Abstract
Age-related changes in the spatial chromatic contrast sensitivity function of detection, measured along S and L – M cone axes, were demonstrated in a companion paper [Hardy et al., J. Opt. Soc. Am. A 22, 49 (2005)]. Here senescent changes in chromatic contrast appearance were assessed by contrast-matching functions (CMFs). Luminance and chromatic CMFs (S and L – M axes) were compared for younger (age 18–31 yr) and older (age 65–75 yr) trichromatic subjects by using stimuli that were perceptually anchored to the same physical standard contrasts. Subjects matched the contrast of test gratings of various spatial frequencies (0.5–8 cycles per degree) to the standard stimuli under natural viewing conditions. Because of changes in the visual system with age, the standard stimuli were closer to threshold for older subjects; however, in general, the shapes of the CMFs were similar for both groups. The results suggest that the perception of relative contrasts across spatial frequencies is stable with age.
1. INTRODUCTION
The contrast sensitivity function (CSF) is a useful tool for characterizing spatial sensitivity. CSFs typically plot the reciprocal of the minimum contrast required to detect sine-wave or Gabor patterns over a range of spatial frequencies. Luminance CSFs generally show bandpass characteristics, while chromatic CSFs tend to be low pass (e.g., Refs. 1–4). To quantify performance at suprathreshold levels, one can measure the contrast-matching function (CMF). Here a contrast at a particular spatial frequency is chosen as the standard, and observers match the perceived contrast of patterns with different spatial frequencies to it. CMFs at contrast levels close to threshold tend to show characteristics similar to those of CSFs. However, when contrast levels are raised, the curves tend to flatten, a phenomenon referred to as contrast constancy.2–4
A number of optical, anatomical, and physiological changes occur in the visual system as a consequence of aging. The crystalline lens becomes denser, resulting in a gradual change in the spectra of light reaching the retina. Age-related miosis results in smaller maximum pupil sizes, with age allowing less light to reach the retina.5 There are also reports of ganglion cell loss in the retina (see Spear6 for a review). Visual-evoked potential research shows declines in amplitude and increases in latency of signals largely originating in area V1.7,8 Some research suggests that there may be greater deterioration of the S-cone pathway relative to the L – M and luminance pathways, although the evidence is equivocal.9–13
Previous research has shown that the color appearance of spatially uniform stimuli remains relatively stable with age. Kraft and Werner14 used a hue-scaling task to investigate differences in color appearance and found that younger and older subjects used similar ratings to describe the same chromatic stimuli. Recently Hardy et al.15 found little change in color naming with age, even for stimuli that were most filtered by the aging lens. Achromatic points and unique hue loci have been shown to be relatively stable throughout the life span.16 Luminance CSFs for older subjects show losses of sensitivity, particularly at the higher frequencies, although much of the higher-frequency loss can be attributed to optical factors.17,18 The shape of luminance CMFs is relatively unaffected by age.19
Our companion paper showed that after carefully controlling for chromatic aberration and retinal illuminance differences, there is no selective loss of sensitivity at any of the spatial frequencies tested.20 In addition, after correction for ocular media optical density, losses in sensitivity were similar for both S and L – M chromatic channels. The effect of aging on chromatic CMFs has not previously been studied and is the focus of this paper. In our companion paper,20 observers viewed the stimuli through a telescope that incorporated an artificial pupil and an achromatizing lens. In the present paper, we were interested in testing suprathreshold performance differences in a more natural environment and allowed subjects to directly view stimuli presented on a computer monitor. Heterochromatic flicker photometry was used to ensure that the achromatic stimuli were equiluminant for each observer. Otherwise, no other modifications were made to compensate for age-related changes in the lens and the pupil. The relative naturalism of the conditions was motivated by the need for a model of chromatic contrast appearance at different spatial scales as a function of age.
We asked subjects to match the perceived contrast of sine-wave gratings of various spatial frequencies to a standard pattern to obtain both luminance and chromatic CMFs. The stimuli were modulated along three cardinal axes of cone space (S, L – M, and luminance). All of the standard stimuli were perceptually anchored to the same physical standards for both old and young subjects. The results suggest that there is little change in the overall shape of the CMFs for the two age groups.
2. METHODS
A. General Procedure
CMFs were obtained for younger and older observers at two different luminance levels (5 and 30 cd/m2). S-axis standards were selected at 2 c/deg, and the standards on the two other axes (L – M and luminance) were acquired for each observer separately by asking them to perceptually match the contrast to that of the S-axis standards. CMFs were produced separately for each of the cardinal axes by asking observers to match the perceived contrast of test stimuli of various spatial frequencies to the perceived contrast of the standards. Therefore all CMF matches were in effect perceptually matched to the same physical S-axis standards for all observers. In addition, for each observer we obtained contrast detection threshold values for the S-axis standard stimulus pattern to allow us to describe our stimuli in relation to thresholds.
B. Subjects
Ten younger (five male/five female, mean age 24 yr, range 18–31 yr) and ten older (four male/six female, mean age 72 yr, range 65–75 yr) color-normal subjects participated in the study. Nine of these subjects (four younger and five older) also participated in our companion study.20 The subjects were screened for the presence of abnormal ocular media and retinal disease by means of a slit lamp examination and by taking fundus photographs of the macula and the optic disk, which were examined by a retinal specialist. Intraocular pressure was normal for all observers (≤22 mm Hg). All observers were normal trichromats based on testing with the Neitz anomaloscope, the HRR pseudoisochromatic plates, and the Farnsworth F-2 plate. All subjects had corrected acuity of 20/20 or better in the tested eye. Those in the younger group wore their corrective lenses. For subjects over age 60 yr, the crystalline lens has little remaining accommodative power.21 Therefore the subjects in the older group wore trial lenses for proper refraction at the 1-m test distance. Written informed consent was obtained following the Tenets of Helsinki and with approval of the Office of Human Research Protection of the University of California, Davis, School of Medicine.
C. Apparatus
The stimuli were presented on a 17-in. CRT monitor (Eizo FlexScan T566) driven by a Macintosh G4 400-MHz computer with 14 bits of color resolution obtained by using the Bits++ digital-to-analog converter (Cambridge Research Systems Ltd.) The refresh rate was 85 Hz. The experimental software was written in MATLAB and used the Psychophysics Toolbox extensions.22,23 The monitor was calibrated by using a Minolta colorimeter (CS-100 Chroma Meter) and procedures set out by Brainard et al.24 Smith and Pokorny25,26 cone fundamentals were used to convert between measured monitor RGB outputs and cone space coordinates. The experiment took place in a windowless room with all lights off. Observers dark adapted for a minimum of 2 min before starting the experiment.
D. Stimuli
All stimuli were vertically oriented sinusoidal gratings. The stimuli produced modulation separately along the cardinal axes of cone space (S, L – M, and luminance axes). Chromaticity coordinates corresponding to CIE illuminant C were used as the white point. Heterochromatic flicker photometry (flicker rate of 14.2 Hz) was used to ensure isoluminance for the chromatic stimuli for each subject. The isoluminant point was determined by eliminating (or minimizing) flicker at two different contrasts for each color axis at both luminance levels (5 and 30 cd/m2). The mean of eight settings (four at each contrast) was used as the isoluminant point for the experimental stimuli. The stimuli were presented on a calibrated CRT at two mean luminance levels and subtended a visual angle of 4°. The phase of the sine wave was chosen randomly on each trial. Subjects were placed 1 m from the monitor, and a chin rest was used to stabilize head position.
Chaparro et al.27 specified contrast modulations by combining contrasts for each cone class. Here we use a similar approach based on a formula suggested by Brainard28:
| (1) |
where ci is the Michelson contrast for each cone class and c̄ is the normalized cone contrast vector length. The results from our experiments are plotted by using this contrast metric.
E. Contrast Detection Thresholds
A temporal two-alternative forced-choice task was used together with the QUEST29 adaptive staircase procedure, which was set to converge at 82% correct. Thresholds were estimated by averaging the results of two randomly interleaved staircases for each luminance level. The stimuli were presented for 0.75 s with an interstimulus interval of 0.75 s. The stimulus was the same size and spatial frequency as those for the standard stimuli (see Subsection 2.F). Thresholds were obtained at two luminance levels (5 and 30 cd/m2).
F. Standard Stimuli
Switkes and Crognale30 have shown that observers can reliably make perceptual matches across luminance-varying and isoluminant sinusoidal gratings. We obtained a full set of standard stimuli by first choosing two cone contrast levels along the S axis and then obtaining contrast matches along the L – M and luminance axes for each observer. The chosen S-axis cone contrasts were 0.30 and 0.50 (the maximum S-axis contrast of the monitor was 0.80). These values equated to 0.173 and 0.289 by using the contrast vector metric [see Eq. (1)]. The standard stimulus patterns had a spatial frequency of 2 c/deg. The standard S-axis stimuli and match gratings were presented in the center of the monitor and continuously alternated (presentation time 0.75 s, interstimulus interval 0.75 s). Observers adjusted the test stimuli until the contrast perceptually matched the contrast of the S-axis standard. Four matches were made for each color axis and for each contrast, and the means for each subject were used as the standard stimulus contrasts for the main experiment (see Subsection 2.G).
G. Contrast-Matching Functions
Subjects adjusted the contrast of the test gratings to perceptually match the contrast of the standards. Spatial frequencies of 0.5, 1, 2, and 4 c/deg were matched to the standard for all axes. To avoid luminance artifacts due to chromatic aberration, we did not use chromatic stimuli with spatial frequencies above 4 c/deg.31,32 For our luminance conditions, we used an additional spatial frequency of 8 c/deg. The stimuli were all vertically oriented sine-wave gratings subtending 4° of visual angle. The same matching procedure was used as that in the standard stimuli experiment (see Subsection 2.F). Four matches to each standard were made for each spatial frequency.
3. RESULTS
A. Contrast Detection Thresholds
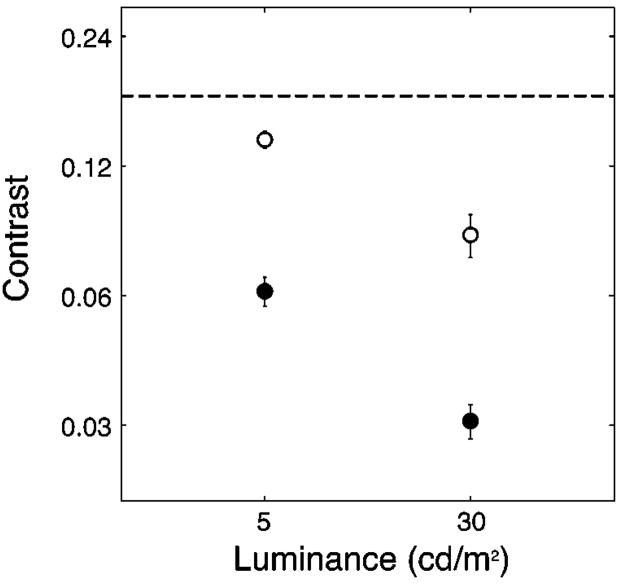
The mean results for both age groups at both luminance levels are shown in Fig. 1 (younger group denoted by solid circles, older group by open circles). The thresholds for the older group are substantially higher than those for the younger group. This is consistent with other threshold measurements made in our laboratory.20 Note that the mean threshold for older subjects for the 5-cd/m2 luminance condition is close to the lower S-axis contrast standard. Thus our standard stimuli were 2.1 and 1.3 times the mean threshold at 5 cd/m2 and 4.7 and 2.8 times the mean threshold at 30 cd/m2 for younger and older observers, respectively.
Fig. 1.
Mean S-axis threshold for younger (solid circles) and older (open circles) observers at two luminance levels (5 and 30 cd/m2). Error bars are ±1 standard error of the mean (SEM). The dashed line indicates the level of the lower-contrast S-axis standard.
B. Standard Stimuli
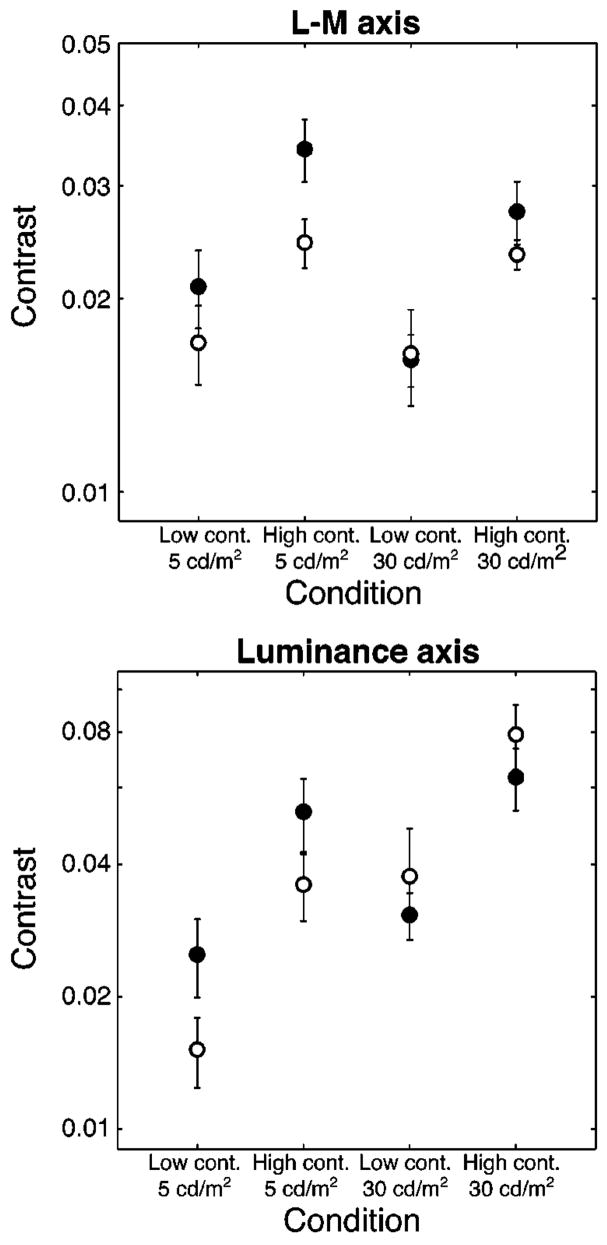
Figure 2 shows the mean settings for both luminance levels for the younger (solid circles) and older (open circles) groups. For the lower-luminance stimuli, the older group matched the S-axis stimuli to lower physical contrast levels on the two other axes. However, at the higher luminance level, the settings were closer for the two age groups.
Fig. 2.
Mean standards for the L – M and luminance axes for younger (solid circles) and older (open circles) observers. These standards were perceptually matched to the two S-axis standards.
C. Contrast-Matching Functions
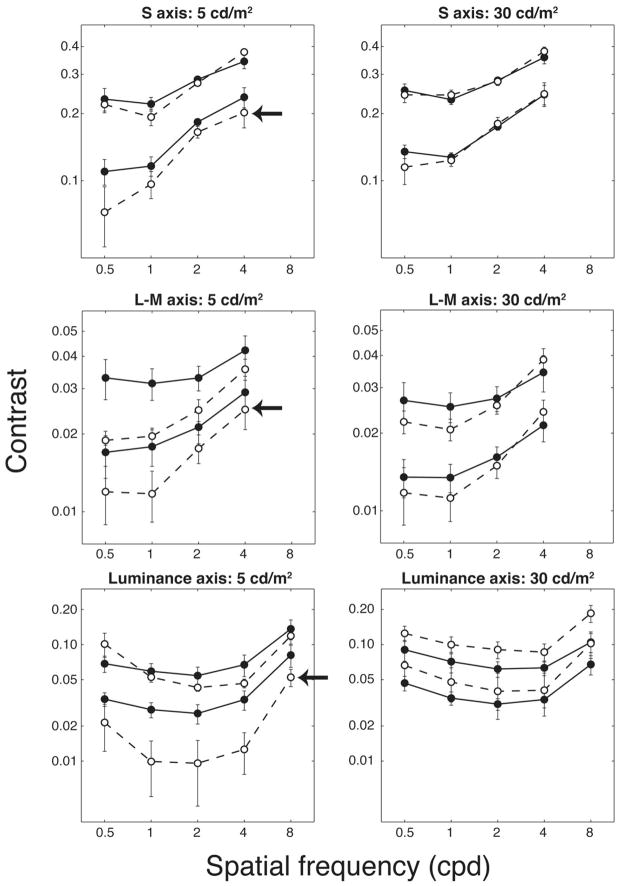
Five of the ten older subjects had S-axis thresholds that were above the contrast level used for one of the S-axis standards (the lower-contrast S-axis standard at the 5-cd/m2 luminance level). Therefore the CMFs for the lower-contrast and lower-luminance conditions for the older group are based on the mean of the remaining five observers. The CMFs for both groups are shown in Fig. 3. Each plotted point is the mean value of the settings made by the observers in each age group. Matches were made at two luminance levels (5 cd/m2, left panels; 30 cd/m2, right panels). For the S-axis CMFs (top panels), the same physical standards were used for both age groups. For the L – M and luminance axes, the standard stimuli were based on perceptual matches made to the S-axis stimuli for each observer. Observers matched the perceived contrast of the standards to stimuli with a range of spatial frequencies (0.5, 1, 2, and 4 c/deg for the S and L – M axes and additionally 8 c/deg for the luminance axis). The CMFs for the chromatic axes (S and L – M) generally show low-pass characteristics, while the CMFs for the luminance axis are bandpass with greatest sensitivity at approximately 2 c/deg.
Fig. 3.
Nonnormalized CMFs for younger (solid circles, solid lines) and older (open circles, dashed lines) observers. The arrows point to curves obtained for the older group by using only five observers (the standards were above threshold for the other five observers). Error bars are ±1 SEM.
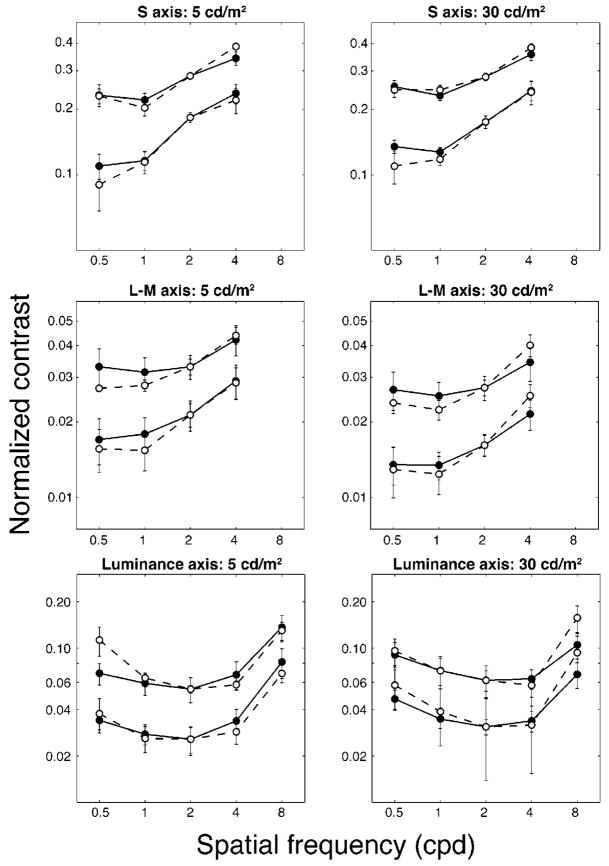
To allow easier comparison of the function shapes, we normalized each CMF separately at 2 c/deg. The log difference of the mean values at 2 c/deg for both groups was added to each of the mean settings made by the older group. The normalized plots are shown in Fig. 4. In general, the shape of the curves is similar for both young and old groups and for both luminance levels. There is, however, some evidence for small losses in sensitivity at higher spatial frequencies. Also, there is a slight flattening of the curves for the younger group relative to the older group.
Fig. 4.
Normalized CMFs for younger (solid circles, solid lines) and older (open circles, dashed lines) observers. The curves were normalized at 2 c/deg. Note that the lower-contrast curves for the older group in the left panels were obtained by using only five observers (the standards were above threshold for the other five observers). Error bars are ±1 SEM.
D. Modeling the Effect of Aging
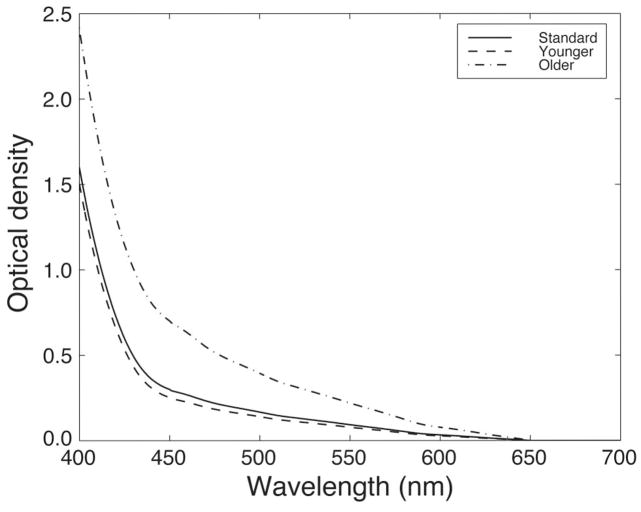
The chromatic stimuli in our experiment were designed to produce modulation along cardinal chromatic axes of cone space (S and L – M axes). We used coordinates based on standard observer color-matching data (see Subsection 2.A). For individual observers, there will generally be small deviations from standard observer matches. One important source of variance results from differences in the light reaching the retina caused by variations in the filtering of the crystalline lens. The optical density of the lens increases with age, leading to increasing levels of light absorption at shorter visible wavelengths. This source of variation is particularly relevant for the present study, where two different age groups were tested. Figure 5 shows the estimated optical density of the lens at age 32 yr (the age of the standard observer33) and for the mean age of both of our subject groups (ages 24 and 72 yr).
Fig. 5.
Optical density of the crystalline lens for a standard observer33 and estimates based on the mean ages for the two age groups used in our study.
Deviations from the standard observer data will produce luminance artifacts in the chromatic stimuli, and these will be greatest for older observers. In our study, each subject performed heterochromatic flicker photometry to counteract this problem. However, the filtering effect also has an impact on cone contrasts. To estimate the changes in contrast, we used the lens aging model of Pokorny et al.33 to estimate the chromaticity of the light after it passed through the crystalline lens. Table 1 shows the estimated change in contrast for younger and older groups for both S and L – M contrasts. These differences are relatively small and should have a minor effect on the results.
Table 1.
Estimated Cone Contrasts Compared with Those of a Standard Observera
| Younger | Standard | Older | ||
|---|---|---|---|---|
| S axis | L | −0.005 | 0.000 | 0.002 |
| M | −0.004 | 0.000 | −0.010 | |
| S | 0.498 | 0.500 | 0.478 | |
| L – M axis | L | 0.030 | 0.030 | 0.033 |
| M | −0.056 | −0.057 | −0.069 | |
| S | 0.003 | 0.000 | −0.023 |
Reference 33.
Age-related miosis causes a reduction in the maximum pupillary size of older observers. With the use of data from Kornzweig,5 the mean pupil sizes for light-adapted observers are approximately 4.6 mm for age 24 yr, 4.3 mm for age 32 yr, and 2.8 mm for age 72 yr. Luminance levels of 5 and 30 cd/m2 were used for our experimental stimuli; however, the differences in pupil size, together with differences in lens absorption, produce large differences in retinal illuminance for our two age groups. The estimated mean retinal illuminance values in photopic trolands are shown in Table 2.
Table 2.
Estimated Retinal Illuminance Values (Photopic Trolands)
| Luminance (cd/m2) | Standard | Younger | Older |
|---|---|---|---|
| 30 | 435.2 | 497.2 | 187.0 |
| 5 | 72.5 | 82.9 | 31.2 |
4. DISCUSSION
The luminance CMFs that we obtained exhibited bandpass characteristics, while the chromatic CMFs were low pass. This is in agreement with previous research on CSFs1 and CMFs matched to low-contrast standards.4,19 In our research, we obtained luminance and chromatic CMFs for young and old age groups. In general, the function shapes were similar for both age groups, with no consistent selective loss at any spatial frequency [although there is some evidence for slight losses for two out of six of our conditions (see Fig. 4)]. Studies of luminance CSFs at threshold levels have shown a loss of sensitivity at higher spatial frequencies for older observers (e.g., Ref. 17). This loss is generally attributed to both optical and neural factors.34 Tulunay-Keesey et al.19 obtained luminance CSFs and CMFs for a range of ages and found a selective loss of sensitivity at threshold levels for older observers but no such loss for CMFs. They suggested that these results could be explained by a contrast gain mechanism that has greater gains for spatial frequencies with high thresholds. The luminance CMFs obtained in our study are in general agreement with those of Tulunay-Keesey et al.19 In our companion paper examining chromatic CSFs,20 we found a general loss of sensitivity but no selective loss at any spatial frequency. The present study is in general agreement, with no selective loss for chromatic CMFs. Therefore a selective contrast gain model is not required to relate these chromatic CSFs and CMFs.
Previous research has shown that although younger and older observers generally describe chromatic stimuli similarly, older subjects have higher thresholds for discriminating colorimetric purity, particularly at lower light levels.14,35 The standard contrasts used in our experiment were closer to threshold levels for our older observers (see Table 2). For the lower-luminance conditions, the older observers matched the perceived contrast of the S-axis standards to L – M and luminance axis stimuli that were lower in contrast than those for younger observers (see Table 1). At the higher luminance level, the perceived contrast of the matches was closer for both groups. Despite differences in the perceived contrast of the stimuli, particularly at the lower luminance level, the similar shapes of the CMFs for young and old suggest that relative contrasts across spatial frequencies remain fairly constant across the life span for both luminance-varying and chromatic-varying stimuli.
Other research has shown that luminance and chromatic CMFs tend to flatten as contrast is increased above threshold.2–4 This phenomenon is known as contrast constancy. Our S-axis standard contrasts were 1.3 and 2.8 times the mean threshold measurements for our older observers and 2.1 and 4.7 times mean threshold for our younger observers. Tiippana et al.4 found increased flattening of the curves at these levels. In general, our results provide little evidence for contrast constancy under the conditions tested. This may be due to differences in methodology, including stimulus size (our stimuli were 4°, theirs were 2.6°), presentation of the test and match stimuli (we used sequential presentation, they used simultaneous presentation), and differences in pattern display times (ours was fixed at 0.75 s, theirs was variable depending on the response of the observer).
Contrast sensitivity varies with retinal illuminance, with decreased sensitivity at lower light levels and greater losses at higher spatial frequencies.36–40 The effect of light levels on CMFs has not previously been studied. In our study, we used two light levels (5 and 30 cd/m2). The additional filtering of the aging lens together with age-related miosis produces a large reduction in retinal illuminance for our older subjects (see Table 2). Despite this, the shapes of the CMFs that we obtained were similar for both light levels and for both age groups (see Fig. 4).
The crystalline lens increasingly filters short-wavelength light with age (see Fig. 5). We used stimuli based on standard observer color-matching data (see Subsection 2.A), and the changes in lens filtering for our age groups introduced luminance and cone contrast differences. Luminance artifacts were minimized by using heterochromatic flicker photometry for each observer. Cone contrast differences remained, although these were relatively small (see Table 1) and should only have a minor impact on the results. Individual differences in macular pigment will also produce some cone contrast artifacts; however, macular pigment density does not appear to change markedly with age, particularly if subjects are healthy and active.10 The older subjects used in our study generally fell into this category, and it is reasonable to assume that the levels of macular pigment were similar for both age groups.
In summary, the luminance and chromatic CMFs that we studied have essentially the same shape for both of our age groups. The standard stimuli that we chose were closer to threshold levels for our older observers, but this had little impact on the shape of the functions. The function shapes were also similar for the two light levels tested. Our companion paper20 showed that there is an overall loss in sensitivity for detection of chromatically modulated stimuli. Here we show that at suprathreshold levels the visual system compensates for this loss in sensitivity, so that younger and older observers experience the same effective contrast at different spatial scales.
Acknowledgments
This work was supported by a National Institute on Aging grant (AG04058) and a Research to Prevent Blindness Jules and Doris Stein Professorship.
Contributor Information
Peter B. Delahunt, Department of Ophthalmology and Section of Neurobiology, Physiology and Behavior, University of California, Davis Medical Center, Sacramento, California 95817
Joseph L. Hardy, Department of Ophthalmology and Section of Neurobiology, Physiology and Behavior, University of California, Davis Medical Center, Sacramento, California 95817
Katsunori Okajima, Imaging Science and Engineering Laboratory, Tokyo Institute of Technology, Yokohama, Kanagawa 226-8503, Japan.
John S. Werner, Department of Ophthalmology and Section of Neurobiology, Physiology and Behavior, University of California, Davis Medical Center, Sacramento, California 95817
References
- 1.Mullen KT. The contrast sensitivity of human colour vision to red–green and blue–yellow chromatic gratings. J Physiol (London) 1985;359:381–400. doi: 10.1113/jphysiol.1985.sp015591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Georgeson MA, Sullivan GD. Contrast constancy: deblurring in human vision by spatial frequency channels. J Physiol (London) 1975;253:627–656. doi: 10.1113/jphysiol.1975.sp011162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vimal RL. Spatial color contrast matching: broad-bandpass functions and the flattening effect. Vision Res. 2000;40:3231–3243. doi: 10.1016/s0042-6989(00)00171-1. [DOI] [PubMed] [Google Scholar]
- 4.Tiippana K, Rovamo J, Nasanen R, Whitaker D, Makela P. Contrast matching across spatial frequencies for isoluminant chromatic gratings. Vision Res. 2000;40:2159–2165. doi: 10.1016/s0042-6989(00)00076-6. [DOI] [PubMed] [Google Scholar]
- 5.Kornzweig AL. Physiological effects of age on the visual process. Sight Sav Rev. 1954;24:130–138. [Google Scholar]
- 6.Spear PD. Neural basis of visual deficits during aging. Vision Res. 1993;33:2589–2609. doi: 10.1016/0042-6989(93)90218-l. [DOI] [PubMed] [Google Scholar]
- 7.Fiorentini A, Porciatti V, Morrone MC, Burr DC. Visual ageing: unspecific decline of the responses to luminance and colour. Vision Res. 1996;36:3557–3566. doi: 10.1016/0042-6989(96)00032-6. [DOI] [PubMed] [Google Scholar]
- 8.Crognale MA. Development, maturation, and aging of chromatic visual pathways: VEP results. J Vision. 2002;2:438–450. doi: 10.1167/2.6.2. [DOI] [PubMed] [Google Scholar]
- 9.Knoblauch K, Vital-Durand F, Barbur JL. Variation of chromatic sensitivity across the life span. Vision Res. 2001;41:23–36. doi: 10.1016/s0042-6989(00)00205-4. [DOI] [PubMed] [Google Scholar]
- 10.Werner JS, Bieber ML, Schefrin BE. Senescence of foveal and parafoveal cone sensitivities and their relations to macular pigment density. J Opt Soc Am A. 2000;17:1918–1932. doi: 10.1364/josaa.17.001918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haegerstrom-Portnoy G, Hewlett SE, Barr SAN. S cone loss with aging. In: Drum B, Verriest G, editors. Colour Vision Deficiencies IX. Kluwer Academic; Dordrecht, The Netherlands: 1989. [Google Scholar]
- 12.Eisner A, Fleming SA, Klein ML, Mauldin WM. Sensitivities in older eyes with good acuity: cross-sectional norms. Invest Ophthalmol Visual Sci. 1987;28:1824–1831. [PubMed] [Google Scholar]
- 13.Werner JS, Steele VG. Sensitivity of human foveal color mechanisms throughout the life span. J Opt Soc Am A. 1988;5:2122–2130. doi: 10.1364/josaa.5.002122. [DOI] [PubMed] [Google Scholar]
- 14.Kraft JM, Werner JS. Aging and the saturation of colors. 2. Scaling of color appearance. J Opt Soc Am A. 1999;16:231–235. doi: 10.1364/josaa.16.000231. [DOI] [PubMed] [Google Scholar]
- 15.Hardy JL, Frederick CM, Kay P, Werner JS. Psychol Sci. Color naming, lens aging, and grue: what the optics of the aging eye can teach us about color language. (to be published) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schefrin BE, Werner JS. Loci of spectral unique hues throughout the life span. J Opt Soc Am A. 1990;7:305–311. doi: 10.1364/josaa.7.000305. [DOI] [PubMed] [Google Scholar]
- 17.Owsley C, Sekuler R, Siemsen D. Contrast sensitivity throughout adulthood. Vision Res. 1983;23:689–700. doi: 10.1016/0042-6989(83)90210-9. [DOI] [PubMed] [Google Scholar]
- 18.Burton KB, Owsley C, Sloane ME. Aging and neural spatial contrast sensitivity: photopic vision. Vision Res. 1993;33:939–946. doi: 10.1016/0042-6989(93)90077-a. [DOI] [PubMed] [Google Scholar]
- 19.Tulunay-Keesey U, Hoeve JNV, Terkla-McGrane C. Threshold and suprathreshold spatiotemporal response throughout adulthood. J Opt Soc Am A. 1988;5:2191–2200. doi: 10.1364/josaa.5.002191. [DOI] [PubMed] [Google Scholar]
- 20.Hardy JL, Delahunt PB, Okajima K, Werner JS. Senescence of spatial chromatic contrast sensitivity. I. Detection under conditions controlling for optical factors. J Opt Soc Am A. 2005;22:49–59. doi: 10.1364/josaa.22.000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glasser A, Campbell MCW. Presbyopia and the optical changes in the human crystalline lens with age. Vision Res. 1998;38:209–229. doi: 10.1016/s0042-6989(97)00102-8. [DOI] [PubMed] [Google Scholar]
- 22.Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- 23.Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [Google Scholar]
- 24.Brainard DH, Peli DG, Robson T. Display characterization. In: Hornak J, editor. The Encyclopedia of Imaging Science and Technology. Wiley; New York: 2002. [Google Scholar]
- 25.Smith V, Pokorny J. Spectral sensitivity of the foveal cone photopigments between 400 and 500 nm. Vision Res. 1975;15:161–171. doi: 10.1016/0042-6989(75)90203-5. [DOI] [PubMed] [Google Scholar]
- 26.Smith VC, Pokorny J. The design and use of a cone chromaticity space: a tutorial. Color Res Appl. 1996;21:375–383. [Google Scholar]
- 27.Chaparro A, Stromeyer CFI, Huang EP, Kronauer RE, Eskew RTJ. Colour is what the eye sees best. Nature (London) 1993;361:348–350. doi: 10.1038/361348a0. [DOI] [PubMed] [Google Scholar]
- 28.Brainard DH. Cone contrast and opponent modulation color spaces. In: Kaiser PK, Boynton RM, editors. Human Color Vision. Optical Society of America; Washington, D.C.: 1996. [Google Scholar]
- 29.Watson AB, Pelli DG. QUEST: a Bayesian adaptive psychometric method. Percept Psychophys. 1983;33:113–120. doi: 10.3758/bf03202828. [DOI] [PubMed] [Google Scholar]
- 30.Switkes E, Crognale MA. Comparison of color and luminance contrast: apples versus oranges? Vision Res. 1999;39:1823–1831. doi: 10.1016/s0042-6989(98)00219-3. [DOI] [PubMed] [Google Scholar]
- 31.Bradley A, Zhang X, Thibos L. Failures of isoluminance caused by ocular chromatic aberrations. Appl Opt. 1992;31:3657–3667. doi: 10.1364/AO.31.003657. [DOI] [PubMed] [Google Scholar]
- 32.Cottaris NP. Artifacts in spatiochromatic stimuli due to variations in preretinal absorption and axial chromatic aberration: implications for color physiology. J Opt Soc Am A. 2003;20:1694–1713. doi: 10.1364/josaa.20.001694. [DOI] [PubMed] [Google Scholar]
- 33.Pokorny J, Smith VC, Lutze M. Aging of the human lens. Appl Opt. 1987;26:1437–1440. doi: 10.1364/AO.26.001437. [DOI] [PubMed] [Google Scholar]
- 34.Losada MA, Navarro R, Santamaria J. Relative contributions of optical and neural limitations to human contrast sensitivity at different luminance levels. Vision Res. 1993;33:2321–2336. doi: 10.1016/0042-6989(93)90109-a. [DOI] [PubMed] [Google Scholar]
- 35.Kraft JM, Werner JS. Aging and the saturation of colors. 1. Colorimetric purity discrimination. J Opt Soc Am A. 1999;16:223–230. doi: 10.1364/josaa.16.000223. [DOI] [PubMed] [Google Scholar]
- 36.Rovamo J, Luntinen O, Nasanen R. Modelling the dependence of contrast sensitivity on grating area and spatial frequency. Vision Res. 1993;33:2773–2788. doi: 10.1016/0042-6989(93)90235-o. [DOI] [PubMed] [Google Scholar]
- 37.Mustonen J, Rovamo J, Nasanen R. The effects of grating area and spatial frequency on contrast sensitivity as a function of light level. Vision Res. 1993;33:2065–2072. doi: 10.1016/0042-6989(93)90005-h. [DOI] [PubMed] [Google Scholar]
- 38.De Valois RL, Morgan H, Snodderly DM. Psychophysical studies of monkey vision. III. Spatial luminance contrast sensitivity tests of macaque and human observers. Vision Res. 1974;14:75–81. doi: 10.1016/0042-6989(74)90118-7. [DOI] [PubMed] [Google Scholar]
- 39.Daitch JM, Green DG. Contrast sensitivity of the human peripheral retina. Vision Res. 1969;9:947–952. doi: 10.1016/0042-6989(69)90100-x. [DOI] [PubMed] [Google Scholar]
- 40.Sloane ME, Owsley C, Alvarez SL. Aging, senile miosis and spatial contrast sensitivity at low luminance. Vision Res. 1988;28:1235–1246. doi: 10.1016/0042-6989(88)90039-9. [DOI] [PubMed] [Google Scholar]