Abstract
A change in the serum creatinine is not sensitive for an early diagnosis of acute kidney injury. We evaluated urinary levels of matrix metalloproteinase-9 (MMP-9), N-acetyl-β-D-glucosaminidase (NAG), and kidney injury molecule-1 (KIM-1) as biomarkers for the detection of acute kidney injury. Urine samples were collected from 44 patients with various acute and chronic kidney diseases, and from 30 normal subjects in a cross-sectional study. A case–control study of children undergoing cardio-pulmonary bypass surgery included urine specimens from each of 20 patients without and with acute kidney injury. Injury was defined as a greater than 50% increase in the serum creatinine within the first 48 h after surgery. The biomarkers were normalized to the urinary creatinine concentration at 12, 24, and 36 h after surgery with the areas under the receiver-operating characteristic curve compared for performance. In the cross-sectional study, the area under the curve for MMP-9 was least sensitive followed by KIM-1 and NAG. Combining all three biomarkers achieved a perfect score diagnosing acute kidney injury. In the case–control study, KIM-1 was better than NAG at all time points, but combining both was no better than KIM-1 alone. Urinary MMP-9 was not a sensitive marker in the case–control study. Our results suggest that urinary biomarkers allow diagnosis of acute kidney injury earlier than a rise in serum creatinine.
Keywords: acute kidney injury, MMP-9, NAG, KIM-1
The absence of sensitive and specific biomarkers for the early detection of acute kidney injury (AKI) has impaired progress in the diagnosis and treatment of patients with AKI and has had a detrimental effect on the design and possibly the outcomes of clinical trials of AKI. Traditional blood (creatinine, blood urea nitrogen) and urinary markers of kidney injury (urinary casts, fractional excretion of Na +) are insensitive for the early diagnosis of AKI.1 AKI is a heterogeneous entity associated with various clinical presentations, treatments, and procedures, and is often seen in the setting of multiple organ failure and sepsis. Many potential therapeutic agents have been tried but with little success. Absence of reliable biomarkers for early detection of injury leads to delay in the introduction of treatment until well into the course of the renal disease. It is possible that a single biomarker will be insufficiently sensitive and specific across the full spectrum of AKI. A panel of biomarkers may be required to optimize the early detection of AKI.
Recently, several protein biomarkers have been evaluated as non-invasive indicators of kidney injury.2 Human kidney injury molecule-1 (KIM-1) is a type 1 transmembrane protein that is not detectable in normal kidney tissue or urine, but is expressed at very high levels in dedifferentiated proximal tubule epithelial cells in human and rodent kidneys after ischemic or toxic injury and in renal cell carcinoma.3–7 We previously reported that a soluble form of cleaved KIM-1 can be detected in the urine of patients with AKI and that KIM-1 is a sensitive urinary biomarker for AKI.4 High urinary KIM-1 expression was also associated with adverse clinical outcomes in patients with AKI.8 N-acetyl-β-D-glucosaminidase (NAG) is a lysosomal enzyme found mainly in proximal tubular cells that has been shown to be a sensitive proximal tubular injury marker in the setting of a wide variety of drugs, environmental toxicants, contrast-induced toxicity, and ischemic acute tubular injury.9–12 Matrix metalloproteinase-9 (MMP-9) is elevated in postischemic kidney tissue in an animal model.13 MMP-9 has not previously been evaluated as a urinary biomarker for AKI. It has been found in disulfide-linked complexes with neutrophil gelatinase-associated lipocalin,14 which has been implicated as an early predictive urinary biomarker of ischemic AKI in pediatric and adult patients after cardiac surgery.15,16 We hypothesized that urine samples from patients with AKI might also contain elevated levels of MMP-9. In this study, we examined the diagnostic utility of urinary MMP-9, NAG, and KIM-1 as biomarkers, singly or in combination, for the detection of AKI in a cross-sectional study in adults and the temporal expression pattern of urinary biomarkers before the development of AKI in a prospective case–control study in children.
RESULTS
Cross-sectional study
Characteristics of patients with AKI
Clinical characteristics of all patients from whom urine was evaluated for the presence of urinary biomarkers are reported in Table 1. Patients with AKI due to ischemia in the setting of sepsis and hypoperfusion (n = 24)/or nephrotoxins (n = 1) and contrast-induced nephropathy (n = 4) were diagnosed based on evidence for tubular cell injury including muddy-brown or granular casts in urine sediments, clinical history, and elevation of serum creatinine. In 12 patients, ischemia was associated with sepsis and in nine other patients, cardiac arrest and cardiogenic shock accompanied myocardium infarctions or various cardiovalvular operations. In the remaining two patients, ischemia developed in the setting of massive hepatic artery bleeding and trauma. One patient developed AKI in the setting of rhabdomyolysis. Eleven of 29 patients required renal replacement therapy. Eight patients with AKI died in hospital. Each patient with contrast-induced nephropathy had (1) exposure to contrast dye with a well-documented elevation of serum creatinine and blood urea nitrogen temporally related to the contrast dye exposure, and (2) rapidly reversible AKI.
Table 1.
Baseline characteristics of patients with renal diseases and control in cross-sectional study
| Control (n=45)
|
||||
|---|---|---|---|---|
| Characteristic | AKI (n=29) | Normal (n=30) | CKD (n=15) | UTI (n=10) |
| Age (years) | 59.0±3.6 | 40.8±2.4 | 69.2±2.4 | 49.8±8.1 |
| Sex (M/F) | 19/10 | 15/15 | 10/5 | 2/8 |
| Baseline serum creatinine (mg per 100 ml) | 1.3±0.1 | 0.9±0.1 | 2.4±0.3 | 0.9±0.1 |
| Peak serum creatinine (mg per 100 ml) | 4.3±0.4 | |||
| Serum creatinine at time of urine collection (mg per 100 ml) | 3.2±0.2 | |||
AKI, acute kidney injury; CKD, chronic kidney disease; UTI, urinary tract infection.
Values are means (±s.e.).
Quantitation of human urinary MMP-9, NAG, and KIM-1 proteins
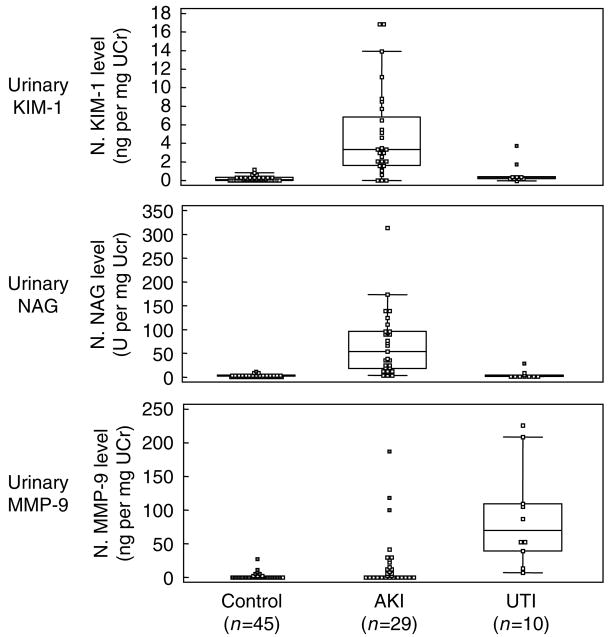
Comparison of median normalized biomarker levels among patients with AKI, control, or urinary tract infection (UTI) in the cross-sectional study is shown in Table 2. Patients with AKI had higher levels of KIM-1 (P<0.001), NAG (P<0.001), and MMP-9 (P<0.001) than control subjects. KIM-1 and NAG levels were higher in patients with AKI than in patients with UTI (P = 0.002, P<0.001, respectively). In contrast, MMP-9 levels were higher in those with UTI (P<0.001). Normalized levels of urinary MMP-9, NAG, and KIM-1 are shown in combined box and whisker and dot plot in Figure 1.
Table 2.
Comparison of various urinary biomarker levels in cross-sectional study
| Group (n) | Normalized KIM-1 (ng mg−1 Ucr) | Normalized NAG (U mg−1 Ucr) | Normalized MMP-9 (ng mg−1 Ucr) |
|---|---|---|---|
| AKI (n=29) | 3.3 (2.1–5.5)*, ^ | 53.6 (20.0–93.5)*, ** | 3.9 (0.2–14.9)* |
| Control (n=45) | 0.1 (0.1–0.2) | 2.7 (2.3–3.8) | 0.0 (0.0–0.3) |
| Normal (n=30) | 0.1 (0.0–0.2) | 2.3 (1.9–3.4) | 0.0 (0.0–0.7) |
| CKD (n=15) | 0.1 (0.1–0.4) | 4.7 (2.4–7.0) | 0.0 (0.0–1.3) |
| UTI (n=10) | 0.4 (0.2–2.0) | 4.1 (2.2–11.4) | 66.9 (11.3–203.3)*, ^^ |
AKI, acute kidney injury; CKD, chronic kidney disease; KIM-1, kidney injury molecule-1; MMP-9, matrix metalloproteinase-9; NAG, N-acetyl-β-D-glucosaminidase; Ucr, urine creatinine; UTI, urinary tract infection.
Values are medians (95% CI for median).
P<0.001 vs control;
P<0.001 vs UTI;
P=0.002 vs UTI;
P<0.001 vs AKI.
Figure 1. Comparison of various urinary biomarkers in cross-sectional study.
Combined box and whisker and dot plot of normalized urinary MMP-9, NAG, and KIM-1 levels is shown among control (non-AKI), AKI, and UTI groups. AKI, acute kidney injury; UTI, urinary tract infection.
Receiver-operating characteristic curves analysis
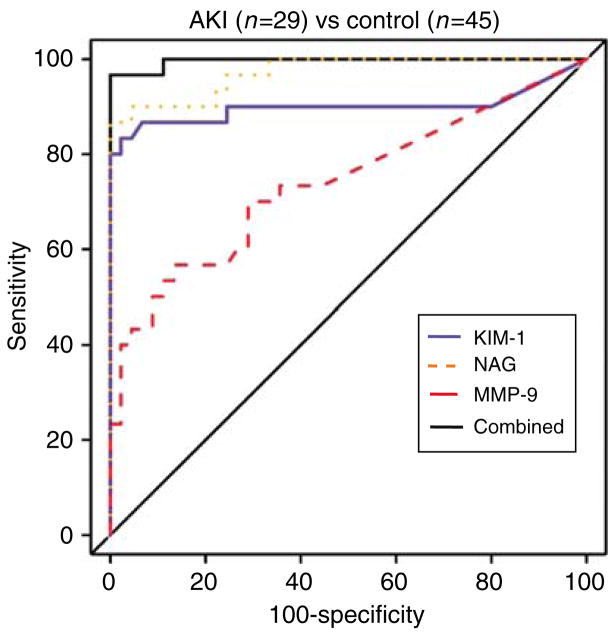
The performance of each biomarker in differentiating patients with AKI from control group is illustrated by receiver-operating characteristic curves (ROC) curves in Figure 2. The areas under the curve (AUCs) and 95% confidence interval for the diagnosis of AKI using normalized MMP-9, NAG, KIM-1, as well as the combination of all three biomarkers, were 0.74 (0.63, 0.83), 0.97 (0.91, 1.00), 0.90 (0.81, 0.96), and 1.00 (0.98, 1.00), respectively. Comparisons of AUCs between each pair of biomarkers was as follows: KIM-1 vs NAG, P = 0.08; KIM-1 vs MMP-9, P = 0.03; and NAG vs MMP-9, P<0.001. Urinary NAG and KIM-1 had the best diagnostic performances for detecting AKI in the cross-sectional study.
Figure 2. ROC analysis for normalized urinary biomarkers in cross-sectional study.
ROC curves of normalized MMP-9, NAG, and KIM-1 as a single test and in combination were plotted. The greater the displacement above and to the left of the line identified, the greater the likelihood that raised values of the test will identify AKI.
Case–control study
Patient characteristics
The clinical and demographic characteristics of 40 pediatric patients from whom urine was evaluated for the presence of urinary biomarkers are reported in Table 3. AKI was diagnosed in eight of 20 patients between 24 and 48 h following cardiopulmonary bypass (CPB), and between 48 and 72 h in the remaining 12 patients. No differences were noted between patients with and without AKI with respect to age, sex, and race. CPB time was significantly longer in patients with AKI (P<0.001). The increases in serum creatinine on postoperative days were statistically significantly different from the baseline and non-AKI groups (P<0.05).
Table 3.
Patient’s characteristic and clinical outcome in case–control study
| AKI (n=20) | Non-AKI (n=20) | |
|---|---|---|
| Age (years) | 2.0±1.2 | 4.4±1.3 |
| Sex (M/F) | 13/7 | 11/9 |
| Serum creatinine (mg per 100 ml) | ||
| Baseline (Pre-Op) | 0.40±0.04 | 0.45±0.04 |
| Post-Op (immediately) | 0.45±0.05 | 0.42±0.04 |
| Post-Op day 1 | 0.59±0.08^, ^^ | 0.41±0.04 |
| Post-Op day 2 | 0.79±0.08*, ** | 0.41±0.04 |
| Post-Op day 3 | 0.65±0.08^, ^^ | 0.42±0.06 |
| Cardiopulmonary bypass time (min) | 179.0±13.6* | 74.5±7.5 |
| Duration of AKI (days) | 2.8±0.4 | NA |
| Renal replacement therapy | None | NA |
AKI, acute kidney injury; NA, not available.
Values are means (±s.e.).
P<0.001 vs non-AKI;
P<0.05 vs non-AKI.
P<0.001 vs baseline;
P<0.05 vs baseline.
Quantitation of serial urinary MMP-9, NAG, and KIM-1 proteins
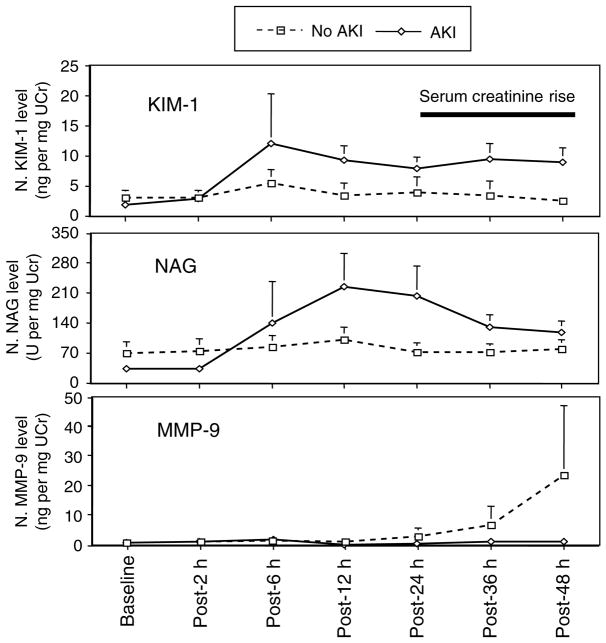
Comparisons of median and mean normalized biomarker levels among patients with and without AKI in the case–control study are shown in Table 4 and Figure 3, respectively. Urinary NAG and KIM-1 were higher among patients with AKI at multiple time points after CPB. Urinary KIM-1 increased at 6–12 h after CPB and remained significantly elevated up to 48 h after CPB. Urinary NAG increased within 6 h and remained elevated up to 48 h after CPB. Urinary MMP-9 was not elevated in patients with or without AKI, and was not a sensitive AKI biomarker in the case–control study.
Table 4.
Comparison of various urinary marker levels in patients with AKI and non-AKI
| Post-Op
|
|||||||
|---|---|---|---|---|---|---|---|
| Pre-Op | 2 h | 6 h | 12 h | 24 h | 36 h | 48 h | |
| Normalized value | |||||||
| KIM-1 (ng mg−1 Ucr) | * | * | * | * | |||
| AKI | 0.2 (0.0–1.2) | 0.6 (0.0–2.3) | 0.7 (0.2–2.7) | 3.6 (1.8–18.0) | 4.6 (1.2–14.9) | 4.0 (1.9–14.5) | 4.7 (3.2–10.8) |
| Non-AKI | 0.4 (0.0–1.9) | 1.1 (0.0–3.9) | 1.4 (0.2–2.6) | 0.4 (0.0–1.6) | 0.5 (0.0–1.6) | 0.6 (0.0–2.3) | 0.9 (0.1–3.1) |
| NAG (U mg−1 Ucr) | ** | ** | ** | ||||
| AKI | 17.6 (5.5–52.9) | 21.1 (8.6–54.8) | 32.0 (10.0–70.9) | 78.1 (46.7–255.2) | 74.1 (47.2–241.8) | 79.1 (53.5–186.9) | 80.9 (46.3–128.5) |
| Non-AKI | 28.6 (15.0–59.5) | 32.1 (18.3–64.6) | 31.9 (21.3–122.0) | 39.7 (22.7–117.0) | 38.6 (19.3–71.0) | 38.1 (15.0–88.9) | 44.8 (14.6–87.7) |
| MMP-9 (ng mg−1 Ucr) | |||||||
| AKI | ND | ND | ND | ND | ND | ND | ND |
| Non-AKI | ND | ND | ND | ND | ND | ND | ND |
AKI, acute kidney injury; ND, non-detectable; Ucr, urine creatinine.
Values are medians (95% CI for median).
P<0.005 vs non-AKI;
P<0.05 vs non-AKI.
Figure 3. Pattern of urinary biomarker expression in case–control study.
Graphs showing mean normalized urine KIM-1, NAG, and MMP-9 concentrations at multiple time points after cardiopulmonary bypass. Error bars are s.e.
ROC analysis of urinary MMP-9, NAG, KIM-1, and combination of NAG and KIM-1 as early biomarkers for detection of AKI
The performance of MMP-9, NAG, and KIM-1 in diagnosing AKI is illustrated in Table 5. At 12, 24, and 36 h after CPB, the AUCs for the diagnosis of AKI using KIM-1 were 0.83, 0.78, and 0.84; the AUCs for MMP-9 were 0.50, 0.53, and 0.53; the AUCs for NAG were 0.69, 0.70, and 0.71; and the AUCs for combination of NAG and KIM-1 were 0.83, 0.79, and 0.85 (Table 5). Table 6 demonstrates the sensitivity; specificity; and positive and negative likelihood ratio at 6, 12, and 24 h after cardiopulmonary bypass for diagnosis of AKI. MMP-9 was excluded in combination of biomarkers because it was not elevated in patients with or without AKI. The performance difference between KIM-1 and NAG by AUC for diagnosis of AKI was not statistically significantly different. There was no added value for combining two biomarkers for detection of AKI in our case–control study.
Table 5.
AUCs for urinary biomarkers at various time points in case–control study
| Time after cardiopulmonary bypass
|
||||||
|---|---|---|---|---|---|---|
| 2 h | 6 h | 12 h | 24 h | 36 h | 48 h | |
| Normalized urinary biomarkers | ||||||
| KIM-1 95% CI | 0.57 (0.50–0.73) | 0.52 (0.50–0.71) | 0.83 (0.67–0.96) | 0.78 (0.61–0.92) | 0.84 (0.69–0.96) | 0.81 (0.65–0.94) |
| NAG 95% CI | 0.65 (0.51–0.73) | 0.58 (0.50–0.76) | 0.69 (0.52–0.85) | 0.70 (0.54–0.87) | 0.71 (0.54–0.87) | 0.66 (0.51–0.84) |
| MMP-9 95% CI | 0.55 (0.50–0.69) | 0.51 (0.50–0.63) | 0.50 (0.50–0.62) | 0.53 (0.50–0.61) | 0.53 (0.50–0.63) | 0.52 (0.50–0.63) |
| Combined (NAG, KIM-1) 95% CI | 0.66 (0.52–0.83) | 0.61 (0.50–0.80) | 0.83 (0.69–0.96) | 0.79 (0.62–0.93) | 0.85 (0.69–0.96) | 0.81 (0.68–0.94) |
CI, confidence interval; KIM-1, kidney injury molecule-1; MMP-9, matrix metalloproteinase-9; NAG, N-acetyl-β-D-glucosaminidase.
Table 6.
Performance of urinary KIM-1 and NAG for diagnosis of AKI at various time points in case–control study
| Sensitivity | Specificity | Positive likelihood ratio | Negative likelihood ratio | |
|---|---|---|---|---|
| Urinary KIM-1 at 6 h | ||||
| >2.0 ng mg−1 Ucr | 65 | 42 | 1.12 | 0.83 |
| >7.0 ng mg−1 Ucr | 85 | 21 | 1.08 | 0.71 |
| >18.0 ng mg−1 Ucr | 90 | 16 | 1.07 | 0.63 |
| Urinary KIM-1 at 12 h | ||||
| >2.0 ng mg−1 Ucr | 74 | 90 | 7.37 | 0.29 |
| >7.0 ng mg−1 Ucr | 32 | 90 | 3.16 | 0.76 |
| >18.0 ng mg−1 Ucr | 21 | 90 | 2.11 | 0.88 |
| Urinary KIM-1 at 24 h | ||||
| >2.0 ng mg−1 Ucr | 65 | 80 | 3.25 | 0.44 |
| >7.0 ng mg−1 Ucr | 35 | 90 | 3.50 | 0.72 |
| >18.0 ng mg−1 Ucr | 20 | 90 | 2.00 | 0.89 |
| Urinary NAG at 6 h | ||||
| >10.0 U mg−1 Ucr | 25 | 84 | 1.58 | 0.89 |
| >25.0 U mg−1 Ucr | 50 | 63 | 1.36 | 0.79 |
| >40.0 U mg−1 Ucr | 55 | 37 | 0.87 | 1.22 |
| Urinary NAG at 12 h | ||||
| >10.0 U mg−1 Ucr | 100 | 10 | 1.11 | 0.00 |
| >25.0 U mg−1 Ucr | 100 | 30 | 1.43 | 0.00 |
| >40.0 U mg−1 Ucr | 84 | 55 | 1.87 | 0.29 |
| Urinary NAG at 24 h | ||||
| >10.0 U mg−1 Ucr | 95 | 20 | 1.19 | 0.25 |
| >25.0 U mg−1 Ucr | 85 | 25 | 1.13 | 0.60 |
| >40.0 U mg−1 Ucr | 80 | 55 | 1.78 | 0.36 |
AKI, acute kidney injury; KIM-1, kidney injury molecule-1; NAG, N-acetyl-β-D-glucosaminidase; Ucr, urine creatinine.
DISCUSSION
The traditional laboratory approach for detection of renal disease does not allow for early detection of acute renal injury. Damage to renal tubules can be insufficient to result in a change in a parameter of kidney function such as serum creatinine. In addition, in cases of more extensive tubular injury, there is a lag in time between the injury and an increase in serum creatinine. Sensitive biologic markers of renal tubular injury are needed in order to detect early kidney injury. In this study, we demonstrate that (1) urinary MMP-9, NAG, and KIM-1 can be detected in AKI, and that the concentration of each marker is significantly higher in urine samples from patients with AKI compared with urine samples from patients with chronic kidney disease (CKD) and normal controls in cross-sectional study; (2) MMP-9 is associated with AKI but does not differentiate patients with AKI from those with UTI; (3) urinary NAG and KIM-1 are elevated well before an increase in serum creatinine in a prospective case–control study in children undergoing CPB; and (4) we cannot conclude whether combining biomarkers enhances diagnostic performance for detection of AKI in the prospective case–control study.
There have been over 16 definitions used for the diagnosis of acute renal failure in previously published studies, with most of them based on serum creatinine values. Recently the Acute Kidney Injury Network has supported the use of the term ‘acute kidney injury’ to reflect the broad spectrum of acute kidney disease, including conditions that do not progress to ‘failure’.17 Nevertheless, in the absence of a more widely accepted biomarker for injury, serum creatinine continues to be used. The change in serum creatinine, however, does not discriminate the time and type of renal insult or the site and extent of glomerular or tubular injury. Levels are relatively insensitive to small changes in GFR and may lag behind changes in GFR by several days. Therefore, it is a challenge to detect acute tubular injury in a timely manner so that intervention can be initiated. Recently, several biomarkers have been explored for the early diagnosis of AKI, including neutrophil gelatinase-associated lipocalin (NGAL), interleukin-18, cystatin C, and KIM-1.2 None of these markers, however, have been systematically evaluated in various clinical settings of AKI. In this study, we demonstrated that urinary MMP-9, NAG, and KIM-1 are useful biomarkers for the identification of established AKI in a cross-sectional study of patients with clinical and laboratory evidence for AKI. Then we determined the temporal expression pattern of urinary MMP-9, NAG, and KIM-1 from the onset of AKI in children undergoing open-heart surgery. Urinary NAG and KIM-1 increased in the urine before an increase in serum creatinine. In a prospective study in children, 15 out of 20 AKI patients’ serum creatinine returned to baseline by 5 days after cardio-pulmonary bypass. Since there was no pathologic evaluation of the kidney, we cannot relate the biomarkers to injury directly, but, given the mean increase of creatinine, the AKI suffered was likely a result of mild to moderate tubular injury.
The heterogeneity of AKI suggests that more than one marker may be necessary to obtain sufficient sensitivity and specificity for AKI screening. Analysis of multiple biomarkers may optimize early detection of AKI as in the case of prostate cancer detection.18 The main challenge is how to combine multiple biomarkers for clinical use. A naive approach to combining multiple biomarkers is to call a result positive if either one or both biomarkers are positive. Such an approach, however, can be inefficient and increase sensitivity at the expense of specificity, or vice versa. In this study, we used a linear combination that maximized the area under ROC for detection of AKI. The usefulness of this approach remains to be validated in future studies.
We recognize that normalization of urinary MMP-9, NAG, and KIM-1 concentrations to urine creatinine concentration is less than ideal for a number of reasons including the non-steady state of creatinine balance in patients with AKI. We analyzed our data using both absolute and normalized values in our study and found the results did not markedly differ.
There are a number of limitations to our observational study. The population studied is not representative of the global group of patients with AKI. The study is based on two different populations, with different characteristics. The cross-section study includes the important group of AKI patients who suffer ischemic events from either sepsis or cardiovascular events, which are the major causes of hospital-acquired AKI.19 The prospective case–control group was restricted to children undergoing open-heart surgery. We excluded prerenal azotemia, diagnosed by clinical criteria, in the cross-sectional study, but it is possible that there might have been cases of prerenal etiologies in the case–control study, given the rapid reversibility of serum creatinine to baseline in AKI group. We did not find that a combination of urinary biomarkers enhances diagnostic performance of AKI in our case–control study, perhaps due to the small sample size. There is currently very limited data available regarding temporal expression patterns of various urinary biomarkers, including NGAL, KIM-1. interleukin-18, MMP-9, and cystatin C, for various clinical settings of AKI from the onset of renal insult. The usefulness of combining multiple biomarkers remains to be validated in further studies. In a follow-up study involving a larger number of adult patients, we are currently evaluating whether a panel of urinary biomarkers enhances the detection of AKI.
This study utilized samples from all of the AKI cases and less than half of the non-AKI cases from a prospective study in children undergoing cardiopulmonary bypass where AKI was defined as an increase of 50% in serum creatinine. It is not possible to determine if any of these patients had prerenal azotemia. In that study, urinary NGAL was measured and found to have an AUC–ROC of 0.99 at 2 h and 1.00 at 4 h following CPB;15 a subsequent study using all of the AKI cases and under half of the non-AKI cases showed urinary interleukin-18 to have an AUC–ROC of 0.61 at 4 h, 0.75 at 12 h, and 0.73 at 24 h following CPB.20 In this study, urinary KIM-1 had an AUC-ROC of 0.57 at 2 h, 0.83 at 12 h, and 0.78 at 24 h. Comparisons among the three biomarkers (NGAL from the original study and interleukin-18 and KIM-1 at later dates using frozen samples), however, should be made with caution for a variety of reasons. The cohorts were all children with a small sample size. Patients with CKD were excluded from the cohort. There were no patients who required dialysis or died. The heterogeneity of AKI was not well addressed. In addition, the urine samples were thawed and refrozen two times before this study and were frozen for about 2 years at −80°C. We do not know the influence of prolonged duration of storage at −80°C and number of freeze–thaw cycles on stability of various urinary biomarkers at the present time. Our unpublished data indicated that urinary KIM-1 remained stable after over 1 year of storage at −80°C without repeat freeze and thaw cycles. It is possible that the poor performance of urinary MMP-9 in the case–control study may be explained by instability of MMP-9 during prolonged freezing and, thus, further study is necessary to determine its utility as AKI biomarker.
In conclusion, we have demonstrated that biomarkers may improve the diagnosis of renal proximal tubule injury and may result in early detection of AKI. Larger prospective studies are necessary to validate the temporal expression pattern of various urinary biomarkers for early detection of AKI, how to combine multiple biomarkers for early detection of AKI, and how this temporal course relates to the onset, severity, and outcome of AKI.
MATERIALS AND METHODS
Patient selection and urine collections
Cross-sectional study
In order to identify biomarkers that are sensitive and specific for the diagnosis of established AKI, 29 patients with various causes of AKI were selected from patients seen for renal consultation at Brigham and Women’s Hospital (19 males, 10 females; mean: 59 years) between November 2004 and March 2005. In this study, all patients with AKI had a 50% or greater increase in serum creatinine from baseline and muddy-brown granular or granular casts in urine sediments on microscopic examination. An additional 15 patients with various CKDs were selected from the renal clinic at Brigham and Women’s Hospital (10 males, five females; mean: 69.2 years). Patients were considered to have CKD if (1) they had a documented renal disease, which was diagnosed by renal biopsy and/or on clinical grounds and (2) the Scr remained relatively stable (± 0.5 mg per 100 ml) for at least 6 months. All patients in this group had stage 3 to 5 CKD as estimated by the Modification of Diet in Renal Disease study equation.21 CKD etiologies represented in this study include focal segmental glomerulosclerosis, hypertensive nephrosclerosis, renal artery stenosis, diabetic nephropathy, and lupus nephritis. Urine samples were also collected from 30 healthy subjects without known renal disease with bland urine sediments and from 10 patients with UTI without evidence of previous renal disease or AKI. All 10 UTI patients had a positive leukocyturia and urine culture with greater than 105 colonies. Relevant clinical information was obtained from the review of patients’ records. Spot urine samples were collected at the time of renal consult or outpatient clinic visit.
Case–control study
In order to study the expression pattern of biomarkers before the development of AKI, we analyzed urine samples that were collected prospectively in patients who were admitted to Cincinnati Children’s Hospital for surgical correction of congenital heart disease between January 2004 and November 2004.15 Exclusion criteria included CKD, diabetes mellitus, peripheral vascular disease, and use of nephrotoxic drugs before or during the study period. Urine samples were collected before surgery and every 2 h for the first 12 h and then once every 12 h. Serum creatinine was measured at baseline, routinely monitored at least twice a day in the immediate postoperative period, and at least daily after postoperative day 3. Postoperative AKI was defined as a 50% or greater increase in serum creatinine from baseline. A total of 280 urine samples were collected from all of the 20 pateints with AKI and randomly selected 20 patients without AKI. Serial urinary MMP-9, NAG, and KIM-1 levels were measured at the various time points in this study. Laboratory personal (A Johnson) was blinded to AKI and non-AKI status.
The institutional review boards of Brigham and Women’s Hospital and the Cincinnati Children’s Hospital Medical Center approved the study.
Urine samples
In the cross-sectional study, urine samples were centrifuged to remove cellular components and debris and the supernatant was stored at −80°C. All urine samples were analyzed by urinary dipstick before centrifugation (Multistix 8 SG; Bayer Corporation, Tarrytown, NY, USA) and by microscopic examination by one of the authors (WK Han) (using Olympus microscope). Urine samples in the case–control study were centrifuged at 2000 g for 5 min and the supernatants were stored at −80°C. The samples were thawed and refrozen two times before this study. The time between initial urine collection and assay at Brigham and Women’s Hospital was about 2 years.
Measurement of urinary MMP-9, NAG, and KIM-1
Urinary soluble KIM-1 protein was quantified by a modified enzyme-linked immunosorbent assay system as previously described.4 Briefly, the wells of an enzyme-linked immunosorbent assay plate (MaxiSorp; Nunc, Naperville, IL, USA) were coated with anti-KIM-1 polyclonal antibody (overnight incubation at 4°C with 200 μl of antibody at 1.0 μg ml−1 in 50 mM carbonate solution). The wells were blocked with a bovine serum albumin solution (1% in phosphate-buffered saline) and were washed four times with PBST (phosphate-buffered saline with 0.05% Tween 20). Urine samples (100 μl) were then added to each well at room temperature for 3 h. After four washes with PBST, biotinylated anti-KIM-1 monoclonal antibody (AWE-2) was added, followed by horseradish peroxidase-conjugated streptavidin and tetramethylbenzidine as substrate. Total urine MMP-9 concentration was measured using a commercially available enzyme-linked immunosorbent assay kit (Quantikine; R&D systems, Minneapolis, MN, USA). NAG was measured by colorimetric assay by using a commercially available kit (Roche Applied Science, Indianapolis, IN, USA). The measured values were normalized to the urinary creatinine concentration. The inter-assay and intra-assay coefficients of variation for MMP-9, NAG, and KIM-1 were less than 10%.
Serum and urinary creatinine determination
Serum and urine creatinine concentrations were measured by the Jaffé assay using Roche/Hitachi 917 and 911 systems (Roche Diagnostics, Indianapolis, IN, USA), respectively.
Statistical analysis
Continuous variables were compared using the Student’s t-test or the non-parametric Mann–Whitney U-test, as appropriate. For analysis of single biomarkers, ROC curves were drawn and the AUCs calculated and compared using the method of Hanley and McNeil22 for the cross-sectional data and comparison for a single time point for the case–control data. Two-tailed P-values less than 0.05 were considered statistically significant. For joint analysis of multiple biomarkers, the linear combination that maximized the area under the ROC curve was found.23 For the cross-sectional study, the formula for multiple biomarkers was [normalized KIM-1 concentration] +0.18[normalized NAG concentration] + 0.1[MMP-9 concentration]. For the case–control study, the formula was [normalized KIM-1 concentration] + 0.25[normalized NAG concentration]. Bootstrap percentile confidence intervals were calculated using 2000 resamples. Statistical analyses were performed using SAS Version 9.1 (SAS Institute, Cary, NC, USA), MedCalc Version 9.3.1 (MedCalc Inc., Mariakerke, Belgium), and R version 2.1.1 (http://www.r-project.org).
Acknowledgments
This work was supported in part by National Institutes of Health grants RO1-DK39773, DK074099 and DK072381 (JVB), RO1-DK53289 (PD), and KO8-DK64075 (WKH). This work was presented in part at the 2005 and 2006 (Philadelphia, PA, USA 8 to 13 November 2005; San Diego, CA, USA 14 to 19 November 2006) American Society of Nephrology annual meetings and published in abstract forms (J Am Soc Nephrol 16: 316A, 2005 and J Am Soc Nephrol 17: 403A, 2006).
References
- 1.Star RA. Treatment of acute renal failure. Kidney Int. 1998;54:1817–1831. doi: 10.1046/j.1523-1755.1998.00210.x. [DOI] [PubMed] [Google Scholar]
- 2.Han WK, Bonventre JV. Biologic markers for the early detection of acute kidney injury. Curr Opin Crit Care. 2004;10:476–482. doi: 10.1097/01.ccx.0000145095.90327.f2. [DOI] [PubMed] [Google Scholar]
- 3.Ichimura T, Bonventre JV, Bailly V, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is upregulated in renal cells after injury. J Biol Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 4.Han WK, Bailly V, Abichandani R, et al. Kidney injury molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 5.Ichimura T, Hung CC, Yang SA, et al. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004;286:F552–F563. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- 6.Han WK, Alinani A, Wu CL, et al. Human kidney injury molecule-1 is a tissue and urinary tumor marker of renal cell carcinoma. J Am Soc Nephrol. 2005;16:1126–1134. doi: 10.1681/ASN.2004070530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin F, Zhang PL, Yang XJ, et al. Human kidney injury molecule-1 (hKIM-1): a useful immunohistochemical marker for diagnosing renal cell carcinoma and ovarian clear cell carcinoma. Am J Surg Pathol. 2007;31:371–381. doi: 10.1097/01.pas.0000213353.95508.67. [DOI] [PubMed] [Google Scholar]
- 8.Liangos O, Perianayagam MC, Vaidya VS, et al. Urinary N-acetyl-beta-(D)-glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J Am Soc Nephrol. 2007;18:904–912. doi: 10.1681/ASN.2006030221. [DOI] [PubMed] [Google Scholar]
- 9.Gibey R, Dupond JL, Alber D, et al. Predictive value of urinary N-acetyl-beta-D-glucosaminidase (NAG), alanine-aminopeptidase (AAP) and beta-2-microglobulin (beta 2M) in evaluating nephrotoxicity of gentamicin. Clin Chim Acta. 1981;116:25–34. doi: 10.1016/0009-8981(81)90165-0. [DOI] [PubMed] [Google Scholar]
- 10.Tolkoff-Rubin NE, Rubin RH, Bonventre JV. Noninvasive renal diagnostic studies. Clin Lab Med. 1988;8:507–526. [PubMed] [Google Scholar]
- 11.Stonard MD, Gore CW, Oliver GJ, et al. Urinary enzymes and protein patterns as indicators of injury to different regions of the kidney. Fundam Appl Toxicol. 1987;9:339–351. doi: 10.1016/0272-0590(87)90056-x. [DOI] [PubMed] [Google Scholar]
- 12.Westhuyzen J, Endre ZH, Reece G, et al. Measurement of tubular enzymuria facilitates early detection of acute renal impairment in the intensive care unit. Nephrol Dial Transplant. 2003;18:543–551. doi: 10.1093/ndt/18.3.543. [DOI] [PubMed] [Google Scholar]
- 13.Basile DP, Fredrich K, Weihrauch D, et al. Angiostatin and matrix metalloprotease expression following ischemic acute renal failure. Am J Physiol Renal Physiol. 2004;286:F893–F902. doi: 10.1152/ajprenal.00328.2003. [DOI] [PubMed] [Google Scholar]
- 14.Lander ES, Linton LM, Birren B, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 15.Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 16.Wagener G, Jan M, Kim M, et al. Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology. 2006;105:485–491. doi: 10.1097/00000542-200609000-00011. [DOI] [PubMed] [Google Scholar]
- 17.American Society of Nephrology Renal Research Report. J Am Soc Nephrol. 2005;16:1886–1903. doi: 10.1681/ASN.2005030285. [DOI] [PubMed] [Google Scholar]
- 18.Mikolajczyk SD, Song Y, Wong JR, et al. Are multiple markers the future of prostate cancer diagnostics? Clin Biochem. 2004;37:519–528. doi: 10.1016/j.clinbiochem.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 19.Hou SH, Bushinsky DA, Wish JB, et al. Hospital-acquired renal insufficiency: a prospective study. Am J Med. 1983;74:243–248. doi: 10.1016/0002-9343(83)90618-6. [DOI] [PubMed] [Google Scholar]
- 20.Parikh CR, Mishra J, Thiessen-Philbrook H, et al. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006;70:199–203. doi: 10.1038/sj.ki.5001527. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 22.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 23.Pepe MS, Cai T, Longton G. Combining predictors for classification using the area under the receiver operating characteristic curve. Biometrics. 2006;62:221–229. doi: 10.1111/j.1541-0420.2005.00420.x. [DOI] [PubMed] [Google Scholar]