Abstract
Objective
To investigate visual-motor integration (VI) skills of prelingually deaf (PLD) children before and after cochlear implantation (CI) and investigate correlations with spoken-language and related processing measures.
Design
Study 1 was a longitudinal study in which VI was tested preimplant. Study 2 was a cross sectional study of school-age children who used a CI for >2 years.
Method
In study 1, a standardized design-copying task was administered preimplant, and spoken-language data were obtained at intervals up to 4 years postimplantation. Analyses were conducted to determine if preimplant VI scores were predictive of various spoken-language measures. In study 2, standardized design copying and speeded maze tracing tasks were administered along with speech perception, vocabulary, and related processing measures.
Results
Whereas preimplant VI scores for children in study 1 fell within the typical range based on age-equivalent norms, postimplant VI standard scores in study 2 were low compared to the normative sample. Postimplant VI scores were inversely related to age at implantation. Preimplant VI scores were robustly predictive of most, but not all, spoken-language outcome scores. Postimplant design copying scores were also correlated with spoken-language and related processing measures whereas maze-tracing scores were less robustly related to these measures.
Conclusions
Early auditory and linguistic experience may impact the development of VI skills. VI is a preimplant predictor of later spoken language outcomes. Design copying and speeded maze tracing tasks appear to tap different sets of cognitive resources in PLD children with CIs.
Keywords: Visual-motor integration, preimplant predictor, children, deaf, cochlear implant, individual differences, language, outcome
INTRODUCTION
For children born with profound hearing loss, or prelingual onset of deafness, a cochlear implant (CI) has been shown to be useful for developing spoken language skills.1 Longitudinal studies of outcomes in this clinical population have demonstrated that CI use leads to gains in open-set spoken word recognition, receptive and expressive language skills, vocabulary knowledge, and improved speech production abilities.2–4
However, spoken-language outcomes in prelingually deaf (PLD) children with CIs are extremely variable.5 Although some children progress very well with their device, other children obtain little benefit other than awareness of sound. Wide variation in outcome presents a challenge to the clinician and researcher in predicting which children will do well with a CI and which children will struggle and why.
An emerging focus in the study of pediatric CI outcomes is the investigation of cognitive and linguistic abilities of PLD children.6 These processes are not specific to audition or to spoken language, yet they may play an important role in perceiving speech, acquiring language, and developing the sensory-motor abilities crucial to producing highly intelligible speech.7 In addition to identifying early predictors of outcome and uncovering sources of individual variability, research on cognitive factors may provide the theoretical basis for the development of new therapeutic interventions for PLD children who, despite having access to sound with a CI, often show significant delays in spoken language acquisition.
Visual-Motor/Spoken Language Links
In the past, researchers recognized that perceptual-motor development and language acquisition proceed in a predictable fashion with behavioral milestones correlated across systems.8 Longitudinal studies have demonstrated that early perceptual-motor development is tightly linked to early speech behavior as well as to later spoken-language development.9,10 Language-related skills such as reading have also been shown to be related to motor skills.11 Although various theories exist to explain these relations, the general conclusion is that spoken-language and perceptual-motor systems share some underlying cortical processing resources.12
One perceptual-motor skill that has been linked to spoken-language is visual-motor integration (VI). Traditionally, VI tasks are figure-copying tasks in which children attempt to copy a series of increasingly complex geometric figures.13 Performance on these design copying tasks has been shown to be correlated with language skills, reading ability, and general academic achievement in typically developing, normal-hearing children14 as well as in hearing-impaired children who use American sign language.15,16 Furthermore, there is evidence that children with hearing impairment may show atypical performance on VI tasks17,18 and on other perceptual-motor tasks involving balance, running, throwing, and figure drawing.18,19 However, early studies included children with neurological and cognitive sequelae, most were conducted before hearing-impaired children could be identified at birth through universal newborn hearing screening, and early studies often tested children who were immersed in a manual language environment in which spoken language was not emphasized. Thus, these studies cannot be generalized to the current population of PLD children who present for a CI.
In one study of VI skills in PLD children, children from 2.5 to 17.5 years old were tested with VI measures before implantation and 6 months after implantation.20 Before implantation, the children showed a 1.5-year delay in performance on the VI task as compared with the normative sample. Postimplantation, the children as a group showed significant improvement in performance, although there was still a developmental lag overall. Although the study involved children who were using now-obsolete cochlear-implant-processing strategies and devices, the findings suggested that VI skills are atypical in PLD children who present for a CI but that they can improve over time.
Although little other research has investigated VI skills in pediatric CI users, several recent studies have explored the issue of whether general motor development is atypical in PLD children with CIs.21–23 For example, Kutz et al. measured fine and gross motor skills in 17 infants and children who presented for CI surgery using a standardized caregiver report.22 The authors reported that motor standard scores fell within the typical range of variation found in normal-hearing infants. In a slightly older population of 5- to 9-year-old children, Schlumberger et al. measured motor skills in three different populations: normal-hearing children, profoundly deaf children with CIs, and profoundly deaf children with hearing aids.23 All three groups performed similarly on simple motor tasks across all ages, and complex motor task performance in the 5- to 7-year-olds was equivalent across the three groups. However, 7- to 9-year-old deaf children in both groups demonstrated poorer performance on complex motor tasks than normal-hearing controls.23
Recently, two CI studies demonstrated relations between preimplant motor development and postimplant spoken-language outcomes.21,24 In both studies, PLD infants and children were assessed prior to implantation using a caregiver report of adaptive behaviors. Whereas motor development scores were within the age-appropriate range, other adaptive behaviors (e.g., socialization) were delayed. Furthermore, children in the upper range of motor development showed better performance on several spoken-language measures than children in the lower half of motor development. The relation between preimplant motor scores and postimplant spoken-language measures tended to be stronger for fine-motor scores than for gross motor scores.24
In this paper, we present results from two studies in which three major questions are addressed. First, are VI skills of PLD children enrolled in a CI program typical as compared to a large normative population? Second, are VI skills related to participant factors such as age at implantation or communication mode? Finally, is VI performance related to performance on spoken-language measures?
STUDY 1
METHOD
Participants
A total of 42 children were identified from the large cohort of pediatric CI patients followed longitudinally by the DeVault Otologic Research Laboratory. Demographic inclusion criteria were: severe or profound deafness by 3 years of age, use of a CI with an up-to-date processing strategy, and implantation by 9 years of age. A review of the medical and surgical records for these children identified 2 participants with known or suspected developmental delay. These children were excluded from the study. The demographic, surgical, and rehabilitative characteristics of the remaining 40 PLD children are summarized in Table I. Children were considered oral (OC) if they used spoken-language exclusively with no reliance on Signed Exact English. Children were considered users of total communication (TC) if they used a combination of oral and Signed Exact English.
TABLE I.
Characteristics of Sample in Study 1.
| Characteristic | Data | Range |
|---|---|---|
| Mean age at onset | 2.70 (5.4)* | 0−18 |
| Mean length of deprivation | 58.2 (19.6)* | 22−107 |
| Mean age at test | 57.6 (19.7)* | 24−103 |
| Mean age at implantation | 60.8 (19.1)* | 27−107 |
| Mean PTA (dB HL) | 107.6 (13.0)* | 58−120 |
| Mean number of electrodes | 20.1 (3.7)* | 8−22 |
| Mean non-verbal IQ score | 103.1 (16.5)* | 71−129 |
| Mean VMI quotient | 0.98 (0.2)* | 0.1−1.5 |
| Ear of implantation | 25 right, 15 left | |
| Mode of communication | 16 OC, 24 TC | |
| Gender | 17 female, 23 male | |
| Etiology | 31 congenital: 2 genetic, 1 LVA, 1 Mondini, 2 AN, 25 unknown | |
| 9 acquired: 8 meningitis, 1 CMV | ||
Note: mean values with standard deviations in parentheses. Mean age variables and length of deprivation are in months.
PTA = pure tone average at 500, 1000, and 2000 Hz; IQ = standard nonverbal scores from WPPSI or WISC; OC = oral communication; TC = total communication; LVA = large vestibular aqueduct; AN = auditory neuropathy; CMV = perinatal CMV infection; VMI = Beery Test of Visuomotor Integration.
Measures
Measures obtained during the preimplant period for these children included tests of speech perception, speech production, language, vocabulary, and lip-reading. In addition, cognitive processing measures were obtained. Several of these measures were selected for analysis in our study.
The Beery Test of Visuomotor Integration (VMI)13 was administered to all children during the preimplant period. This test contains a sequence of 24 geometric forms of increasing complexity ranging from a simple vertical line to a complex three-dimensional star. Children are asked to copy each item as accurately as they can. Instructions were given in the child's preferred mode of communication (spoken English or Signed Exact English).
For each item, children received 1 to 4 points based on set criteria.13 Raw scores were converted to age-equivalent scores using normative tables. To derive scores that reflected VMI performance relative to chronological age, we computed age quotients by dividing age equivalent scores by chronological age at testing. These derived age quotients (VMIq) were used in subsequent analyses. Although the VMIq is a continuous measure of age-relative visual-motor skill, a limitation of this measure is that the distribution of these scores cannot easily be compared to the normative sample. For instance, an age-equivalent score below 1.0 (e.g., 0.85) may be within the normal range of development.
A number of standard clinical spoken-language measures were routinely administered at 6-month intervals in this longitudinal study. Open-set word recognition was measured using the Phonetically Balanced-Kindergarten (PBK) test.25 For this test, a list of 50 phonetically-balanced monosyllabic words was administered via live-voice presentation in the auditory-only modality. Children heard a spoken word and were asked to repeat the word aloud to the examiner. The PBK was scored by percentage of words (PBK-w) and phonemes (PBK-p) spoken correctly.
Open-set phrase comprehension was assessed with the Common Phrases (CP) test,26 via auditory-only, live voice. Children were presented with a list of 10 every-day phrases such as, “open the door,” and were asked to repeat each phrase aloud to the examiner. Phrases were scored as correct if the child exactly repeated what was presented or responded with an appropriate action (e.g., opening the door). The CP was scored as percent phrases correct.
Speech intelligibility, or the ability of naïve adults to understand the child's speech, was assessed using the Beginner's Intelligibility Test (BIT).27 Audio recordings of children repeating a list of 10 sentences were presented to naïve adult listeners who were asked to transcribe what they thought the children had said. Intelligibility scores were based on percentage of words correctly transcribed by the adult listeners. No partial credit was given.
Vocabulary knowledge was assessed with the Peabody Picture Vocabulary Test-III (PPVT).28 Vocabulary items were presented in the child's preferred communication modality (either spoken English or Signed Exact English) and the child was asked to choose from four pictures, one of which correctly corresponded to the meaning of the word. Age-equivalent scores were used in analyses.
The Reynell Developmental Language Scales-3rd Edition (RDLS)29 was administered to assess receptive and expressive language skills in the child's preferred mode of communication. The receptive scales (RDLS-r) measured 10 skills, including word recognition, sentence comprehension, and verbal comprehension of ideational content. The expressive language scales (RDLS-e) assessed skills such as spontaneous expression of speech and picture description. Raw scores and age-equivalent scores were used in analyses.
Language measures were administered during the preimplant period (within 6 months before CI surgery) and at 6-month intervals after surgery. Scores were collapsed into one of five intervals of CI use: preimplant, 1 year post, 2 years post, 3 years post, and 4 years post. Not all children could be tested at each interval, creating missing data cells for several reasons (e.g., fatigue, children moved away from the area).
Statistical Analyses
To determine whether participants’ mean VMI scores were significantly different from the normative population, we compared age-equivalent scores computed from the VMI norms to the chronological ages using a paired t-test. To determine whether VMIq was related to demographic, surgical, or rehabilitative variables, we conducted simple bivariate correlations between VMIq and continuous participant variables (e.g., age, degree of hearing loss, number of active electrodes). One-tailed tests were used when there was a predicted direction to the correlation. We predicted negative correlations between VMIq and age at test, length of deprivation, and unaided pure tone average at 500, 1000, and 2000 Hz (PTA). We predicted positive correlations between VMIq and number of active electrodes. For non-continuous participant variables (i.e., gender, communication mode, etc.) we used independent samples t-tests to assess possible effects on VMIq. One-tailed t-tests were used for communication mode as we predicted that children from OC environments would show higher VMIq scores than children from TC environments.
To determine if preimplant VMI skills were related to postimplant spoken language measures, we used SAS to create a set of mixed-effects models.30 Models for each spoken-language measure, including estimates of main effects (and interactions) of length of CI use and preimplant VMI score, were created using maximum-likelihood estimation.31 The benefit of using mixed-effects models over traditional ANOVA methods is that the former can accommodate differing data collection schedules. Therefore, subjects with missing outcome data at one or more test intervals are not excluded in mixed-effects models. Exclusion of such subjects is required by analysis of variance (ANOVA) methods and can lead to skewed results and underestimates of variability.31 Scores from participants tested at two or more time intervals were used to compute an estimation of the mean score at each time interval. Scores from all participants (including those tested at only one interval) were used to compute the variance of the mean score at each time interval. For each model, VMIq score was the predictor variable, interval of test was the repeated measures variable, and the given outcome measure was the dependent variable.
RESULTS
VMI performance
None of the correlations between VMIq and continuous variables reached significance. Independent samples t-tests revealed no significant effects of the of the non-continuous participant variables (e.g., gender, communication modality) on VMIq.
If average VMI performance of PLD children with CIs is similar to their age-matched normal hearing peers, we would expect that mean age equivalent score (VMI-e) would be close to the mean age at testing of the sample. Mean VMI-e was 55.05 months (SD = 19.69) while the mean of the sample was 57.57 months (SD = 19.14). This difference was not statistically significant, repeated measures t (39) = 1.24, P = .223. Thus, mean performance on the VMI task was similar to what would be expected from a sample of age matched normal hearing peers.
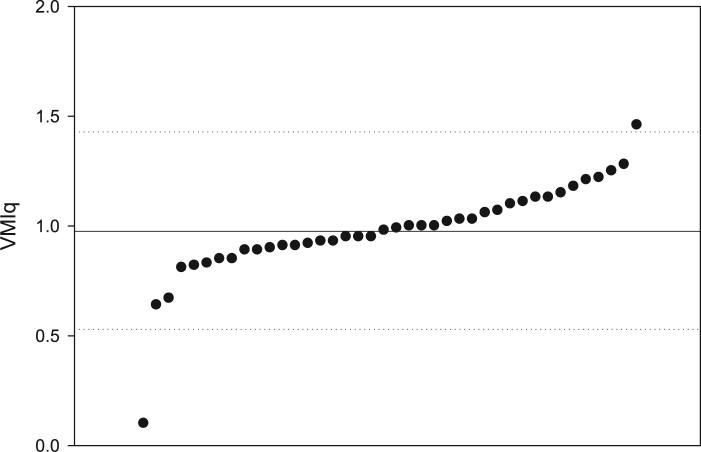
Mean preimplant VMIq for the 40 deaf children was 0.98 (median = 0.98, SD = 0.22, range = 0.10−1.46). This mean was similar to the score expected if age-equivalent performance was equal to chronological age. As shown in the scatterplot of individual VMIq scores in Figure 1, all but two participants fell within 2 SD of the sample mean. Due to possible skewing effects, the two outliers were excluded from all further analyses, reducing the sample size to 38.
Fig. 1.
Distribution of Beery Test of Visuomotor Integration quotient scores (VMIq). Individual VMIq scores are shown by dots from lowest to highest. The solid line represents the sample mean and the dotted lines represent two standard deviations above and below the mean.
VMIq scores and language measures
For each outcome measure, a model was constructed in which VMIq was the predictor variable and interval of CI use was the repeated-measure variable. Age of implantation was included as a second predictor variable. For all language outcome measures, length of CI use was a significant predictor; performance significantly increased with increased CI experience, P < .0001. As shown in Table II, VMIq was a significant predictor of percentage of correct scores on the PBK, CP, and BIT tests. In each case, children with higher preimplant VMIq showed higher percentage of correct scores on the above mentioned tasks. However, VMIq was not an independent predictor of age equivalent scores on the RDLS or PPVT.
TABLE II.
SAS Mixed Procedure Results.
| Dependent Variable | Predictor Variable |
|
|---|---|---|
| Main Effect of VMIq | Effect Size | |
| PBK-p | F (25.6) = 14.26, P = .0009 | 22.217 (SE = 5.88) |
| PBK-w | F (25.8) = 7.32, P < .0119 | 16.176 (SE = 5.98) |
| CP-a | F (21.4) = 7.49, P = .0122 | 29.702 (SE = 10.86) |
| BIT* | F (33.0) = 10.71, P = .0025 | 21.743 (SE = 10.13) |
| PPVT | F (30.8) = 0.79, P = .3804 | NA |
| RDLS-r* | F (34.8) = 0.76, P = .3904 | NA |
| RDLS-e | F (27.4) = 1.15, P = .2927 | NA |
Note: F statistics for main effects of preimplant VMIq on spoken-language measures.
Effect size is calculated as the amount of change in dependent variable with each 1 SD increase in the predictor variable (standard error is shown in parentheses).
Indicates significant interaction between VMIq and length of use. Significant interactions for BIT: F (55.7) = 6.03, P < .0001 and for RDLS-r: F(62.2) = 3.40, P = .0141.
VMI = Beery Test of Visuomotor Integration; PBK-w = phonetically balanced kindergarten test percentage of words correct, PBK-p = phonetically balanced kindergarten test percentage phonemes correct, CP = common phrases test; RDLS-r = Reynell developmental language scales receptive age equivalent; RDLS-e = Reynell developmental language scales expressive age equivalent; PPVT = Peabody picture vocabulary test age equivalent.
In two of the models, there was a significant interaction between length of CI use and preimplant VMIq score. Relations between preimplant VMIq and BIT scores tend to be more positive with subsequent postimplant test intervals. This result indicates that children with higher preimplant VMIq scores show greater improvement in BIT scores with CI use than children with lower preimplant VMIq scores.
DISCUSSION
Study 1 revealed several important findings regarding VI skills in PLD children with CIs. First, preimplant VMI scores of PLD children in our sample were not atypical in comparison with normative data based on a comparison of mean age-equivalent scores and mean chronological age at test. This result contradicts earlier reports that the performance of deaf children on a similar VI task was delayed compared to normal hearing children.17,20 This discrepancy may be due to the fact that, in contrast with previous studies, our sample of deaf children was likely to have been diagnosed earlier and to have received earlier audiologic and speech pathology intervention, as well as the fact that children with gross cognitive or motor delays were excluded from our study.
Because chronological age was not correlated with preimplant VMIq score, we cannot conclude from study 1 that deaf children with longer periods of auditory deprivation show more delayed VMI skills than children with shorter periods of auditory deprivation. Preimplant VMIq was not significantly correlated with any of the other participant variables.
The longitudinal analyses revealed that VMIq score was a robust preimplant predictor of postimplant performance on tests of speech perception, sentence comprehension, and speech intelligibility. This relationship was independent of length of CI use, which was a significant predictor in all models. Children with larger preimplant VMIq quotients had greater percentage of correct scores on these measures across intervals of CI use. For speech intelligibility, higher VMIq scores were associated with greater increases in BIT score over subsequent CI intervals than lower VMIq scores were. Thus, VMIq may not only predict overall performance on a given outcome measure, but may also predict rate of improvement with CI experience.
VMIq was not an independent predictor of RDLS-e, RDLS-r, or PPVT scores. It is not clear why the language and vocabulary measures did not show a similar relation as the other measures. One important difference between the PBK, BIT, and CP tests, compared to the RDLS and PPVT, is that the former tests are administered in the auditory-only format whereas the latter are administered in the child's best mode of communication. Perhaps the relations between VMI and language tasks are heavily influenced by the degree to which the tasks require auditory listening skills or require sub-vocal verbal mediation? Future research with different types of language and vocabulary tasks may be informative.
An important limitation of study 1 is that children were only tested on the VMI at quite early ages before implantation. Variability of VMI skills in PLD children and their relation to spoken-language might not be fully appreciated until later ages when children are older and have more experience with their CI. As a result, we conducted a study with PLD children who had used their implants for several years. We also used different visual-motor measures to see if the relations detected in study 1 generalized to other visual-motor tasks.
STUDY 2
METHOD
Participants
A total of 26 school-aged children (16 males and 10 females), ages 6 to 14 years, were recruited for this study. Criteria for inclusion in the study were: PLD prior to age 4, implantation prior to age 6 years, and use of a CI for at least 2 years. Participant characteristics, demographic data, and educational data were obtained by caregiver report. For several children, demographic information was unavailable (e.g., incomplete questionnaires). Age of implantation ranged from 1 to 6 years. Duration of CI use varied from 3 to 11 years. For 23 children, caregivers reported congenital onset of bilateral deafness, with three children losing hearing at ages 1, 2, and 3.5 years, respectively.
All of the children were in mainstream educational environments. Twenty-five participants were in oral educational environments (auditory-verbal or auditory-oral) and five were in total communication environments. All were children of hearing parents. Etiology of deafness for 20 of the children was unknown. For the remaining six children, reported etiology included Mondini malformation (n = 3), meningitis (n = 1), and genetic (n = 2). Average length of CI use was 6.21 years and varied from 3.5 years to 11.8 years.
Procedure
All measures were collected as part of a larger study of neuropsychological functioning, phonological, and reading skills of PLD children with CIs.32 Each participant was tested in a single 1.5-hour testing session during which several standardized measures of nonverbal psychological development, speech perception and related processing measures were administered. Included in these measures were two tests of visual-motor integration (VI).
VI measures
These tests were taken from a standardized battery of neuropsychologic functions widely used in clinical settings, the NEPSY,33 which assesses neuropsychological function of children between 3 and 12 years of age. The results are expressed raw scores, age-equivalent scores, and standardized scores (i.e., M = 10, SD = 3). Two different VI measures were administered.
The first VI measure, Design Copying (DC), was similar to the VMI measure used in study 1. This test was a pencil-and-paper test of a child's ability to copy two-dimensional geometrical figures of increasing complexity with no time limits. There are 16 items from a simple vertical line to a complex closed-staircase pattern. Points for each item were summed to obtain the DC raw score (DC-r). Age equivalent scores (DC-e) and standardized scores (DC-s) were obtained.
The second VI measure, visual-motor precision (VMP), was a timed maze-tracing task containing two mazes, a simple maze and a complex maze. Children were instructed to draw a line down the track as quickly as they could without crossing the lines or rotating the paper. Composite raw scores for each maze reflected number of errors (number of times the line crossed the track) and speed (time to complete the task). Fewer errors and faster speed led to higher raw scores. Data from each maze were used to compute a total VMP raw score (VMP-r), an age-equivalent score (VMP-e), and a standard score (VMP-s).
Several subset measures were computed from the VMP task as well, due to the possibility that aspects of speed and errors could be assessed independently. The NEPSY includes supplemental percentile scores individually for time to completion (the inverse of speed) and number of errors for each maze as a function of chronological age.33 Therefore, for each participant, we computed four percentile group ranks from the VMP mazes: simple maze speed, simple maze error, complex maze speed, and complex maze error. The groups were ranked 1 through 5 corresponding to the following percentile ranges, respectively: >75%, 26 to 75%, 11 to 25%, 2 to 10%, and ≤2%. Thus, 1 reflects best performance relative to age, whereas 5 reflects poorest performance relative to age.
Speech perception and related processing measures
Open set word recognition was assessed using the PBK, described in study 1. The Forward Digit Span (Fdig) and Backward Digit Span (Bdig) subtests of the Wechsler Intelligence Scale for Children-3rd Edition34 were also administered. Fdig was included as a measure of verbal rehearsal, and backward digit span was included as a measure of working memory capacity. The nonword repetition subtest of the Woodcock Reading Mastery Tests-Revised35 was also administered. The mean accuracy rating was obtained as a measure of phonological accuracy in repeating auditorily presented nonwords.
The McGarr Sentence Intelligibility Test36 was used as a measure of speaking rate (Mrate). Seven-syllable sentences were presented in the child's preferred mode of communication as well as orthographically, and children were asked to repeat the sentences aloud as intelligibly as possible. The children's utterances were recorded and then later scored for length of utterance in seconds.
RESULTS
Design-copying
Mean observed raw scores, age equivalent scores, and standard scores are shown in Table III. If average DC performance of PLD children with CIs is similar to their age-matched normal hearing peers, we would expect the mean age equivalent score (DC-e) to be close to the mean age of the sample. Mean DC-e was 8.14 years (SD = 0.69) while the mean age of the sample was 9.13 years (SD = 2.7). This difference was statistically significant, repeated measures t25 = 2.405, P = .024. Mean DC-s was 8.2 (SD = 3.4) and ranged from 1 to 14. Thus, while most children fell within normal limits, the mean performance on the DC task was lower than would be expected from a sample of age-matched normal-hearing peers.
TABLE III.
Mean Design Copying (DC) and Visual-Motor Precision (VMP) Scores.
| Score | DC Task | VMP Task |
|---|---|---|
| Mean raw score | 46.8 (SD = 9.8) | 20.4 (SD = 9.6) |
| Mean age equivalent score | 8.15 (SD = 0.6)* | 7.17 (SD = 2.95)† |
| Mean standard score | 8.2 (SD = 3.4) | 7.4 (SD = 7.4) |
Age-equivalent scores were obtained for the NEPSY norms based on the raw scores. For each raw score, the NEPSY provides an age-range. The upper limit of this range was used as the age-equivalent score for each participant's raw score. Standard deviations are given in parentheses.
Indicates a significant difference between mean age equivalent score and age of sample, P < .05
Indicates a significant difference between mean age equivalent score and age of sample, P < .001.
Visual-motor precision
Mean observed raw scores, age equivalent scores, and standardized scores are shown in Table III. Two children did not complete the VMP task, and therefore, the sample size was reduced to 24. Again, age equivalent scores were compared to the mean age of the sample to determine if performance was similar to normal hearing peers. Mean VMP-e was 7.17 years (SD = 2.95) and mean age of the sample of 24 was 9.35 years (SD = 2.44). This difference was statistically significant, repeated measures t (25) = 3.363, P = .003. Mean VMP-s was 7.4 (SD = 3.4) and ranged from 1 to 15. These results mirrored the DC results: Most children fell within normal limits while average VMP performance was lower than expected from age-matched peers.
Participant variables and visual-motor scores
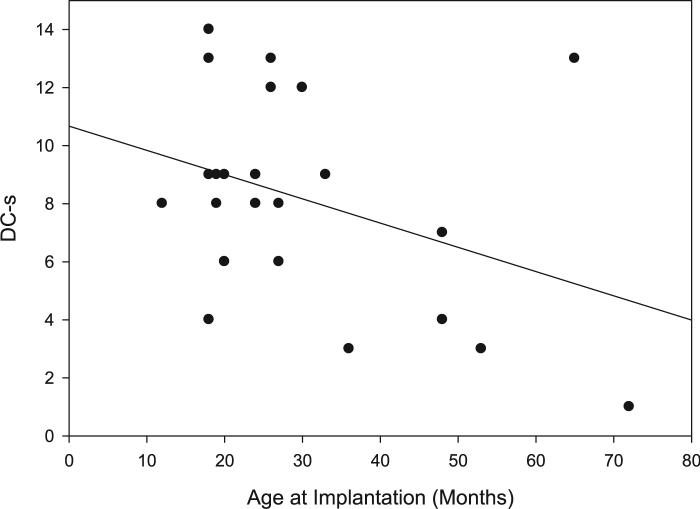
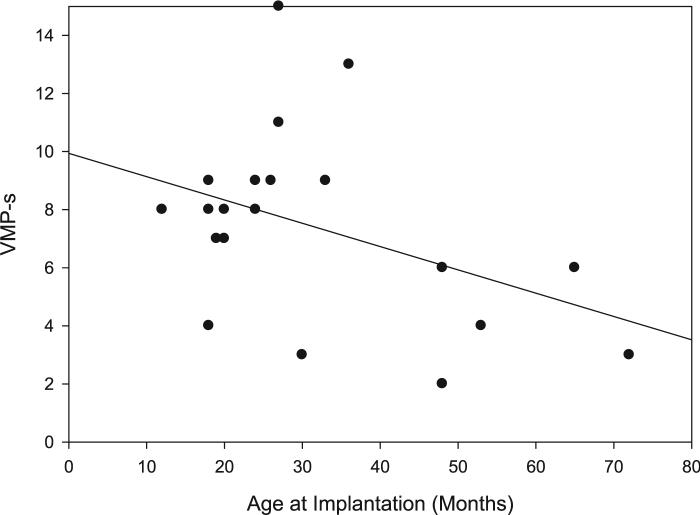
Correlations were carried out using standardized visual-motor scores (i.e., DC-s and VMP-s). One-tailed tests were used when there was a predicted directionality for a correlation. The only participant factor found to correlate significantly with both DC-s and VMP-s was age at implantation, as shown in Table IV. Children who received a CI at an earlier age tended to show greater standardized DC and VMP scores than children implanted at later ages (Figs. 2, 3).
TABLE IV.
Correlations with Age at Implantation, Speech, and Processing Measures.
| Design Copying Scaled Score | Visual-Motor Precision Scaled Score | |
|---|---|---|
| Age at implantation | r = −0.375, P = .035 | r = −0.414, P = .028 |
| Forward digit span total score | r = 0.172, P = .201 | r = 0.004, P = .493 |
| Backward digit span total score | r = 0.594, P = .001 | r = −0.023, P = .459 |
| PBK-p | r = 0.600, P = .001 | r = 0.296, P = .090 |
| PBK-w | r = 0.538, P = .003 | r = 0.418, P = .027 |
| Nonword repetition | r = 0.365, P = .033 | r = 0.350, P = .047 |
| Mrate | r = −0.113, P = .304 | r = −0.247, P = .140 |
Significance levels given for one-tailed tests.
r = bivariate Pearson coefficient; PBK-p = percentage of phonemes correct; PBK-w = percentage of words correct; Nonword repetition = mean accuracy rating from the nonword repetition subtest of the Woodcock Reading Mastery Tests-Revised35; Mrate = mean sentence utterance length on McGarr sentences.
Fig. 2.
Design copying scaled score as a function of age at implantation. Individual scores are shown with a line of best fit.
Fig. 3.
Visual-motor precision scaled score as a function of age at implantation. Individual scores are shown with a line of best fit.
Visual-motor scores and language measures
Again, several correlations were carried out. For those correlations found to be significant, partial correlations were conducted to control for the effect of age at implantation. The correlation matrix is shown in Table IV. We predicted positive correlations between VI scores and speech perception and related processing scores so one-tailed tests were used. DC-s showed significant correlations with PBK percentage of correct phonemes and percentage of correct words, as well as with backward digit-span scores. Moderate and significant correlations were seen between DC-s and non-word repetition scores. Each of these relations remained significant in partial correlations controlling for age at implantation. Two correlations involving VMP-s were also significant. VMP-s scores were significantly correlated with PBK word correct scores, and non-word repetition scores.
Sub-analyses of VMP task
Two-tailed correlations between age at implantation, number of errors (percentile group), and time to completion (percentile group) for each maze were carried out individually. Results for both mazes (simple and complex) were similar; for simplicity, only the complex results are discussed although both are shown in Table V. The correlation between age at implantation and time to completion percentile group was r = −0.387, P = .075. Thus, there was a trend for later implanted children to complete the VMP task more quickly with respect to age than earlier implanted children did. In contrast, a significant positive correlation was found between age at implantation and number of errors percentile group, r = 0.539, P = .010. Later implanted children made more errors with respect to age than did earlier implanted children. Taken together, these correlations suggest that the benefit to earlier implantation seen for overall VMP task performance is carried by the tendency of earlier-implanted children to be less error prone than later-implanted children. Conversely, earlier implantation does not appear to lead to faster performance on the VMP task.
TABLE V.
Visual-Motor Precision Scaled Score Subtests: Correlations With Age at Implantation.
| Simple Maze | Complex Maze | |
|---|---|---|
| Time (seconds) | r = −0.402, P = .064 | r = −0.416, P = .054 |
| Total # errors | r = 0.043, P = .850 | r = 0.093, P = .680 |
| Time percentile group | r = −0.308, P = .164 | r = −0.387, P = .075 |
| Errors percentile group | r = 0.393, P = .070 | r = 0.539, P = .010 |
Note: Significance levels given for one-tailed tests.
r = bivariate Pearson coefficient.
DISCUSSION
Overall, mean performance of the sample on both DC and VMP tasks was below that of normal hearing peers based on the NEPSY norms. Unlike study 1, in which mean VMIq scores were not significantly below the expected performance based on norms, these results support earlier findings that VI skills of deaf children are delayed compared to normal hearing children.17,20 It is possible that, when tested early prior to implantation, design copying tests are not sensitive enough to pick up differences between PLD children and normal-hearing peers. Another possibility is that VI skills show a slower developmental trajectory in PLD children, compared to normal hearing children and, thus, delays become more apparent at later ages.
It is important to note that other cofounding variables might explain these findings, such as undetected motor and/or cognitive impairments in the PLD sample or difficulties in administering standardized behavioral tests to PLD children. Further longitudinal studies in carefully selected samples of PLD children with CIs are needed to more fully understand the development of VI in this population.
Similar to the VMIq results in study 1, DC standard scores were significantly correlated with speech perception percentage of correct scores. These results are similar to correlations between DC standard score and PPVT standard score reported earlier from the same population of CI users.37 DC standard scores were also related to working memory and non-word repetition scores. The former correlation suggests that the design copying VI tasks may recruit working memory, and the latter suggests that DC is influenced by subvocal rehearsal and/or the ability to rapidly encode and manipulate phonological units. These results support previous findings that working memory and subvocal rehearsal play a role in speech perception with a CI.38–40
In contrast to study 1, longer periods without a CI were associated with greater delays on the DC and VMP. Children implanted at later ages showed lower DC and VMP standard scores than children implanted at earlier ages showed. Although these correlations cannot prove causation, they suggest that a period of auditory deprivation may lead to atypical development of a non-verbal skill such as VI. While neuroimaging work has begun to reveal mechanisms of auditory cortical plasticity underlying speech-perception and production outcomes,41,42 we currently know little about how non-verbal processes are affected and/or altered by deafness. In one recent paper, increased preimplant PET activity in frontal and parietal cortex, areas involved in behavioral control and visual-spatial processing, was found to be a predictor of postimplant speech perception scores.43 Further neuroimaging and behavioral studies are needed to understand the relations between frontal and parietal cortical function and other spoken-language measures.
One interesting finding from study 2 is that, in contrast to the DC task, the speeded maze tracing task was not robustly related to speech perception scores, nor was it significantly correlated with DC scores. Furthermore, working memory did not appear to be strongly recruited during the VMP task, based on the lack of correlation with backward digit span. The VMP is a different type of VI task, one that involves a tradeoff between speed and precision, and it likely recruits attention and behavioral inhibition systems more strongly than the DC. The analyses of speed and error measures of the VMP with reference to age at implantation revealed an important pattern: Children implanted earlier (who tended to have higher overall VMP-s) made fewer errors, but they completed the mazes more slowly than children who were implanted later.
One interpretation of the latter findings is that early auditory and linguistic experience influences the development of attentional and behavioral inhibition systems. Several investigators have reported that deaf children with CIs show more age-typical performance on visual-only tests of sustained attention than deaf children without CIs who use hearing aids.44,45 Sustained attention has also been shown to improve with length of CI use.46 Furthermore, the ability of PLD children with CIs to delay premature behavioral responses has been shown to increase with CI use and to be related to performance on several spoken-language measures.47 Our findings with the VMP task support and extend these earlier findings.
CONCLUSIONS
Visual-motor integration skills in PLD children appear to be related to early auditory and linguistic experience. Although the present studies do not prove a causal relationship, these findings are consistent with the hypothesis that early sensory experience can impact the development of information-processing resources that are independent of the sensory domain. The underlying neurocognitive mechanisms behind these findings are still unknown, although our study suggests that working memory, subvocal rehearsal, and behavioral inhibition capacities may play a role. Further prospective research is necessary to uncover how visual-motor performance relates to underlying cognitive mechanisms and how spoken-language outcomes develop in PLD children with CIs.
Our results raise the possibility that preimplant VI tests, such as design copying tasks, can be used clinically to predict benefit from CI. However, it should be stressed that work with larger samples is needed to understand how much outcome variance can be attributed to preimplant VI performance. Nevertheless, these tests, which can be easily administered to deaf children because they require no auditory processing, should be considered as potential additions to neuropsychological assessment batteries used with this population.
Acknowledgments
The authors thank Sujuan Gao, PhD, and Amy Rong Qi, PhD, for statistical assistance.
Supported by NIH-NIDCD Training Grant T32 DC00012 and NIH NIDCD Research Grant RO1 DC00064 to Indiana University.
BIBLIOGRAPHY
- 1.Gates G, Miyamoto R. Cochlear implants. New Engl J Med. 2003;349:421–423. doi: 10.1056/NEJMp038107. [DOI] [PubMed] [Google Scholar]
- 2.Svirsky M, Robbins A, Kirk K, Pisoni D, Miyamoto R. Language development in profoundly deaf children with cochlear implants. Psychol Sci. 2000;11:153–158. doi: 10.1111/1467-9280.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Svirsky M, Sloan R, Caldwell M, Miyamoto R. Speech intelligibility of prelingually deaf children with multichannel cochlear implants. Ann Otol Rhinol Laryngol. 2000;185(Suppl):123–125. doi: 10.1177/0003489400109s1254. [DOI] [PubMed] [Google Scholar]
- 4.Tyler R, Parkinson AJ, Fryauf-Bertchy H, et al. Speech perception by prelingually deaf children using cochlear implants. Otolaryngol Head Neck Surg. 1997;117:180–187. doi: 10.1016/s0194-5998(97)70172-4. [DOI] [PubMed] [Google Scholar]
- 5.Sarant J, Blamey P, Dowell R, Clark G, Gibson W. Variation in speech perception scores among children with cochlear implants. Ear Hear. 2001;22:18–28. doi: 10.1097/00003446-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Pisoni D. Cognitive factors and cochlear implants: some thoughts on perception, learning, and memory in speech perception. Ear Hear. 2000;21:70–78. doi: 10.1097/00003446-200002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pisoni D, Cleary M. Auditory prosthesis. In: Zeng FG, Popper AN, Fay RR, editors. Springer Handbook of Auditory Research. Springer Verlag; New York, NY: 2004. [Google Scholar]
- 8.Lenneberg E. Biological Foundations of Language. John Wiley & Sons; New York, NY: 1967. [Google Scholar]
- 9.Locke J, Bekken K, McMinn-Larson L, Wein D. Emergent control of manual and vocal-motor activity in relation to the development of speech. Brain Lang. 1995;51:498–508. doi: 10.1006/brln.1995.1073. [DOI] [PubMed] [Google Scholar]
- 10.Siegel LS, Saigal S, Rosenbaum P, et al. Predictors of development in preterm and full-term infants: a model for detecting the at risk child. J Pediatr Psychol. 1982;7:135–148. doi: 10.1093/jpepsy/7.2.135. [DOI] [PubMed] [Google Scholar]
- 11.Wolff P, Michel G, Ovrut M, Drake C. Rate and timing precision of motor coordination in developmental dyslexia. Development Psychol. 1990;26:349–359. [Google Scholar]
- 12.Carello C, LeVasseur V, Schmidt R. Movement sequencing and phonological fluency in (putatively) nonimpaired readers. Psychol Sci. 2002;13:375–379. doi: 10.1111/1467-9280.00467. [DOI] [PubMed] [Google Scholar]
- 13.Beery K. The VMI Developmental Test of Visualmotor Integration. 3rd Revision Ed. Modern Curriculum Press; Cleveland, OH: 1989. [Google Scholar]
- 14.Taylor K. Relationship between visual motor integration skill and academic performance in kindergarten through third grade. Optom Vision Sci. 1999;76:69–73. doi: 10.1097/00006324-199903000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Bachara G, Phelan W. Visual perception and language levels of deaf children. Percept Motor Skills. 1980;51:272. doi: 10.2466/pms.1980.51.1.272. [DOI] [PubMed] [Google Scholar]
- 16.Spencer P, Delk L. Hearing-impaired students’ performance on tests of visual processing: relationships with reading performance. Am Ann Deaf. 1985;134:333–337. doi: 10.1353/aad.2012.0539. [DOI] [PubMed] [Google Scholar]
- 17.Erden Z, Otman S, Tunay V. Is visual perception of hearing-impaired children different from healthy children? Int J Pediat Otorhinolaryngol. 2004;68:281–285. doi: 10.1016/j.ijporl.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Savelsbergh G, Netelenbos J, Whiting H. Auditory perception and the control of spatially coordinated action of deaf and hearing children. J Child Psychol Psychiatry. 1991;32:489–500. doi: 10.1111/j.1469-7610.1991.tb00326.x. [DOI] [PubMed] [Google Scholar]
- 19.Wiegersma P, Van der Velde A. Motor development of deaf children. J Child Psychol Psychiatry. 1983;24:103–111. doi: 10.1111/j.1469-7610.1983.tb00107.x. [DOI] [PubMed] [Google Scholar]
- 20.Tiber N. A psychological evaluation of cochlear implants in children. Ear Hear. 1985;6:48S–51S. doi: 10.1097/00003446-198505001-00009. [DOI] [PubMed] [Google Scholar]
- 21.Horn D, Pisoni D, Sanders M, Miyamoto R. Behavioral assessment of pre-lingually deaf children prior to cochlear implantation. Laryngoscope. 2005;115:1603–1611. doi: 10.1097/01.mlg.0000171018.97692.c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kutz W, Wright C, Krull K, Manolidis S. Neuropsychological testing in the screening for cochlear implant candidacy. Laryngoscope. 2003;113:763–766. doi: 10.1097/00005537-200304000-00035. [DOI] [PubMed] [Google Scholar]
- 23.Schlumberger E, Narbona J, Manrique M. Non-verbal development of children with deafness with and without cochlear implants. Dev Med Child Neurol. 2004;46:599–606. doi: 10.1017/s001216220400101x. [DOI] [PubMed] [Google Scholar]
- 24.Horn D, Pisoni D, Miyamoto R. Divergence of fine and gross motor skills in prelingually deaf children: implications for cochlear implantation. Laryngoscope. 2006;116:1500–1506. doi: 10.1097/01.mlg.0000230404.84242.4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haskins H. A phonetically balanced test of speech discrimination for children [master's thesis] Northwestern University; Evanston, IL: 1949. [Google Scholar]
- 26.Osberger MJ, Miyamoto RT, Zimmerman-Phillips S, et al. Independent evaluation of the speech perception abilities of children with the Nucleus-22 channel cochlear implant system. Ear Hear. 1991;12:S66–S80. doi: 10.1097/00003446-199108001-00009. [DOI] [PubMed] [Google Scholar]
- 27.Osberger M, Robbins A, Todd S, Riley A. Speech intelligibility of children with cochlear implants. Volta Review. 1994;96:169–80. [Google Scholar]
- 28.Dunn L, Dunn L. Peabody Picture Vocabulary Test. 3rd Edition American Guidance Service; Circle Pines, MN: 1997. [Google Scholar]
- 29.Reynell JK, Huntley M. Reynell Developmental Language Scales. NFER-Nelson; Windsor, UK: 1985. [Google Scholar]
- 30.Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. Sage Publications; Thousand Oaks, CA: 2002. [Google Scholar]
- 31.Schafer J, Graham J. Missing data: our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- 32.Dillon C. Research on Spoken Language Processing, Technical Report No. 14. Indiana University; Bloomington, IN: 2005. Phonological processing skills and the development of reading in deaf children who use cochlear implants. [Google Scholar]
- 33.Korkman M, Kirk U, Kemp S. NEPSY: A Developmental Neuropsychological Assessment. The Psychological Corporation; San Antonio, TX: 1998. [Google Scholar]
- 34.Wechsler D. The Wechsler Intelligence Scale for Children—Third Edition. The Psychological Corporation; San Antonio, TX: 1991. [Google Scholar]
- 35.Woodcock R. Woodcock Reading Mastery Tests-Revised. DLM Teaching Resources; Allen, TX: 1998. [Google Scholar]
- 36.McGarr N. The intelligibility of deaf speech to experienced and inexperienced listeners. J Speech Hear Res. 1983;26:451–458. doi: 10.1044/jshr.2603.451. [DOI] [PubMed] [Google Scholar]
- 37.Fagan MK, Pisoni DM, Horn DL, Dillon CM. Neuropsychological correlates of vocabulary, reading, and working memory in deaf children with cochlear implants. J Deaf Stud Deaf Educ. 2007 June 7; doi: 10.1093/deafed/enm023. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burkholder R, Pisoni D. Speech timing and working memory in profoundly deaf children after cochlear implantation. J Exp Child Psychol. 2003;85:63–88. doi: 10.1016/s0022-0965(03)00033-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cleary M, Pisoni D, Geers A. Some measures of verbal and spatial working memory in eight- and nine-year-old hearing-impaired children with cochlear implants. Ear Hear. 2001;22:395–411. doi: 10.1097/00003446-200110000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dillon C, Burkholder R, Cleary M, Pisoni D. Nonword repetition by children with cochlear implants: accuracy ratings from normal-hearing listeners. J Speech Lang Hear Res. 2004;47:1103–1116. doi: 10.1044/1092-4388(2004/082). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee DS, Lee JS, Oh SH, et al. Cross-modal plasticity and cochlear implants. Nature. 2001;409:149–150. doi: 10.1038/35051653. [DOI] [PubMed] [Google Scholar]
- 42.Sharma A, Dorman M, Spahr A, Todd N. Early cochlear implantation in children allows normal development of central auditory pathways. Ann Otol Rhinol Laryngol. 2002;189(Suppl):38–41. doi: 10.1177/00034894021110s508. [DOI] [PubMed] [Google Scholar]
- 43.Lee HJ, Kang E, Oh SH, et al. Preoperative differences of cerebral metabolism relate to the outcome of cochlear implants in congenitally deaf children. Hear Res. 2005;203:2–9. doi: 10.1016/j.heares.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 44.Quittner A, Smith L, Osberger M, Mitchell T, Katz D. The impact of audition on the development of visual attention. Psychol Sci. 1994;5:347–353. [Google Scholar]
- 45.Smith L, Quittner A, Osberger M, Miyamoto R. Audition and visual attention: the developmental trajectory in deaf and hearing populations. Dev Psychol. 1998;34:840–850. doi: 10.1037//0012-1649.34.5.840. [DOI] [PubMed] [Google Scholar]
- 46.Horn D, Davis R, Pisoni D, Miyamoto R. Development of visual attention skills in prelingually deaf children who use cochlear implants. Ear Hear. 2005;26:389–408. doi: 10.1097/00003446-200508000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horn D, Davis R, Pisoni D, Miyamoto R. Behavioral inhibition and clinical outcomes in children with cochlear implants. Laryngoscope. 2005;115:595–600. doi: 10.1097/01.mlg.0000161340.00258.1d. [DOI] [PMC free article] [PubMed] [Google Scholar]