (IL-23) and IL-17A regulate granulopoiesis through Granulocyte-Colony Stimulating Factor (G-CSF), the main granulopoietic cytokine. IL-23 is secreted by activated macrophages and dendritic cells and promotes the expansion of three subsets of IL-17A-expressing neutrophil-regulatory T (Tn) cells; CD4−CD8−αβlow, CD4+CD8−αβ+ (Th17) and γδ+ T cells. Here we investigate the effects of IL-17A on circulating neutrophil levels using IL-17 receptor (Il17ra−/−) deficient mice and Il17ra−/−Itgb2−/− mice that lack both IL-17R and all four β2 integrins. IL-17R deficiency conferred a reduction in neutrophil numbers and G-CSF levels, as did antibody blockade against IL-17A in WT mice. Bone marrow (BM) transplantation revealed that IL-17R expression on non-hemopoietic cells had the greatest effects on regulating blood neutrophil counts. Although circulating neutrophil numbers were reduced, IL-17A expression, secretion and the number of IL-17A-producing Tn cells were elevated in Il17ra−/− and Il17ra−/−Itgb2−/−mice suggesting a negative feedback effect through IL-17R. The negative regulation of IL-17A-producing T cells and IL-17A and IL-17F gene expression through the interactions of IL-17A or IL-17F with IL-17R was confirmed in splenocyte cultures in vitro. We conclude that IL-17A regulates blood neutrophil counts by inducing G-CSF production mainly in non-hemopoietic cells. IL-17A controls the expansion of IL-17A-producing Tn cell populations through IL-17R.
Keywords: Granulopoiesis, IL-17 Receptor, Gamma-delta T cells, Neutrophilia, Neutropenia
Introduction
Interleukin-17A (IL-17A) has biological roles at the interface between the adaptive and innate immune systems.(1,2) IL-17A contributes to the pathogenesis of multiple autoimmune diseases including rheumatoid arthritis (3–6), experimental autoimmune encephalitis (7,8) and inflammatory bowel disease (9,10) through the actions of CD4+ Th17 cells. IL-17A also controls host responses against extracellular pathogens by regulating the expression of many inflammatory mediators including IL-6, CCL2, CXCL1, CXCL6 and CXCL8, as well as G-CSF, granulocyte-macrophage-colony stimulating factor (GM-CSF) and matrix metalloproteinases.(1,8,11)
IL-17A is secreted primarily by three subsets of activated memory T cells which we have collectively named neutrophil-regulatory Tn cells; CD4+CD8−αβ+ (also known as Th17 cells), CD4−CD8−αβlow T cells and γδ+ T cells.(12,13) Although most studies have focused on Th17 cells (14,15), γδ+ T cells are the most abundant IL-17A-producing cells in normal wild-type (WT) mice.(12,13) The nuclear receptor RORγt and the transcription factor Stat3 have been identified as key regulators involved in the polarization of naive CD4+ T cells into Th17 cells following stimulation with IL-6, IL-21 and TGF-β1.(8,15–22) Replacing the coding region of the RORγt gene with that of green fluorescent protein (GFP) locus revealed three GFP+ cell populations in these mice (19), analogous to the three populations characterized by Stark et al., 2005.(12) The polarization of Th17 cells can be suppressed by the cytokines interferon-γ (INF-γ) and IL-4 as well as IL-27 and IL-2.(8,23–25)
In mice, IL-17A and IL-17F have a single known homodimeric receptor, IL-17R, which is ubiquitously expressed.(26) In humans, IL-17A and IL-17F can bind to IL-17R alone and to a heterodimeric complex of IL-17R and IL-17RC.(27) IL-17R-deficient mice have much higher mortality rates following infection with Klebsiella pneumoniae or Toxoplasma gondii due to defective neutrophil recruitment to the lung, although the cellular source of IL-17A in these murine models was not determined.(28–30)
In addition to its role in host defense against infections, our group (12,31) and others (28–30,32,33) have shown that IL-17A is a potent regulator of granulopoiesis and neutrophil recruitment under normal and inflammatory conditions. Organ-specific overexpression of IL-17A increases circulating neutrophil numbers and recruitment into the organs by induction of CXCL2, IL-1β and G-CSF.(32–34) We have previously shown that blood neutrophil numbers correlate with serum IL-17A and G-CSF levels in normal and adhesion molecule-deficient mice and that G-CSF acts downstream of IL-17A.(31) Neutrophilia is induced by bacterial infection, but is also commonly found in adhesion molecule-deficient mice with defective neutrophil trafficking.(31,35) One such mouse with a severe neutrophil trafficking defect is the Itgb2−/− mouse (36), which lacks all four β2 integrins and mimics many aspects of the human leukocyte adhesion deficiency-I (LAD-I).(37) Itgb2−/− mice have 10–20-fold elevated neutrophil counts as neutrophils are unable to traffic into many tissues.(31,36)
Here, we investigate the role of IL-17R in regulating blood neutrophil numbers using IL-17R-deficient mice with and without neutrophilia. Specifically, we focus on the effects of defective IL-17R signaling on hemopoietic and non-hemopoietic cells, and identify a “short-loop” negative regulatory pathway by which IL-17A regulates the polarization of IL-17A-producing Tn cells through its cognate receptor.
Materials and Methods
Animals
Mice lacking the β2 integrin subunit (Itgb2−/−) (36), IL-17 receptor (Il17ra−/−) (29) and wild-type (WT) C57Bl/6 mice (Jackson Labs, Bar Harbor, ME) were used between 8 and 16 weeks of age and kept in specific-pathogen-free conditions in a barrier facility. Mice deficient in both CD18 and IL-17R were generated by breeding Itgb2+/− with Il17ra−/− mice using a standard four generation scheme. All mice were on a C57Bl/6 background for at least ten generations and animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Virginia. For antibody blockade of IL-17A (TC11-18H10.1; Biolegend, San Diego, CA), 100 μg of antibody or rat IgG1 isotype control (ebioscience, San Diego, CA) per mouse was injected i.v. into WT mice and blood neutrophil counts assessed after 24 h. Blood counts were taken via tail bleed into EDTA coated capillary tubes, analyzed by automatic analyzer (Hemavet 850, CDC Technologies Inc., Oxford, CT) and confirmed by Kimura-stained manual counts using a hemocytometer.
Bone marrow transplantation
Bone marrow (BM) was isolated from Itgb2−/− and Il17ra−/−Itgb2−/− mice as previously described.(38) Briefly, 107 BM cells resuspended in 500 μl sterile PBS were injected intravenously into lethally irradiated, WT or Il17ra−/− recipients under sterile conditions. Mice were given autoclaved water supplemented with antibiotics (5 mM sulfamethoxazole, 0.86 mM trimethoprim (Sigma, St. Louis, MO). All experiments were conducted 8–9 weeks post BM transplantation. Completeness of reconstitution was monitored by amplifying splenocyte DNA of female mice reconstituted with bone marrow from male mice by real-time PCR using primers targeted against the Y6 gene located on the Y chromosome. Reconstitution was approximately 95% (data not shown).
Antibodies and recombinant proteins
Monoclonal antibodies were from BD-PharMingen, San Diego, CA: Purified or APC-conjugated anti-CD3e (145-2C11), APC-CY7 or PerCP-conjugated anti-CD4 (RM4-5), anti-CD16/CD32 (2.4G2; The Lymphocyte Culture Center, University of Virginia, USA), purified anti-CD28 (37.51), FITC or APC-conjugated anti-TCR β chain (H57-597), FITC or biotin-conjugated anti- γδ TCR (GL3), purified or PE-conjugated anti-IL-17A (TC11-18H10.1) and PerCP-conjugated Streptavidin used for the detection of the biotin-conjugated primary Ab. IL-6 (50 ng/ml; R&D systems, Minneapolis, MN), IL-23 (20 ng/ml; R&D systems), transforming growth factor (TGF)-β1 (1 ng/ml; Peprotech Inc., New Jersey) and IL-17A (10 ng/ml; R&D systems) were used at the concentrations indicated.
Flow cytometry
Animals were anesthetized with an i.p. injection of a cocktail containing ketamine hydrochloride (Sanofi Winthrop Pharmaceuticals, 125 mg/kg, New York, NY), xylazine (TranquiVed, Phoenix Scientific, Inc., 12.5 mg/kg, St. Joseph, MO), and atropine sulfate (Fujisawa USA, Inc., 0.025 mg/kg, Deerfield, IL) then killed by cervical dislocation. Single cell suspensions of the spleen, mesenteric lymph nodes (MLN) and Peyer’s patches were prepared by straining tissues through a 70 μm screen (BD Falcon, San Jose, CA). Red blood cells were lysed and white blood cells washed twice with PBS-FBS. Lamina propria (LP) lymphocytes were prepared as previously described.(39) For flow cytometry, cells were cultured in RPMI-1640 containing 10 % FBS, 1X nonessential amino acids (Gibco), 10 mM HEPES, 2 mM L-glutamine (Gibco), 1 mM sodium pyruvate (Gibco) and 1 % penicillin/streptomycin for 5 h with 10 ng/ml PMA (Sigma-Aldrich), 500 ng/ml calcium ionophore (Sigma-Aldrich) and GolgiStop (BD-Pharmingen).
Following stimulation, cells were re-suspended at 107/ml. Fcγ III/II receptors were blocked with 0.5 μg anti-CD16/CD32 (2.G42) and the cell suspension was incubated with an optimal concentration of mAbs for 20 min at 4°C in staining buffer (5 % FBS in PBS) and washed. When biotinylated mAbs were used, streptavidin-PerCP was added for 20 min at 4°C, and cells washed twice in staining buffer. Intracellular staining was performed using Fix & Perm® cell permeablisation reagents (Caltag Laboratories, Burlingame, CA) according to manufacturer’s instructions. Flow cytometry analysis was performed on a Becton Dickinson FACS Calibur dual laser and data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR). Gates were set by isotype controls. Alternatively, cells were visualized using Amnis Imagestream100™.
Lymphocyte culture
Splenocytes from WT or Il17ra−/− mice were isolated under sterile conditions and cultured on plate-absorbed purified anti-CD3ε (10 μg/ml) and soluble anti-CD28 (1 μg/ml), in the presence or absence of IL-23 (20 ng/ml), IL-6 (50 ng/ml) and TGF-β1 (1 ng/ml) for 3 days.(17) The cell suspensions were then transferred to fresh, non-treated plates for 3 days. On day 6, the splenocytes were reactivated for 5 h with 10 ng/ml PMA (Sigma-Aldrich), 500 ng/ml calcium ionophore (Sigma-Aldrich) and GolgiStop (BD-Pharmingen). Alternatively, splenocytes from Itgb2−/− mice were treated with 10 ng/ml IL-17A or IL-17F before being prepared for RNA isolation. IL-17A and G-CSF protein in serum or cell culture supernatants were measured by Quantikine M mouse ELISA kits (R&D systems, Minneapolis, MN) according to manufacturer’s directions.
mRNA quantification
RNA was extracted from isolated splenocytes or tissue following homogenization in Trizol® (Invitrogen), according to manufacturer’s instructions. Reverse transcription and PCR steps were performed using QuantiTect SYBR Green RT-PCR Kit (Qiagen,Valencia, CA) as previously described.(12) IL-17A primer sequences were as follows:
| Sense: | ATCCCTCAAAGCTCAGCGTGTC |
| Anti-Sense: | GGGTCTTCATTGCGGTGGAGAG |
FAM-labeled mouse IL-17F and IL-22 Assay on Demand probes and Vic-labeled TaqMan® Ribosomal RNA control reagent (Applied Biosystems [ABI], CA, USA) were performed using the Quantitech Probe One-Step RT-PCR kit (Qiagen). 1 μg of total RNA was used for all tissues. Values were determined using iCycler iQ Real-Time Detection System Software v3.0 (Qiagen, Valencia, CA). The corresponding values were normalized to 18s RNA and then normalized to individual WT mouse organs as the calibrator control (always equal to 1), thereby expressing the values as relative quantification (RQ) values.
Statistical Analysis
Data were expressed as mean ± SEM. Statistical significance between groups was set at p<0.05 using unpaired t tests, non-parametric Mann Whitney test or One-Way ANOVA with Bonferroni’s post-hoc tests.
Results
IL-17R in non-hemopoietic cells regulates peripheral blood neutrophil counts
Circulating neutrophil numbers were reduced by approximately 37 % in Il17ra−/− mice compared to WT mice, confirming previous studies (Figure 1a).(12) In order to assess the effects of IL-17R deficiency in cases of sever neutrophilia, Il17ra−/− mice were crossed with neutrophilic, adhesion molecule-deficient (Itgb2−/−) mice. Neutrophil counts were significantly attenuated in Il17ra−/−Itgb2−/− mice by approximately 56 % compared to Itgb2−/− mice, but absence of IL-17R signaling did not completely rectify the neutrophilia (Figure 1b). Under barrier vivarium conditions, Il17ra−/− mice did not show any overt phenotype, as previously reported.(29) However, Il17ra−/−Itgb2−/− mice were consistently smaller in size than Itgb2−/− mice and weighed significantly less (10.5 ± 0.5 g versus 14.7 ± 1.2 g; p<0.05). Il17ra−/−Itgb2−/− mice exhibited splenomegaly and extramedullary hematopoiesis causing an expansion in splenic red pulp and subsequent decrease in white pulp. A large infiltration of neutrophils was also found in the lung tissue (data not shown). Furthermore, Il17ra−/−Itgb2−/− mice failed to thrive, and many had to be euthanized between 12–14 weeks of age. Antibody blockade against IL-17A in WT mice caused a significant reduction in neutrophil numbers, by approximately 39%, confirming that IL-17A signaling via IL-17R regulates blood neutrophil numbers in WT mice (Figure 1c).
Figure 1. Blood neutrophil counts are reduced in IL-17R-deficient mice.
Blood neutrophil counts were measured in (A) IL-17R-deficient mice (n=14) compared to WT mice (n=15), in (B) Il17ra−/−Itgb2−/− mice (n=7) compared to Itgb2−/− mice (n=22) and in (C) WT mice following antibody treatment against IL-17A for 24h (n=4–8; *p<0.05 by nonparametric Mann-Whitney test). BM transplantations (D) from Il17ra−/−Itgb2−/− or Itgb2−/− donors into irradiated WT or Il17ra−/− recipients (n=5–10) were performed and blood counts assessed after 6 weeks. †p<0.05 from all other groups, #p<0.05 compared to Itgb2−/− BM into WT, §p<0.05 compared to Il17ra−/−Itgb2−/− BM into WT mice.
Since β2-integrins are only expressed on BM-derived cells (except glia cells in the brain), blood cells of lethally irradiated mice reconstituted with Itgb2−/− BM are completely devoid of all four β2-integrins.(40) To investigate whether the expression of IL-17R on hemopoietic or non-hemopoietic cells was important in regulating circulating neutrophil numbers, BM cells were isolated from Itgb2−/− or Il17ra−/−Itgb2−/− mice and injected i.v. into lethally irradiated WT or Il17ra−/− mice. Mice were analyzed 6–7 weeks after BM transfer. Il17ra−/− mice reconstituted with Itgb2−/− BM had much lower neutrophil counts than WT recipients of Itgb2−/− BM (Figure 1c). Their blood neutrophil counts were not significantly different from those of Il17ra−/− mice reconstituted with Itgb2−/−Il17ra−/− BM. These findings demonstrate that IL-17R on non-hemopoietic cells has a dominant effect on neutrophil counts. WT mice reconstituted with Itgb2−/−Il17ra−/−BM have high neutrophil counts but not as high as WT mice reconstituted with Itgb2−/− BM (Figure 1c). This suggests that IL-17R on hemopoietic cells also contributes to neutrophil homeostasis, but less than IL-17R on non-hemopoietic cells.
Systemic levels of IL-17A and tissue expression of IL-17A are elevated in IL-17R-deficient mice
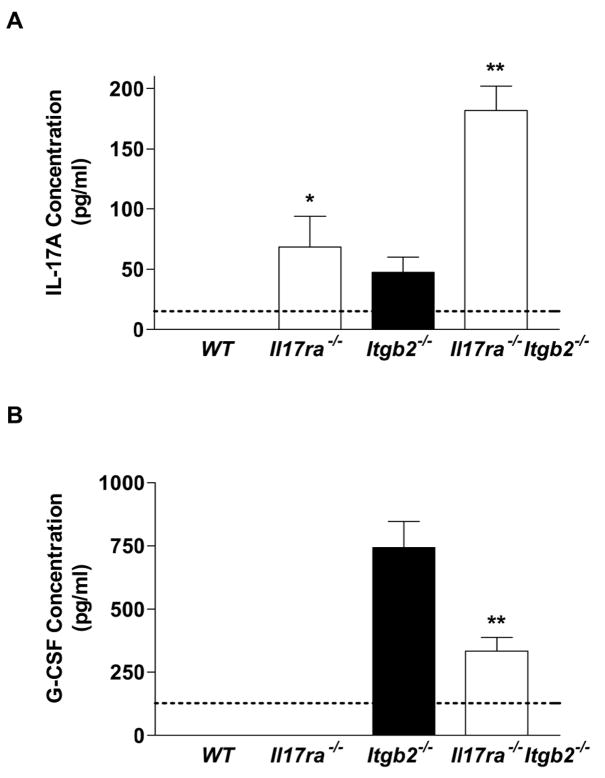
Blood sera from Il17ra−/− and Il17ra−/−Itgb2−/− mice and their respective control groups were analyzed for IL-17A protein by ELISA. IL-17A was not detectable in WT mice, but was elevated in the sera of Il17ra−/− mice (70 ± 22 pg/ml). A significant further elevation in systemic IL-17A levels was seen in Il17ra−/−Itgb2−/− mice compared to the Itgb2−/− control group (Figure 2a). This suggests that IL-17A secretion may be controlled by an IL-17R-dependent process and this mechanism is defective in Il17ra−/− mice.
Figure 2. Effects of IL-17R deficiency on serum cytokine levels.
Analysis of IL-17A (n=3–6) (A) and G-CSF (n=6) (B) in the serum of WT, Il17ra−/−, Itgb2−/− and Il17ra−/−Itgb2−/− mice. Detection limit of each ELISA assay indicated by dotted line. *p<0.05, **p<0.01 by nonparametric Mann-Whitney test.
G-CSF levels were not detectable in WT or Il17ra−/− mice but were elevated in Itgb2−/− mice. G-CSF levels were significantly reduced in Il17ra−/−Itgb2−/− mice, suggesting that G-CSF is regulated by IL-17A in vivo through IL-17R (Figure 2b). IL-17A has also been reported to induce CCL2, CXCL1 and IL-6 release from stromal cells.(11) However, circulating levels of CCL2, CXCL1 and IL-6 were not significantly affected in Il17ra−/− mice on either a WT or Itgb2−/− background (data not shown). This excludes a role for these cytokines in the regulation of blood neutrophil numbers and suggests that G-CSF is the major cytokine controlling neutrophil production.
Elevated levels of IL-17A and IL-17A-producing Tn cells in IL-17R-deficient mice
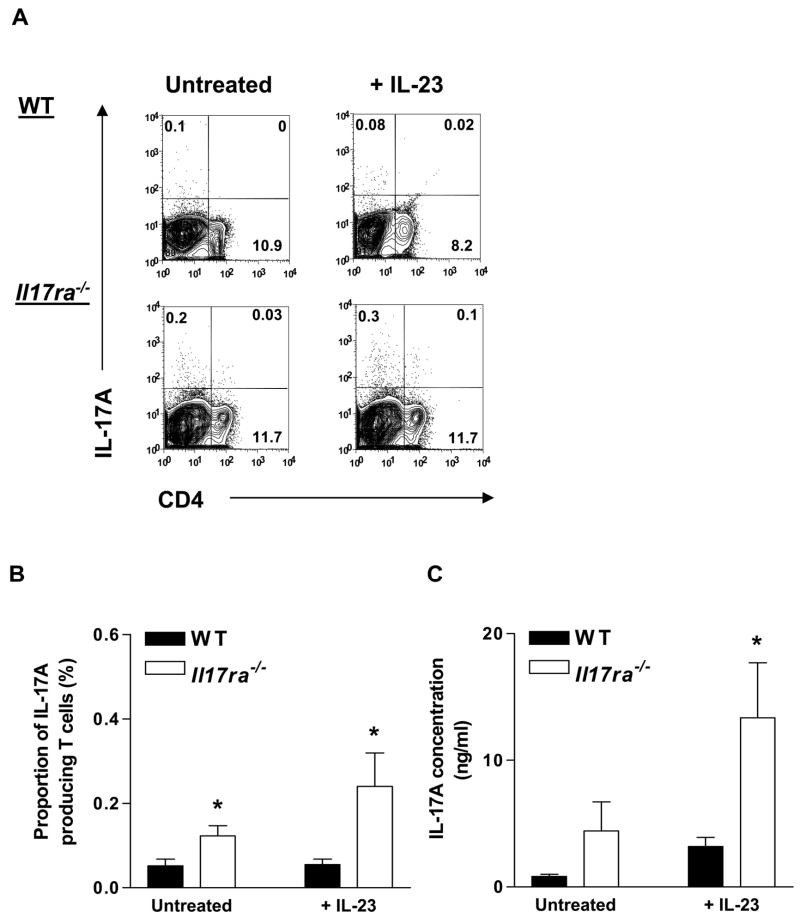
To investigate the sources of the elevated systemic levels of IL-17A seen in Il17ra−/− mice, we cultured splenocytes from WT and Il17ra−/− mice on plate-absorbed anti-CD3ε and soluble anti-CD28 for 3 days in the presence or absence of IL-23, followed by a rest period of 3 days. On day 6, splenocyte cultures were reactivated with a PMA/ionomycin and GolgiStop cocktail. In mixed splenocytes, only a small percentage of all cells polarized into IL-17A producers, less than the percentage previously reported for purified naïve CD4+ cell cultures.(17,18,41) The number of IL-17A-producing Tn cells in Il17ra−/− splenocyte cultures was significantly increased compared to WT splenocytes cultures. This difference was further enhanced by treatment with IL-23 (Figure 3a and b). Concomitantly, IL-17A secretion into the cell culture supernatants was also significantly increased in Il17ra−/− splenocyte cultures (Figure 3c).
Figure 3. Effects of IL-17R deficiency on the number of IL-17A-producing Tn cells and IL-17A secretion.
Splenocytes from Il17ra−/− and WT mice were stimulated for 3 days on plate-adsorbed anti-CD3ε and soluble anti-CD28 antibodies in the presence or absence of IL-23 (20 ng/ml), followed by a 3 day rest. Cells were reactivated by PMA/ionomycin in the presence of GolgiStop for 5 h before intracellular staining for IL-17A. (A) Representative plots from WT and Il17ra−/− mice. (B) IL-17A-producing Tn cells (n=3–4) as a proportion (%) of the gated lymphocyte population in Il17ra−/− splenocytes (open bars) compared to WT splenocytes (filled bars). (C) IL-17A protein in the supernatants (n=3–5). *p<0.05 by unpaired t test.
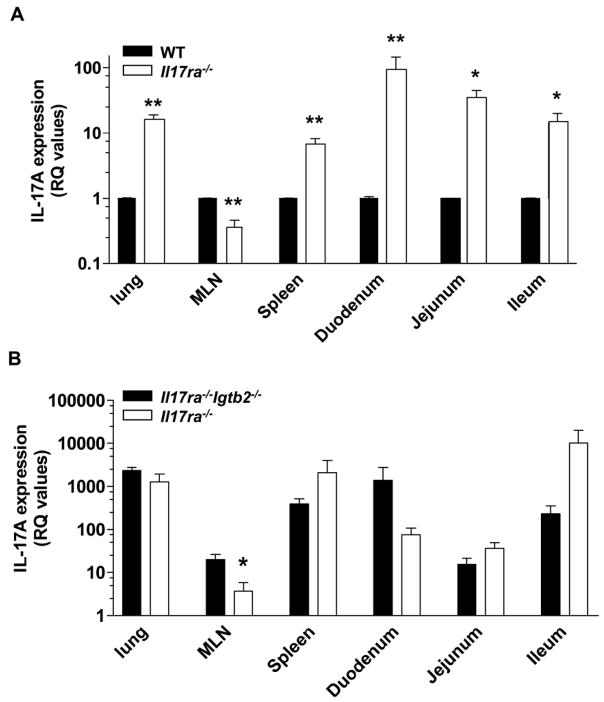
In vitro, IL-17A-producing T cells can only be detected following stimulation using PMA/ionomycin. However, detectable serum levels of IL-17A in Il17ra−/−, Itgb2−/−and Il17ra−/−Itgb2−/− mice suggests that some T cells actively secrete IL-17A in vivo. To investigate whether basal levels of IL-17A mRNA expression matched the increase in IL-17A protein seen in sera of IL-17R-deficient mice, we monitored IL-17A mRNA expression in various organs (Figure 4a). In IL-17R-deficient mice, an increase in IL-17A mRNA expression was found in the lung, spleen and small intestines, but not the MLN (Figure 4a). As reported previously, IL-17A message was elevated in the tissue of Itgb2−/−mice.(12) The levels of IL-17A mRNA were also high in all organs tested (excepting the MLN) of Il17ra−/−Itgb2−/− mice (Figure 4b).
Figure 4. IL-17A is upregulated in the tissue of IL-17R-deficient mice.
Basal expression of IL-17A was examined in organs of (A) IL-17R-deficient mice (open bars) compared to WT (filled bars) (n=3–4) and (B) Il17ra−/−Itgb2−/− mice (open bars) compared to Itgb2−/− mice (filled bars) (n=3–5). For the Il17ra−/−, Itgb2−/− and Il17ra−/−Itgb2−/− mice, the relative quantification (RQ) values were expressed as a fold-increase compared to IL-17A expression in WT mice (the calibrator control which is equal to 1). *p<0.05, **p<0.01 by unpaired students t test
IL-17A-producing Tn cell populations are expanded in IL-17R-deficient mice
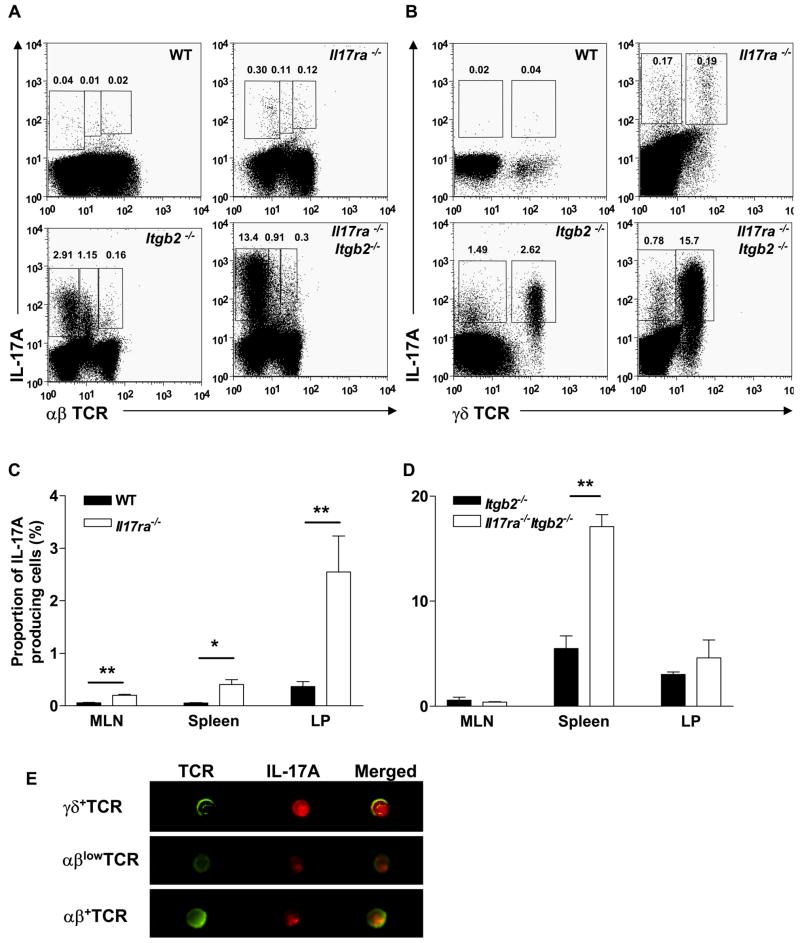
To investigate the source of elevated IL-17A found in IL-17R-deficient mice, the numbers of IL-17A-producing T cells were assessed in various organs. IL-17A-secreting T cells were significantly expanded in the spleen, MLN and LP of Il17ra−/− mice compared to WT mice, with the majority of IL-17A-producing Tn cells found in the LP (Figure 5a, b and c). The proportion (%) of Tn cell subsets remained similar between Il17ra−/− and WT mice, with γδ+ T cells being the major Tn cell population, followed by Th17 cells and then CD4−CD8−αβlow T cells (Figure 5b and Table 1a). Representative Th17, CD4−CD8−αβlow and γδ+ T cells were analyzed using Amnis technology (Figure 5d).
Figure 5. IL-17A-producing Tn cell populations are expanded in IL-17R-deficient mice.
(A) αβhigh and αβlow T cells and (B) γδ+ T cells in the spleen of WT, IL17R−/−, Itgb2−/−and Il17ra−/−Itgb2−/− mice. IL-17A-producing Tn cell populations are elevated in the MLN, spleen and LP of Il17ra−/− mice compared to WT mice (n=4–12) (C), and in the spleen of Il17ra−/−Itgb2−/− mice compared to Itgb2−/− mice (n=3–13) (D). Representative images of IL-17A-producing Tn cell subsets taken by Amnis (E). *p<0.05, **p<0.01 by unpaired student’s t test.
Table 1. IL-17A-producing Tn cells in IL-17R-deficient mice.
1 *p<0.05 by nonparametric Mann-Whitney test.
| A | ||||||
|---|---|---|---|---|---|---|
| Organs | % of IL-17A-producing cells | |||||
| γδ+ | CD4−αβlow | Th17 | ||||
| WT | Il17ra−/− | WT | Il17ra−/− | WT | Il17ra−/− | |
| MLN | 54 ± 17 | 33 ± 7 | 17 ± 5 | 28 ± 4 | 29 ± 7 | 39 ± 5 |
| Spleen | 59 ± 10 | 54 ± 2 | 25 ± 17 | 22 ± 7 | 16 ± 4 | 24 ± 9 |
| LP | 73 ± 16 | 65 ± 14 | 10 ± 6 | 13 ± 6 | 17 ± 9 | 22 ± 11 |
| B | ||||||
| Organs | % of IL-17A-producing cells | |||||
| γδ+ | CD4− αβlow | Th17 | ||||
| Itgb2−/− | Il17ra−/−Itgb2−/− | Itgb2−/− | Il17ra−/−Itgb2−/− | Itgb2−/− | Il17ra−/−Itgb2−/− | |
| MLN | 57 ± 5 | 43 ± 11 | 24 ± 10 | 17 ± 1 | 19 ± 6 | *40 ± 5 |
| Spleen | 79 ± 15 | 95 ± 10 | 17 ± 6 | 3 ± 1 | 4 ± 1 | 2 ± 1 |
| LP | 74 ± 9 | 70 ± 32 | 17 ± 7 | 12 ± 4 | 9 ± 3 | 18 ± 11 |
Cells were gated by forward-side scatter for lymphocytes.
On the neutrophilic (Itgb2−/−) background, most Tn cells were found in the spleen and LP (Figure 5e). The expansion of IL-17A-producing T cells found in the spleen of Itgb2−/− mice was further exacerbated in Il17ra−/−Itgb2−/− mice. γδ+ T cells were the major Tn cell population in both Itgb2−/− and Il17ra−/−Itgb2−/− mice in all organs assessed. The population of CD4−CD8−αβlow T cells was also expanded above those of Th17 cells (Table 1b).
IL-17R regulates the polarization of IL-17A-producing Tn cells
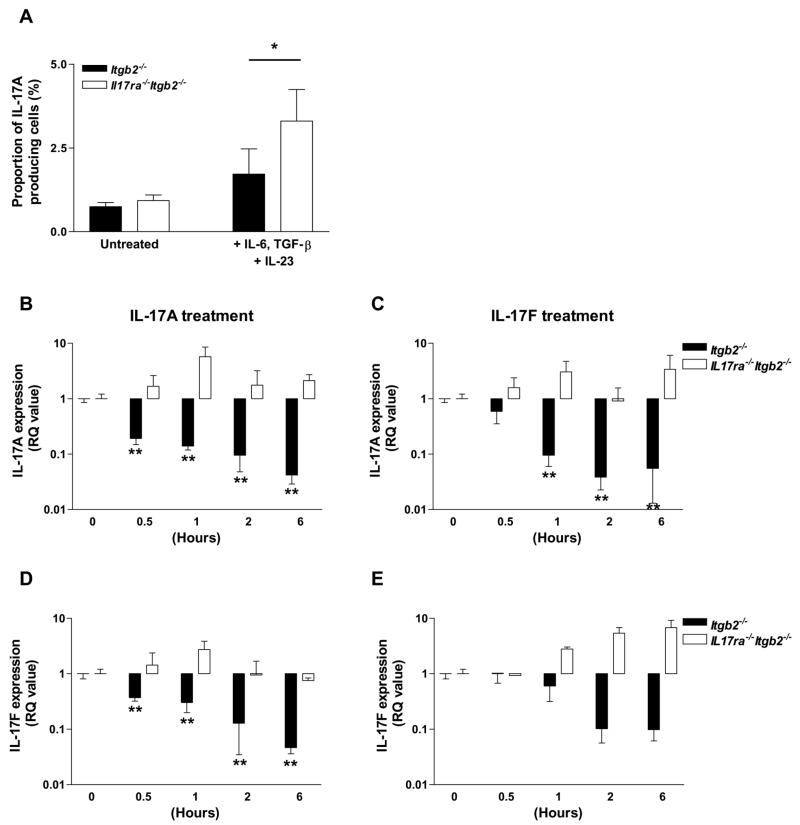
The expansion of IL-17A-producing Tn cells in IL-17R-deficient mice compared to the control groups led us to hypothesize that IL-17A may negatively regulate the expansion of these cells through IL-17R signaling in an autocrine or paracrine manner. To test this hypothesis, splenocytes were isolated from Itgb2−/− or Il17ra−/−Itgb2−/− mice and cultured as before. IL-17A-producing Tn cells were expanded in Il17ra−/−Itgb2−/−splenocyte cultures compared to Itgb2−/− cultures by 48% (Figure 6a). IL-17A secretion in the cell culture supernatants was also elevated following cytokine treatment (data not shown).
Figure 6. IL-17A and F negatively regulate their own expression and expansion of IL-17A-producing Tn cells.
Unfractionated splenocytes from Itgb2−/− or Il17ra−/−Itgb2−/− mice were cultured on anti-CD3ε and soluble anti-CD28, in the presence or absence of IL-6, TGF-β and IL-23 for 3 days followed by a 3 day rest. Cells were reactivated by PMA/ionomycin in the presence of GolgiStop for 5 h before intracellular staining for IL-17A. Proportion of IL-17A-producing Tn cells (A) in cytokine-stimulated Il17ra−/−Itgb2−/−cultures (open bars) compared to Itgb2−/− cultures (closed bars) (n=3–4). *p<0.05 by unpaired student’s t test. IL-17A (B; p<0.0001 and C; p<0.001 by one-way ANOVA), and IL-17F (D; p<0.0001 and E; p<0.01 by one-way ANOVA) gene expression was determined in unfractionated Itgb2−/− (n=3–4) or Il17ra−/−Itgb2−/− (n=2) splenocyte cultures treated with IL-17A (10 ng/ml) or IL-17F (10 ng/ml) from 0–6h. *p<0.05, **p<0.01 compared to untreated samples (0h) by Bonferroni’s post-hoc test.
Treatment of unfractionated Itgb2−/− but not Il17ra−/−Itgb2−/− splenocytes with recombinant IL-17A or IL-17F for 0–6h caused a significant reduction in IL-17A gene expression compared to untreated cells (Figure 6b and c). Furthermore, IL-17A and IL-17F treatments caused a reduction in IL-17F gene expression in Itgb2−/− but not Il17ra−/−Itgb2−/− splenocytes (Figure 6D and E). IL-22 mRNA was also down regulated by 90–99% in response to IL-17A or F (data not shown). Taken together, these data show that both IL-17A and IL-17F can inhibit their own or each others production through a short feedback loop that requires IL-17R. Whether this is a T cell autonomous or paracrine process remains to be determined.
Discussion
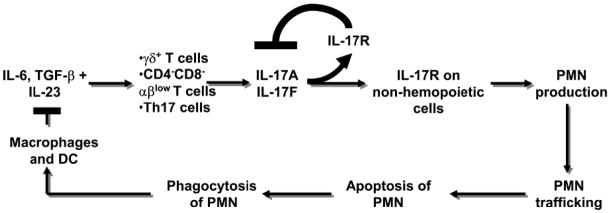
Mice lacking IL-17R have reduced neutrophil numbers and G-CSF levels. Most of this effect is due to IL-17R expression on non-hemopoietic cells. IL-17A expression, secretion and the percentage of IL-17A-producing Tn cells were expanded in IL-17R-deficient mice and IL-17A or IL-17F treatment inhibited IL-17A gene expression. This suggests a short feedback loop by which IL-17A (and probably IL-17F) blunts the expansion of IL-17A-producing T cells in vivo and in vitro. Taken together, our data show a key role for IL-17R signaling in neutrophil homeostasis and in controlling the expansion of IL-17A-producing Tn cells (Figure 7).
Figure 7. Neutrostat feedback loop.
Cytokines released by macrophages and dendritic cells (DC) induce the release of IL-17A from three Tn cell subsets. IL-17A binds to IL-17R on non-hemopoietic cells in the bone marrow inducing G-CSF release and elevating blood neutrophil (PMN) counts. PMN traffic into the tissue, undergo apoptosis and become engulfed by tissue resident macrophages and DC. Phagocytosis of apoptotic PMN curbs IL-23 release from these cells, closing the “long” feedback loop.(12,13) The present findings add a “short” feedback loop (bold lines) which negatively regulates IL-17A and IL-17F expression via IL-17R.
The IL-17R-deficient mouse was first generated by Ye et al., (2001) to investigate the role of IL-17R signaling in response to infection by Klebsiella pneumoniae. These mice failed to recruit neutrophils into the lung, resulting in 100 % mortality. Circulating neutrophil counts in Il17ra−/− mice and the cellular source of IL-17A were not reported.(29) As part of its protective role against bacteria, IL-17A has been shown to regulate circulating blood neutrophil counts, with neutrophil numbers positively correlating with IL-17A serum levels in neutrophilic mice.(31) Previous work has shown that irradiated Il17ra−/− mice show increased mortality and a dose-dependent decrease in blood neutrophil counts and BM and spleen colony forming units compared to WT mice.(42) These findings suggest that IL-17R on stromal cells is required for hemopoietic recovery. Previous studies have demonstrated that various bone marrow (BM) stromal cell lines respond to IL-17A by secreting G-CSF and up-regulating the expression of stem cell factor and thus must express IL-17R.(34) IL-17R expression has also been demonstrated on fibroblast-like cells, mononuclear cells, polymorphonuclear cells, endothelial cells, BM cells, and bone lining cells in the joints of mice.(6) Lubberts et al. used BM chimeric mice with IL-17R expression on either hemopoietic or non-hemopoietic cells to demonstrate that IL-17R signaling on non-hemopoietic cells was an essential component of the destructive synovitis found in adjuvant induce arthritis models.(6) This fits well with the present data showing that IL-17R on non-hemopoietic cells is important in regulating the granulopoietic process.
We have previously demonstrated that IL-23 regulates both IL-17A and G-CSF secretion in neutrophilic mice.(43) IL-17A regulates G-CSF release from BM-derived stromal cell lines and increases surface expression of stem cell factor (SCF), the ligand for c-Kit, in vitro.(34) Here, we demonstrate that G-CSF secretion was significantly reduced in IL-17R-deficient mice, which became apparent on a neutrophilic background. Mice lacking G-CSF have chronic neutropenia.(44) Therefore, the reduction in neutrophil numbers in Il17ra−/−Itgb2−/− mice probably reflects the lack of G-CSF secretion by BM stromal cells in these mice. Even though large amounts of IL-17A are made, IL-17A is ineffective because the receptor is absent on the relevant cells. However, removing IL-17A signaling in Il17ra−/−Itgb2−/− mice did not reduce neutrophil counts to normal WT levels, indicating that other, IL-17R independent mechanisms can regulate neutrophil numbers in these mice. Such mechanisms might include alternative cytokine pathways.
The increased expression and systemic secretion of IL-17A in IL-17R-deficient mice suggests a possible compensatory mechanism in these mice involving IL-17A. Consistent with our data, Ye et al., 2001 found elevated IL-17A levels in the bronchoaveolar lavage fluid of Il17ra−/− mice in response to K. pneumoniae infection compared to the control group.(29) Furthermore, the expansion of IL-17A-producing Tn cell numbers in IL-17R-deficient mice (between 3–20 fold) suggests that IL-17A may be able to negatively regulate the polarization of IL-17A-producing cells through IL-17R. The cytokines INF-γ, IL-2, IL-4 and IL-27 have previously been described to limit the polarization of Th17 cells in vitro and in vivo.(8,23–25) Here we demonstrate the expansion of IL-17A-producing Tn cells in vivo using IL-17R-deficient mice. IL-17R also binds two other ligands, IL-17F and IL-17A/F heterodimers. All three ligands can induce downstream signaling events and the release of inflammatory mediators in vitro and in vivo.(26,27,45–48) Therefore, it is plausible that these ligands may also inhibit the expansion of IL-17A-producing T cells via IL-17R. Indeed, treatment of Itgb2−/− but not Il17ra−/−Itgb2−/− splenocytes with recombinant IL-17A or IL-17F significantly inhibited IL-17A and IL-17F mRNA expression as well as IL-22, but the effects of IL-17A treatment was stronger and more sustained. Taken together, our results suggest that IL-17A is able to moderate its own secretion by inhibiting the expansion of IL-17A-producing Tn cells.
It is clear that the IL-23/IL-17A/G-CSF axis is responsible for most of the neutrophilia seen in mice lacking β2 integrins.(12,31,43) This pathway may therefore also be of key importance in the neutrophilia seen in human leukocyte adhesion deficiency syndromes.(37) The ability of IL-17A to negatively regulate the expansion of IL-17A-producing Tn cells leads us to question the usefulness of anti-IL-17R therapies for autoimmune diseases because they may lead to unwanted expansion of IL-17A-producing T cells. Additionally, if such therapies reduce the circulating neutrophil counts, they can significantly compromise host defense in the patients receiving therapies. It is likely that alternative ways of curbing the pro-inflammatory effects of IL-17A can be found that circumvent these problems.
Acknowledgments
We wish to thank Jacques Peschon (Amgen, Seattle, WA 98119) for generously providing us with the Il17ra−/− mice.
Footnotes
This work was supported by grants from the National Institutes of Health HL73361 (K.L.), T32 GM 08715-01A1 (M.A.S.) and Deutsche Forschungsgemeinschaft AZ428/2-1 (A.Z.).
Disclosure
The authors have no competing financial interests.
Reference List
- 1.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 3.Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. PNAS. 2003;100:5986–5990. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of Immune Induction of Collagen-Induced Arthritis in IL-17-Deficient Mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 5.Miossec P. IL-17 in rheumatoid arthritis: a new target for treatment or just another cytokine? Joint Bone Spine. 2004;71:87–90. doi: 10.1016/j.jbspin.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Lubberts E, Schwarzenberger P, Huang W, Schurr JR, Peschon JJ, van den Berg WB, Kolls JK. Requirement of IL-17 Receptor Signaling in Radiation-Resistant Cells in the Joint for Full Progression of Destructive Synovitis. J Immunol. 2005;175:3360–3368. doi: 10.4049/jimmunol.175.5.3360. [DOI] [PubMed] [Google Scholar]
- 7.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, Mckenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, it-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Ley K, Smith E, Stark MA. IL-17A-producing neutrophil-regulatory Tn lymphocytes. Immunol Res. 2006;34:229–242. doi: 10.1385/IR:34:3:229. [DOI] [PubMed] [Google Scholar]
- 14.Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat Rev Immunol. 2006;6:329–334. doi: 10.1038/nri1807. [DOI] [PubMed] [Google Scholar]
- 15.Steinman L. A brief history of TH17, the first major revision in the TH1/TH2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 16.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 17.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-[beta] induces development of the TH17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 19.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 20.Nishihara M, Ogura H, Ueda N, Tsuruoka M, Kitabayashi C, Tsuji F, Aono H, Ishihara K, Huseby E, Betz UAK, Murakami M, Hirano T. IL-6-gp130-STAT3 in T cells directs the development of IL-17+ Th with a minimum effect on that of Treg in the steady state. Int Immunol. 2007;19:695–702. doi: 10.1093/intimm/dxm045. [DOI] [PubMed] [Google Scholar]
- 21.Zhou L, I, Ivanov I, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 22.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 23.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJM, O’Shea JJ, Hennighausen L, Ernst M, Hunter CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 24.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 25.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O’Shea JJ. Interleukin-2 Signaling via STAT5 Constrains T Helper 17 Cell Generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, Cohen JI, Spriggs MK. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 27.Toy D, Kugler D, Wolfson M, Bos TV, Gurgel J, Derry J, Tocker J, Peschon J. Cutting Edge: Interleukin 17 Signals through a Heteromeric Receptor Complex. J Immunol. 2006;177:36–39. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- 28.Happel KI, Zheng M, Young E, Quinton LJ, Lockhart E, Ramsay AJ, Shellito JE, Schurr JR, Bagby GJ, Nelson S, Kolls JK. Cutting Edge: Roles of Toll-Like Receptor 4 and IL-23 in IL-17 Expression in Response to Klebsiella pneumoniae Infection. J Immunol. 2003;170:4432–4436. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly MN, Kolls JK, Happel K, Schwartzman JD, Schwarzenberger P, Combe C, Moretto M, Khan IA. Interleukin-17/interleukin-17 receptor-mediated signaling is important for generation of an optimal polymorphonuclear response against Toxoplasma gondii infection. Infect Immun. 2005;73:617–621. doi: 10.1128/IAI.73.1.617-621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forlow SB, Schurr JR, Kolls JK, Bagby GJ, Schwarzenberger PO, Ley K. Increased granulopoiesis through interleukin-17 and granulocyte colony-stimulating factor in leukocyte adhesion molecule-deficient mice. Blood. 2001;98:3309–3314. doi: 10.1182/blood.v98.12.3309. [DOI] [PubMed] [Google Scholar]
- 32.Schwarzenberger P, La Russa V, Miller A, Ye P, Huang W, Zieske A, Nelson S, Bagby GJ, Stoltz D, Mynatt RL, Spriggs M, Kolls JK. IL-17 Stimulates Granulopoiesis in Mice: Use of an Alternate, Novel Gene Therapy-Derived Method for In Vivo Evaluation of Cytokines. J Immunol. 1998;161:6383–6389. [PubMed] [Google Scholar]
- 33.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001;25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 34.Schwarzenberger P, Huang W, Ye P, Oliver P, Manuel M, Zhang Z, Bagby G, Nelson S, Kolls JK. Requirement of Endogenous Stem Cell Factor and Granulocyte-Colony-Stimulating Factor for IL-17-Mediated Granulopoiesis. J Immunol. 2000;164:4783–4789. doi: 10.4049/jimmunol.164.9.4783. [DOI] [PubMed] [Google Scholar]
- 35.Mizgerd JP, Kubo H, Kutkoski GJ, Bhagwan SD, Scharffetter-Kochanek K, Beaudet AL, Doerschuk CM. Neutrophil Emigration in the Skin, Lungs, and Peritoneum: Different Requirements for CD11/CD18 Revealed by CD18-deficient Mice. J Exp Med. 1997;186:1357–1364. doi: 10.1084/jem.186.8.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scharffetter-Kochanek K, Lu H, Norman K, van Nood N, Munoz F, Grabbe S, McArthur M, Lorenzo I, Kaplan S, Ley K, Smith CW, Montgomery CA, Rich S, Beaudet AL. Spontaneous skin ulceration and defective T cell function in CD18 null mice. J Exp Med. 1998;188:119–131. doi: 10.1084/jem.188.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hogg N, Bates PA. Genetic analysis of integrin function in man: LAD-1 and other syndromes. Matrix Biology. 2000;19:211–222. doi: 10.1016/s0945-053x(00)00066-4. [DOI] [PubMed] [Google Scholar]
- 38.Zarbock A, Singbartl K, Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest. 2006;116:3211–3219. doi: 10.1172/JCI29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rivera-Nieves J, Olson T, Bamias G, Bruce A, Solga M, Knight RF, Hoang S, Cominelli F, Ley K. L-selectin, alpha 4 beta 1, and alpha 4 beta 7 integrins participate in CD4+ T cell recruitment to chronically inflamed small intestine. J Immunol. 2005;174:2343–2352. doi: 10.4049/jimmunol.174.4.2343. [DOI] [PubMed] [Google Scholar]
- 40.Simon SI, Green CE. Molecular mechanics and dynamics of leukocyte recruitment during inflammation. Annu Rev Biomed Eng. 2005;7:151–185. doi: 10.1146/annurev.bioeng.7.060804.100423. [DOI] [PubMed] [Google Scholar]
- 41.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 42.Tan W, Huang W, Zhong Q, Schwarzenberger P. IL-17 receptor knockout mice have enhanced myelotoxicity and impaired hemopoietic recovery following gamma irradiation. J Immunol. 2006;176:6186–6193. doi: 10.4049/jimmunol.176.10.6186. [DOI] [PubMed] [Google Scholar]
- 43.Smith E, Zarbock A, Stark MA, Burcin TL, Bruce AC, Foley P, Ley K. IL-23 Is Required for Neutrophil Homeostasis in Normal and Neutrophilic Mice. J Immunol. 2007;179:8274–8279. doi: 10.4049/jimmunol.179.12.8274. [DOI] [PubMed] [Google Scholar]
- 44.Lieschke GJ, Grail D, Hodgson G, Metcalf D, Stanley E, Cheers C, Fowler KJ, Basu S, Zhan YF, Dunn AR. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 1994;84:1737–1746. [PubMed] [Google Scholar]
- 45.McAllister F, Henry A, Kreindler JL, Dubin PJ, Ulrich L, Steele C, Finder JD, Pilewski JM, Carreno BM, Goldman SJ, Pirhonen J, Kolls JK. Role of IL-17A, IL-17F, and the IL-17 Receptor in Regulating Growth-Related Oncogene-{alpha} and Granulocyte Colony-Stimulating Factor in Bronchial Epithelium: Implications for Airway Inflammation in Cystic Fibrosis. J Immunol. 2005;175:404–412. doi: 10.4049/jimmunol.175.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rong Z, Cheng L, Ren Y, Li Z, Li Y, Li X, Li H, Fu XY, Chang Z. Interleukin-17F signaling requires ubiquitination of interleukin-17 receptor via TRAF6. Cellular Signalling. 2007;19:1514–1520. doi: 10.1016/j.cellsig.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 47.Wright JF, Guo Y, Quazi A, Luxenberg DP, Bennett F, Ross JF, Qiu Y, Whitters MJ, Tomkinson KN, Dunussi-Joannopoulos K, Carreno BM, Collins M, Wolfman NM. Identification of an Interleukin 17F/17A Heterodimer in Activated Human CD4+ T Cells. J Biol Chem. 2007;282:13447–13455. doi: 10.1074/jbc.M700499200. [DOI] [PubMed] [Google Scholar]
- 48.Liang SC, Long AJ, Bennett F, Whitters MJ, Karim R, Collins M, Goldman SJ, Dunussi-Joannopoulos K, Williams CMM, Wright JF, Fouser LA. An IL-17F/A Heterodimer Protein Is Produced by Mouse Th17 Cells and Induces Airway Neutrophil Recruitment. J Immunol. 2007;179:7791–7799. doi: 10.4049/jimmunol.179.11.7791. [DOI] [PubMed] [Google Scholar]