Abstract
Context
Basic and observational studies suggest vitamins E or C may reduce risk of cardiovascular disease (CVD). However, few long-term trials have evaluated men at initially low risk of CVD, and no previous trial in men has examined vitamin C alone in the prevention of CVD.
Objective
To test whether long-term vitamin E or C supplementation decreases risk of major cardiovascular events among men.
Design, Setting, and Participants
The Physicians’ Health Study II (PHS II) is a randomized, double-blind, placebo-controlled factorial trial of vitamins E and C that began in 1997 and continued until its scheduled completion on August 31, 2007. We enrolled 14,641 U.S. male physicians initially aged ≥50 years, including 754 (5.1%) men with prevalent CVD at randomization.
Intervention
Individual supplements of 400 IU vitamin E every other day and 500 mg vitamin C daily.
Main Outcome Measures
A composite endpoint of major cardiovascular events (nonfatal myocardial infarction (MI), nonfatal stroke, and CVD death).
Results
During a mean follow-up of 8.0 years, there were 1,245 confirmed major cardiovascular events. Compared with placebo, vitamin E had no effect on the incidence of major cardiovascular events (both active and placebo vitamin E groups, 10.9 events per 1,000 person-years; hazard ratio [HR], 1.01; 95% confidence interval [CI], 0.90–1.13; P=0.86), as well as total MI (HR, 0.90; 95% CI, 0.75–1.07; P=0.22), total stroke (HR, 1.07; 95% CI, 0.89–1.29; P=0.45), and cardiovascular mortality (HR, 1.07; 95% CI, 0.90–1.29; P=0.43). There was also no significant effect of vitamin C on major cardiovascular events (active and placebo vitamin E groups, 10.8 and 10.9 events per 1,000 person-years, respectively; HR, 0.99; 95% CI, 0.89–1.11; P=0.91), as well as total MI (HR, 1.04; 95% CI, 0.87–1.24; P=0.65), total stroke (HR, 0.89; 95% CI, 0.74–1.07; P=0.21), and cardiovascular mortality (HR, 1.02; 95% CI, 0.85–1.21; P=0.86). Neither vitamin E (HR, 1.07; 95% CI, 0.97–1.18; P=0.15) nor vitamin C (HR, 1.07; 95% CI, 0.97–1.18; P=0.16) had a significant effect on total mortality, but vitamin E was associated with an increased risk of hemorrhagic stroke (HR, 1.74; 95% CI, 1.04–2.91; P=0.036).
Conclusions
In this large, long-term trial of male physicians, neither vitamin E nor C supplementation reduced the risk of major cardiovascular events. These data provide no support for the use of these supplements for the prevention of CVD in middle-aged and older men.
Keywords: vitamin E, vitamin C, cardiovascular disease, randomized clinical trial, men
INTRODUCTION
Despite uncertainty regarding long-term health benefits, most US adults have taken a vitamin supplement in the past year.1 In the 1999–2000 National Health and Examination Survey, 12.7% and 12.4% of US adults took vitamin E and C supplements, respectively.2 With annual vitamin supplement sales in the billons of US dollars,3 vitamin supplementation has broad public health implications.
Basic research studies suggest that vitamins E, C, and other antioxidants reduce cardiovascular disease (CVD) by trapping organic free radicals and/or deactivating excited oxygen molecules to prevent tissue damage.4 Antioxidants may slow or prevent atherosclerotic plaque formation by inhibiting low-density lipoprotein cholesterol oxidation,5 modifying platelet activity,6, 7 reducing thrombotic potential,8 and modifying vascular reactivity.9, 10 Some,11–13 but not all,14 prospective cohort studies support a role for vitamin E in CVD prevention. Dietary and supplemental vitamin C have been inconsistently associated with CVD, including significant15 and non-significant16 inverse associations as well no association.17, 18 In a pooled analysis of 9 cohorts, vitamin C supplement use exceeding 700 mg/day was significantly associated with a 25% reduction in coronary heart disease risk.19.
Initial clinical trials of individual vitamin E use among male smokers in the ATBC trial showed both possible benefits on prostate cancer20 and risks on hemorrhagic stroke,21 in addition to secondary prevention trials such as CHAOS22 that indicated possible CVD reductions. Yet even as largely negative trials of vitamin E later emerged among subjects with multiple coronary risk factors or preexisting CVD,23–28 vitamin E and other supplement use has remained surprisingly prevalent among healthy individuals who report its regular use as part of their routine health regimen.29 There have been fewer long-term primary prevention trials of individual vitamin E use among participants at initially low risk of CVD, for which there has been no effect on CVD30, 31 with comparatively less data in men.30
Vitamin C has typically been incorporated into antioxidant cocktails with vitamin E, β-carotene, and other vitamins and minerals in large-scale clinical trials that reported no significant cardiovascular effects.32–35 Individual vitamin C use has only been evaluated among 8,171 women at high risk for CVD, finding no effect of 500 mg vitamin C daily on CVD.28 Therefore, the clinical utility of individual vitamin C use in preventing CVD among those at low initial risk of CVD remains uncertain. Further, because vitamin C may potentially interact with vitamin E,36 it is important to evaluate the effect of their interaction on CVD.
Given these persistent gaps in knowledge and ongoing debate regarding the roles of vitamins E and C for CVD prevention, we designed the Physicians’ Health Study II (PHS II) to provide novel and clinically relevant information on the individual effects of vitamin E and vitamin C supplementation over a median follow-up of 8 years on the risk of major cardiovascular events among 14,641 male physicians at lower initial risk of CVD compared with most previous trials.
METHODS
Study Design
The PHS II was a randomized, double-blind, placebo-controlled, 2×2×2×2 factorial trial evaluating the balance of risks and benefits of vitamin E (400 IU synthetic α-tocopherol or its placebo on alternate days; BASF Corporation), vitamin C (500 mg synthetic ascorbic acid or its placebo daily; BASF Corporation), and a multivitamin (Centrum Silver or its placebo daily; Wyeth Pharmaceuticals) in the prevention of cancer and CVD among 14,641 male physicians aged ≥50 years.37 A fourth randomized component, β-carotene (50 mg Lurotin or placebo on alternate days; BASF Corporation), was scheduled to stop in March 2003, while the Data and Safety Monitoring Board recommended that the vitamin E, C, and multivitamin components continue.
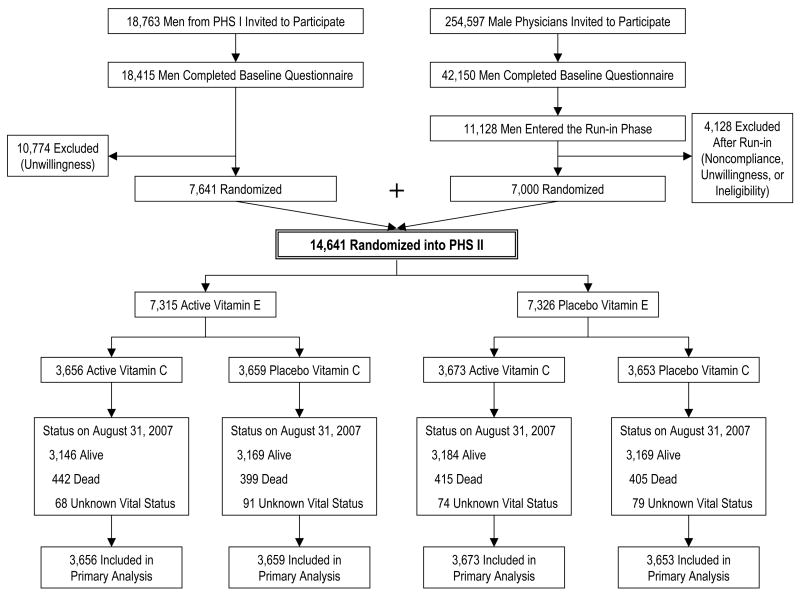
The PHS II study design has previously been described.37 Recruitment, enrollment, and randomization of men into PHS II occurred in two phases (Figure 1). Starting in July 1997, 18,763 living PHS I participants38, 39 were invited to participate in PHS II. Men were ineligible if they reported a history of cirrhosis, active liver disease, were on anticoagulants, or reported a serious illness that might preclude participation. Men with a history of myocardial infarction (MI), stroke, or cancer were eligible to enroll in PHS II. Subjects also must have been willing to forego during the course of PHS II any current use of multivitamins or individual supplements containing more than 100% of the RDA of vitamin E, vitamin C, β-carotene, or vitamin A. A total of 7,641 (41%) willing participants from PHS I were randomized into PHS II and retained their original β-carotene treatment assignment.
Figure 1.
Flow diagram of participants from screening to completion of the vitamin E and vitamin C components of the Physicians’ Health Study (PHS) II.
In 1999, invitational letters and baseline questionnaires were mailed to 254,597 US male physicians aged ≥50 years identified from a list provided by the American Medical Association, excluding PHS I participants. Between July 1999 and July 2001, 42,150 men completed a baseline questionnaire. Of these, 16,743 participants were willing to participate in PHS II, of whom 11,128 were eligible following the same eligibility criteria as PHS I participants. A 12-week run-in period excluded non-compliers who typically emerge during the first several months of participation.40 Of 11,128 physicians who entered the run-in phase, 7,000 (63%) willing and eligible men took at least two-thirds of their pills and were randomized into PHS II.
Thus, 14,641 men (7,641 from PHS I and 7,000 new physicians) were randomized into PHS II in blocks of 16, stratified by age, prior diagnosis of CVD, prior diagnosis of cancer, and, for the 7,641 PHS I participants, their original β-carotene treatment assignment. Men were randomly assigned to vitamin E or its placebo, to vitamin C or its placebo, and to active or placebo β-carotene and multivitamin. There were 754 (5.1%) men with prevalent CVD (nonfatal MI and stroke) randomized into PHS II. All participants provided informed consent and the Institutional Review Board at Brigham and Women’s Hospital approved the research protocol.
Study Treatment, Follow-up, and Compliance
Participants were sent monthly calendar packs, containing vitamin E or placebo (taken every other day), and vitamin C or placebo (taken daily), every six months for the first year and annually thereafter. Participants were also sent annual questionnaires asking about compliance, potential adverse events, the occurrence of new endpoints, and updated risk factors. Treatment and follow-up continued in blinded fashion through August 31, 2007, the scheduled end of the vitamin E and C components of PHS II. The multivitamin component is still ongoing. Analyses include follow-up and validation of reported endpoints through September 2008. Morbidity and mortality follow-up were extremely high, at 95.3% and 97.7%, respectively. Morbidity and mortality follow-up as a percentage of person-time each exceeded 99.9%, with only 1,055 and 289 person-years of morbidity and mortality follow-up lost through August 31, 2007.
Compliance was defined from participant self-reports as taking at least two thirds of the study agents. For the active vitamin E and its placebo, compliance at 4 years was 78% and 77%, respectively (P=0.12), and at the end of follow-up (mean of 8 years), 72% and 70% (P=0.004). For the active vitamin C and its placebo, compliance at 4 years was 78% and 78%, respectively (P=0.99), and at the end of follow-up, 71% and 71% (P=0.54). There were no differences between groups in average rates of individual non-trial vitamin E (3.2% active, 3.1% placebo) or vitamin C supplement (3.8% active, 4.4% placebo) use for ≥31 days/year (“drop-ins”) at the end of the trial (each P>0.05).
Confirmation of End Points
The primary cardiovascular endpoint, major cardiovascular events, was a composite endpoint that included nonfatal MI, nonfatal stroke, and cardiovascular mortality. For each endpoint reported by participants by follow-up questionnaire, letter, telephone call, and other correspondence, we requested permission from the participant to examine relevant medical records. Once consent was obtained, records were requested from the hospital or attending physician and reviewed by an Endpoints Committee of physicians blinded to randomized treatment assignment.
The diagnosis of MI was confirmed by evidence of symptoms in the presence of either diagnostic elevations of cardiac enzymes or diagnostic changes on electrocardiograms. For fatal events, the diagnosis of MI was also accepted based on autopsy findings.38 We confirmed diagnoses of stroke that were defined as a typical neurologic deficit of sudden or rapid onset and vascular origin, and lasted >24 hours. Stroke was classified according to National Survey of Stroke criteria into ischemic, hemorrhagic and unknown subtype,41 with high interobserver agreement.42
Participant deaths were usually reported by family members or postal authorities. Following a report of a participant death, permission was requested to obtain death certificates and/or autopsy reports from next of kin or from the state vital records bureau in which the participant died. Total mortality was confirmed by the Endpoints Committee or by death certificate. CVD mortality was additionally documented by convincing evidence of a cardiovascular mechanism from all available sources, including death certificates, hospital records, and for deaths outside the hospital, observers’ impressions. For men with unknown vital status, we used web searches to identify deaths along with National Death Index searches that included data through 2006. By the end of the vitamin E and C components of PHS II, mortality follow-up as a percentage of person-time exceeded 99.9%. Endpoint data were also collected on participant self-reports of congestive heart failure, angina pectoris, and revascularization (including coronary artery bypass graft and percutaneous coronary intervention).
Statistical Analyses
All primary analyses were based upon the intention-to-treat principle, in which all 14,641 randomized participants were classified according to their randomized vitamin E or C treatment assignments and were followed until the occurrence of a disease endpoint, death, loss to follow-up, or the end of the vitamin E and C components of PHS II on August 31, 2007, whichever came first. All data were analyzed using SAS version 9.1 (SAS Institute Inc, Cary, NC), with statistical significance set at P<0.05 using 2-sided tests. The PHS II was designed to have 80% power to detect a 16% relative reduction in the hazard of our primary endpoint of major cardiovascular events, based upon historical event rates observed in PHS physicians.
We first compared baseline characteristics by vitamin E or C treatment assignment to evaluate whether randomization equally distributed baseline characteristics. We used Cox proportional hazards models to calculate the HRs and 95% confidence intervals (CIs) comparing event rates in the vitamin E and placebo groups, and the vitamin C and placebo groups, for each pre-specified endpoint, adjusting for variables of PHS II study design: age, PHS cohort (original PHS I participant, new PHS II participant), and randomized β-carotene, vitamin E or vitamin C, and multivitamin assignments. We tested the proportional hazards assumption by modeling interaction terms separately for vitamin E or C with the logarithm of time, and these assumptions were not violated (P>0.05). We then investigated whether vitamin E or C compliance impacted our primary results through sensitivity analyses that censored follow-up when a participant reported taking less than two thirds of either vitamin E or vitamin C over the previous year. The effect of vitamin E or C on major cardiovascular events was also examined separately among 13,887 men without and 754 with baseline CVD (including MI or stroke). Finally, we conducted subgroup analyses stratified by major coronary risk factors, and assessed effect modification by using interaction terms between subgroup indicators and either vitamin E or C assignment.
RESULTS
PHS II randomized 14,641 men with a mean (SD) age of 64.3 years, with a mean follow-up of 8.0 years (median [interquartile range], 7.6 [7.1–9.6] years; maximum, 10.0 years; total follow-up, 117,711 person-years). Randomization equally distributed all baseline characteristics between vitamin E or vitamin C and their placebo groups (Table 1; all P>0.05). At the end of PHS II, there were 1,245 confirmed major cardiovascular events, including 511 total MIs, 464 total strokes, and 509 cardiovascular deaths, with some men experiencing multiple events. A total of 1,661 men died during follow-up.
Table 1.
Baseline characteristics according to vitamin E and vitamin C treatment assignment in 14,641 men from the Physicians’ Health Study (PHS) II.
| Vitamin E a |
Vitamin C a |
|||
|---|---|---|---|---|
| Self-reported baseline characteristics | Active (n=7,315) | Placebo (n=7,326) | Active (n=7,329) | Placebo (n=7,312) |
| Age (mean ± SD, years) | 64.2 ± 9.1 | 64.3 ± 9.2 | 64.3 ± 9.2 | 64.3 ± 9.1 |
| Age (No. (%)) | ||||
| 50–59 years | 2,940 (40.2) | 2,951 (40.3) | 2,953 (40.3) | 2,938 (40.2) |
| 60–69 years | 2,349 (32.1) | 2,347 (32.0) | 2,348 (32.0) | 2,348 (32.1) |
| ≥70 years | 2,026 (27.7) | 2,028 (27.7) | 2,028 (27.7) | 2,026 (27.7) |
|
| ||||
| Body mass index (mean ± SD, kg/m2) | 26.0 ± 3.6 | 26.0 ± 3.7 | 26.0 ± 3.6 | 26.0 ± 3.7 |
|
| ||||
| Body mass index (No. (%)) | ||||
| <25 kg/m2 | 3,055 (41.8) | 2,996 (40.9) | 3,019 (41.2) | 3,032 (41.5) |
| 25 to <30 kg/m2 | 3,433 (47.0) | 3,499 (47.8) | 3,479 (47.5) | 3,453 (47.3) |
| ≥30 kg/m2 | 815 (11.2) | 823 (11.3) | 823 (11.2) | 815 (11.2) |
|
| ||||
| Cigarette smoking (No. (%)) | ||||
| Never | 4,104 (56.1) | 4,148 (56.7) | 4,135 (56.5) | 4,117 (56.4) |
| Former | 2,967 (40.6) | 2,885 (39.4) | 2,908 (39.7) | 2,944 (40.3) |
| Current | 239 (3.3) | 285 (3.9) | 280 (3.8) | 244 (3.3) |
|
| ||||
| Exercise ≥1 time/wk (No. (%)) | ||||
| No | 2,739 (38.4) | 2,766 (38.7) | 2,759 (38.5) | 2,746 (38.6) |
| Yes | 4,389 (61.6) | 4,383 (61.3) | 4,408 (61.5) | 4,364 (61.4) |
|
| ||||
| Alcohol consumption (No. (%)) | ||||
| Rarely/never | 1,372 (18.9) | 1,358 (18.7) | 1,364 (18.7) | 1,366 (18.8) |
| ≥1 drink/month | 5,893 (81.1) | 5,923 (81.4) | 5,920 (81.3) | 5,896 (81.2) |
|
| ||||
| Current aspirin use (No. (%)) | ||||
| No | 1,627 (22.6) | 1,634 (22.6) | 1,638 (22.6) | 1,623 (22.6) |
| Yes | 5,578 (77.4) | 5,589 (77.4) | 5,605 (77.4) | 5,562 (77.4) |
|
| ||||
| History of hypertension (No. (%)) b | ||||
| No | 4,219 (58.0) | 4,187 (57.5) | 4,252 (58.3) | 4,154 (57.1) |
| Yes | 3,058 (42.0) | 3,098 (42.5) | 3,039 (41.7) | 3,117 (42.9) |
|
| ||||
| History of high cholesterol (No. (%)) c | ||||
| No | 4,490 (63.4) | 4,476 (63.2) | 4,494 (63.1) | 4,472(63.5) |
| Yes | 2,589 (36.6) | 2,601 (36.8) | 2,624 (36.9) | 2,566 (36.5) |
|
| ||||
| History of diabetes (No. (%)) | ||||
| No | 6,850 (93.7) | 6,871 (93.9) | 6,880 (94.0) | 6,841 (93.7) |
| Yes | 461 (6.3) | 444 (6.1) | 442 (6.0) | 463 (6.3) |
|
| ||||
| Parental history of MI <60 years (No. (%)) d | ||||
| No | 5,928 (89.5) | 5,941 (89.9) | 5,954 (89.5) | 5,915 (89.9) |
| Yes | 697 (10.5) | 665 (10.1) | 700 (10.5) | 662 (10.1) |
|
| ||||
| Self-reported history of CVD (No. (%)) e | ||||
| No | 6,940 (94.9) | 6,947 (94.8) | 6,945 (94.8) | 6,942 (94.9) |
| Yes | 375 (5.1) | 379 (5.2) | 384 (5.2) | 370 (5.1) |
Abbreviations: CVD, cardiovascular disease; MI, myocardial infarction; SD, standard deviation
P>0.05 for all comparisons between active and placebo groups of vitamin E and vitamin C.
History of hypertension was defined as self-reported systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or past/current treatment for hypertension.
History of high cholesterol was defined as self-reported total cholesterol ≥240 mg/dL or past/current treatment for high cholesterol.
Excludes 1,410 men with missing information on parental history of MI <60 years.
History of CVD included nonfatal myocardial infarction or nonfatal stroke.
Vitamin E and Major Cardiovascular Events
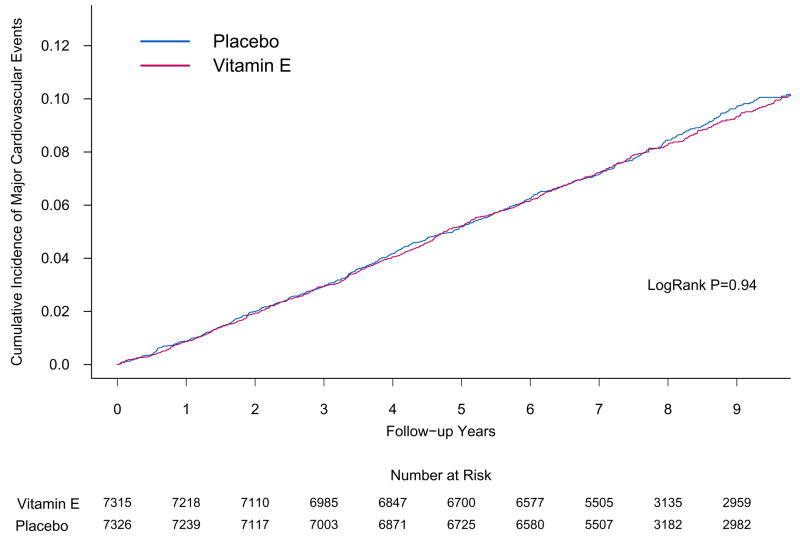
The overall rates of major cardiovascular events were 10.9 and 10.9 per 1,000 person-years in the active and placebo vitamin E groups, respectively. There was no effect of vitamin E on the primary endpoint of major cardiovascular events (HR, 1.01; 95% CI, 0.90–1.13; P=0.86) (Table 2). The cumulative incidence curves indicate that this lack of effect did not vary for up to 10 years of treatment and follow-up (log-rank P=0.94) (Figure 2). Compared with placebo, vitamin E did not reduce the incidence of individual cardiovascular events, including total MI (HR, 0.90; 95% CI, 0.75–1.07; P=0.22) and total stroke (HR, 1.07; 95% CI, 0.89–1.29; P=0.45). Among stroke subtypes, however, there were 39 hemorrhagic strokes in the active vitamin E group and 23 hemorrhagic strokes in the placebo vitamin E group (HR, 1.74; 95% CI, 1.04–2.91; P=0.036). Based on concerns raised in HOPE-TOO,27 we examined the rates of congestive heart failure by treatment group, finding no effect (HR, 1.02; 95% CI, 0.87–1.20; P=0.80). Finally, there was no significant effect of vitamin E on cardiovascular (HR, 1.07; 95% CI, 0.90–1.28; P=0.43) or total (HR, 1.07; 95% CI, 0.97–1.18; P=0.15) mortality. Censoring participants at the time of vitamin E non-compliance did not impact our results for major cardiovascular events (HR, 0.97; 95% CI, 0.85–1.11; P=0.68).
Table 2.
Association between randomized vitamin E and vitamin C assignment and the risk of major cardiovascular events and mortality in the Physicians’ Health Study II.a
| Vitamin E | Vitamin C | |||||
|---|---|---|---|---|---|---|
| Outcome | Active (n=7,315) | Placebo (n=7,326) | Hazard Ratio b (95% CI) | Active (n=7,329) | Placebo (n=7,312) | Hazard Ratio b (95% CI) |
| Major cardiovascular events c | 620 d | 625 | 1.01 (0.90–1.13) | 619 | 626 | 0.99 (0.89–1.11) |
| Total myocardial infarction e | 240 | 271 | 0.90 (0.75–1.07) | 260 | 251 | 1.04 (0.87–1.24) |
| Myocardial infarction death | 22 | 30 | 0.75 (0.43–1.31) | 30 | 22 | 1.37 (0.79–2.38) |
| Total stroke e | 237 | 227 | 1.07 (0.89–1.29) | 218 | 246 | 0.89 (0.74–1.07) |
| Stroke death | 45 | 56 | 0.86 (0.58–1.27) | 44 | 57 | 0.77 (0.52–1.14) |
| Ischemic stroke f | 191 | 196 | 1.00 (0.82–1.22) | 180 | 207 | 0.87 (0.71–1.07) |
| Hemorrhagic stroke f | 39 | 23 | 1.74 (1.04–2.91) | 30 | 32 | 0.95 (0.57–1.56) |
| Cardiovascular death | 258 | 251 | 1.07 (0.90–1.28) | 256 | 253 | 1.02 (0.85–1.21) |
| Congestive heart failure g | 289 | 294 | 1.02 (0.87–1.20) | 293 | 290 | 1.02 (0.87–1.20) |
| Angina g | 689 | 736 | 0.94 (0.85–1.05) | 686 | 739 | 0.92 (0.83–1.02) |
| Revascularization gh | 667 | 707 | 0.95 (0.86–1.06) | 674 | 700 | 0.96 (0.86–1.07) |
| Total mortality | 841 | 820 | 1.07 (0.97–1.18) | 857 | 804 | 1.07 (0.97–1.18) |
Abbreviation: CI, confidence interval
Mean follow-up of 8.0 years for all 14,641 men through August 31, 2007.
Adjusted for age, PHS cohort (original PHS I participant, new PHS participant), randomized beta-carotene assignment, randomized multivitamin assignment, and either randomized vitamin E or vitamin C assignment and stratified on baseline CVD.
Defined as a composite endpoint consisting of the first of any of the following individual events: nonfatal myocardial infarction, nonfatal stroke, and cardiovascular death. The individual events do not sum to the total because each individual analysis assesses the first event that occurs during follow-up. Therefore, a participant who (e.g.) has a myocardial infarction then dies of cardiovascular disease would be counted for both individual events, but only once for the primary endpoint of major cardiovascular events.
Number of events.
Includes both nonfatal and fatal events.
Stroke type was unknown for 7 men in the active vitamin E group and 8 men in the placebo E group; as well as 8 men in the active vitamin C group and 7 men in the placebo vitamin C group.
Self-reported.
Includes both coronary artery bypass graft and percutaneous transluminal coronary angioplasty.
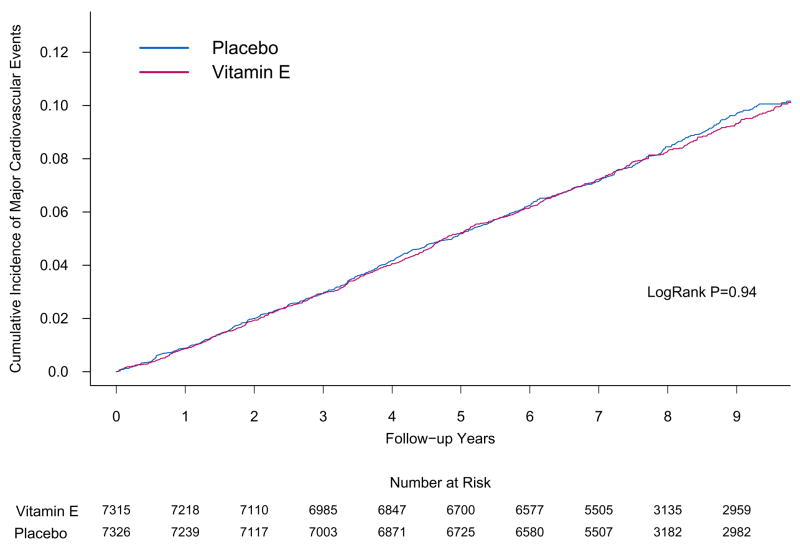
Figure 2.
Cumulative incidence rates of major cardiovascular events by either randomized vitamin E (part A) or vitamin C (part B) assignment in the Physicians’ Health Study II.
We next examined the association between vitamin E and major cardiovascular events among the 13,887 men without and 754 men with a baseline history of CVD (including MI or stroke). Vitamin E had no effect on the primary prevention of major cardiovascular events (532 events in active vitamin E and 520 events in placebo vitamin E) (HR, 1.05; 95% CI, 0.93–1.19; P=0.42), total MI (HR, 0.90; 95% CI, 0.75–1.08; P=0.25), total stroke (HR, 1.15; 95% CI, 0.95–1.41; P=0.16), cardiovascular mortality (HR, 1.16; 95% CI, 0.95–1.42; P=0.13), and total mortality (HR, 1.10; 95% CI, 0.99–1.22; P=0.07). A statistically significant increased risk of hemorrhagic stroke remained (HR, 1.99, 95% CI, 1.13–3.52; P=0.017). In analyses among the 754 men with baseline CVD, there was a suggestion of a lower risk of major cardiovascular events (HR, 0.82; 95% CI, 0.62–1.09; P=0.18), total MI (HR, 0.88; 95% CI, 0.50–1.55; P=0.67), total stroke (HR, 0.74; 95% CI, 0.47–1.16; P=0.18), cardiovascular mortality (HR, 0.83; 95% CI, 0.57–1.19; P=0.31), and total mortality (HR, 0.91; 95% CI, 0.70–1.17; P=0.45). There were only 4 and 5 cases of hemorrhagic stroke in the active and placebo vitamin E groups (P=0.62). Expanding our definition of baseline CVD to add angina pectoris or revascularization, among 1,419 men vitamin E still had no effect on major cardiovascular events (HR, 0.88; 95% CI, 0.70–1.10; P=0.26).
Finally, we evaluated whether coronary risk factors and each of the other randomized interventions from the PHS II modified the effect of vitamin E on major cardiovascular events (Table 3). Parental history of MI <60 years significantly modified (P interaction=0.042) the effect of vitamin E on major cardiovascular events, with non-significant reductions among men who took vitamin E and had a parental history of MI <60 years. Otherwise, we found no other significant effect modification by coronary risk factors on major cardiovascular events. In addition, there was no effect modification by randomized β-carotene or the ongoing multivitamin treatment assignment.
Table 3.
Association between randomized vitamin E and vitamin C assignment and risk of major cardiovascular events (nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death) according to baseline characteristics and treatment assignment in the Physicians’ Health Study II.a
| Vitamin E
|
Vitamin C
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Group | Active | Placebo | Hazard Ratio b (95% CI) | P Interaction | Active | Placebo | Hazard Ratio b (95% CI) | P Interaction |
| Age, years | 0.21 | 0.67 | ||||||
| 50–59 | 70 c | 87 | 0.81 (0.59–1.10) | 78 | 79 | 0.98 (0.72–1.34) | ||
| 60–69 | 166 | 162 | 1.03 (0.83–1.28) | 159 | 169 | 0.95 (0.76–1.18) | ||
| ≥70 | 384 | 376 | 1.05 (0.91–1.21) | 382 | 378 | 1.02 (0.89–1.18) | ||
| Body mass index, kg/m2 | 0.42 | 0.73 | ||||||
| <25 | 242 | 267 | 0.92 (0.77–1.10) | 249 | 260 | 0.98 (0.82–1.17) | ||
| 25–29 | 307 | 285 | 1.13 (0.96–1.33) | 297 | 295 | 0.99 (0.84–1.16) | ||
| ≥30 | 67 | 71 | 0.88 (0.63–1.23) | 70 | 68 | 1.08 (0.77–1.51) | ||
| Smoking status | 0.76 | 0.45 | ||||||
| Never | 290 | 300 | 1.01 (0.86–1.19) | 294 | 296 | 1.02 (0.87–1.19) | ||
| Former | 302 | 286 | 1.04 (0.88–1.22) | 294 | 294 | 1.00 (0.85–1.17) | ||
| Current | 28 | 39 | 0.91 (0.56–1.48) | 31 | 36 | 0.72 (0.45–1.18) | ||
| Exercise ≥1 time/week | 0.94 | 0.84 | ||||||
| No | 269 | 268 | 1.02 (0.86–1.21) | 267 | 270 | 0.98 (0.83–1.17) | ||
| Yes | 330 | 330 | 1.03 (0.88–1.20) | 331 | 329 | 1.01 (0.87–1.18) | ||
| Alcohol consumption | 0.94 | 0.51 | ||||||
| Rarely/never | 136 | 134 | 1.02 (0.81–1.30) | 132 | 138 | 0.93 (0.74–1.19) | ||
| ≥1 drink/month | 477 | 486 | 1.01 (0.89–1.14) | 481 | 482 | 1.01 (0.89–1.14) | ||
| Current aspirin use | 0.48 | 0.47 | ||||||
| No | 130 | 123 | 1.10 (0.86–1.41) | 128 | 125 | 1.07 (0.83–1.36) | ||
| Yes | 470 | 483 | 0.99 (0.87–1.13) | 474 | 479 | 0.98 (0.87–1.12) | ||
| History of hypertension d | 0.92 | 0.68 | ||||||
| No | 214 | 211 | 1.02 (0.85–1.24) | 219 | 206 | 1.03 (0.85–1.25) | ||
| Yes | 403 | 414 | 1.00 (0.88–1.15) | 399 | 418 | 0.99 (0.86–1.13) | ||
| History of high cholesterol e | 0.98 | 0.76 | ||||||
| No | 360 | 359 | 1.00 (0.87–1.16) | 360 | 359 | 1.01 (0.88–1.17) | ||
| Yes | 250 | 257 | 1.03 (0.86–1.22) | 253 | 254 | 0.98 (0.82–1.16) | ||
| History of diabetes | 0.69 | 0.84 | ||||||
| No | 527 | 540 | 1.00 (0.89–1.13) | 532 | 535 | 0.99 (0.88–1.12) | ||
| Yes | 92 | 85 | 1.07 (0.80–1.44) | 87 | 90 | 1.04 (0.77–1.40) | ||
| Parental history of MI <60 yearsf | 0.042 | 0.86 | ||||||
| No | 494 | 473 | 1.07 (0.94–1.21) | 482 | 485 | 0.99 (0.88–1.13) | ||
| Yes | 57 | 73 | 0.79 (0.56–1.12) | 67 | 63 | 0.99 (0.70–1.40) | ||
| History of CVD g | 0.10 | 0.85 | ||||||
| No | 532 | 520 | 1.05 (0.93–1.19) | 525 | 527 | 1.00 (0.89–1.13) | ||
| Yes | 88 | 105 | 0.82 (0.62–1.09) | 94 | 99 | 0.96 (0.72–1.27) | ||
| Randomized to vitamin C or E h | 0.77 | 0.77 | ||||||
| Placebo | 310 | 316 | 1.00 (0.85–1.17) | 309 | 316 | 0.97 (0.83–1.14) | ||
| Active | 310 | 309 | 1.03 (0.88–1.21) | 310 | 310 | 1.01 (0.86–1.18) | ||
| Randomized to β-carotene | 0.41 | 0.82 | ||||||
| Placebo | 304 | 317 | 0.97 (0.83–1.14) | 309 | 312 | 1.00 (0.85–1.17) | ||
| Active | 316 | 308 | 1.06 (0.91–1.24) | 310 | 314 | 0.99 (0.84–1.15) | ||
Abbreviation: CI, confidence interval
Mean follow-up of 8.0 years for all 14,641 men through August 31, 2007.
Adjusted for age, PHS cohort (original PHS I participant, new PHS participant), randomized beta-carotene assignment, randomized multivitamin assignment, and either randomized vitamin E or vitamin C assignment.
Number of events.
History of hypertension was defined as self-reported systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or past/current treatment for hypertension.
History of high cholesterol was defined as self-reported total cholesterol ≥240 mg/dL or past/current treatment for high cholesterol.
Excludes 1,410 men with missing information on parental history of MI <60 years.
History of cardiovascular disease (CVD) included nonfatal myocardial infarction or nonfatal stroke.
For analyses of vitamin E, stratified by vitamin C; for analyses of vitamin C, stratified by vitamin E.
Vitamin C and Major Cardiovascular Events
The overall rates of major cardiovascular events for the active and placebo vitamin C groups were 10.8 and 10.9 per 1,000 person-years, respectively. There was no effect of vitamin C on the primary endpoint of major cardiovascular events (HR, 0.99; 95% CI, 0.89–1.11; P=0.91) (Table 2). The cumulative incidence curves showed no difference between groups in the HRs over time (log-rank P=0.86) (Figure 2). Vitamin C also had no effect on individual cardiovascular endpoints, including total MI (HR, 1.04; 95% CI, 0.87–1.24; P=0.65), total stroke (HR, 0.89; 95% CI, 0.74–1.07; P=0.21), and cardiovascular mortality (HR, 1.02; 95% CI, 0.85–1.21; P=0.86), as well as total mortality (HR, 1.07; 95% CI, 0.97–1.18; P=0.16) There was also no effect of vitamin C on hemorrhagic stroke. Censoring for noncompliance with vitamin C did not appreciably affect our findings for major cardiovascular events (HR, 0.98; 95% CI, 0.86–1.13; P=0.81).
When we examined vitamin C among 13,887 men without and 754 men with a baseline history of CVD, the lack of effect between vitamin C and major cardiovascular events remained. Vitamin C had no effect on the primary prevention of major cardiovascular events (525 events in active vitamin C and 527 events in placebo vitamin C (HR, 1.00; 95% CI, 0.88–1.13; P=0.98), total MI (HR, 1.47; 95% CI, 0.82–2.63; P=0.19), total stroke (HR, 0.86; 95% CI, 0.69–1.07; P=0.18), cardiovascular mortality (HR, 0.99; 95% CI, 0.81–1.20; P=0.88), and total mortality (HR, 1.08; 95% CI, 0.97–1.20; P=0.15) In the 754 men with a history of CVD at baseline, vitamin C did not impact incident major cardiovascular events (HR, 0.96; 95% CI, 0.72–1.27; P=0.77). For total MI, there were 18 and 31 cases in the active and placebo vitamin C groups (HR, 0.57; 95% CI, 0.32–1.02; P=0.06). Adding angina and revascularization to our definition of baseline CVD did not change the lack of effect on major cardiovascular events (HR, 1.03; 95% CI, 0.82–1.29; P=0.81), and the effect on total MI weakened somewhat (HR, 0.71; 95% CI, 0.47–1.07; P=0.10).
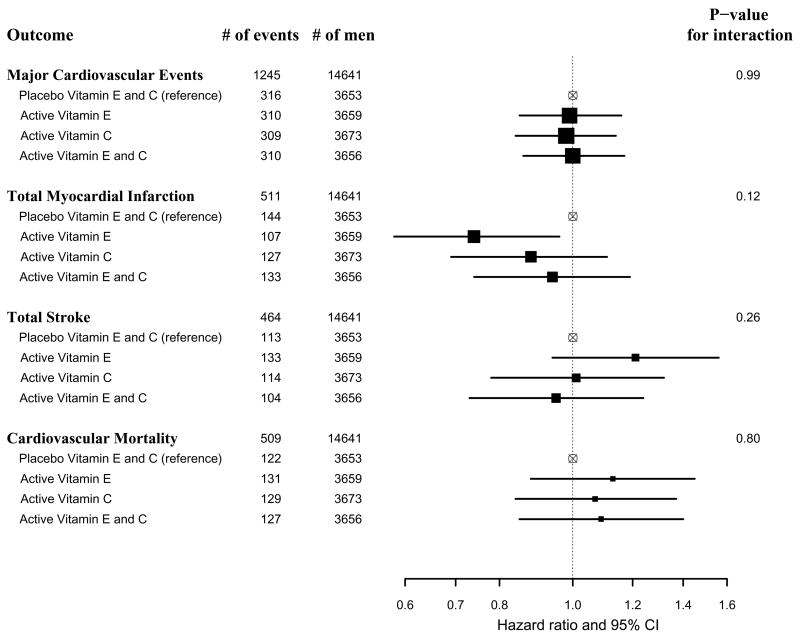
We then considered whether the effect of vitamin C on major cardiovascular events was modified by baseline coronary risk factors or other PHS II randomized interventions (Table 3). We found no significant effect modification between vitamin C and various baseline factors, randomized β-carotene, or the ongoing multivitamin treatment assignment on major cardiovascular events. When we examined the 2-way interaction between randomized vitamin E and vitamin C assignments, we found no significant interactions for major cardiovascular events (P interaction = 0.99), total MI (P interaction = 0.12), total stroke (P interaction = 0.26), or CVD mortality (P interaction = 0.80) (Figure 3). Men assigned to active vitamin E only had a lower risk of total MI than those assigned to placebo vitamins E and C (HR, 0.74; 95% CI, 0.58–0.96), but no reduction was seen in those receiving both active vitamin E and C (HR, 0.94; 95% CI, 0.74–1.19).
Figure 3.
Hazard ratios (HRs) and 95% confidence intervals (CIs) of major cardiovascular events, total myocardial infarction, total stroke, and cardiovascular mortality comparing combinations of active vitamin E and/or active vitamin C groups with the placebo vitamin E and placebo vitamin C group in the Physicians’ Health Study II.
Adverse Effects
We next considered whether vitamin E or vitamin C treatment increased potential adverse effects such as bleeding (including hematuria, easy bruising, and epistaxis), since vitamin E may potentially inhibit platelet function,6 along with gastrointestinal symptoms (peptic ulcer, constipation, diarrhea, gastritis, and nausea), fatigue, drowsiness, skin discoloration or rashes, and migraine. We observed no significant differences in adverse effects, including hematuria, easy bruising, and epistaxis, for active vitamin E or C compared with placebo.
DISCUSSION
In this large-scale, randomized clinical trial among middle-aged and older men, long-term vitamin E and vitamin C supplement use did not reduce the primary endpoint of incident major cardiovascular events. We also found that neither vitamin E nor C reduced total MI, total stroke, cardiovascular death, congestive heart failure, total mortality, angina, or coronary revascularization. We did find an increase in hemorrhagic stroke for vitamin E.
Randomized Trials of Vitamin E
Our finding that vitamin E has no effect on major cardiovascular events, including among a small subgroup of 754 men with a baseline history of CVD, is consistent with the majority of previous clinical trials conducted among higher risk individuals with22, 24, 26, 28 or without20, 25, 26, 28 pre-existing CVD. Though some of these trials report possible reductions in composite cardiovascular endpoints,22, 25, 28 meta-analyses indicate no overall benefit for vitamin E in the secondary prevention of CVD.43–45
The strength of PHS II is that the majority (94.9%) of participants were of initially low initial risk of CVD, a previously understudied population. Primary prevention trials such as the Chinese Cancer Prevention Study32 and SU.VI.MAX35 included 30 mg/day vitamin E as part of a vitamin cocktail, with no effect on CVD. The Primary Prevention Project examined the individual effect of 300 mg/day vitamin E among 1,912 men and 2,583 women (mean age, 64.4 years) with at least one cardiovascular risk factor for 3.6 years, finding no effect on pre-specified cardiovascular endpoints.46 The Women’s Health Study (WHS) tested 600 IU vitamin E every other day among 39,876 women at low or usual risk of CVD for 10 years and also reported no overall effect on major cardiovascular events.31 Moreover, results in PHS II did not corroborate the significant 24% reduction in cardiovascular death or the significant 26% reduction in major cardiovascular events among women aged ≥65 years in WHS. At present, the Selenium and Vitamin E Cancer Prevention Trial (SELECT) is the only ongoing, large-scale clinical trial testing 400 IU/day vitamin E, with CVD as a secondary endpoint.47
Although vitamin E has appeared relatively safe with few documented side effects,48 we observed a 74% increase in the risk of hemorrhagic stroke in the vitamin E treatment group consistent with results among the male smokers in the ATBC trial,21 but not observed in other primary30, 31 and secondary24, 26–28 prevention trials testing individual vitamin E supplement use. Vitamin E did not increase the incidence of reported congestive heart failure in PHS II, in contrast to increased risk reported by HOPE-TOO.27 Finally, while meta-analyses of clinical trials have reinforced the lack of effect between vitamin E and CVD,43 possible slight but significant increases in total mortality43–45 have been reported. However, in PHS II we found no significant effect between vitamin E and total mortality after up to 10 years of treatment and follow-up.
The source, type, and dose of vitamin E used in PHS II warrant discussion. We used synthetic vitamin E (all-rac-alpha-tocopheryl acetate) in PHS II, similar to earlier trials,20, 24 whereas more recent trials have used natural source vitamin E (HRR-alpha-tocopheryl acetate).26–28, 31 However, neither form of vitamin E appears more or less associated with CVD, consistent with the observation that both vitamin E sources have similar antioxidant properties.49 Second, PHS II and other prevention trials have used α-tocopherol, whereas the γ-tocopherol isomer may also have a role in CVD prevention,50 as it has greater efficacy than α-tocopherol to inhibit lipid peroxidation51 and may be suppressed in the presence of α-tocopherol.52 Finally, our dose of 400 IU/day of vitamin E is lower than that used in some other trials of individual vitamin E use, but remains far greater than usual dietary levels and can only be achieved through supplementation.53
Randomized Trials of Vitamin C
The PHS II represents the first large-scale, long-term trial of individual vitamin C supplementation in the prevention of CVD in men. The Women’s Antioxidant Cardiovascular Study (WACS) also tested 500 mg/day vitamin C in 8,171 women at higher risk of CVD, and there was no effect on major cardiovascular endpoints.28 Other primary and secondary prevention trials have considered vitamin C as part of a vitamin cocktail, in which no cardiovascular benefits were observed.32–35 Trials of intermediate cardiovascular endpoints have yielded inconsistent results. A cocktail that included vitamin C had no effect on the rate and severity of restenosis in one trial.54 In contrast, 500 mg/day vitamin C given to patients with percutaneous transluminal coronary angioplasty reduced restenosis rates.55 In 520 subjects with hypercholesterolemia, 6 years of a vitamin C and E cocktail reduced the progression of atherosclerosis.56 Yet among postmenopausal women, a vitamin E and C combination provided no cardiovascular benefits.57 Our observation that vitamin C use among 754 men with pre-existing CVD non-significantly reduced total MI by 46% was interesting, but confined to a small subgroup. Among 5,238 women with prior CVD in WACS, there was no effect of vitamin C on major CVD (HR, 1.05; 95% CI, 0.93–1.18; P=0.43).28 Finally, in PHS II long-term vitamin C supplementation had no significant effect on total and cardiovascular mortality.
The dose of vitamin C used in PHS II, 500 mg/day, greatly exceeds usual dietary vitamin C levels58 and can only be achieved through supplementation. While even higher doses of vitamin C, generally tolerated up to a level of 2,000 mg/day,59 may be considered for CVD prevention, limits in gastrointestinal absorption and other physiological restrictions may hinder vitamin C bioavailability attainable by dietary and supplemental vitamin C intake.60 Despite the lack of effect for vitamin C on CVD in PHS II, more comprehensive clinical trial data on individual vitamin C supplemental use at different doses and in other populations remain lacking.
Potential Limitations
Compliance is of potential concern in any clinical trial. However, compliance remained high in PHS II during up to 10 years of follow-up, with low drop-in use in active and placebo vitamin E and C. Sensitivity analyses that censored follow-up time upon noncompliance did not alter our findings. Although PHS II represents one of the longest trials to date of individual vitamin E and vitamin C use on CVD, an even longer period of vitamin supplementation may be necessary to cover the critical etiologic window or provide a sufficient cumulative dose capable of preventing CVD. Finally, vitamins E and C represent two parts of a broader spectrum of essential vitamins and minerals with possible roles in CVD prevention. The randomized multivitamin component of PHS II still continues, with total treatment and follow-up planned to last more than a decade.
Summary
In this large-scale trial after a mean of 8 years of treatment and follow-up in 14,641 men, neither vitamin E nor vitamin C supplementation reduced the risk of major cardiovascular events. These data provide no support for the use of these supplements in the prevention of CVD in middle-aged and older men.
Acknowledgments
Supported by grants CA 97193, CA 34944, CA 40360, HL 26490, and HL 34595 from the National Institutes of Health (Bethesda, MD), and an investigator-initiated grant from BASF Corporation (Florham Park, NJ). Study agents and packaging were provided by BASF Corporation, Wyeth Pharmaceuticals (Madison, NJ), and DSM Nutritional Products, Inc. (formerly Roche Vitamins) (Parsippany, NJ).
We are deeply indebted to the 14,641 physician participants for their longstanding dedication and conscientious collaboration. We would also like to acknowledge the exemplary contributions of the staff of the Physicians’ Health Study, under the leadership of Charlene Belanger: Jose Carrion, Beth Holman, Kenneth Breen, Mary Breen, Mary G. Breen, Ivan Fitchorov, Andrea Hrbek, Tony Laurinaitis, Chandra McCarthy, Geneva McNair, Leslie Power, Philomena Quinn, Harriet Samuelson, Fred Schwerin, Joanne Smith, Miriam Schvartz, Michelle Sheehey, Martin Van Denburgh, and Phyllis Johnson Wojciechowski. Finally, we are grateful for the efforts of the Physicians’ Health Study Endpoint Committee, including Drs. Samuel Goldhaber, Carlos Kase, Meir Stampfer, and James Taylor over the course of PHS II.
AUTHOR CONTRIBUTIONS
Drs. Sesso and Gaziano had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses
Study concept and design: Sesso, Buring, Belanger, Manson, Glynn, Gaziano
Acquisition of data: Sesso, Buring, Christen, Kurth, Belanger, MacFadyen, Manson, Glynn, Gaziano
Analysis and interpretation of data: Sesso, Buring, Christen, Kurth, Bubes, Manson, Glynn, Gaziano
Drafting of the manuscript: Sesso
Critical revision of the manuscript for important intellectual content: Sesso, Buring, Christen, Kurth, Bubes, Manson, Glynn, Gaziano
Statistical analysis: Sesso, Bubes, Glynn
Obtained funding: Sesso, Buring, Gaziano
Administrative, technical, or material support: Sesso, Buring, Kurth, Belanger, MacFadyen, Bubes, Manson, Gaziano Study supervision: Sesso, Buring, Belanger, MacFadyen, Kurth, Bubes, Manson, Gaziano
FINANCIAL DISCLOSURES
Howard D. Sesso: Dr. Sesso has received investigator-initiated research funding from the National Institutes of Health, American Heart Association, American Cancer Society, California Strawberry Commission, Roche Vitamins, Inc (now DSM Nutritional Products, Inc), and Cambridge Theranostics, Ltd.
Julie E. Buring: Dr. Buring has received study agents and packaging from Bayer Healthcare and the Natural Source Vitamin E Association, as well as research funding from the National Institutes of Health.
William G. Christen: Dr. Christen has received research funding support from the National Institutes of Health, Harvard University (Clinical Nutrition Research Center), and DSM Nutritional Products, Inc (Roche).
Tobias Kurth: Dr. Kurth has received investigator-initiated research funding from Bayer AG, the National Institutes of Health, McNeil Consumer & Specialty Pharmaceuticals, Merck, and Wyeth Consumer Healthcare; he is a consultant to i3 Drug Safety, and received an honorarium from Genzyme for educational lectures and Organon for contributing to an expert panel.
Charlene Belanger: None
Jean MacFadyen: None
Vadim Bubes: None
JoAnn E. Manson: Dr. Manson has received investigator-initiated research funding from the National Institutes of Health and assistance with study pills and packaging from BASF and Cognis.
Robert J. Glynn: Dr. Glynn has received investigator-initiated research funding from the National Institutes of Health, Bristol-Meyers Squibb and Astra Zeneca
J. Michael Gaziano: Dr. Gaziano has received investigator-initiated research funding from the National Institutes of Health, the Veterans Administration, Veroscience and Amgen. He has served as a consultant or received honorarium from Bayer AG and Pfizer and has served as an expert witness for Merck.
ROLE OF THE SPONSORS
BASF, Wyeth Pharmaceuticals, and DSM Nutritional Products, Inc had no role in the study design; conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
DATA AND SAFETY MONITORING BOARD
Voting Members included Lawrence Cohen, Theodore Colton, I. Craig Henderson, Ross Prentice, and Nanette Wenger (Chair); Ex-Officio Members included Frederick Ferris, Peter Greenwald, Natalie Kurinij, Howard Parnes, Eleanor Schron, and Alan Zonderman.
References
- 1.Timbo BB, Ross MP, McCarthy PV, Lin CT. Dietary supplements in a national survey: Prevalence of use and reports of adverse events. J Am Diet Assoc. 2006;106(12):1966–1974. doi: 10.1016/j.jada.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, Picciano MF. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999–2000. Am J Epidemiol. 2004;160(4):339–349. doi: 10.1093/aje/kwh207. [DOI] [PubMed] [Google Scholar]
- 3.Muth MK, Anderson DW, Domanico JL, Smith JB, Wendling B. Economic characterization of the dietary supplement industry. Washington DC: Center for Food Safety and Administration, Food and Drug Administration; 1999. [Google Scholar]
- 4.Packer L. Protective role of vitamin E in biological systems. Am J Clin Nutr. 1991;53(4 Suppl):1050S–1055S. doi: 10.1093/ajcn/53.4.1050S. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg D, Lewis A. Conner Memorial Lecture. Oxidative modification of LDL and atherogenesis. Circulation. 1997;95(4):1062–1071. doi: 10.1161/01.cir.95.4.1062. [DOI] [PubMed] [Google Scholar]
- 6.Steiner M. Vitamin E, a modifier of platelet function: rationale and use in cardiovascular and cerebrovascular disease. Nutr Rev. 1999;57(10):306–309. doi: 10.1111/j.1753-4887.1999.tb06903.x. [DOI] [PubMed] [Google Scholar]
- 7.Mabile L, Bruckdorfer KR, Rice-Evans C. Moderate supplementation with natural alpha-tocopherol decreases platelet aggregation and low-density lipoprotein oxidation. Atherosclerosis. 1999;147(1):177–185. doi: 10.1016/s0021-9150(99)00169-0. [DOI] [PubMed] [Google Scholar]
- 8.Mehta J, Li D, Mehta JL. Vitamins C and E prolong time to arterial thrombosis in rats. J Nutr. 1999;129(1):109–112. doi: 10.1093/jn/129.1.109. [DOI] [PubMed] [Google Scholar]
- 9.Andrews TJ, Laight DW, Anggard EE, Carrier MJ. Investigation of endothelial hyperreactivity in the obese Zucker rat in- situ: reversal by vitamin E. J Pharm Pharmacol. 2000;52(1):83–86. doi: 10.1211/0022357001773544. [DOI] [PubMed] [Google Scholar]
- 10.Koh KK, Blum A, Hathaway L, et al. Vascular effects of estrogen and vitamin E therapies in postmenopausal women. Circulation. 1999;100(18):1851–1857. doi: 10.1161/01.cir.100.18.1851. [DOI] [PubMed] [Google Scholar]
- 11.Rimm EB, Stampfer MJ, Ascherio A, Giovannucci E, Colditz GA, Willett WC. Vitamin E consumption and the risk of coronary heart disease in men. N Engl J Med. 1993;328(20):1450–1456. doi: 10.1056/NEJM199305203282004. [DOI] [PubMed] [Google Scholar]
- 12.Stampfer MJ, Hennekens CH, Manson JE, Colditz GA, Rosner B, Willett WC. Vitamin E consumption and the risk of coronary disease in women. N Engl J Med. 1993;328(20):1444–1449. doi: 10.1056/NEJM199305203282003. [DOI] [PubMed] [Google Scholar]
- 13.Kushi LH, Folsom AR, Prineas RJ, Mink PJ, Wu Y, Bostick RM. Dietary antioxidant vitamins and death from coronary heart disease in postmenopausal women. N Engl J Med. 1996;334(18):1156–1162. doi: 10.1056/NEJM199605023341803. [DOI] [PubMed] [Google Scholar]
- 14.Klipstein-Grobusch K, Geleijnse JM, den Breeijen JH, et al. Dietary antioxidants and risk of myocardial infarction in the elderly: the Rotterdam Study. Am J Clin Nutr. 1999;69(2):261–266. doi: 10.1093/ajcn/69.2.261. [DOI] [PubMed] [Google Scholar]
- 15.Enstrom JE, Kanim LE, Klein MA. Vitamin C intake and mortality among a sample of the United States population. Epidemiology. 1992;3(3):194–202. doi: 10.1097/00001648-199205000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Ascherio A, Rimm EB, Hernan MA, et al. Relation of consumption of vitamin E, vitamin C, and carotenoids to risk for stroke among men in the United States. Ann Intern Med. 1999;130(12):963–970. doi: 10.7326/0003-4819-130-12-199906150-00003. [DOI] [PubMed] [Google Scholar]
- 17.Messerer M, Hakansson N, Wolk A, Akesson A. Dietary supplement use and mortality in a cohort of Swedish men. Br J Nutr. 2008;99(3):626–631. doi: 10.1017/S0007114507812049. [DOI] [PubMed] [Google Scholar]
- 18.Buijsse B, Feskens EJ, Kwape L, Kok FJ, Kromhout D. Both alpha- and beta-carotene, but not tocopherols and vitamin C, are inversely related to 15-year cardiovascular mortality in Dutch elderly men. J Nutr. 2008;138(2):344–350. doi: 10.1093/jn/138.2.344. [DOI] [PubMed] [Google Scholar]
- 19.Knekt P, Ritz J, Pereira MA, et al. Antioxidant vitamins and coronary heart disease risk: a pooled analysis of 9 cohorts. Am J Clin Nutr. 2004;80(6):1508–1520. doi: 10.1093/ajcn/80.6.1508. [DOI] [PubMed] [Google Scholar]
- 20.The effect of vitamin E beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330(15):1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 21.Leppala JM, Virtamo J, Fogelholm R, et al. Controlled trial of alpha-tocopherol and beta-carotene supplements on stroke incidence and mortality in male smokers. Arterioscler Thromb Vasc Biol. 2000;20(1):230–235. doi: 10.1161/01.atv.20.1.230. [DOI] [PubMed] [Google Scholar]
- 22.Stephens NG, Parsons A, Schofield PM, Kelly F, Cheeseman K, Mitchinson MJ. Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS) Lancet. 1996;347(9004):781–786. doi: 10.1016/s0140-6736(96)90866-1. [DOI] [PubMed] [Google Scholar]
- 23.Virtamo J, Rapola JM, Ripatti S, et al. Effect of vitamin E and beta carotene on the incidence of primary nonfatal myocardial infarction and fatal coronary heart disease. Arch Intern Med. 1998;158(6):668–675. doi: 10.1001/archinte.158.6.668. [DOI] [PubMed] [Google Scholar]
- 24.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354(9177):447–455. [PubMed] [Google Scholar]
- 25.Boaz M, Smetana S, Weinstein T, et al. Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): randomised placebo-controlled trial. Lancet. 2000;356(9237):1213–1218. doi: 10.1016/s0140-6736(00)02783-5. [DOI] [PubMed] [Google Scholar]
- 26.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342(3):154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 27.Lonn E, Bosch J, Yusuf S, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293(11):1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 28.Cook NR, Albert CM, Gaziano JM, et al. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women's Antioxidant Cardiovascular Study. Arch Intern Med. 2007;167(15):1610–1618. doi: 10.1001/archinte.167.15.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blendon RJ, DesRoches CM, Benson JM, Brodie M, Altman DE. Americans’ views on the use and regulation of dietary supplements. Arch Intern Med. 2001;161(6):805–810. doi: 10.1001/archinte.161.6.805. [DOI] [PubMed] [Google Scholar]
- 30.de Gaetano G. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Collaborative Group of the Primary Prevention Project. Lancet. 2001;357(9250):89–95. doi: 10.1016/s0140-6736(00)03539-x. [DOI] [PubMed] [Google Scholar]
- 31.Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294(1):56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 32.Blot WJ, Li JY, Taylor PR, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease- specific mortality in the general population. J Natl Cancer Inst. 1993;85(18):1483–1492. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- 33.Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345(22):1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 34.MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):23–33. doi: 10.1016/S0140-6736(02)09328-5. [DOI] [PubMed] [Google Scholar]
- 35.Hercberg S, Galan P, Preziosi P, et al. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164(21):2335–2342. doi: 10.1001/archinte.164.21.2335. [DOI] [PubMed] [Google Scholar]
- 36.Niki E. Interaction of ascorbate and alpha-tocopherol. Ann N Y Acad Sci. 1987:498186–199. doi: 10.1111/j.1749-6632.1987.tb23761.x. [DOI] [PubMed] [Google Scholar]
- 37.Christen WG, Gaziano JM, Hennekens CH. Design of Physicians’ Health Study II--a randomized trial of beta- carotene, vitamins E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials. Ann Epidemiol. 2000;10(2):125–134. doi: 10.1016/s1047-2797(99)00042-3. [DOI] [PubMed] [Google Scholar]
- 38.Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med. 1989;321(3):129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 39.Hennekens CH, Buring JE, Manson JE, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334(18):1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 40.Lang JM, Buring JE, Rosner B, Cook N, Hennekens CH. Estimating the effect of the run-in on the power of the Physicians’ Health Study. Stat Med. 1991;10(10):1585–1593. doi: 10.1002/sim.4780101010. [DOI] [PubMed] [Google Scholar]
- 41.Walker AE, Robins M, Weinfeld FD. The national survey of stroke. Clinical findings. Stroke. 1981:12113–144. [PubMed] [Google Scholar]
- 42.Berger K, Kase CS, Buring JE. Interobserver agreement in the classification of stroke in the physicians’ health study. Stroke. 1996;27(2):238–242. doi: 10.1161/01.str.27.2.238. [DOI] [PubMed] [Google Scholar]
- 43.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297(8):842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 44.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2008;(2):CD007176. doi: 10.1002/14651858.CD007176. [DOI] [PubMed] [Google Scholar]
- 45.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142(1):37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 46.Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Lancet. 2001:35789–95. doi: 10.1016/s0140-6736(00)03539-x. [DOI] [PubMed] [Google Scholar]
- 47.Lippman SM, Goodman PJ, Klein EA, et al. Designing the Selenium and Vitamin E Cancer Prevention Trial (SELECT) J Natl Cancer Inst. 2005;97(2):94–102. doi: 10.1093/jnci/dji009. [DOI] [PubMed] [Google Scholar]
- 48.Gilman AS, Goodman LS, Rall TW, Murad F. The Pharmacological Basis of Therapeutics. 7. London: MacMillan; 1985. pp. 1586–1589. [Google Scholar]
- 49.Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med. 2007;43(1):4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dietrich M, Traber MG, Jacques PF, Cross CE, Hu Y, Block G. Does gamma-tocopherol play a role in the primary prevention of heart disease and cancer? A review. J Am Coll Nutr. 2006;25(4):292–299. doi: 10.1080/07315724.2006.10719538. [DOI] [PubMed] [Google Scholar]
- 51.Hensley K, Benaksas EJ, Bolli R, et al. New perspectives on vitamin E: gamma-tocopherol and carboxyelthylhydroxychroman metabolites in biology and medicine. Free Radic Biol Med. 2004;36(1):1–15. doi: 10.1016/j.freeradbiomed.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 52.Robinson I, de Serna DG, Gutierrez A, Schade DS. Vitamin E in humans: an explanation of clinical trial failure. Endocr Pract. 2006;12(5):576–582. doi: 10.4158/EP.12.5.576. [DOI] [PubMed] [Google Scholar]
- 53.Ford ES, Ajani UA, Mokdad AH. Brief communication: The prevalence of high intake of vitamin E from the use of supplements among U.S. adults. Ann Intern Med. 2005;143(2):116–120. doi: 10.7326/0003-4819-143-2-200507190-00010. [DOI] [PubMed] [Google Scholar]
- 54.Tardif JC, Cote G, Lesperance J, et al. Probucol and multivitamins in the prevention of restenosis after coronary angioplasty. Multivitamins and Probucol Study Group. N Engl J Med. 1997;337(6):365–372. doi: 10.1056/NEJM199708073370601. [DOI] [PubMed] [Google Scholar]
- 55.Tomoda H, Yoshitake M, Morimoto K, Aoki N. Possible prevention of postangioplasty restenosis by ascorbic acid. Am J Cardiol. 1996;78(11):1284–1286. doi: 10.1016/s0002-9149(96)00613-3. [DOI] [PubMed] [Google Scholar]
- 56.Salonen RM, Nyyssonen K, Kaikkonen J, et al. Six-year effect of combined vitamin C and E supplementation on atherosclerotic progression: the Antioxidant Supplementation in Atherosclerosis Prevention (ASAP) Study. Circulation. 2003;107(7):947–953. doi: 10.1161/01.cir.0000050626.25057.51. [DOI] [PubMed] [Google Scholar]
- 57.Waters DD, Alderman EL, Hsia J, et al. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: a randomized controlled trial. JAMA. 2002;288(19):2432–2440. doi: 10.1001/jama.288.19.2432. [DOI] [PubMed] [Google Scholar]
- 58.Hampl JS, Taylor CA, Johnston CS. Vitamin C deficiency and depletion in the United States: the Third National Health and Nutrition Examination Survey, 1988 to 1994. Am J Public Health. 2004;94(5):870–875. doi: 10.2105/ajph.94.5.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes -Food and Nutrition Board. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Beta-carotene, and Other Carotenoids. Washington, DC: National Academy Press; 2000. [Google Scholar]
- 60.Li Y, Schellhorn HE. New developments and novel therapeutic perspectives for vitamin C. J Nutr. 2007;137(10):2171–2184. doi: 10.1093/jn/137.10.2171. [DOI] [PubMed] [Google Scholar]