Abstract
Developmental language disorders are characterized by a maturational trajectory that deviates or lags that of normal children. Given the wide variation in the rate of normal language development, diagnosis and classification of these disorders poses severe problems for the clinician. Our laboratory has been searching for anatomical signatures that could aid the development of a neurobiologically based classification. Quantitative analysis of the magnetic resonance imaging (MRI) brain scans of a series of samples of children and adults with reading and language disorders has identified two clusters with contrasting anatomical and reading profiles. Individuals with small symmetrical brain structures tend to have deficits in multiple domains of written and oral language while those with larger asymmetrical structures are more likely to have the isolated phonological deficits seen in adults with compensated dyslexia. Surprisingly, the anatomical risk factors that define these clusters do not form a continuum of increasing severity but deviate in opposite directions from normal. Individuals with moderate brain size and asymmetry typically demonstrate the best over-all performance. Further research should determine if phonological impairments in the two clusters are associated with differing genetic and environmental risk factors requiring different types of intervention.
Broca’s insight that the seemingly symmetrical cerebral hemispheres are actually stunningly different in function has stood the test of almost two centuries of research and clinical observation. His initial observation that aphasia follows damage to the left hemisphere but not the right fostered the idea of the “cerebral dominance” of the speaking left hemisphere. This metaphor has given way to views of a more balanced partnership. The left hemisphere and the right hemisphere appear to have different modes of information processing, modes that favor the development of different types of skills. Tool use and expressive language are favored by left hemisphere processing strategies while visuospatial attention and the interpretation of the ambiguous messages in metaphor, emotion and pragmatics appear to be better served by the right hemisphere (Ehrsson, Kito, Sadato, Passingham, & Naito, 2005; Hagler, Riecke, & Sereno, 2007; Halderman & Chiarello, 2005; Kacinik & Chiarello, 2005; Naito et al., 2005).
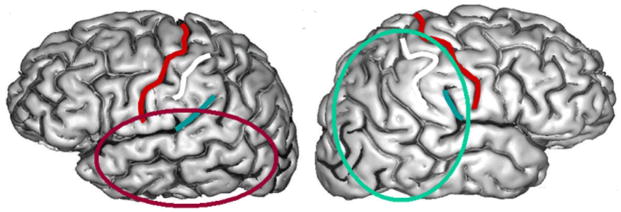
Why do the two hemispheres have different information processing biases? One possibility, depicted in Figure 1, is that interhemispheric specializations reflect differential development of the neural substrates for intrahemispheric specializations. Temporal lobe biases derive from auditory processing maps where temporal resolution and digital analysis is at a premium (Belin, Zilbovicius, Fontaine, Crozier, & Thivard, 1998). The parietal lobe biases derive from somatosensory and visual maps concerned with spatial resolution. Because of the well known asymmetry of the Sylvian fissure, temporal lobe information processing maps dominate left hemisphere processing, while parietal lobe maps dominate right hemisphere processing. This binary view may be oversimplified, but considerable experimental evidence is consistent with the idea that visuospatial functions are better developed in the right hemisphere (Corbetta, Miezin, Shulman, & Peterson, 1993; Heilman, Bowers, Valenstein, & Watson, 1986) while fine-grained temporal discriminations are the domain of the left (Belin et al., 1998; Robin, Tranel, & Damasio, 1990). The hypothesis being proposed here is that these functional dissociations have an anatomical basis, due to asymmetrical development of parietal maps in the right hemisphere and temporal maps in the left hemisphere. Apparent interhemisphere differences in processing strategy are due to differential development of corresponding areas in the left and right hemisphere that are associated with well known anatomical asymmetries.
Figure 1.
Information processing maps in the temporal lobes (red oval) are differentiated from perisylvian auditory maps that are biased toward fast automatic digital processing in the time (temporal) domain, while parietal information processing maps (turquoise oval) differentiate from somatosensory and visual maps that are biased towards slower spatial reconstruction. Because of the anatomical asymmetry of the Sylvian fissure, temporal maps dominate left hemisphere processing and parietal maps dominate right hemisphere processing. Central sulcus, outlined in red, separates frontal and parietal lobes.
Anatomical asymmetries
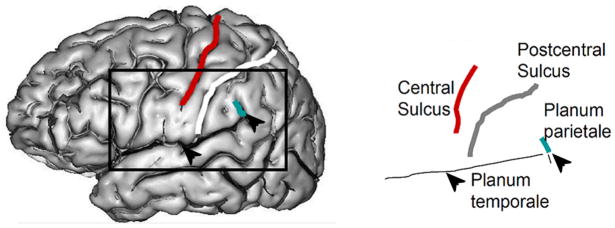
The size of the temporal and parietal lobes typically differs on the left and the right due to reciprocal development of the posterior horizontal and ascending branches of the Sylvian fissure (Steinmetz, Rademacher et al., 1990). The literature describing these differences was old but neglected when Geschwind and Levitsky published their landmark paper on 100 post mortem brains (Geschwind & Levitsky, 1968). Two-thirds of the brains had longer plana temporale on the left and only one-eighth had longer plana on the right (see Figure 2 for location of the planum temporale). Subsequently, Steinmetz and colleagues (Steinmetz, Ebeling, Huang, & Kahn, 1990) elaborated on Rubens’ description of a shape asymmetry in the Sylvian fissure (Rubens, Mahwold, & Hutton, 1976). They found that there is more likely to be an extra gyrus in the upper bank of the Sylvian fissures in the left hemisphere than the right. Conversely, there is more likely to be a missing gyrus in the upper bank of the right. They speculated that the neuropsychological profile of those with anomalous formations might differ.
Figure 2.
Location of the planum temporale in the posterior Sylvian fissure (arrows) and the planum parietale (depicted in blue). In the most common variant, the central sulcus separates the precentral and postcentral gyri and the planum parietale rises in the supramarginal gyrus, posterior to the postcentral sulcus.
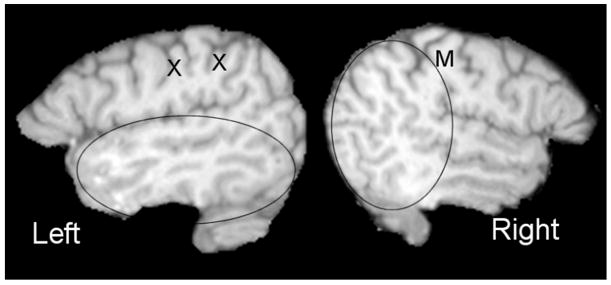
The MRI images from an unusual individual shown in Figure 3 demonstrate how differing numbers of gyri in the upper bank of the Sylvian fissure can be associated with differential development of the temporal and parietal lobes. In the left hemisphere, there are extra gyri between the motor and sensory strips (X) and between the sensory strip and the gyrus containing the terminating ramus of the Sylvian fissure. The extension of the Sylvian fissure is associated with a relatively long temporal lobe and a foreshortened parietal lobe. In the right hemisphere, there is a missing gyrus. The ascending ramus of the Sylvian fissure (parietal planum) rises directly posterior to the central sulcus, rather than posterior to the postcentral sulcus, as it does in most brains (Figure 2). The missing gyrus is associated with an expansion of the parietal lobe at the expense of the temporal lobe. In the next section we will explain how individual differences in these asymmetries might be related to dyslexia.
Figure 3.
Sagittal MRI images of the left and right hemispheres of a severely dyslexic man illustrating anomalous Sylvian fissure variants in both the left and right hemisphere. The X’s depict anomalous extra gyri interspersed between the central sulcus and the parietal planum. On the right, the planum parietale terminating the Sylvian fissure rises directly posterior to the central sulcus rather than the postcentral sulcus (compare the number of sulci descending from the superior surface anterior to the planum parietale on the left and right). These anomalies increase the asymmetries between the temporal and parietale lobes in the two hemispheres, leading, it is argued, to inefficient bottom up processing.
The brain used in Figure 3 to illustrate the Steinmetz classification is from a severely dyslexic man (Leonard et al., 1993). He has a long planum temporale with no planum parietale in the left hemisphere and the reverse condition in the right, leading to extreme asymmetries of the planum temporale and planum parietale in opposite directions. His mother had been told he was mentally retarded, might have autism, and could not be educated. She persevered, however, finally found professionals who could treat him for his severe dyslexia, and he is now a successful builder of expensive homes and an ardent advocate for children with dyslexia. His success in a career requiring good spatial ability bears out Steinmetz’ prediction that anomalous fissure development might be associated with neuropsychological profile.
There are two other anecdotal accounts of men in whom expanded parietal lobes are associated with unusually developed visualization ability. Witelson and colleagues have examined photographs of the brain of Albert Einstein (Witelson, Kigar, & Harvey, 1999). They report that the ascending ramus of the Sylvian fissure rises posterior to the central sulcus in both the left and right hemisphere, leading to truncation of the temporal lobes and expansion of the parietal lobes, bilaterally. Einstein was a “late talker” and, throughout his career, had difficulty with mathematics facts (hypothesized to be automatically processed by the temporal lobe), in contrast to his unusual ability to visualize complex spatiotemporal relationships processed in the parietal lobe (West, 1997).
Recently, we have encountered another young man whose MRI also demonstrated a missing gyrus above the Sylvian fissure in the right hemisphere. Like the man whose MRI is shown in Figure 3, he had been tested for mental retardation on entering school and had struggled with educational requirements, never learning his times tables (mathematics facts) and failing English every year in high school. In community college, however, he discovered a facility with the visualization of complex statistical equations, and now is embarked on a professional career (Chiarello, Lombardino, Kacinik, Otto, & Leonard, 2006).
We had encountered this young man because he had been a participant in a psychological experiment requiring rapid (automatic) identification of words presented to either the left or right hemisphere. His accuracy in identifying words presented to the right hemisphere (the hemisphere with the truncated temporal lobe) was abysmal (25%) in contrast to his near perfect performance when words were presented to the left hemisphere. This asymmetry in rapid identification contrasted sharply with his normal ability to generate verbs to nouns presented in either hemisphere (Chiarello et al., 2006). Verb generation is slow and effortful, in contrast to automatic word naming. The dissociation between accurate performance on slow effortful tasks and inaccurate performance on automatic tasks on words presented to the right but not the left hemisphere may be associated with differing parietal lobe/temporal lobe balance in the two hemispheres. This dissociation may also underlie the ability of “compensated” dyslexic individuals to comprehend the “gist” of text in spite of an inability to read single words (Bruck, 1992).
The idea that superior spatial ability is found in some dyslexics is not new, but it has not previously been associated with a shortened Sylvian fissure in the right hemisphere. On the contrary, a pervasive theme in dyslexia neurobiology has been the idea of compromised lateralization, that is, decreased, not increased asymmetry between the two hemispheres. In the following section we show that both these ideas are correct. Dyslexia can be associated with both decreased and increased asymmetry. However, the two groups of dyslexic individuals have fundamentally different reading profiles and may form clusters on the opposite sides of normal.
Asymmetry and dyslexia
In 1978, Galaburda, Geschwind and colleagues proposed that learning disabilities such as dyslexia were associated with fundamental disruptions in brain asymmetry (Galaburda, LeMay, Kemper, & Geschwind, 1978). Although the hypothesis was not new, recent anatomical work on cerebral asymmetry had at last made the hypothesis testable. Galaburda was able to acquire a series of post mortem specimens and in a series of papers published during the 80’s, reported that eight of eight cases had symmetry of the planum temporale, due to an enlarged planum in the right hemisphere (Galaburda, 1989; Galaburda, Sherman, Rosen, Aboitiz, & Geschwind, 1985).
Galaburda’s findings were very exciting because they suggested a one-to-one correspondence between a congenital developmental disability and a structural anomaly in the brain. The planum temporale is located directly posterior to the primary auditory cortex on Heschl’s gyrus (see Figure 2), and contains auditory association cortex that could contribute to the representation of phonemes whose decoding is required for fluent reading. A disruption of the normal asymmetry in this region would be expected to disrupt efficient coding. This kind of direct structure/function correspondence is commonly seen in adult neurology. Pediatric neurology, by contrast, is dominated by tales of plasticity. Given that extensive damage to the left hemisphere is consistent with the normal development of language (Thal et al., 1991) surely the developing brain could compensate for modest variation in region size. The uniform relationship between neuropathology and developmental disability described by Galaburda and colleagues was somewhat unexpected.
There have been no subsequent reports of post mortem examinations of individuals with dyslexia, as autopsies are expensive and increasingly rare. All subsequent structural examinations have been made in vivo, with MRI. The first paper to usher in this new era was by Hynd and colleagues (Hynd, Semrud-Clikeman, Lorys, Novey, & Eliopulos, 1990). This study confirmed Galaburda’s finding: only one of ten children with dyslexia had leftward asymmetry. Two additional papers provided additional support (Duara, Kusch, Gross-Glenn, & et al., 1991; Larsen, Høien, Lundberg, & Ødegaard, 1990). Oddly, however, in both the Hynd and the Larsen study, the reduction in asymmetry was due to a small planum temporale in the left hemisphere rather than a larger planum temporale on the right. Still, the appearance of these papers was very exciting because it provided hope that this noninvasive technology might provide a neurobiological marker to aid clinical diagnosis.
Subsequent work has not fulfilled these optimistic expectations. Although review articles and media reports still link planar symmetry with dyslexia, most controlled studies have not found such an association (Best & Demb, 1999; Eckert et al., 2003; Leonard et al., 2001; Leonard et al., 2002; Leonard et al., 1993; Rumsey et al., 1997; Schultz et al., 1994). In fact, two of the studies from our lab (published in 1993 & 2001) have found the opposite trend -- an exaggerated leftward asymmetry in individuals with dyslexia.
The 1993 study included the individual whose MRI is depicted in Figure 3. This individual was part of quite a heterogeneous group. The diagnosis of dyslexia relied on clinical history because we were collecting pilot data for a grant application and had no funds for comprehensive assessment. We provided scores on only two assessment measures: the Woodcock Johnson test of nonsense word reading called Word Attack (WA) (Woodcock & Johnson, 1989) and the Lindamood test of Auditory Conceptualization (LAC) (Lindamood & Lindamood, 1979). Although these scores ranged widely, our clinicians were confident in the diagnoses, because all cases were highly achieving individuals who reported struggling with reading and writing and never read for pleasure. Few of them would have qualified for a rigorous “research” diagnosis of dyslexia, however.
We made two kinds of MRI assessments in this study. We measured the length of the planum temporal (PT) and planum parietale (PP) in the region where PT was most asymmetric (roughly halfway between the insula and the surface of the brain). The first row in Table 1 shows that the groups did not differ substantially on planum temporale asymmetry. A majority of each group had the expected leftward asymmetry of the planum temporale. We also evaluated the Sylvian fissure using the Steinmetz classification described above. We found that both the dyslexic individuals and their family members tended to have extra gyri in the upper bank of the Sylvian fissure on the left, leading to enlargement of the temporal lobe on the left.
Table 1.
Samples with reading disability.
| Sample | Controls M/F | Exptl M/F | Age Range(yrs) | Selection criteria for experimental group | Proportion with L PT > R PT | |
|---|---|---|---|---|---|---|
| UF0 | 5/7 | 4/6 relatives | 7/2 | 6 –65 | No specified criteria; Clinical impression of reading disability gained from history | E: 100
C: .75 R: .80 |
| UF1 | 7/8 | 7/6 | 18 –33 | Certified for extra time at University; Diagnosis of dyslexia verified by Speech/Language Pathologist specializing in reading | E: .85
C: .73 |
|
| UF2 | 59/44 | 7/7 | 6 – 12 | Member of control group at first assessment at 5–6 yrs; in remedial education at second assessment at 8–11 yrs | E: .79
C: .75 |
|
| 12/9 | 6 – 13 | SLI: At least two oral language scores > 1 S.D. below average; Nonverbal IQ within normal limits | SLI: .57 | |||
| UW1 | 15/11 | 13/4 | 9–13 | Discrepancy between estimated full scale IQ and reading scores | E: .47
C: .69 |
|
| UW2 | 12/10 | 9 – 13 | Discrepancy between estimated full scale IQ and reading scores | E: .68
C: .67 |
||
| GT | 14/8 | 11 – 16 | Identified in schools as need remedial help in reading | E: .91 | ||
| UF 3 | 11/0 | 11/0 | 20 – 50 | Discrepancy between estimated full scale IQ and reading scores | E: .55
C: .55 |
Not all the dyslexic individuals had enlarged temporal lobes, however. Two individuals had small plana temporale and large plana parietale in both left and right hemispheres. Although they did not have missing gyri, the short plana temporale were still associated (as in Einstein’s case) with enlarged parietal lobes in both left and right hemispheres. Both individuals had chosen professions dependent on visuospatial processing and had been highly successful in spite of great difficulties with written language. These individuals provide further support for the idea that the combination of special talent in visuospatial function and dyslexia can be associated with enlargement of the parietal lobe at the expense of the temporal lobe. This kind of bilateral enlargement is quite rare. In a current sample of 200 college students, only 9 individuals had plana parietale that were larger than their plana temporale in both hemispheres (Leonard, unpublished data).
Although this group of dyslexic individuals had a heterogeneous assortment of anatomical anomalies, one consistent theme emerged. The more severely affected individuals had both more anomalies and more bilateral anomalies. This was our first hint that learning disability might be the result of an accumulation of anatomical risk factors exceeding some threshold.
At the same time that we were collecting data for this study, we were also studying normal development and special language impairment (SLI) (Gauger, Lombardino, & Leonard, 1997). The anomalies in the children with SLI were somewhat different from those described above. Although one of 21 children with SLI had a missing gyrus in the upper bank of the Sylvian fissure in the right hemisphere, extra gyri in the left hemisphere were rare, and overall the children with SLI had diminished not increased asymmetry of the planum temporale (Leonard et al., 2002). In our studies of normal children we saw a linear relationship between reading skills and planar asymmetry, such that poor readers had symmetry or rightward asymmetry of the planum temporale while good readers had leftward asymmetry (Eckert, Lombardino, & Leonard, 2001; Leonard et al., 1996).
We found these results very puzzling. It seemed quite paradoxical that children with poor reading aptitude would have rightward asymmetry but that adults with dyslexia would not. We began to wonder if the children with poor reading ability in our “normal” samples might differ in some way from the individuals in our dyslexia study. Most of the dyslexic individuals were very high functioning, “compensated” adults, who were successful professionals. Their reading problems were “unexpected” given their level of achievement. We speculated that perhaps symmetrical plana temporale were more likely to characterize the reading scores in children whose reading difficulties were not unexpected given their overall cognitive and language profile.
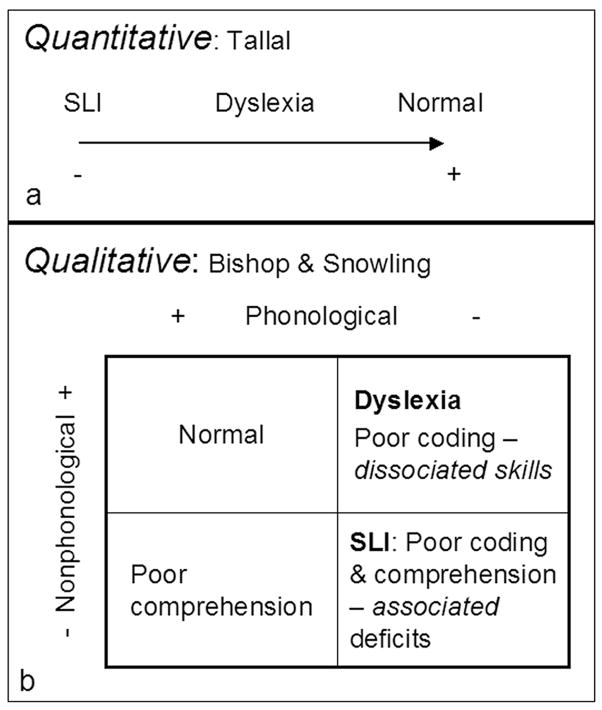
These results were relevant to an ongoing controversy over the relationship between reading and oral language impairments. In a series of papers Paula Tallal had developed a unifying hypothesis for their neurological basis (Stark & Tallal, 1988; Tallal, 1980; Tallal, Miller, & Fitch, 1993), proposing that SLI and dyslexia lay along a continuum of severity due to a left hemisphere defect in fast auditory processing (see Figure 4). This hypothesis would predict that poor readers with oral language deficits would have more severe neuroanatomical anomalies than poor readings with isolated deficits in phonological processing. The hypothesis would also predict that reduced planar asymmetry and compromised auditory processing would be associated in dyslexia. Our findings that children with poor reading skills had reduced planar asymmetry provided partial confirmatory evidence for the hypothesis. However, the finding of exaggerated leftward asymmetry in adult dyslexics did not, unless one revised the hypothesis to postulate that good auditory discrimination is associated with an optimal asymmetry and that deviations in either direction are associated with reduced ability.
Figure 4.
Contrasting views of the relation between reading and oral language deficits. A: Tallal proposes that auditory processing problems lead to phonological processing problems which lead to reading and oral language deficits. The severity of the auditory processing problem is expected to correlate with the severity of the reading and language deficits. B: The multidimensional view of Bishop & Snowling (2005). In this view, comprehension and phonological deficits have independent causes. Children with SLI have deficits in both phonological and comprehension, while some children with reading deficits actually have better phonological processing than comprehension.
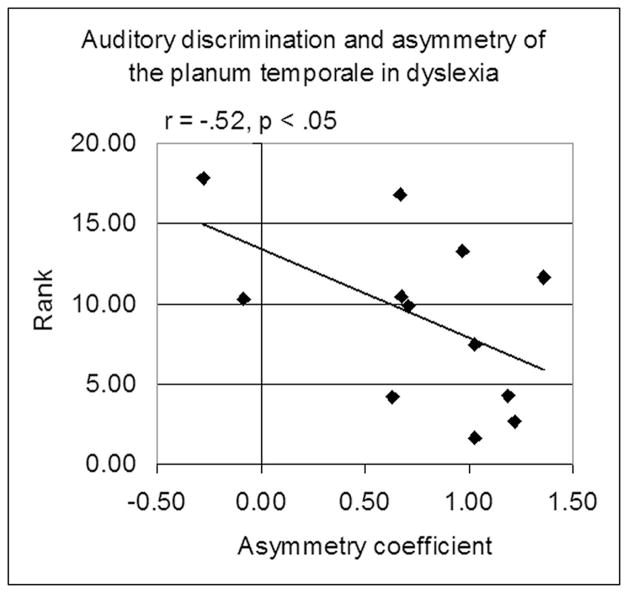
We were able to test this hypothesis in a study of college students with dyslexia where we performed extensive anatomical cognitive, reading and auditory assessments, including tests of gap detection, duration detection and pitch discrimination. The hypothesis was not supported. Auditory ability was not related either to the severity of the reading deficit or the degree of planar asymmetry. Figure 5 graphs the relationship between the average rank on the three auditory tests (low rank = low performance) and the coefficient of planar asymmetry. Not only was this hypothesis not supported, there was actually a significant relationship in the opposite direction: better auditory discrimination performance was associated with rightward (negative) not leftward asymmetry of the planum temporale.
Figure 5.
Contrary to the prediction that anomalous asymmetry of the planum would be associated with worse auditory processing, auditory function is actually better in the students wih anomalous (negative) asymmetry. Auditory procedures are described in King, Lombardino, Crandell & Leonard (2003).
This study provided additional findings that were not predicted by the single dimension view. The dyslexic students with exaggerated planar asymmetry had very poor auditory discrimination and phonological decoding scores but their reading comprehension was equal to that of the controls. Reading comprehension appeared to be independent of phonological decoding skill in these students. By contrast, the two dyslexic students with rightward asymmetry of the planum temporale had relatively good auditory discrimination, but only average phonological decoding and average comprehension, substantially below the levels of the controls. Clearly the relationship between auditory discrimination, asymmetry and reading disability was not simple. Our results were not compatible with a simple linear relation between audition, phonology and comprehension.
These results were more compatible with the view of language disorders proposed by Bishop & Snowling (2004) depicted in the bottom panel of Figure 4. In this view, children with dyslexia have phonological deficits while children with oral language impairments (SLI) have deficits on both phonological and nonphonological dimensions. Children with deficits limited to nonphonological dimensions are labeled “comprehension only”. Although this model was developed to distinguish SLI from dyslexia, we think it may also be applicable to the discrimination of different types of reading disability in children who do not qualify for a diagnosis of oral language impairment. In other words, dyslexia is heterogeneous and this heterogeneity is associated with heterogeneous neural substrates. The etiology and neural substrate of dyslexia that is associated with good comprehension may be different from that in which a number of reading functions are affected. The evidence for that proposition will be presented in the final section.
Anatomy and dyslexia
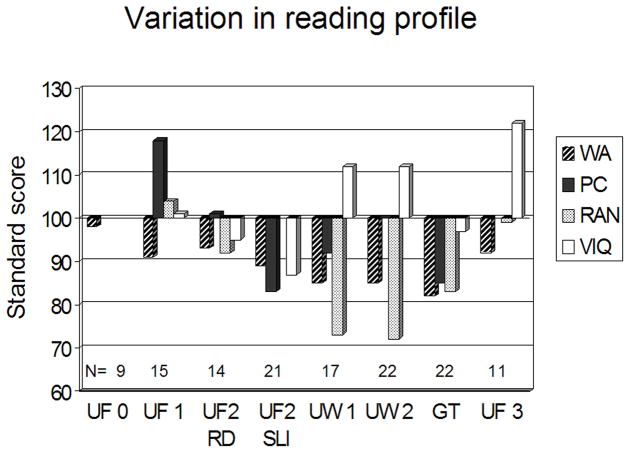
The reading profiles of a number of samples whose MRI scans have been processed in our laboratory are presented in Figure 6 (Table 1 gives brief demographic descriptions of each sample). They are ordered chronologically from left to right, starting with the 1993 study discussed above. Three overall generalizations can be made: 1) Most samples do not display the dissociation between comprehension (PC) and phonological decoding (WA) seen in the college students discussed above (UF1); and 2) Some samples are characterized by uniformly depressed scores (multiple deficits seen in UF2 and GT) while others (UW and UF3) are characterized by depressed reading scores in the presence of superior verbal IQ; 3) Most surprisingly, comprehension is strongly dissociated from verbal IQ in opposite directions in different samples (PC > IQ in UF 1; IQ > PC in UW).
Figure 6.
The reading profiles of the individuals in the samples discussed in this article. WA: Woodcock Johnson (WJ) Word Attack; PC: WJ Passage Comprehension; RAN: rapid naming of colors, objects, letters and/or numbers; VIQ: estimated verbal intelligence quotient; RD: reading disability; Samples are described in Table 1.
These samples also differ in the relationship between anatomy and reading skill. In UF1, UF2 and GT, an anatomical risk index comprised of seven measurements of anatomical size and asymmetry predicted reading and language function. Individuals with larger auditory and cerebellar structures on the left and larger cerebral hemispheres overall on the right have better cognitive function than those with the opposite configuration. In the samples with superior IQ (UW and UF3), by contrast, this brain/behavior relation is not seen. After briefly summarizing how we developed the anatomical risk index, we will speculate briefly on possible reasons for the inconsistency in brain behavior relations among samples.
Anatomical risk index
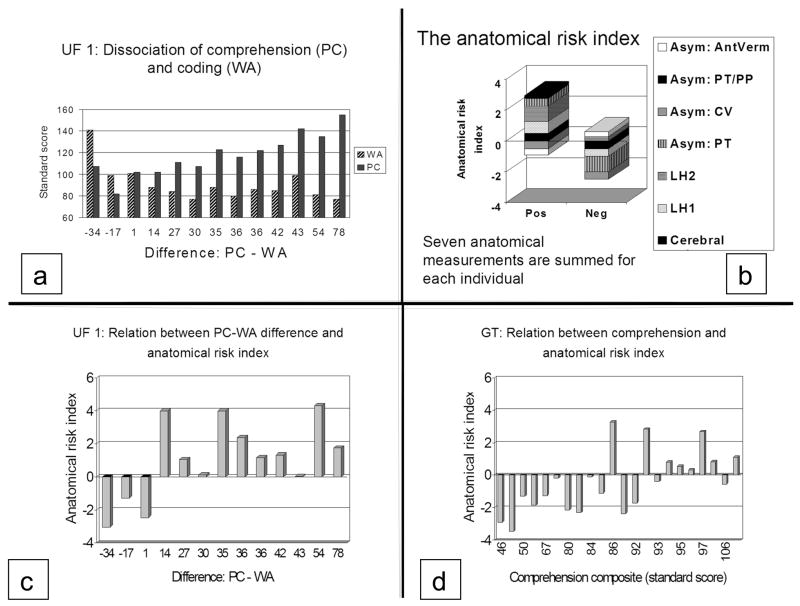
We were stimulated to develop the anatomical risk index when we studied the anatomical characteristics of the 13 students with dyslexia in UF 1. Figure 7a shows that these students showed a broad range of PC scores (82 – 156). The anatomical measures we collected included measures of cerebral and cerebellar volume and surface area measures of perisylvian structures including Heschl’s gyrus, planum temporale and planum parietale. Cerebellar volume was included because of evidence that eye movements and motor coordination might be disrupted in dyslexia (the cerebellar hypothesis (Fawcett, Nicolson, & Dean, 1996)).
Figure 7.
Results from anatomical risk index studies. A: College students ranked in order of their discrepancy between PC and WA. B. Graphic depiction of how the seven anatomical measurements are added together to produce positive and negative indices. AntVerm: anterior lobe of the cerebellar vermis; PT/PP: summed asymmetries of the planum temporale and planum parietale; CV: cerebral volume (adjusted for sex); LH2: surface area of second Heschl’s gyrus (left); LH: surface area of first Heschl’s gyrus (left). C. Relationship between anatomical risk index and PC-WA discrepancy. Students ordered as in 7a. Students with negative or small discrepancies have negative anatomical risk indices. Students with large positive discrepancies have positive anatomical risk indices. D. GT sample: children with poor comprehension tend to have negative anatomical risk indices while children with normal comprehension tend to have positive anatomical risk indices.
The anatomical characteristics of students whose PC scores were better than WA were strikingly different from those with discrepancies in the opposite direction (negative discrepancies). The students with small or negative discrepancies (left side of Fig 7a) had symmetrical plana temporale (like children with poor reading ability) while the ones with positive discrepancies had extreme planum temporale asymmetry (like the dyslexics in UF 0). The students with negative discrepancies also tended to have rightward asymmetry of the cerebral hemispheres, leftward asymmetry of the anterior lobe of the cerebellum, duplicated Heschl’s gyri on the left, and leftward asymmetry of both the planum temporale and planum parietale. The students with positive discrepancies did not have these characteristics (Leonard et al., 2001). The difference in anatomical characteristics between individuals with positive and negative discrepancies reinforced our feeling that poor readers were not all the same and that it was important to distinguish between individuals with isolated phonological deficits and those with more extensive deficits.
We decided to see if we could identify a set of anatomical measurements that, when combined, would discriminate between individuals with mild and severe deficits (Leonard et al., 2002). From a review of our previous studies as well as the literature we chose seven anatomical measures (planum temporale asymmetry, summed planum temporale and planum parietale asymmetry, cerebral asymmetry, anterior cerebellar asymmetry, cerebral size, primary and secondary Heschl’s gyrus size) and entered them into a discriminant analysis.
Figure 7b presents graphic examples of how these measurements are combined. Negative indices are produced when individuals have low cerebral volume, small Heschl’s gyri, symmetrical plana, leftward asymmetry of the cerebrum and rightward asymmetry of the anterior cerebellum. Individuals with negative risk indices tend to have multiple deficits. Positive risk indices are produced when individuals have higher cerebral volumes, larger Heschl’s gyri, asymmetrical plana, rightward asymmetry of the cerebrum and leftward asymmetry of the anterior cerebellum. Positive indices characterize individuals with mild dyslexia or isolated phonological deficits.
In Figure 7c, the dyslexic college students are ordered in the same way as in Figure 7a, with the individuals with small discrepancies on the left. It can be seen that these individuals have negative anatomical risk indices, while most of the students with positive discrepancies have positive risk indices. Since the weights for the individual measurements making up the risk index were derived in this sample (as well as in UF 2), this relationship simply demonstrates the success of the discriminant analysis.
The validity of the index was tested in three ways. First it was calculated in 103 normal children who were not involved in the development of the index. Their indices were normally distributed, clustering near 0. Interestingly, children with positive indices showed evidence of decreasing phonological decoding ability relative to their peers, over time, suggesting they might have compromised phonological decoding skill (Leonard et al., 2002). Children with negative indices did not have multiple deficits, however. The fact that negative indices were not associated with impairments in a normal sample suggests that a negative index is not sufficient for developing a disability. Deviance in other anatomical structures or a deficient environment may also be required.
In another attempt at validation we applied this index to a new sample of reading impaired children recruited for a functional imaging study on the effects of intervention. In Figure 7d the children in this sample are ordered by their comprehension scores (average of written and oral comprehension), rather than their discrepancies, because there were few children with discrepant PC and WA scores in this sample. Once again there is a strong relationship between anatomy and behavior. Children with low comprehension scores have negative anatomical risk indices while children with better ability have positive anatomical risk indices (Leonard, Eckert, Givens, Berninger, & Eden, 2006).
In this sample, there was a high correlation between all measures of reading and language and virtually all these measures correlated with the anatomical risk index. Interestingly, however, some measures, notably WA, had a curvilinear relation – that is children with strongly positive anatomical risk indices had worse scores than children with moderate risk indices. Since normal children have anatomical risk indices that cluster around zero (Leonard et al., 2002), this finding suggests that the two types of dyslexia may form anatomical clusters on either side of normal. Children with multiple deficits tend to have negative anatomical risk indices, children with lower phonological scores than expected for their general ability level tend to have positive risk indices, while those with consistently high profiles would cluster around zero. The fact that the anatomical substrates for these two profiles differ in opposite directions from average anatomy suggests that children with these two profiles might have qualitatively different disorders. It is interesting to speculate that they might respond to different types of intervention and show different activation profiles in functional imaging experiments.
Why is variation in structural anatomy associated with variation in reading ability? When we initiated these studies, we were relying on a neuropsychological model where different functions were associated with different brain regions. We reasoned that larger perisylvian regions would support higher resolution processing of acoustic contrasts and phonemes and thus larger size would be associated with better function. The failure of so many of our expected structure/function correlations has led us to realize that this was an overly simplistic model. Each of these brain regions is only a node in an extensive network. The shape and size of these regions is an index of network connectivity (Fox et al., 2001; Van Essen, 1997).
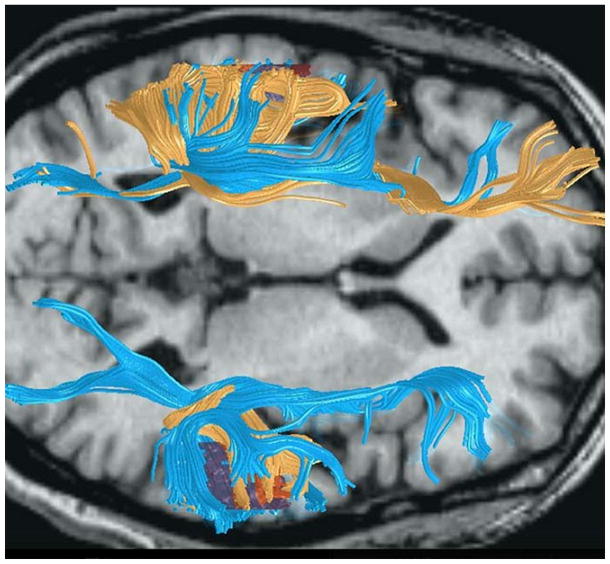
In Figure 8, diffusion tensor images of the fiber connections of two individuals with different planum temporale anatomy are overlain to demonstrate how different anatomy can be associated with different connectivity. The fibers depicted in yellow come from an individual with normal leftward asymmetry of the planum temporale. In the top of the figure, there is abundant connectivity between the planum, to the left, and the frontal lobe, to the right (presumably representing the arcuate fasciculus connecting Broca’s and Wernicke’s areas). The fibers depicted in blue come from an individual with large plana temporale in both hemispheres. In this individual, there are strong connections with the frontal lobe on both sides.
Figure 8.
This horizontal MRI image shows the overlain fiber tracings from diffusion tensor imaging of two men with different structural anatomy. The fibers depicted in yellow come from a man with normal leftward planar asymmetry. The fibers traced in blue come from a man with symmetrical plana temporale due to a large planum temporale in the right hemisphere (bottom of figure). Note the absence of connections between the temporal and frontal lobes in the right hemisphere in the man with normal asymmetry. We hypothesize that the parietal lobe dominates frontal circuits on the right when temporal lobe connections are missing. Image modified from (Leonard, Eckert, & Kuldau, 2006), used with permission of Blackwell Publishing.
Clearly information flow among different regions could be expected to be somewhat different in people with differing neural architectures. It might be interesting to keep track of structural anatomy while performing functional connectivity experiments. It might turn out that the connectivity among regions active in information processing (Binder et al., 1999; Fox et al., 2005) might vary in individuals with different structural anatomy.
That’s the positive interpretation of our studies. But it’s important to note that the anatomical risk index does not provide a marker for dyslexia or SLI. Many perfectly healthy individuals with normal reading ability have negative and positive risk indices. Furthermore, the anatomical risk index did not discriminate between dyslexic and control individuals who have superior verbal IQ, as do the individuals in the UW and UF3 samples. At present we have no explanation of these failures. We did find anatomical measures that discriminated the children in UW 1 from healthy controls. The children with dyslexia had smaller right anterior lobes of the cerebellum (like the discrepant college students in UF 1) and smaller cerebral hemispheres than controls (like the children with SLI) (Eckert et al., 2003). But these measures were not reduced in children in the UW 2 sample, who were recruited one year after UW 1. Although these children were chosen, specifically, for spelling difficulties, their reading profiles look almost identical to those in UW 1 (Figure 6). Clearly, many factors play a role in the development of a reading disability. The anatomical risk index may capture a few of these factors. We feel the most important message of our work is caution. Caution against broad generalizations. Caution against combining brain images from different individuals, together, in imaging experiments to gain an average response. Caution, above all, against one-size-fits-all educational prescriptions. Individual differences in both cognitive and neural architecture should be taken seriously.
Acknowledgments
Work described was supported by NIH grants NIDCD R01 02922 (CML), HD P50 33812 to Virginia W. Berninger, and NSF SBR-9729009 and NIDCD R01 006957 to Christine Chiarello and a Veterans Administration Merit Review award to John M. Kuldau.
This work depended on the collaboration of many investigators and students. I would like to particularly thank Kytja Voeller, Linda Lombardino, Mark Eckert, John Kranzler, Virginia Berninger, Wayne King, Cecile Mohr, Alan Freeman, Laurie Gauger, Jennifer Mockler, Sharyl Williams, Lisa Rowe, Susan Smith and the experimental participants and their families.
Contributor Information
Christiana M. Leonard, Department of Neuroscience, McKnight Brain Institute, University of Florida
Mark A. Eckert, Department of Otolaryngology, Medical University of Charleston
References
- Belin P, Zilbovicius M, Fontaine A, Crozier S, Thivard L. Lateralization of speech and auditory temporal processing. Journal of Cognitive Neuroscience. 1998;10:536–540. doi: 10.1162/089892998562834. [DOI] [PubMed] [Google Scholar]
- Best M, Demb J. Normal planum temporale asymmetry in dyslexics with a magnocellular deficit. Neuroreport. 1999;10:607–612. doi: 10.1097/00001756-199902250-00030. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Bishop DV, Snowling MJ. Developmental dyslexia and specific language impairment: same or different? Psychol Bull. 2004;130:858–886. doi: 10.1037/0033-2909.130.6.858. [DOI] [PubMed] [Google Scholar]
- Bruck M. Persistence of dyslexics’ phonological awareness deficits. Developmental Psychology. 1992;28:874–886. [Google Scholar]
- Chiarello C, Lombardino LJ, Kacinik NA, Otto R, Leonard CM. Neuroanatomical and behavioral asymmetry in an adult compensated dyslexic. Brain Lang. 2006;98:169–181. doi: 10.1016/j.bandl.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Shulman GL, Peterson SE. A PET study of spatial attention. Journal of Neuroscience. 1993;13:1202–1226. doi: 10.1523/JNEUROSCI.13-03-01202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duara R, Kusch A, Gross-Glenn K, et al. Neuroanatomic differences between dyslexic and normal readers on magnetic resonance imaging scans. Archives of Neurology. 1991;48:410–416. doi: 10.1001/archneur.1991.00530160078018. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Richards TL, Aylward EH, Thomson J, Berninger VW. Anatomical correlates of dyslexia: frontal and cerebellar findings. Brain. 2003;126:482–494. doi: 10.1093/brain/awg026. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Lombardino LJ, Leonard CM. Planar asymmetry tips the phonological playground and environment raises the bar. Child Dev. 2001;72:988–1002. doi: 10.1111/1467-8624.00330. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Kito T, Sadato N, Passingham RE, Naito E. Neural substrate of body size: illusory feeling of shrinking of the waist. PLoS Biol. 2005;3:e412. doi: 10.1371/journal.pbio.0030412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett AJ, Nicolson RI, Dean P. Impaired performance of children with dyslexia on a range of cerebellar tasks. Annals of Dyslexia. 1996;46:259–283. doi: 10.1007/BF02648179. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Huang A, Parsons LM, Xiong JH, Zamarippa F, Rainey L, et al. Location-probability profiles for the mouth region of human primary motosensory cortex: model and validation. Neuroimage. 2001;13:196–209. doi: 10.1006/nimg.2000.0659. [DOI] [PubMed] [Google Scholar]
- Galaburda AM. Ordinary and extraordinary brain development: Anatomical variation in developmental dyslexia. Annals of Dyslexia. 1989;39:67–79. doi: 10.1007/BF02656901. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, LeMay M, Kemper TL, Geschwind N. Right-left asymmetrics in the brain. Science. 1978;199:852–856. doi: 10.1126/science.341314. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, Sherman GF, Rosen GD, Aboitiz F, Geschwind N. Developmental dyslexia: Four consecutive cases with cortical anomalies. Annals of Neurology. 1985;18:222–233. doi: 10.1002/ana.410180210. [DOI] [PubMed] [Google Scholar]
- Gauger LM, Lombardino LJ, Leonard CM. Brain morphology in children with specific language impairment. Journal of Speech, Language, and Hearing Research. 1997;40:1272–1284. doi: 10.1044/jslhr.4006.1272. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Levitsky W. Human brain: left-right asymmetries in temporal speech region. Science. 1968;161:186–187. doi: 10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- Hagler DJ, Jr, Riecke L, Sereno MI. Parietal and superior frontal visuospatial maps activated by pointing and saccades. Neuroimage. 2007 Feb 8; doi: 10.1016/j.neuroimage.2007.01.033. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halderman LK, Chiarello C. Cerebral asymmetries in early orthographic and phonological reading processes: evidence from backward masking. Brain Lang. 2005;95:342–352. doi: 10.1016/j.bandl.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Bowers D, Valenstein E, Watson RT. The right hemisphere: neuropsychological functions. Journal of Neurosurgery. 1986;64:693–704. doi: 10.3171/jns.1986.64.5.0693. [DOI] [PubMed] [Google Scholar]
- Hynd GW, Semrud-Clikeman M, Lorys AR, Novey ES, Eliopulos D. Brain morphology in developmental dyslexia and attention deficit disorder/hyperactivity. Archives of Neurology. 1990;47:919–926. doi: 10.1001/archneur.1990.00530080107018. [DOI] [PubMed] [Google Scholar]
- Kacinik NA, Chiarello C. Understanding metaphors: Is the right hemisphere uniquely involved? Brain Lang. 2005 doi: 10.1016/j.bandl.2005.10.010. [DOI] [PubMed] [Google Scholar]
- King WM, Lombardino LJ, Crandell CC, Leonard CM. Comorbid auditory processing disorder in developmental dyslexia. Ear Hear. 2003;24:448–456. doi: 10.1097/01.AUD.0000090437.10978.1A. [DOI] [PubMed] [Google Scholar]
- Larsen JP, Høien T, Lundberg I, Ødegaard H. MRI evaluation of the size and symmetry of the planum temporale in adolescents with developmental dyslexia. Brain and Language. 1990;39:289–301. doi: 10.1016/0093-934x(90)90015-9. [DOI] [PubMed] [Google Scholar]
- Leonard C, Eckert M, Givens B, Berninger V, Eden G. Individual differences in anatomy predict reading and oral language impairments in children. Brain. 2006;129:3329–3342. doi: 10.1093/brain/awl262. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Eckert MA, Kuldau JM. Exploiting human anatomical variability as a link between genome and cognome. Genes Brain Behav. 2006;5(Suppl 1):64–77. doi: 10.1111/j.1601-183X.2006.00196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CM, Eckert MA, Lombardino LJ, Oakland T, Kranzler J, Mohr CM, et al. Anatomical risk factors for phonological dyslexia. Cerebral Cortex. 2001;11:148–157. doi: 10.1093/cercor/11.2.148. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Lombardino LJ, Mercado LR, Browd SR, Breier JI, Agee OF. Cerebral asymmetry and cognitive development in children: A magnetic resonance imaging study. Psychological Science. 1996;7:79–85. [Google Scholar]
- Leonard CM, Lombardino LJ, Walsh K, Eckert MA, Mockler JL, Rowe LA, et al. Anatomical risk factors that distinguish dyslexia from SLI predict reading skill in normal children. Journal of Communication Disorders. 2002;35:501–531. doi: 10.1016/s0021-9924(02)00120-x. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Voeller KS, Lombardino LJ, Morris MK, Alexander AW, Andersen HG, et al. Anomalous cerebral structure in dyslexia revealed with magnetic resonance imaging. Archives of Neurology. 1993;50:461–469. doi: 10.1001/archneur.1993.00540050013008. [DOI] [PubMed] [Google Scholar]
- Lindamood CH, Lindamood PC. Lindamood Auditory Conceptualization Test. Boston: Teaching Resources Corporation; 1979. [Google Scholar]
- Naito E, Roland PE, Grefkes C, Choi HJ, Eickhoff S, Geyer S, et al. Dominance of the right hemisphere and role of area 2 in human kinesthesia. J Neurophysiol. 2005;93:1020–1034. doi: 10.1152/jn.00637.2004. [DOI] [PubMed] [Google Scholar]
- Robin DA, Tranel D, Damasio H. Auditory perception of temporal and spectral events in patients with focal left and right cerebral lesions. Brain and Language. 1990;39:539–555. doi: 10.1016/0093-934x(90)90161-9. [DOI] [PubMed] [Google Scholar]
- Rubens AB, Mahwold MW, Hutton JT. Asymmetry of the lateral sylvian fissures in man. Neurology. 1976;26:620–624. doi: 10.1212/wnl.26.7.620. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Donahue BC, Brady DR, Nace K, Giedd JN, Andreason P. A magnetic resonance imaging study of planum temporale asymmetry in men with developmental dyslexia. Archives of Neurology. 1997;54:1481–1489. doi: 10.1001/archneur.1997.00550240035010. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Cho NK, Staib LH, Kier LE, Fletcher JM, Shaywitz SE, et al. Brain morphology in normal and dyslexic children: The influence of sex and age. Annals of Neurology. 1994;35:732–742. doi: 10.1002/ana.410350615. [DOI] [PubMed] [Google Scholar]
- Stark R, Tallal P. Language, speech, and reading disorders in children: Neuropsychological studies. Boston MA: College-Hill; 1988. [Google Scholar]
- Steinmetz H, Ebeling U, Huang Y, Kahn T. Sulcus topography of the parietal opercular region: An anatomic and MR study. Brain and Language. 1990;38:515–533. doi: 10.1016/0093-934x(90)90135-4. [DOI] [PubMed] [Google Scholar]
- Steinmetz H, Rademacher J, Jancke L, Huang Y, Thron A, Zilles K. Total surface of temporoparietal intrasylvian cortex; diverging left-right asymmetries. Brain and Language. 1990;39:357–372. doi: 10.1016/0093-934x(90)90145-7. [DOI] [PubMed] [Google Scholar]
- Tallal P. Auditory temporal perception: phonics and reading disabilities in children. Brain and Language. 1980;9:182–198. doi: 10.1016/0093-934x(80)90139-x. [DOI] [PubMed] [Google Scholar]
- Tallal P, Miller S, Fitch RH. Temporal processing in the nervous system; implications for the development of phonological systems. Annals of the New York Academy of Sciences. 1993;682:27–47. doi: 10.1111/j.1749-6632.1993.tb22957.x. [DOI] [PubMed] [Google Scholar]
- Thal D, Marchman V, Stiles J, Aram D, Trauner D, Nass R, et al. Early lexical development in children with focal brain injury. Brain and Language. 1991;40:491–527. doi: 10.1016/0093-934x(91)90145-q. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385:313–318. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- West TG. The Mind’s Eye. Amherst NY: Prometheus Books; 1997. [Google Scholar]
- Witelson SF, Kigar D, Harvey T. The exceptional brain of Albert Einstein. Lancet. 1999;353:2149–2153. doi: 10.1016/S0140-6736(98)10327-6. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, Johnson RB. Woodcock-Johnson tests of achievement: standard and supplemental batteries. Allen, TX: DLM/Teaching Resources; 1989. [Google Scholar]