Abstract
The importance of NADPH oxidase (Nox) in hypoxic responses in hypoxia-sensing cells including pulmonary artery smooth muscle cells (PASMCs) remains uncertain. In this study, using Western blot analysis we found that the major Nox subunits Nox1, Nox4, p22phox, p47phox, and p67phox were equivalently expressed in mouse pulmonary and systemic (mesenteric) arteries. However, acute hypoxia significantly increased Nox activity and translocation of p47phox protein to the plasma membrane in pulmonary, but not mesenteric arteries. The Nox inhibitor apocynin and p47phox gene deletion attenuated hypoxic increase in intracellular reactive oxygen species and Ca2+ concentration ([ROS]i and [Ca2+]i) as well as contraction in mouse PASMCs, and abolished hypoxic activation of Nox in pulmonary arteries. The conventional/novel protein kinase C (PKC) inhibitor chelerythrine, specific PKCε translocation peptide inhibitor and PKCε gene deletion, but not the conventional PKC inhibitor GÖ6976, prevented hypoxic increase in Nox activity in pulmonary arteries and in [ROS]i in PASMCs. The PKC activator phorbol-12-myristate-13-acetate could increase Nox activity in pulmonary and mesenteric arteries. Inhibition of mitochondrial ROS generation with rotenone or myxothiazol prevented hypoxic activation of Nox. Glutathione peroxidase-1 (Gpx1) gene overexpression to enhance H2O2 removal significantly inhibited hypoxic activation of Nox, whereas Gpx1 gene deletion had an opposite effect. Exogenous H2O2 increased Nox activity in pulmonary and mesenteric arteries. These findings suggest that acute hypoxia may distinctively activate Nox to increase [ROS]i through mitochondrial ROS–PKCε signaling axis, providing a positive feedback mechanism to contribute to hypoxic increase in [ROS]i and [Ca2+]i as well as contraction in PASMCs.
Keywords: hypoxia, NADPH oxidase, reactive oxygen species, intracellular calcium, mitochondria, protein kinase C, pulmonary artery
Introduction
It is well known that hypoxia causes vasoconstriction in pulmonary arteries (HPV) to ensure the adequate matching of regional alveolar ventilation and pulmonary perfusion, thereby allowing sufficient oxygenation of the blood in the lung. In contrast, systemic arteries normally do not contract or even dilate in response to hypoxia to retain fairly constant blood flow in important organs. Consistent with the privileged importance of intracellular Ca2+ concentration ([Ca2+]i) in the regulation of contraction in pulmonary and systemic (e.g., mesenteric) artery smooth muscle cells (PASMCs and MASMCs), the differential contractile responsiveness to hypoxia in pulmonary and systemic arteries is related to the distinct change in [Ca2+]i in vascular SMCs. Vadula et al have reported that hypoxia greatly increases [Ca2+]i in pulmonary, but not in cerebral (systemic) artery SMCs [1]. Supportedly, we have shown that hypoxia results in a large increase in [Ca2+]i and contraction in PASMCs, but not in MASMCs [2,3]. However, the cellular and molecular mechanisms responsible for the heterogeneity of hypoxic increase in [Ca2+]i and contraction in pulmonary and systemic artery SMCs remain elusive.
Intracellular reactive oxygen species (ROS) functions as an important signaling molecule in vascular SMCs, mediating numerous physiological and pathological processes. There are several sources for generation of intracellular ROS; among them, mitochondria appear to be one of the major sources in vascular SMCs. Archer and his colleagues have reported that hypoxia may reduce the generation of mitochondrial ROS, thus causing inhibition of voltage-dependent K+ currents and contraction in PASMCs, while hypoxia is likely to produce the opposite effects in renal (systemic) artery SMCs [4]. We and many other research groups have also demonstrated that mitochondrial ROS is important for hypoxic responses in PASMCs, but hypoxia increases, rather than decreases the production of mitochondrial and/or intracellular ROS [3,5–15]. Our recent studies further indicate that hypoxia increases mitochondrial ROS generation and subsequently activates PKCε in pulmonary, but not mesenteric artery SMCs; pharmacological and genetic inhibition of mitochondrial ROS generation block hypoxia-induced activation of PKCε, increase in [Ca2+]i, and contraction in PASMCs [3,14]. Thus, the differential effect of hypoxia on mitochondrial ROS-PKCε signaling axis may contribute to the diversity of hypoxic responses in pulmonary and systemic arteries.
NADPH oxidase (Nox) is believed to be another important source in the generation of intracellular ROS in vascular SMCs. The active form of Nox is normally composed of various subunits depending on the cell types. In phagocytic cells, Nox is well characterized to include the membrane-bound subunits p22phox and gp91phox (Nox2) subunits, as well as the cytosolic subunits p47phox and p67phox; the association of these cytosolic and membrane-bound subunits is required for assembly of the active Nox. There are a number of reports showing that Nox inhibition reduces hypoxic responses in PASMCs [5,16–24]; however, the specific role of Nox has been disputed [22,25].
In the present study, thus, we first attempted to investigate whether acute hypoxia could produce a differential effect on the Nox activity in freshly isolated, endothelium-denuded mouse pulmonary and mesenteric (systemic) arteries using a biochemical approach. In next series of experiments, we examined the potential contribution of Nox to hypoxia-induced increase in intracellular ROS concentration ([ROS]i) and [Ca2+]i in freshly isolated mouse PASMCs using pharmacological inhibitors and gene deletion mice. Finally, we performed studies to test the role of mitochondrial ROS and PKCε in hypoxic activation of Nox in mouse pulmonary arteries. Our data reveal that the major Nox membrane-bound subunits p22phox, Nox1 and Nox4, as well as the cytosolic subunits p47phox and p67phox are comparably expressed in both mouse pulmonary and mesenteric arteries. Acute hypoxia activates Nox in pulmonary, but not mesenteric arteries. The activation of Nox is important for hypoxia-induced increase in [ROS]i and [Ca2+]i as well as contraction in PASMCs, which is mediated by the mitochondrial ROS-PKCε signaling axis.
Experimental materials and methods
Reagents
5,6-Chloromethyl-2,7-dichlorodihydrofluorescein diacetate (CM-DCF-DA) and fura-2/AM were obtained from Molecular Probes (Eugene, OR); PKCε translocation peptide inhibitor and chelerythrine from Calbiochem (La Jolla, CA); antibodies against the Nox subunits Nox1, Nox2 (gp91phox), Nox4, p22phox, p47phox, and p67phox from Santa Cruz (Santa Cruz, CA); and rotenone, myxothiazol, antimycin-A, hydrogen peroxide, phorbol-12-myristate-13-acetate (PMA), and apocynin from Sigma (St. Louis, MO).
Cell and tissue preparation
All animal experiments were approved by the Institutional Animal Care and Use Committee of Albany Medical College. Freshly isolated resistance (third and smaller branches) pulmonary and mesenteric artery SMCs and tissues were prepared from Swiss-Webster mice (Taconic, Germantown, NY), as we reported previously [3,14]. In brief, mice were euthanized by an intraperitoneal injection of sodium pentobarbital. Resistance pulmonary and mesenteric arteries were carefully dissected free of endothelium and connective tissues in ice-cold, physiological saline solution (PSS) gassed with 20%O2, 5%CO2 and 75%N2 (termed normoxia). For assessing Nox activity, isolated arteries were subjected to lysis. To obtain isolated, single SMCs, the arteries were cut into small pieces and then incubated in low Ca2+ (100 μM) PSS containing (mg/ml) 1.5 papain, 0.4 dithioerythritol, and 1.0 bovine serum albumin (BSA) for 20 min, followed by low Ca2+ PSS containing (mg/ml) 1.0 collagenase II, 1.0 collagenase H, 1.0 dithiothreitol, and 1.0 BSA for 10–15 min. Finally, tissues were washed in ice-cold low Ca2+ PSS for 3 times. Single cells were harvested by gentle trituration and kept on ice for daily use. To examine the effects of pharmacological reagents, control experiments were carried out in cells or tissues from the same mice.
To obtain acute hypoxic responses, isolated tissues or single cells were exposed to PSS bubbled with 1%O2, 5%CO2 and 94%N2 (hypoxia) for 5 min. In control experiments, tissues or cells were treated identically, but not exposed to hypoxia. Unless indicated otherwise, reagents were applied to tissues or cells for 10 min before the start of hypoxia.
Pulmonary and mesenteric artery tissues and SMCs from glutathione peroxidase-1 gene deletion (Gpx1−/−), Gpx1 gene overexpression, PKCε−/− and p47phox−/− mice were prepared by using the same protocol as described above. Gpx1−/− and Gpx1 gene overexpression mice were generated and maintained as reported previously [26,27]. PKCε−/− mice [B6.129S4-Prkcetm1Msg/J] and p47phox−/− mice [B6.129S2-Ncf1<tm1shl> N14] were purchased from Taconic and Jackson Laboratory (Bar Harbor, ME), respectively. For experiments using genetically manipulated mice, control cells and tissues were obtained from wild-type mice with the same genetic background, age and sex.
Western blot analysis
Western blot analysis was performed, as we described before [3]. Membrane and cytoplasmic fraction from pulmonary and mesenteric artery tissues were resolved by using the standard SDS-PAGE procedure. Proteins were transferred to a polyvinylidene fluoride membrane (Bio-Rad, Hercules, CA). The nonspecific binding sites on the membrane were blocked by Tris buffer containing 5% nonfat dry milk for overnight at 4°C. The samples were incubated with primary antibodies for 1 h, washed 3 times for 10 min with 20 mM Tris, 150 mM NaCl, and 0.2% Tween 20 (TBST), incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h, and washed 3 times with TBST. Blots were visualized by using a Chemiluminescence Detection Kit (Santa Cruz) and then analyzed by using Multi Gauge software version 3.0 (Fujifilm Science Systems, Tokyo, Japan). The Nox subunit expression levels were normalized to β-actin expression levels.
Measurement of NADPH oxidase activity
Nox activity was measured using the standard cytochrome c reduction method, as described previously [28]. Isolated pulmonary and mesenteric arteries were homogenized in a RIPA buffer. The homogenate was subjected to centrifugation at 250×g for 10 minutes to remove cellular debris. Supernatant was then centrifuged at 20000 g for 20 min at 4 °C to eliminate mitochondria, lysosomes, peroxisomes, Golgi membranes and rough endoplasmic reticulum. The resulting supernatant was centrifuged at 100,000 g for 60 min at 4 °C. The pelleted plasma membrane fraction containing Nox was dissolved in a buffer containing 8 mM piperazine-N,N′-bis 2-ethanesulfonic acid (pH 7.2), 100 mM KCl, 3 mM NaCl, 3.5 mM MgCl2, 1.25 mM EGTA and proteolytic inhibitors. In a multi-well plate, the solubilized membrane fraction samples were added by flavin adenine dinucleotide (FAD, 0.01 mM), acetylated cytochrome c (0.1 mM), and GTPγS (0.01 mM, as an activating agent for NADPH oxidase). Superoxide dismutase (SOD, 100 U/ml) was included to block cytochrome c reduction in half of the samples. After the samples were kept at room temperature for 2 min, sodium dodecyl sulphate (0.1 mM) as an additional activating agent was added. Following incubation for 3 min, NADPH (0.2 mM) was added to initiate cytochrome c reduction. Absorbance at 550 nm (A550) in samples un-incubated and incubated with SOD was measured for 10 min on a FlexStation-III microplate reader (Molecular Device, Sunnyvale, CA). The superoxide-dependent cytochrome c reduction was calculated by subtracting the value of A550 in samples incubated with SOD from that in samples un-incubated with SOD.
Measurement of intercellular ROS
Measurement of intracellular ROS was conducted by using the fluorescent dye chloromethyl-dihydrodichlorofluorescein diacetate (CM-H2DCF/DA), as described previously [3,14]. Freshly isolated cells were loaded with 5 μM CM-H2DCF/DA at 35 °C for 30 min, and then perfused with pre-warmed, dye-free bath solution for 15 min to washout extracellular dye and to allow the conversion of intracellular dye into its non-ester form. The dye was excited at 488 nm, and DCF fluorescence at 510 nm was collected with an LSM510 laser scanning confocal microscope (Thornwood, NY). The DCF fluorescent intensity was expressed in arbitrary units (a.u.) after background subtraction.
Measurement of [Ca2+]i
[Ca2+]i was measured using a dual excitation wavelength fluorescence method, with a TILLvisION digital imaging system (TILL Photonics GmbH, Munich, Germany) and a Nikon inverted microscope with a ×20 oil immersion objective, as reported previously [3,14]. Cells were loaded with 5 μM fura-2/AM at room temperature for 30 min followed by 20 min of washout to ensure proper deesterification. [Ca2+]i was determined from the ratio of dye fluorescence intensity at 340 and 380 nm, with an emission wavelength at 510 nm. Background fluorescence intensity was corrected.
Measurement of single cell contraction
Contraction in single freshly isolated cells was measured by assessing cell length shortening, as described previously [3]. Transmitted light x-y images were taken using a Zeiss LSM510 laser scanning confocal microscope. Cell length in each image was determined at its longest axis.
Statistical analysis
Data were expressed as mean ± SE of at least three independent experiments. Student’s t-test was used for determining the significance of differences between two groups, whereas one-way ANOVA with Bonferroni post hoc test was used for multiple comparisons. P < 0.05 was accepted as a level of statistical significance.
RESULTS
Major NADPH oxidase subunits are equivalently expressed in pulmonary and mesenteric arteries
Previous studies have shown that Nox is highly expressed in both pulmonary and systemic arteries [37]; thus, it is very unlikely that the heterogeneity of hypoxic responses in pulmonary and mesenteric arteries is related to the inherent nature of distinct expression of Nox in these two different tissues. To exclude this possibility, nevertheless, we investigated the expression of the major phagocytic Nox subunits, including the membrane-bound subunits gp91phox (Nox2) and p22phox, as well as the cytosolic subunits p47phox and p67phox in mouse pulmonary and mesenteric arteries using Western blot analysis. Our data indicate that p22phox, p47phox and p67phox were expressed with comparable expression levels in pulmonary and mesenteric arteries (Figure 1A).
Figure 1.
Major NADPH oxidase subunit proteins are comparably expressed in mouse pulmonary and mesenteric arteries. A: Comparable expression of major phagocytic Nox subunits p22phox, p47phox, and p67phox proteins in pulmonary and mesenteric arteries. Protein expression was determined using SDS-PAGE and immunobloted with a specific antibody against each of individual Nox subunits. Bar graph shows quantification of Nox subunit protein expression levels that were normalized to β-actin expression levels. Data are presented as means ± S.E. from 4 independent experiments. B: The phagocytic Nox subunit gp91phox (Nox2) was undetected, but its analogues Nox1 and Nox4 were expressed with equivalent expression levels in pulmonary and mesenteric arteries. Data were obtained from 3 independent experiments.
However, as shown in Figure 1B, gp91phox (Nox2) was not detected in both pulmonary and mesenteric arteries, although its expression was found in isolated rat aortic SMCs as reported previously [29]. We then wondered whether the gp91phox analogues Nox1 and Nox4 were expressed to form the functional Nox in mouse pulmonary arteries, as they have been previously found in mouse lungs [21,30]. Our results indicate that the expression of Nox1 and Nox4 proteins were observed with an equivalent level in both pulmonary and mesenteric arteries.
Hypoxia significantly increases NADPH oxidase activity in pulmonary, but not mesenteric arteries
In these series of experiments, we first examined and compared the effect of hypoxia on Nox activity in mouse pulmonary and mesenteric arteries using the standard cytochrome c reduction method, as described previously [28]. Acute hypoxic exposure for 5 min caused a significant increase in Nox activity in pulmonary arteries. In 4 independent experiments, the mean increase in Nox activity was 1.7 fold, compared with control (P < 0.05; Figure 2A). In contrast, acute hypoxia for 5 min had no effect in mesenteric arteries (Figure 2B).
Figure 2.
Acute hypoxia uniquely activates NADPH oxidase in mouse pulmonary arteries. A: Hypoxic exposure for 5 min causes a large increase in the activity of Nox in pulmonary arteries. Nox activity was determined by the difference in absorbance at 550 nm (A550) due to cytochrome c reduction in the presence and absence of SOD, as described in the Methods section. Data are presented as means ± S.E. from 4 independent experiments. *P < 0.05 compared with control (normoxia). B: Hypoxia has no effect on Nox activity in mesenteric arteries. C: Hypoxia induces the translocation of p47phox to the plasma membrane of pulmonary arteries. The amount of p47phox protein in the plasma membrane fraction from pulmonary arteries was determined using SDS–PAGE and immunobloted with anti-p47phox antibody. Bar graph shows quantitative assessment of p47phox expression levels normalized to β-actin levels. Data are presented as means ± SE from 4 independent experiments. *P < 0.05 compared with normoxia.
In support of the hypoxic increase in Nox activity in PASMCs, using Western blot analysis we found that hypoxic exposure for 5 min resulted in an increase in the amount of the essential Nox subunit p47phox in membrane fraction from pulmonary arteries (Figure 2C). This result indicates its translocation to plasma membrane to assemble more active forms of Nox.
Pharmacological and genetic inhibition of NADPH oxidase attenuate hypoxic increase in [ROS]i in pulmonary artery smooth muscle cells
Apocynin is thought to be a specific Nox inhibitor by preventing the association of p47phox with the membrane-bound Nox subunits [31]; thus, we examined the effect of apocynin on hypoxic increase in [ROS]i in PASMCs to determine the potential functional role of Nox in hypoxic responses. Consistent with our previous reports [3,14], hypoxic exposure for 5 min caused a significant increase in [ROS]i. However, treatment with apocynin (1 μM) for 10 min significantly inhibited hypoxia-induced increase in [ROS]i (Figure 3A). Genetic inhibition of Nox with p47phox gene deletion suppressed hypoxic increase in [ROS]i as well. The mean increase in DCF fluorescence following hypoxic stimulation was significantly lower in p47phox−/− cells than that in control (p47phox+/+) cells (Figure 3A).
Figure 3.
Pharmacological and genetic inhibition of NADPH oxidase significantly attenuate hypoxic increase in [ROS]i in freshly isolated mouse pulmonary artery smooth muscle cells. A: Effect of the Nox inhibitor apocynin and p47phox gene deletion on hypoxic increase in [ROS]i (DCF fluorescence). DCF fluorescence was recorded before (normoxia) and after hypoxia for 5 min in control (p47phox+/+) cells, p47phox+/+ cells pretreated with apocynin (1 μM) for 10 min, and p47phox−/− cells. Data are presented as mean ± S.E. from 21 – 23 cells in 4 independent experiments. *P < 0.05 compared with normoxia (before hypoxia). B: Effect of apocynin on hypoxic increase in Nox activity in mouse pulmonary arteries. The activity of Nox was determined in arteries treated with normoxia, hypoxia for 5 min and apocynin (1 μM) for 10 min plus hypoxia for 5 min. Data are presented as means ± S.E. from 3 independent experiments. *P < 0.05 compared with control (normoxia); †P< 0.05 compared with hypoxia. C: Effect of p47phox gene deletion on hypoxic increase in Nox activity. Data are presented as means ± S.E. from 3 independent experiments. *P < 0.05 compared with control (normoxia, p47phox+/+); †P< 0.05 compared with hypoxia (p47phox+/+).
In parallel to the effect on the hypoxic increase in [ROS]i, pharmacological and genetic inhibition of Nox completely abolished the hypoxic increase in Nox activity. As shown in Figure 3B, treatment with apocynin (1 μM) for 10 min eliminated hypoxia-induced increase in Nox activity in pulmonary arteries. Similarly, hypoxia failed to increase the activity of Nox in p47phox−/− pulmonary arteries (Figure 3C).
Pharmacological and genetic inhibition of NADPH oxidase diminish hypoxic increase in [Ca2+]i in pulmonary artery smooth muscle cells
In these series of experiments, we investigated the potential involvement of Nox in hypoxia-induced increase in [Ca2+]i using the same pharmacological and genetic approaches as described above. In isolated PASMCs, hypoxic challenge for 5 min resulted in a significant increase in [Ca2+]i. A mean increase in [Ca2+]i by hypoxia was 342.4 ± 17.3 nM. In cells pretreated with apocynin (1 μM) for 10 min, hypoxia-induced increase in [Ca2+]i was 199.5 ± 10.0 nM, approximating a decrease by 42% (P < 0.05; Figure 4A). However, apocynin did not significantly affect the resting level of [Ca2+]i. The mean resting level of [Ca2+]i was 112.5 ± 14.6 and 135.23 ± 26.9 nM before and after application of apocynin, respectively. Nox inhibition by p47phox gene deletion also attenuated hypoxic increase in [Ca2+]i in PASMCs. The mean increase in [Ca2+]i following hypoxic stimulation was decreased by 61% in p47phox−/− cells (Figure 4A). In addition, p47phox gene deletion did not alter the resting level of [Ca2+]i, either.
Figure 4.
Pharmacological and genetic inhibition of NADPH oxidase diminish hypoxic increase in [Ca2+]i and contraction in freshly isolated mouse pulmonary artery smooth muscle cells. A: Effect of the Nox inhibitor apocynin and p47phox gene deletion on hypoxic increase in [Ca2+]i. Original recordings of hypoxic increase in [Ca2+]i were made in a cell treated without and with apocynin (1 μM) for 10 min. Bar graph summarizes the effect of apocynin and p47phox gene deletion on hypoxia-induced increase in [Ca2+]i. Data are presented as mean ± S.E. from 4 or 5 independent experiments. *P < 0.05 compared with control (untreated with apocynin) or PKCε+/+. B: Effect of treatment with apocynin (1μM) for 10 min and p47phox gene deletion on hypoxia-induced contraction. The hypoxic contraction was determined as the difference in cell shortening before and after hypoxic exposure for 5 min. *P < 0.05 compared with control (untreated with apocynin) or PKCε+/+.
Comparable to the effect on hypoxic increases in [Ca2+]i, pharmacological inhibition of Nox significantly reduced hypoxic contraction in PASMCs. As shown in Figure 4B, hypoxia caused cell length shortening by 9.7 ± 0.6% in control PASMCs (n = 23) and 4.5 ± 0.4% in PASMCs pretreated with apocynin (1 μM) for 10 min (n = 35, P < 0.05). Similarly, hypoxia-evoked cell shortening was suppressed in p47phox−/− cells, relative to p47phox+/+ cells.
In contrast to the effect hypoxic responses, treatment with apocynin did not affect an increase in [Ca2+]i induced by caffeine, a contractile agent, in PASMCs. The mean increase in [Ca2+]i by caffeine (10 mM) was 1179 ± 92 nM in control cells (n = 56) and 1159 ± 88 nM in cells pretreated with apocynin (1 μM) for 10 min (n = 51), respectively. Genetic inhibition of Nox by p47phox gene deletion also had no effect on caffeine-induced increase in [Ca2+]i (1243 ± 100 nM in 76 p47phox+/+ cells v.s. 1374 ± 140 nM in 52 p47phox−/− cells).
Hypoxic increase in NADPH oxidase activity is mediated by PKCε in pulmonary arteries
We have recently shown that acute hypoxia stimulates PKCε in pulmonary arteries [3]. This finding, together with the well-known fact that PKC can greatly enhance Nox activity, led us to hypothesize that hypoxia may activate Nox by stimulating PKCε. To test this hypothesis, we examined the effect of pharmacological and genetic inhibition of PKCε on the hypoxic activation of Nox in mouse pulmonary arteries. After treatment with the conventional/novel PKC inhibitor chelerythrine (10 μM) for 10 min, hypoxic challenge for 5 min failed to increase Nox activity (Figure 5A). Similarly, a specific PKCε translocation peptide inhibitor (10 μM) also abolished the hypoxic effect. On the contrary, GÖ6976 (0.1 μM), an inhibitor shown to block conventional, but not novel PKC isozymes, had no effect on hypoxia-induced increase in Nox activity. We also measured and compared Nox activity in pulmonary arteries from PKCε−/− and PKCε+/+ mice. As summarized in Figure 5B, hypoxia-induced increase in Nox activity was completely blocked in PKCε−/− mouse resistance pulmonary arteries. In contrast to the inhibitory effect of PKCε inhibition, stimulation of PKC with PMA (1 μM) largely increased Nox activity in pulmonary arteries, which was similar to the hypoxic effect. PMA increased Nox activity in mesenteric arteries as well (Figure 5C).
Figure 5.
Hypoxia-induced activation of NADPH oxidase is mediated by PKCε in mouse pulmonary arteries. A: Treatment with the conventional/novel PKC inhibitor chelerythrine (10 μM) and PKCε translocation peptide inhibitor (10 μM) for 10 min reduce hypoxia-induced increase in the Nox activity in pulmonary arteries, whereas GÖ6976 (100 nM) has no effect. *P < 0.05 compared with control (without hypoxia); †P < 0.05 compared with hypoxia alone. B: PKCε gene deletion prevents hypoxic increase in Nox activity in pulmonary arteries. *P < 0.05 compared with control (without hypoxia); †P < 0.05 compared with hypoxia alone. C: The PKC activator phorbol 12-myristate 13-acetate (PMA) augments the activity of Nox in pulmonary (left) and mesenteric arteries (right). Data are presented as means ± S.E. from 3 or 4 independent experiments. *P < 0.05 compared with control (without PMA).
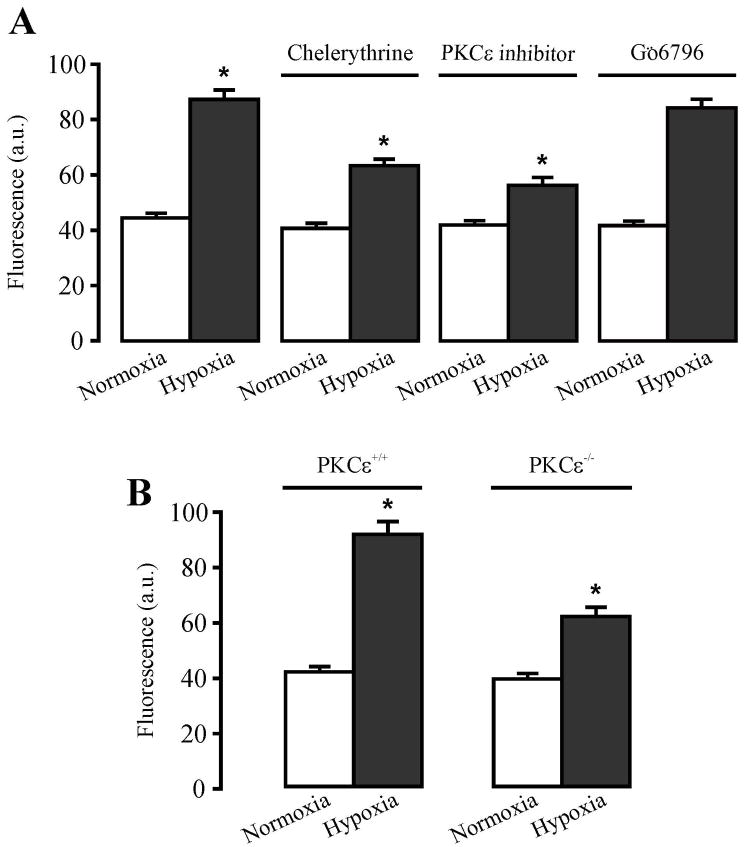
We next examined the effect of pharmacological and genetic inhibition of PKCε on the hypoxic increase in [ROS]i in isolated PASMCs. Treatment with chelerythrine (10 μM) or a specific PKCε translocation peptide inhibitor (10μM)for 10 min significantly suppressed hypoxia-induced increase in DCF fluorescence ([ROS]i) (Figure 6A). In contrast, GÖ6976 (0.1 μM) did not alter hypoxia-induced increase in [ROS]i. In complement of the effect of pharmacological inhibitors, we found that hypoxic increase in [ROS]i was significantly lower in PKCε −/− cells than that in PKCε+/+ cells (Figure 6B).
Figure 6.
Pharmacological and genetic inhibition of PKCε reduce hypoxic increase in [ROS]i in mouse pulmonary artery smooth muscle cells. A: Effect of chelerythrine, PKCε translocation peptide inhibitor and GÖ6976 on hypoxic increase in [ROS]i (DCF fluorescence). DCF fluorescence was recorded in freshly isolated PAMSCs before and after hypoxia for 5 min in the absence or presence of chelerythrine (10 μM), PKCε translocation peptide inhibitor (10 μM) and GÖ6976 (100 nM) 10 min. Data are presented as mean ± SE from 25 cells in 4 independent experiments. *P < 0.05 compared with normoxia (before hypoxia). B: Effect of PKCε gene deletion on hypoxic increase in [ROS]i. Data are presented as means ± SE from 25 cells in 4 independent experiments. *P < 0.05 compared with normoxia (before hypoxia).
Mitochondrial ROS is involved in hypoxic increase in NADPH oxidase activity in pulmonary arteries
Our previous work has revealed that the mitochondrial complex 1 inhibitor rotenone and complex III pre-ubisemiquinone site inhibitor myxothiazol block hypoxia-induced increase in [ROS]i and [Ca2+]i in PASMCs [3,14]. Thus, we sought to examine the effect of rotenone and myxothiazol on hypoxic increase in Nox activity in mouse pulmonary arteries. Our data indicate that hypoxia-induced increase in Nox activity was significantly attenuated following treatment with either rotenone (12.7 μM) or myxothiazol (10 μM) for 10 min (Figure 7A). We have also found that treatment with the complex III post-ubisemiquinone site inhibitor antimycin-A, unlike hypoxia, had no effect on the activity of Nox in pulmonary arteries. In 4 independent experiments, the mean value of A550 in arteries treated without and with antimycin-A (5.7 μM) for 10 min was 0.061 ± 0.008 and 0.067 ± 0.005 o.d. units, respectively.
Figure 7.
Pharmacological and genetic inhibition of mitochondrial ROS generation prevent hypoxia-induced increase in NADPH oxidase activity in mouse pulmonary arteries. A: Inhibition of mitochondrial ROS generation with rotenone (12.7 μM) or myxothiazol (10 μM) for 10 min blocks hypoxia-induced increase in Nox activity. Data are presented as means ± S.E. from 3 independent experiments. *P < 0.05 compared with control (normoxia); †P< 0.05 compared with hypoxia. B: Gpx1 gene overexpression (Gpx1-Tg) to enhance mitochondrial and cytosolic H2O2 decomposition prevents (left), while Gpx1 gene deletion (Gpx1−/−) to inhibit mitochondrial and cytosolic breakdown H2O2 augments (right), hypoxia-induced increase in Nox activity in pulmonary arteries. *P < 0.05 compared with normoxia, and †P < 0.05 compared with hypoxia alone. (C): Application of H2O2 for 5 min increased the activity of Nox in pulmonary (left) and mesenteric arteries (right). Data are presented as means ± S.E. from 3 independent experiments, *P < 0.05 compared with control (untreated with H2O2).
To further confirm the involvement of mitochondrial ROS, we next investigated the effect of genetic modifications of mitochondrial and cytosolic H2O2 generation on hypoxic activation of Nox in pulmonary arteries. As shown in Figure 7B, Gpx1 gene overexpression to enhance H2O2 decomposition in mitochondria and cytosol significantly inhibited hypoxia-induced increase in Nox activity. In contrast, Gpx1 gene deletion to inhibit H2O2 breakdown had an opposite effect.
The role of mitochondrial ROS in hypoxic activation of Nox was further investigated by examining the effect of exogenous H2O2 on Nox activity. Under normoxic conditions, application of H2O2 (5.1 μM) led to a great increase in Nox activity in pulmonary arteries (Figure 7C). Likewise, H2O2 also increased Nox activity in mesenteric arteries.
Discussion
In the present study we have for the first time demonstrated: 1) the major Nox subunit Nox1, Nox4, p22phox, p47phox, and p67phox proteins are equally expressed in both pulmonary and mesenteric (systemic) arteries; 2) acute hypoxia stimulates Nox in pulmonary, but not mesenteric arteries; 3) Nox plays an important role in hypoxia-induced increase in [ROS]i and [Ca2+]i as well as contraction in PASMCs; and 4) hypoxia-induced activation of Nox in pulmonary arteries is mediated by the mitochondrial ROS-PKCε signaling axis. Collectively, these data support the concept that mitochondrial ROS-PKCε dependent activation of NADPH oxidase is involved in the signal transduction of HPV, and may contribute to the differential responsiveness to hypoxia in pulmonary and systemic systems.
Previous studies using RT-PCR have reported the expression of gp91phox (Nox2), p22phox, p47phox and p67phox, Nox1 and Nox4 mRNAs in mouse lung tissues [30]. Immunofluorescence staining and Western blot analysis show the presence of Nox2 and p22phox protein in mouse lungs [25]. In this study, we systemically examined and compared the expression of the major Nox subunits in endothelium-denuded mouse pulmonary and mesenteric arteries using Western blot analysis. The experimental results indicate that the well-characterized, major phagocytic Nox membrane-bound subunit p22phox as well as the cytosolic subunits p47phox and p67phox are expressed in pulmonary arteries (Figure 1A). These three major subunits are also present in mesenteric arteries, with equivalent expression abundance to that in pulmonary arteries. However, the phagocytic Nox membrane-bound subunit Nox2 protein expression is not detected in pulmonary and mesenteric arteries (Figure 1B). This result is different from a previous report by Archer et al, showing Nox2 protein expression in mouse lungs [25]. Since lungs are composed of various types of cells, it is possible that Nox2 is highly expressed in other types of cells, rather in PASMCs. Supportedly, Archer et al have found that Nox2 is most abundant in the alveolar macrophages and airway epithelium, and in greater quantities in the large pulmonary veins. Similarly, a previous study using immunohistological staining has shown that Nox2 is primarily located in mouse pulmonary vascular endothelial cells [30]. Additionally, Archer et al have shown that hypoxic vasoconstriction in isolated lungs and hypoxic inhibition of voltage-dependent K+ currents in pulmonary artery SMCs are unaffected in Nox2−/− mice [25]. These data are virtually consistent with our finding that Nox2 protein is unable to be observed in endothelium-denuded mouse pulmonary arteries, further suggesting that Nox2 in PASMCs may play an insignificant role in HPV in mice. Interestingly, it has been reported that Nox2 mRNA and protein are presented in bovine pulmonary arteries [32], reflecting that Nox2 expression may vary in pulmonary arteries from different animal species. In spite of the absence of Nox2 protein expression, we have discovered that its analogues Nox1 and Nox4 proteins are observed in mouse pulmonary and mesenteric arteries (Figure 1B). A recent study has shown that Nox4, but not Nox2, mRNA and protein expression are upregulated in pulmonary arteries from mice with hypoxic pulmonary hypertension and humans with idiopathic pulmonary hypertension [30]. The potential functional importance of the Nox2 analogues is reinforced by a report that siRNA-mediated Nox4 gene knockdown inhibits proliferation and ROS generation in human PASMCs [33]. Taken together, our studies confirm the concept that the membrane-bound and cytosolic Nox that are required to assemble the active Nox are equally expressed in both pulmonary and systemic artery SMCs.
It has been reported that Nox inhibition by diphenylene iodonium (DPI), iodonium diphenyl, or 4-(2-aminoethyl)-benzenesulfonyl fluoride reduces hypoxia-induced increase in [ROS]i, inhibition of voltage-dependent K+ currents, increase in [Ca2+]i, and contraction in PASMCs [5,16–22]. The specificity of iodonium compounds as Nox inhibitors has been disputed, since these agents can inhibit mitochondrial electron transport chain in heart cells and voltage-dependent Ca2+ currents in PASMCs [22,34]. While gp91phox gene deletion mice show normal or reduced hypoxic responses [23,25], HPV is inhibited in p47phox gene deletion (p47phox−/−) mice [24]. As such, the significance of Nox in hypoxic responses in PASMCs is still uncertain. Furthermore, no biochemical evidence is available to support the importance of Nox in hypoxic responses in vascular SMCs. Using biochemical measurement of Nox activity, here we have observed that acute hypoxia for 5 min causes an increase in Nox activity in endothelium-denuded mouse pulmonary, but not systemic (mesenteric) arteries (Figure 2A and 2B), indicating that hypoxia activates Nox only in pulmonary, but not systemic artery SMCs. The hypoxic activation of Nox in PASMCs is further supported by the finding that hypoxic exposure significantly increases the translocation of p47phox, a key component to form the active Nox, to the plasma membrane (Figure 2C).
Nox-dependent ROS generation has been described in PASMCs. An early study has disclosed that hypoxic increase in [ROS]i is inhibited by the Nox inhibitor DPI in calf PASMCs [5,18]. In this study, we have found that the Nox inhibitor apocynin significantly attenuates hypoxic increase in [ROS]i in freshly isolated mouse PASMCs (Figure 3A). Similarly, p47phox gene deletion attenuates hypoxic increase in [ROS]i as well (Figure 3B). Furthermore, our data indicate that apocynin and p47phox gene deletion both completely abolish hypoxic increase in the activity of Nox (Figure 3C). In support of our results, a study using well-documented hypoxia-sensing, carotid body chemoreceptor cells demonstrates that hypoxia fails to increase intracellular ROS generation in p47phox−/− mice [35]. Interestingly, agonist stimulation of intracellular ROS generation is decreased in p47phox−/− mouse systemic (aortic) SMCs [36]. Moreover, apocynin attenuates intracellular ROS generation in aortic SMCs from DOCA salt-induced hypertensive rats [37]. However, it should be noted that hypoxia may inhibit the Nox-dependent generation of intracellular ROS in a microsome-enriched fraction of calf pulmonary arteries [18].
It is well accepted that an increase in [Ca2+]i in PASMCs serves as a key signaling step to mediate HPV. The hypoxic increase in [Ca2+]i may result from extracellular Ca2+ influx due to inhibition of voltage-dependent K+ channels, in addition to Ca2+ release from the sarcoplasmic reticulum though ryanodine receptors/Ca2+ release channels. Previous studies have reported that pharmacological inhibition of Nox suppresses hypoxic inhibition of voltage-dependent K+ currents [22], increase in [Ca2+]i and contraction [20] in single PASMCs, as well as HPV in pulmonary arteries and lungs [5,16–19,21]. Consistent with these reports, we have demonstrated that the Nox blocker apocynin significantly reduces hypoxia-induced increase in [Ca2+]i in freshly isolated mouse PASMCS. Targeted deletion of p47phox gene produces a similar inhibitory effect (Figure 4A). However, neither apocynin nor p47phox gene deletion affects the resting level of [Ca2+]i and increases in [Ca2+]i evoked by caffeine (a contractile agent). We have also found that pharmacological and genetic inhibition of Nox with apocynin and p47phox gene deletion reduce hypoxia-induced contraction (cell shortening) in PASMCs (Figure 4B). Thus, hypoxia may uniquely activate Nox to enhance intracellular ROS generation, contributing to hypoxic increase in [ROS]i and [Ca2+]i as well as contraction in PASMCs.
Extensive studies have shown that PKC is involved in hypoxic responses in PASMCs. The PKC inhibitors H7, bisindolylmaleimide, calphostin C, and chelerythrine prevent, whereas the PKC activators PMA, thymelation and farnesylthiotriazole mimic and subsequently reduce, HPV in isolated canine and rabbit lungs, as well as isolated rat pulmonary arteries [38–42]. In extension of these findings, our recent studies using complementary biochemical, pharmacological and genetic approaches demonstrate that PKCε is a major isoform of PKC to mediate hypoxic increase in [Ca2+]i and contraction in freshly isolated mouse PASMCs [3]. Consistent with our results, it has been reported that HPV is inhibited in isolated lungs from PKCε−/− mice [43]. While the signaling mechanisms downstream of PKCε in hypoxic responses in PASMCs are unclear, it has been documented that PKC can activate Nox to increase intracellular generation of ROS, participating in a variety of cellular responses in vascular SMCs [37,44]. In the present study, thus, we have tested the possibility that hypoxic activation of Nox may be mediated by PKCε in PASMCs. Our data indicate that the conventional/novel PKC inhibitor chelerythrine, specific PKCε translocation peptide inhibitor and PKCε gene deletion all block hypoxia-induced increase in Nox activity in mouse pulmonary arteries, while the conventional PKC blocker G_6796 has no effect (Figure 5A and 5B). In addition, we have also found that the PKC activator PMA leads to an increase in Nox activity in mouse pulmonary arteries (Figure 5C). PMA increases Nox activity as well in mesenteric arteries. The degree of the increase in Nox activity by PMA is virtually identical in both arteries, which is consistent with the equivalent expression levels of Nox subunits Nox1, Nox4, P22phox, p47phox and p67phox (Figure 1). In agreement with the effect on hypoxic activation of Nox, chelerythrine, specific PKCε translocation peptide inhibitor and PKCε gene deletion considerably reduce hypoxia-induced increase in [ROS]i (Figure 6) and [Ca2+]i as well in mouse PASMCs [3,14]. This is the first report demonstrating the PKCε-dependent Nox activation in pulmonary arteries, as a mediator of hypoxic-induced increase in [ROS]i and [Ca2+]i as well as constriction in PASMCs.
Numerous investigators have demonstrated that mitochondria are involved in hypoxia-evoked contraction in isolated PASMCs, pulmonary arteries and lungs [4,7,8,13,24,45–48]. Using pharmacological inhibitors and genetically manipulated mice, we have previously disclosed that mitochondrial ROS signaling pathway is uniquely activated to mediate hypoxic increase in [ROS]i and [Ca2+]i as well as contraction in PAMSCs [3,14]. Our recent work further points out that hypoxic activation of PKCε is secondary to the increased generation of mitochondrial ROS [3]. In the present study, we have observed that inhibition of mitochondrial ROS generation with rotenone and myxothiazol both significantly prevent hypoxia-induced increase in Nox activity in mouse pulmonary arteries (Figure 7A). Over-expression of Gpx1 to enhance ROS removal in mitochondria and the cytosol significantly inhibits hypoxia-induced increase in Nox activity, whereas Gpx1 gene deletion has the opposite effect (Figure 7B). Consistent with these results, exogenous application of H2O2 mimics hypoxic response, resulting in an increase in Nox activity in pulmonary arteries. H2O2 significantly increases Nox activity as well in mesenteric arteries (Figure 7C). Previous studies have reported H2O2 induces an increase in [Ca2+]i in cultured rat PASMCs [13,46,49] and vasoconstriction in rat pulmonary arteries [50–58]. Moreover, we have recently demonstrated that mitochondrial inhibition almost fully abolishes hypoxic increase in [ROS]i and activation of PKCε in PASMCs [3,14]. Additionally, our data indicate that hypoxic increase in ROS generation in mitochondrial areas precedes that in non-mitochondrial areas (cytosolic regions) [14]. These results, together with the fact that PKCε mediates hypoxic activation of Nox in pulmonary arteries (Figure 5) and increase in [ROS]i in isolated PASMCs (Figure 6), suggest that hypoxia may initially increase mitochondrial ROS generation, activate PKCε and then enhance Nox activity to cause a further increase in intracellular ROS generation, providing a positive feedback mechanism to mediate hypoxic increase in [Ca2+]i and contraction in PASMCs. This hypoxic signaling mechanism appears to be unique in PASMCs, because hypoxia has been shown to cause an increase in [ROS]i and activation of PKCε in PASMCs, but not in mesenteric SMCs [3].
The mitochondrial complex I, II and III are all likely to be involved in hypoxic responses in PASMCs; however, the effect of the complex III post-ubisemiquinone site inhibitor antimycin-A is uncertain. It has been reported that antimycin-A mimics and subsequently blocks HPV in isolated rat lungs [4;45]; does not mimic, but suppresses HPV in isolated rabbit lungs [46]; and neither mimics nor inhibits hypoxic contraction in isolated rat lungs and PASMCs [7;13;46]. Our recent data indicate that this inhibitor has no effect on the resting and hypoxic increase in [ROS]i and [Ca2+]i in freshly isolated mouse PASMCs [14]. In the current study, we have found that antimycin-A does not alter the activity of Nox in mouse pulmonary arteries. These results are consistent with our previous findings, further supporting the view that the mitochondrial electron transport chain molecules prior to the complex III ubisemiquinone site may serve as a functional unit to sense acute hypoxia, thereby initiating hypoxic responses in freshly isolated mouse PASMCs [7;13;14;46;47].
In summary, in this study we present biochemical, pharmacological and genetic evidence that acute hypoxia distinctively augments Nox activity through mitochondrial ROS–PKCε signaling pathway, which provides a positive feedback mechanism to enhance hypoxic generation of intracellular ROS, contributing to the hypoxic increase in [Ca2+]i and contraction in PASMCs.
Acknowledgments
The authors thank Ms. Jodi Heim and Ms. Krista Wardsworth for technical assistance. This work was supported by AHA Scientist Development Grant 0630236N (Y.-M.Z.) and 0730242N (Q.-H.L.), EHS Center Grant P30ES06639 (Y.-S.H.), and AHA Established Investigator Award 0340160N, and NIH R01HL064043 and R01HL075190 (Y.-X.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vadula MS, Kleinman JG, Madden JA. Effect of hypoxia and norepinephrine on cytoplasmic free Ca2+ in pulmonary and cerebral arterial myocytes. Am J Physiol. 1993;265:L591–L597. doi: 10.1152/ajplung.1993.265.6.L591. [DOI] [PubMed] [Google Scholar]
- 2.Wang YX, Zheng YM, Abdullaev II, Kotlikoff MI. Metabolic inhibition with cyanide induces intracellular calcium release in pulmonary artery myocytes and Xenopus oocytes. Am J Physiol Cell Physiol. 2003;284:C378–C388. doi: 10.1152/ajpcell.00260.2002. [DOI] [PubMed] [Google Scholar]
- 3.Rathore R, Zheng YM, Li XQWang QS, Liu QH, Ginnan R, Singer HA, Ho YS, Wang YX. Mitochondrial ROS-PKCε signaling axis is uniquely involved in hypoxic increase in [Ca2+]i in pulmonary artery smooth muscle cells. Biochem Biophys Res Commun. 2006;351:784–790. doi: 10.1016/j.bbrc.2006.10.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michelakis ED, Hampl V, Nsair A, Wu X, Harry G, Haromy A, Gurtu R, Archer SL. Diversity in mitochondrial function explains differences in vascular oxygen sensing. Circ Res. 2002;90:1307–1315. doi: 10.1161/01.res.0000024689.07590.c2. [DOI] [PubMed] [Google Scholar]
- 5.Marshall C, Mamary AJ, Verhoeven AJ, Marshall BE. Pulmonary artery NADPH-oxidase is activated in hypoxic pulmonary vasoconstriction. Am J Respir Cell Mol Biol. 1996;15:633–644. doi: 10.1165/ajrcmb.15.5.8918370. [DOI] [PubMed] [Google Scholar]
- 6.Killilea DW, Hester R, Balczon R, Babal P, Gillespie MN. Free radical production in hypoxic pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L408–L412. doi: 10.1152/ajplung.2000.279.2.L408. [DOI] [PubMed] [Google Scholar]
- 7.Waypa GB, Chandel NS, Schumacker PT. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res. 2001;88:1259–1266. doi: 10.1161/hh1201.091960. [DOI] [PubMed] [Google Scholar]
- 8.Paddenberg R, Ishaq B, Goldenberg A, Faulhammer P, Rose F, Weissmann N, Braun-Dullaeus RC, Kummer W. Essential role of complex II of the respiratory chain in hypoxia-induced ROS generation in the pulmonary vasculature. Am J Physiol Lung Cell Mol Physiol. 2003;284:L710–L719. doi: 10.1152/ajplung.00149.2002. [DOI] [PubMed] [Google Scholar]
- 9.Wedgwood S, Black SM. Role of reactive oxygen species in vascular remodeling associated with pulmonary hypertension. Antioxid Redox Signal. 2003;5:759–769. doi: 10.1089/152308603770380061. [DOI] [PubMed] [Google Scholar]
- 10.Liu JQ, Sham JS, Shimoda LA, Kuppusamy P, Sylvester JT. Hypoxic constriction and reactive oxygen species in porcine distal pulmonary arteries. Am J Physiol Lung Cell Mol Physiol. 2003;285:L322–L333. doi: 10.1152/ajplung.00337.2002. [DOI] [PubMed] [Google Scholar]
- 11.Brennan LA, Steinhorn RH, Wedgwood S, Mata-Greenwood E, Roark EA, Russell JA, Black SM. Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: a role for NADPH oxidase. Circ Res. 2003;92:683–691. doi: 10.1161/01.RES.0000063424.28903.BB. [DOI] [PubMed] [Google Scholar]
- 12.Jernigan NL, Resta TC, Walker BR. Contribution of oxygen radicals to altered NO-dependent pulmonary vasodilation in acute and chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2004;286:L947–L955. doi: 10.1152/ajplung.00215.2003. [DOI] [PubMed] [Google Scholar]
- 13.Waypa GB, Marks JD, Mack MM, Boriboun C, Mungai PT, Schumacker PT. Mitochondrial reactive oxygen species trigger calcium increases during hypoxia in pulmonary arterial myocytes. Circ Res. 2002;91:719–726. doi: 10.1161/01.res.0000036751.04896.f1. [DOI] [PubMed] [Google Scholar]
- 14.Wang QS, Zheng YM, Dong L, Ho YS, Guo Z, Wang YX. Role of mitochondrial reactive oxygen species in hypoxia-dependent increase in intracellular calcium in pulmonary artery myocytes. Free Radic Biol Med. 2007;42:642–653. doi: 10.1016/j.freeradbiomed.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weissmann N, Schermuly RT, Ghofrani HA, Hanze J, Goyal P, Grimminger F, Seeger W. Hypoxic pulmonary vasoconstriction--triggered by an increase in reactive oxygen species? Novartis Found Symp. 2006;272:196–208. [PubMed] [Google Scholar]
- 16.Thomas HMIII, Carson RC, Fried ED, Novitch RS. Inhibition of hypoxic pulmonary vasoconstriction by diphenyleneiodonium. Biochem Pharmacol. 1991;42:R9–R12. doi: 10.1016/0006-2952(91)90440-g. [DOI] [PubMed] [Google Scholar]
- 17.Grimminger F, Weissmann N, Spriestersbach R, Becker E, Rosseau S, Seeger W. Effects of NADPH oxidase inhibitors on hypoxic vasoconstriction in buffer-perfused rabbit lungs. Am J Physiol. 1995;268:L747–L752. doi: 10.1152/ajplung.1995.268.5.L747. [DOI] [PubMed] [Google Scholar]
- 18.Mohazzab KM, Fayngersh RP, Kaminski PM, Wolin MS. Potential role of NADH oxidoreductase-derived reactive O2 species in calf pulmonary arterial PO2-elicited responses. Am J Physiol. 1995;269:L637–L644. doi: 10.1152/ajplung.1995.269.5.L637. [DOI] [PubMed] [Google Scholar]
- 19.Mohazzab KM, Wolin MS. Properties of a superoxide anion-generating microsomal NADH oxidoreductase, a potential pulmonary artery PO2 sensor. Am J Physiol. 1994;267:L823–L831. doi: 10.1152/ajplung.1994.267.6.L823. [DOI] [PubMed] [Google Scholar]
- 20.Zhang F, Carson RC, Zhang H, Gibson G, Thomas HMIII. Pulmonary artery smooth muscle cell [Ca2+]i and contraction: responses to diphenyleneiodonium and hypoxia. Am J Physiol. 1997;273:L603–L611. doi: 10.1152/ajplung.1997.273.3.L603. [DOI] [PubMed] [Google Scholar]
- 21.Weissmann N, Tadic A, Hanze J, Rose F, Winterhalder S, Nollen M, Schermuly RT, Ghofrani HA, Seeger W, Grimminger F. Hypoxic vasoconstriction in intact lungs: a role for NADPH oxidase- derived H2O2? Am J Physiol Lung Cell Mol Physiol. 2000;279:L683–L690. doi: 10.1152/ajplung.2000.279.4.L683. [DOI] [PubMed] [Google Scholar]
- 22.Weir EK, Wyatt CN, Reeve HL, Huang J, Archer SL, Peers C. Diphenyleneiodonium inhibits both potassium and calcium currents in isolated pulmonary artery smooth muscle cells. J Appl Physiol. 1994;76:2611–2615. doi: 10.1152/jappl.1994.76.6.2611. [DOI] [PubMed] [Google Scholar]
- 23.Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox) Am J Physiol Lung Cell Mol Physiol. 2006;290:L2–10. doi: 10.1152/ajplung.00135.2005. [DOI] [PubMed] [Google Scholar]
- 24.Weissmann N, Zeller S, Schafer RUTurowski C, Ay M, Quanz K, Ghofrani HA, Schermuly RT, Fink L, Seeger W, Grimminger F. Impact of mitochondria and NADPH oxidases on acute and sustained hypoxic pulmonary vasoconstriction. Am J Respir Cell Mol Biol. 2006;34:505–513. doi: 10.1165/rcmb.2005-0337OC. [DOI] [PubMed] [Google Scholar]
- 25.Archer SL, Reeve HL, Michelakis E, Puttagunta L, Waite R, Nelson DP, Dinauer MC, Weir EK. O2 sensing is preserved in mice lacking the gp91phox subunit of NADPH oxidase. Proc Natl Acad Sci USA. 1999;96:7944–7949. doi: 10.1073/pnas.96.14.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho YS, Magnenat JL, Bronson RT, Cao J, Gargano M, Sugawara M, Funk CD. Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia. J Biol Chem. 1997;272:16644–16651. doi: 10.1074/jbc.272.26.16644. [DOI] [PubMed] [Google Scholar]
- 27.Xiong Y, Shie FS, Zhang J, Lee CP, Ho YS. The protective role of cellular glutathione peroxidase against trauma-induced mitochondrial dysfunction in the mouse brain. J Stroke Cerebrovasc Dis. 2004;13:129–137. doi: 10.1016/j.jstrokecerebrovasdis.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Miller FJ, Jr, Griendling KK. Functional evaluation of nonphagocytic NAD(P)H oxidases. Methods Enzymol. 2002;353:220–233. doi: 10.1016/s0076-6879(02)53050-0. [DOI] [PubMed] [Google Scholar]
- 29.Datla SR, Dusting GJ, Mori TA, Taylor CJ, Croft KD, Jiang F. Induction of heme oxygenase-1 in vivo suppresses NADPH oxidase derived oxidative stress. Hypertension. 2007;50:636–642. doi: 10.1161/HYPERTENSIONAHA.107.092296. [DOI] [PubMed] [Google Scholar]
- 30.Mittal M, Roth M, Konig P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MA, Fink L, Hanze J, Seeger W, Grimminger F, Schmidt HH, Weissmann N. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res. 2007;101:258–267. doi: 10.1161/CIRCRESAHA.107.148015. [DOI] [PubMed] [Google Scholar]
- 31.Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol. 1994;11:95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- 32.Gupte SA, Kaminski PM, Floyd B, Agarwal R, Ali N, Ahmad M, Edwards J, Wolin MS. Cytosolic NADPH may regulate differences in basal Nox oxidase-derived superoxide generation in bovine coronary and pulmonary arteries. Am J Physiol Heart Circ Physiol. 2005;288:H13–H21. doi: 10.1152/ajpheart.00629.2004. [DOI] [PubMed] [Google Scholar]
- 33.Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, Hoidal JR. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L661–L673. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- 34.Ragan CI, Bloxham DP. Specific labelling of a constituent polypeptide of bovine heart mitochondrial reduced nicotinamide-adenine dinucleotide-ubiquinone reductase by the inhibitor diphenyleneiodonium. Biochem J. 1977;163:605–615. doi: 10.1042/bj1630605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He L, Dinger B, Sanders K, Hoidal J, Obeso A, Stensaas L, Fidone S, Gonzalez C. Effect of p47phox gene deletion on ROS production and oxygen sensing in mouse carotid body chemoreceptor cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L916–L924. doi: 10.1152/ajplung.00015.2005. [DOI] [PubMed] [Google Scholar]
- 36.Brandes RP, Miller FJ, Beer S, Haendeler J, Hoffmann J, Ha T, Holland SM, Gorlach A, Busse R. The vascular NADPH oxidase subunit p47phox is involved in redox-mediated gene expression. Free Radic Biol Med. 2002;32:1116–1122. doi: 10.1016/s0891-5849(02)00789-x. [DOI] [PubMed] [Google Scholar]
- 37.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 38.Orton EC, Raffestin B, McMurtry IF. Protein kinase C influences rat pulmonary vascular reactivity. Am Rev Respir Dis. 1990;141:654–658. doi: 10.1164/ajrccm/141.3.654. [DOI] [PubMed] [Google Scholar]
- 39.Barman SA. Potassium channels modulate canine pulmonary vasoreactivity to protein kinase C activation. Am J Physiol. 1999;277:L558–L565. doi: 10.1152/ajplung.1999.277.3.L558. [DOI] [PubMed] [Google Scholar]
- 40.Jin N, Packer CS, Rhoades RA. Pulmonary arterial hypoxic contraction: signal transduction. Am J Physiol. 1992;263:L73–L78. doi: 10.1152/ajplung.1992.263.1.L73. [DOI] [PubMed] [Google Scholar]
- 41.Weissmann N, Voswinckel R, Hardebusch T, Rosseau S, Ghofrani HA, Schermuly R, Seeger W, Grimminger F. Evidence for a role of protein kinase C in hypoxic pulmonary vasoconstriction. Am J Physiol. 1999;276:L90–L95. doi: 10.1152/ajplung.1999.276.1.L90. [DOI] [PubMed] [Google Scholar]
- 42.Tsai BM, Wang M, Pitcher JM, Meldrum KK, Meldrum DR. Hypoxic pulmonary vasoconstriction and pulmonary artery tissue cytokine expression are mediated by protein kinase C. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1215–L1219. doi: 10.1152/ajplung.00179.2004. [DOI] [PubMed] [Google Scholar]
- 43.Littler CM, Morris KG, Jr, Fagan KA, McMurtry IF, Messing RO, Dempsey EC. Protein kinase C-ε-null mice have decreased hypoxic pulmonary vasoconstriction. Am J Physiol Heart Circ Physiol. 2003;284:H1321–H1331. doi: 10.1152/ajpheart.00795.2002. [DOI] [PubMed] [Google Scholar]
- 44.Lyle AN, Griendling KK. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology (Bethesda) 2006;21:269–280. doi: 10.1152/physiol.00004.2006. [DOI] [PubMed] [Google Scholar]
- 45.Archer SL, Huang J, Henry T, Peterson D, Weir EK. A redox-based O2 sensor in rat pulmonary vasculature. Circ Res. 1993;73:1100–1112. doi: 10.1161/01.res.73.6.1100. [DOI] [PubMed] [Google Scholar]
- 46.Waypa GB, Guzy R, Mungai PT, Mack MM, Marks JD, Roe MW, Schumacker PT. Increases in mitochondrial reactive oxygen species trigger hypoxia-induced calcium responses in pulmonary artery smooth muscle cells. Circ Res. 2006;99:970–978. doi: 10.1161/01.RES.0000247068.75808.3f. [DOI] [PubMed] [Google Scholar]
- 47.Leach RM, Hill HM, Snetkov VA, Robertson TP, Ward JP. Divergent roles of glycolysis and the mitochondrial electron transport chain in hypoxic pulmonary vasoconstriction of the rat: identity of the hypoxic sensor. J Physiol. 2001;536:211–224. doi: 10.1111/j.1469-7793.2001.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weissmann N, Ebert N, Ahrens M, Ghofrani HA, Schermuly RT, Hanze J, Fink L, Rose F, Conzen J, Seeger W, Grimminger F. Effects of mitochondrial inhibitors and uncouplers on hypoxic vasoconstriction in rabbit lungs. Am J Respir Cell Mol Biol. 2003;29:721–732. doi: 10.1165/rcmb.2002-0217OC. [DOI] [PubMed] [Google Scholar]
- 49.Lin MJ, Yang XR, Cao YN, Sham JS. Hydrogen peroxide-induced Ca2+ mobilization in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1598–L1608. doi: 10.1152/ajplung.00323.2006. [DOI] [PubMed] [Google Scholar]
- 50.Rhoades RA, Packer CS, Roepke DA, Jin N, Meiss RA. Reactive oxygen species alter contractile properties of pulmonary arterial smooth muscle. Can J Physiol Pharmacol. 1990;68:1581–1589. doi: 10.1139/y90-241. [DOI] [PubMed] [Google Scholar]
- 51.Jin N, Rhoades RA. Activation of tyrosine kinases in H2O2-induced contraction in pulmonary artery. Am J Physiol. 1997;272:H2686–H2692. doi: 10.1152/ajpheart.1997.272.6.H2686. [DOI] [PubMed] [Google Scholar]
- 52.Kjaeve J, Vaage J, Bjertnaes L. Toxic oxygen metabolites induce vasoconstriction and bronchoconstriction in isolated, plasma-perfused rat lungs. Acta Anaesthesiol Scand. 1991;35:65–70. doi: 10.1111/j.1399-6576.1991.tb03243.x. [DOI] [PubMed] [Google Scholar]
- 53.Jones RD, Thompson JS, Morice AH. The effect of hydrogen peroxide on hypoxia, prostaglandin F2α and potassium chloride induced contractions in isolated rat pulmonary arteries. Pulm Pharmacol Ther. 1997;10:37–42. doi: 10.1006/pupt.1997.0071. [DOI] [PubMed] [Google Scholar]
- 54.Burghuber OC, Strife R, Zirolli J, Mathias MM, Murphy RC, Reeves JT, Voelkel NF. Hydrogen peroxide induced pulmonary vasoconstriction in isolated rat lungs is attenuated by U60,257, a leucotriene synthesis blocker. Wien Klin Wochenschr. 1986;98:117–119. [PubMed] [Google Scholar]
- 55.Seeger W, Suttorp N, Schmidt F, Neuhof H. The glutathione redox cycle as a defense system against hydrogen-peroxide-induced prostanoid formation and vasoconstriction in rabbit lungs. Am Rev Respir Dis. 1986;133:1029–1036. doi: 10.1164/arrd.1986.133.6.1029. [DOI] [PubMed] [Google Scholar]
- 56.Sheehan DW, Giese EC, Gugino SF, Russell JA. Characterization and mechanisms of H2O2-induced contractions of pulmonary arteries. Am J Physiol. 1993;264:H1542–H1547. doi: 10.1152/ajpheart.1993.264.5.H1542. [DOI] [PubMed] [Google Scholar]
- 57.Yamaguchi K, Asano K, Mori M, Takasugi T, Fujita H, Suzuki Y, Kawashiro T. Constriction and dilatation of pulmonary arterial ring by hydrogen peroxide: importance of prostanoids. Adv Exp Med Biol. 1994;361:457–463. doi: 10.1007/978-1-4615-1875-4_80. [DOI] [PubMed] [Google Scholar]
- 58.Wilhelm J, Herget J. Role of ion fluxes in hydrogen peroxide pulmonary vasoconstriction. Physiol Res. 1995;44:31–37. [PubMed] [Google Scholar]