Summary
The anti-Hu syndrome is the most common paraneoplastic neurologic syndrome but the exact mechanism of immune mediated neuronal injury remains unknown. Anti-Hu antibodies do not appear to play a pivotal role in the pathogenesis of the disease. To assess cell-mediated immunity, we selected 51 peptides from the Hu-D sequence and tested their ability to bind to six common HLA class I molecules. Stable complexes with purified HLA molecules were obtained with 19/51 (37%) selected peptides. Subsequently, the ability of the 19 HLA-binding peptides to stimulate T cells from 10 patients and 10 control subjects was evaluated by detecting IFN-γ secretion. An anti-peptide T-cell response was observed in 7/10 Hu-positive patients but also in 3/10 control subjects. Overall, a significant T-cell activation occurred in response to 74% (14 out of 19) of the selected peptides in the Hu-positive patients vs. 16% (3 out of 19) in the control group (p < 0.001). In addition, T cells of patients tested within 3 months of the onset of anti-Hu syndrome responded to 82% (14 out of 17) of assessed Hu-D peptides vs. 37% (7 out of 19) in patients tested 1 year or more after developing the syndrome (p < 0.01). Thus, the present study suggests a role of cellular immunity during the course of anti-Hu syndrome.
Keywords: anti-Hu syndrome, cellular immunity, ELISPOT assay, HLA/peptide complexes, Hu-D epitopes, IFN-γ, paraneoplastic disorders, T lymphocytes
Introduction
The term ‘anti-Hu syndrome’ is used to describe patients with paraneoplastic encephalomyelitis and sensory neuronopathy associated with anti-Hu antibodies. Most of these patients have small-cell lung cancer (SCLC) and develop high titers of serum and CSF antibodies to a group of proteins of 35–40 kDa, known as Hu antigens. These Hu proteins are normally expressed in the nucleus and, to a lesser extent, in the cytoplasm of all neurons in the central and peripheral nervous system [1,2]. However, one of those antigens, Hu-D, is consistently expressed in SCLC [3]. Ectopic expression by tumors of so-called onconeural antigens is present in all patients with antibody-positive paraneoplastic neurologic syndromes (PNS). The antigen expressed by the tumor is strictly identical to its neuronal counterpart [4]. Though anti-Hu syndrome is the most common PNS of the central nervous system (CNS), the exact mechanism of immune mediated neuronal injury remains unknown. The most prevalent hypothesis is that Hu neuronal proteins, when expressed by a tumor, are identified, for still unknown reasons, as non-self by the immune system. As a result, they trigger an immune response which is subsequently misdirected to identical neuronal antigens, eventually leading to neuron death [1,3]. Except for a few in vitro cytotoxicity studies suggesting a cytotoxic effect of anti-Hu antibodies on granule cells of the cerebellum [5] and neurons of the myenteric plexus [6], there is no further evidence of a primary pathogenic role of humoral immunity. In particular, passive transfer of IgG from anti-Hu patients to animals did not cause the disorder, and animal immunization with Hu-D protein or cDNA resulted in synthesis of high titers of antibodies but without modeling the disease [7,8]. There is increasing evidence that cellular immunity is involved in the pathogenesis of the disease [9–12]. We have previously shown that the Hu-D fusion protein is a specific target of patients’ auto-reactive circulating CD4+ T cells, presumably of Th1 subtype [13]. Second, other investigators have demonstrated that activated circulating CD8+ T cells from patients with anti-Hu syndrome cause lysis of autologous fibroblasts previously injected with recombinant Hu-D protein [14]. In a separate work, those same investigators found similar results using MHC class I-restricted Hu-D peptides instead of the whole protein [15]. Finally, cytotoxic responses were elicited with Hu-D peptides in transgenic mice expressing human HLA-A2 molecules [16].
To confirm the presence of circulating Hu-D specific T cells in patients with anti-Hu syndrome, we examined the ex vivo T cell response induced by Hu-D peptides selected for their ability to bind to various HLA class I molecules.
Material and methods
Peripheral blood mononuclear cells and cell culture medium
Blood samples were collected after informed consent from 10 Hu-positive patients and 10 Hu-negative donors (control group including seven healthy volunteers and three patients with hemochromatosis). All Hu-positive patients had SCLC and PNS, except for one ‘cured’ patient (No 3, Table 1) whose antibody titers became negative and remained so at 3-year follow-up. Nine Hu-positive patients had not received immunosuppressive or anti-tumor treatment for at least 6 months before blood was collected; one patient had been treated with corticosteroids several days before the blood sample was taken.
Table 1.
HLA typing
| Hu-positive patients | HLA typing |
|---|---|
| 1 | A24 A29/B39 B62 |
| 2 | A1 A3/B65 B1508 |
| 3 | A1 A2/B18 B51 |
| 4 | A24 A26/B38 B35 |
| 5 | A2 A32/B18 B35 |
| 6 | A2 A24/B44 B18 |
| 7 | A1 A2/B8 |
| 8 | A2 A24/B18 B13 |
| 9 | A24 A11/B44 B51 |
| 10 | A2/B57 B60 |
| Volunteer donors | |
| HC 37 | A3 A11/B18 |
| HC 63 | A24/B7 B35 |
| HC 67 | A1 A25/B8 B18 |
| BT 08 | A1 A24/B35 |
| BT 10 | A2 A11/B35 B44 |
| BT 12 | A24 A30/B7 B13 |
| BT 15 | A2 A3/B7 B35 |
| BT 33 | A1 A29/B7 B18 |
| BT 34 | A1 A2/B5 B8 |
| BT 35 | A2 A28/B8 B18 |
HC – hemochromatosis patients, ‘BT’ letters refer to volunteer donors in the blood bank.
The 10 Hu-negative blood bank volunteer donors were selected on their HLA profile. Those displaying similar panels of HLA molecules that Hu-positive patients were used as controls (Table 1).
PBMCs were isolated from heparinized blood by density gradient centrifugation using Ficoll Hypaque 1.077. Cells recovered from the interface were washed twice with isotonic saline serum, suspended in complete medium and counted in trypan blue. Complete medium consisted of RPMI 1640 supplemented with 10% human AB serum, 2 mM L-glutamine, 1% non-essential amino acids, 100 IU/ml penicillin, 100 μg/ml streptomycin, 1 mM sodium pyruvate and 10 nM Hepes. PBMCs were used freshly for ELISPOT assays or kept frozen at −180 °C in fetal calf serum (FCS) containing 10% dimethylsulfoxide (DMSO) and tested after thawing.
HLA typing
HLA typing was performed serologically by the standard NIH microlymphocytotoxicity test as previously described [17].
Synthetic peptides
From the Hu-D sequence we selected 8–11 amino acid long peptides containing anchor residues required for binding to common MHC class I molecules. We found 129 peptides of 8–11 residues containing anchor motifs to class I molecules and among them we selected a set of 51 peptides whose target HLA molecules were frequent in our Hu-positive patients.
These 51 peptides were synthesized (Chiron Mimitopes, Clayton, Australia), dissolved in DMSO at a concentration of 1 mg/ml before further dilution in complete medium for individual experiments and stored at −20 °C.
HLA source
HLA molecules were purified from human lymphoblastoid cell lines transformed by Epstein-Barr virus [18]. Purified HLA (50–100 μg) class I molecules (stored frozen at −80 °C) diluted in 500 μl PBS, 0.1% NP-40 were denatured in 12.5 mM NaOH pH 11.7, 1.5 M urea, 1% bovine serum albumin (BSA) for 1 h at 4 °C. The preparation was run through G25/PD10 Sephadex column (Pharmacia, Peapack, NJ) equilibrated in PBS containing 0.05% Tween 20 (PBS-T), 2 mM EDTA, 0.1% NP-40 to separate heavy chains and β2-microglobulin from endogenous peptides. Thereafter, exogenous β2-microglobulin (2 μg/ml, Sigma, St Louis, MO) and CHAPS (6 mM, Sigma) were added to purified and denatured HLA class I molecules before addition of Hu-D or control peptides.
Peptide binding assay
Aliquots (1 μg) of purified and denatured HLA class I molecules were incubated in Eppendorf microtubes with or without exogenous peptide for 2 h at room temperature. Peptides were assayed at three dilutions ranging from 10−4 to 10−8 M and the mixture was diluted to 200 μl in PBS-T, 1% BSA, 1 mM PMSF (p-amidino-phenyl-methyl-sulfonyl-fluoride), 10 μg/ml trypsin inhibitor. Wells of microtiter plates (Nunc Maxisorp, Taastrop, Denmark) were coated with monoclonal anti-HLA heavy chain antibodies at a concentration of 10 μg/ml in PBS for 2 h at 37 °C. HLA-A1 molecules were detected with mAb B9.12.1, HLA-A2 molecules with mAb BB7.2, HLA-A24 molecules with mAb A11.1, HLA-B35 molecules with mAb PA2.6 and HLA-B8, - B18 and -B51 with mAb B1.23.2. Antibodies were obtained from American Type Culture Collection (ATCC) or as previously described [19]. Thereafter, the plates were washed three times with PBS-T before being saturated overnight at 4 °C with 100 μl/well PBS-T, 1% BSA. One hundred μl HLA molecules pre-incubated with peptides were added in duplicate to antibody-coated wells and incubated for 24 h at 4 °C. The plates were then washed three times with PBS-T. The wells were filled for 1 h at 37 °C with 100 μl anti-β2 microglobulin antibodies M28 coupled to alkaline phosphatase to detect correctly folded HLA complexes. Enzymatic activity was revealed using MUP substrate and the fluorescence was measured at 355/460 nm in a Fluoscan reader (VICTOR; Wallac, Evry, France).
ELISPOT assay
IFN-γ secretion from T cells stimulated by Hu-D peptides was detected by an ex vivo ELISPOT assay. Ninety six-well nitrocellulose-bottomed plates (Millititer, Millipore, Bedford, MA) were coated with 100 μl of monoclonal anti-human IFN-γ antibodies (clone 1-D1K, Mabtech, Nacka, Sweden) at a concentration of 1 μg/ml in carbonate-bicarbonate buffer 0.1 M pH 9.6 and incubated overnight at 4 °C. Unbound antibodies were removed by three successive washings with sterile PBS. The wells were then blocked with 200 μl complete medium for 2 h at 37 °C. The blocking medium was discarded and the wells were each filled in duplicate or triplicate with 100 μl of complete medium containing 3 × 105 freshly isolated PBMCs plus 1 μg/ml synthetic peptide in a final volume of 200 μl/well. The release of IFN-γ by PBMCs in response to a non-specific stimulator such as phorbol myristate acetate (PMA)–ionomycine (50 ng/ml PMA plus 500 ng/ml ionomycine) was included as a positive control. Negative assay control consisted in the cytokine release in the presence of an irrelevant peptide.
The plates were incubated for 20 h at 37 °C in a humidified atmosphere with 5% CO2. After incubation the plates were extensively washed with PBS-T and 100 μl biotinylated anti-human IFN-γ antibodies (clone 7-B6-1, Mabtech) were added to each well at a concentration of 1 μg/ml in PBS-T, BSA 1%. The plates were incubated 2 h at room temperature. Thereafter they were vigorously rinsed five times with PBS-T and each well was exposed for 45 min at room temperature to 100 μl Extravidin-Alkaline Phosphatase diluted 1/6000 in PBS-T, BSA 1%. After another step washing with PBS-T, 100 μl/well of BCIP/NBT substrate (Biorad, Hercules, CA) were added for 30 min. Color development was stopped by washing under running tap water. After drying overnight at room temperature, colored spots were counted using a computer-assisted image analysis system (KS ELIspot, Zeiss, Thornwood, NY).
Statistical analysis
The results were analyzed using a K2 test. For the purpose of statistical analysis, T cell response to a given peptide was considered positive when the number of spots was at least twice the number of spots measured in the presence of an irrelevant peptide [20]. Differences with a p value of <0.05 were considered significant.
Results
Binding of Hu-D peptides to purified HLA molecules
We analyzed the HLA class I expression profile of 10 Hu-positive patients and selected blood bank volunteer donors displaying equivalent panels of HLA molecules (Table 1). For the most frequent molecules detected in our patients (HLA-A1, -A2, -A24, -B8, -B18 and -B35/51), we identified in the Hu-D sequence 51 8- to 10-mer peptides containing HLA-binding motifs. The HLA binding capacity of the synthetic peptides was tested at various concentrations (from 10−4 to 10−8 M) and we present herein the most significant results that were obtained at 10−6 M, as already described [21]. For each HLA molecule a positive control (a known epitope) and a negative one (an irrelevant epitope) were added to the test. For example, peptide PB1 591–599 from Influenza virus was used as a positive control in the HLA-A1 binding test and gave 100% binding. Nine HLA-A1-restricted Hu-D peptides were tested and three of them bound significantly to HLA-A1 molecules (58–66, 147–155 and 245–254) (Table 2). Similar studies using appropriate positive and negative controls and the adequate HLA molecules were performed for each Hu-D peptide (Table 2). Twenty peptides were tested for their binding capacity to HLA-A2 and seven of them were binders (64–72, 86–95, 111–119, 163–171, 248–256, 315–323 and 362–370). Among 12 peptides tested for HLAA24 binding, seven formed stable complexes with purified HLA molecules (88–95, 134–143, 151–158, 154–163, 176–184, 341–348 and 347–355). Two out of nine HLA-B18-restricted peptides promoted significant HLA assembly (245–254 and 347–355). Among 15 peptides containing HLA-B8 anchor residues, none was binder (not shown). In addition, two peptides were positive in the HLA-B35 binding test (272–281 and 305–313) and were also retained for further studies.
Table 2.
HLA binding of Hu-D peptides
| Peptide | Sequence | HLA binding
|
|||
|---|---|---|---|---|---|
| A1 | A2 | A24 | B18 | ||
| 58–66 | TQEEFRSLF | 62 | 9 | ||
| 61–70 | EFRSLFGSI | 0 | |||
| 64–72 | SLFGSIGEI | 38 | |||
| 86–95 | SLGYGFVNYI | 36 | |||
| 88–95 | GYGFVNYI | 89 | |||
| 102–11 | KAINTLNGL | 0 | |||
| 109–117 | GLRLQTKTI | 5 | |||
| 111–119 | RLQTKTIKV | 24 | |||
| 128–136 | SIRDANLYV | 8 | |||
| 134–143 | LYVSGLPKTM | 21 | |||
| 135–143 | YVSGLPKTM | 5 | |||
| 142–151 | TMTQKELEQL | 18 | |||
| 145–152 | QKELEQLF | 0 | 0 | ||
| 147–155 | ELEQLFSQY | 53 | 6 | ||
| 150–158 | QLFSQYGRI | 18 | |||
| 151–158 | LFSQYGRI | 35 | |||
| 154–163 | QYGRIITSRI | 109 | |||
| 157–165 | RIITSRILV | 12 | |||
| 163–171 | ILVDQVTGV | 54 | |||
| 167–175 | QVTGVSRGV | 8 | |||
| 176–184 | GFIRFDKRI | 27 | |||
| 170–178 | GVSRGVGFI | 15 | |||
| 177–184 | FIRFDKRI | 12 | |||
| 201–209 | ATEPITVKF | 18 | 5 | ||
| 243–250 | RFRLDNLL | 8 | |||
| 245–254 | RLDNLLNMAY | 124 | 67 | ||
| 248–256 | NLLNMAYGV | 42 | |||
| 253–260 | AYGVKRLM | 12 | |||
| 272–281 | RFSPITIDGM | 9 | |||
| 277–285 | TIDGMTSLV | 13 | |||
| 303–312 | NLSPDSDESV | 0 | |||
| 305–313 | SPDSDESVL | 6 | 9 | ||
| 308–317 | SDESVLWQLF | 0 | 18 | ||
| 315–323 | QLFGPFGAV | 21 | |||
| 341–348 | GFVTMTNY | 26 | |||
| 347–355 | NYDEAAMAI | 0 | 20 | 21 | |
| 355–363 | IASLNGYRL | 3 | |||
| 360–368 | GYRLGDRVL | 3 | |||
| 362–370 | RLGDRVLQV | 31 | |||
| 363–372 | LGDRVLQVSF | 19 | |||
Results correspond to a peptide concentration of 10−6 M. HLA binding of each Hu-D peptide is expressed as % of a positive control. Significant bindings (at least 20% of control binding) are presented in bold characters. Positive control peptides were: for HLA-A1 binding, peptide 591–599 from PB1 protein of Influenza virus (VSDGGPNLY) which gave 1050 fluorescence units (FU) (=100% binding); for HLA-A2 binding, peptide 58–66 from matrix of Influenza virus (GILGFVFTL) which gave 4930 FU (100%); for HLA-A24 binding, peptide 419–427 from LMP-2 protein of Epstein Barr virus (EBV) (TYGPVFMCL) which gave 2915 FU (100%); for HLA-B18 binding, peptide 44–52 from HPV E7 (QAEPDRAHY) which gave 1900 FU (100%).
Overall, 19 out of the 51 tested Hu-D peptides (37%) formed stable complexes with purified HLA class I molecules.
Recognition of Hu-D peptides by T cells
The 19 Hu-D peptides that bound significantly to HLA class I molecules were each added to PBMCs of patients and healthy donors, and ex vivo reactivity of T cells against them was tested in the ELISPOT assay detecting IFN-γ secretion. As presented in Table 3, three Hu-D peptides gave no response (64–72, 272–281 and 305–313) and one (347–355) triggered a significant cytokine secretion from one control subject but not from Hu-positive patients. Two Hu-D peptides (147–155 and 245–254) were able to stimulate T cells from both Hu-positive patients and healthy donors. The remaining 13 Hu-D peptides only activated T cells from Hu-positive patients. Overall, responses were obtained with T cells from 7/10 Hu-positive patients (patients 1–3 and 6–9) as compared to 3/10 control subjects. Thus, a significant T-cell activation occurred in response to 74% (14 out of 19) of the peptides in the Hu-positive patients vs. 16% (3 out of 19) in the control group (p < 0.001). It should also be noted that when a given peptide was recognized by PBMCs from both Hu-positive patients and volunteer donors, peptide recognition intensity (i.e. ratio [number of spots for 3 × 105 PBMCs in response to that peptide/number of spots for 3 × 105 PBMCs in response to an irrelevant peptide]) did not differ significantly between these two groups. For example, Hu-D peptide 245–254 activated T cells from two Hu-positive patients as well as from two control subjects. Peptide recognition intensity was 2.96 and 2.23 for the responding Hu-positive patients, respectively, and 2.45 and 2.24 for the two volunteer donors, respectively.
Table 3.
Reactivity of T cells from Hu-positive patients and healthy donors
| Hu-D peptides | Hu+ patients | Hu− donors |
|---|---|---|
| 58–66 | 1/3 | 0/4 |
| 64–72 | 0/6 | 0/4 |
| 86–95 | 1/6 | 0/4 |
| 88–95 | 2/5 | 0/3 |
| 111–119 | 1/6 | 0/4 |
| 134–143 | 2/5 | 0/3 |
| 147–155 | 2/3 | 1/4 |
| 151–158 | 2/5 | 0/3 |
| 154–163 | 4/5 | 0/3 |
| 163–171 | 1/6 | 0/4 |
| 176–184 | 4/5 | 0/3 |
| 245–254 | 2/6 | 2/6 |
| 248–256 | 2/6 | 0/4 |
| 272–281 | 0/2 | 0/4 |
| 305–313 | 0/2 | 0/4 |
| 315–323 | 2/6 | 0/4 |
| 341–348 | 3/5 | 0/3 |
| 347–355 | 0/4 | 1/4 |
| 362–370 | 2/6 | 0/4 |
Results are expressed as numbers of donors having T cells secreting IFN-γ in the presence of one Hu-D peptide in the ELISPOT assay. Results presented in bold characters correspond to peptides recognized by T cells of Hu-positive patients only.
Correlation between PBMC response and clinical course of the disease
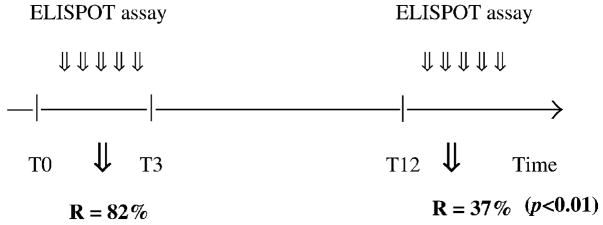
The peptide recognition rate was higher in patients whose anti-Hu syndrome had developed less than 3 months before our test (patients 2 and 6–10) than in patients whose disease had developed more than a year before PBMC testing (patients 1 and 3–5). In the first group, 82% of the tested peptides were recognized by the patients’ lymphocytes, while in the second group only 37% of the peptides were identified (p < 0.01) (Figure 1).
Figure 1.
PBMC response according to time. T – time in months, T0 – diagnosis of anti-Hu syndrome, R – peptide recognition rate.
These findings suggest a correlation between peptide recognition and the stage of the paraneoplastic disorder. In contrast, no correlation was found between the titer of anti-Hu antibodies and the likelihood of peptide recognition or the clinical status (not shown).
Discussion
In this work, a set of 19 Hu-D peptides able to bind to a panel of HLA class I molecules was identified. In addition, circulating T lymphocytes from Hu-positive patients recognized much more frequently Hu-D peptides than those from Hu-negative controls. Indeed, among the 19 Hu-D peptides tested, 74% induced a significant IFN-γ secretion from T cells of Hu-positive patients as compared with 16% in the control group (p < 0.001). The short exposure needed to target peptides during the ELISPOT assay indicates that the stimulated T cells were capable of rapid effector functions [22,23]. This finding implies a previous presentation of Hu-D peptides to circulating T cells. Presentation can occur at the tumor cell surface, as supported by studies showing frequent co-expression of Hu-D protein and HLA class I molecules by tumor cells of Hu-positive patients [24]. It can also be achieved by dendritic cells in the nearby lymph nodes in the mediastinum of patients.
The fact that a few Hu-D peptides were recognized by T cells of both Hu-positive patients and control subjects without significantly differing response intensity is not surprising. Similar findings have been previously made in other studies comparing T cell response to specific tumor antigens in patients with melanomas and in controls [25–27]. Possible mechanisms include cross-reactivity with an epitope derived from a different antigen [28], non-specific T cell activation (i.e. after a recent viral infection), or the presence of self-reactive T cells responsive to Hu-D epitopes in some healthy individuals.
Consistent with the common phenomenon of intra-molecular epitope spreading described by Bach et al. [29] T cells of Hu-positive patients often recognized several epitopes within the Hu-D antigen.
It is likely that responding lymphocytes were of CD8+ subtype since the Hu-D peptides tested were short (8–11 amino acids) and contained anchor residues to MHC class I molecules [30]. However, some of the peptides may have fitted in MHC class II molecules, therefore stimulating CD4+ T cells. Separation of CD8+ and CD4+ T cells using monoclonal antibodies coupled with magnetic beads would ensure that the release of IFN-γ is due to specific HLA class I/CD8 interaction [31].
The lack of PBMC response in 3/10 Hu-positive patients could reflect incomplete peptide analysis because less than half of the potential epitopes and only seven MHC haplotypes (based on their frequency in the general population and in Hu-positive patients) were studied.
Our control group comprises only ‘healthy’ subjects. It would be of interest to assess INF-γ secretion from PBMCs of patients suffering from SCLC with neither PNS nor circulating anti-Hu antibodies.
Even though our findings indicate T cell activation, they do not prove cytotoxic functions of the activated cells. The ability of those cells to lyse autologous Hu-D expressing SCLC tumor cells has yet to be demonstrated.
It is of interest to note that the clinico-pathological picture and the activity of the T-cell immune response apparently follow a similar course during anti-Hu syndrome. Clinically, during the early active phase of the disease patients develop a rapidly progressive neurologic disorder with prominent inflammatory infiltrates in the neuraxis. At this stage (which usually includes the first 3 months of the disease), we found the concomitant presence of a strong systemic Hu-D-specific cell-mediated immune response (82% of the Hu-D peptides tested during this period elicited IFN-γ secretion). In contrast, in later stages of the disorder (i.e. 1 year after symptom development) symptoms usually stabilize and inflammatory cells in the CSF and the nervous system fade away. In this phase of the disease, called ‘burnt out’ period [32], we found that the immune response also tends to disappear, and the number of actively targeted Hu-D peptides was significantly smaller (37%, p < 0.01). These findings may point to a model of disorder characterized by an initial phase of antigen-driven activation and expansion of T cells with effector functions [10,12], followed by a second phase in which the antigen-specific T cells apoptose as the amount of antigen declines [33].
Taken together, the results of this study may indicate a role of cellular immunity in the pathogenesis of anti-Hu syndrome. T cell reactivity to Hu-D peptides merits to be further explored to ascertain the nature of the effector cells and to elucidate the mechanisms underlying neuronal injury by cytotoxic T cells.
Acknowledgments
This work was supported by AP-HP and INSERM; and RO1CANS89054 (JD).
References
- 1.Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349:1543–1554. doi: 10.1056/NEJMra023009. [DOI] [PubMed] [Google Scholar]
- 2.Altermatt HJ, Rodriguez M, Scheithauer BW, Lennon VA. Paraneoplastic anti-Purkinje and type 1 anti-neuronal nuclear auto-antibodies bind selectively to central, peripheral, and autonomic nervous system cells. Lab Invest. 1991;65:412–420. [PubMed] [Google Scholar]
- 3.Manley GT, Sillevis Smitt P, Dalmau J, Posner JB. Hu antigens: reactivity with Hu antibodies, tumor expression, and major immunogenic sites. Ann Neurol. 1995;38:102–110. doi: 10.1002/ana.410380117. [DOI] [PubMed] [Google Scholar]
- 4.Carpentier AF, Voltz R, DesChamps T, Posner JB, Dalmau J, Rosenfeld MR. Absence of HuD gene mutations in paraneoplastic small cell lung cancer tissue. Neurology. 1998;50:1919. doi: 10.1212/wnl.50.6.1919. [DOI] [PubMed] [Google Scholar]
- 5.Greenlee JE, Parks NT, Jaeckle KA. Type 2a (‘anti-Hu’) antineuronal antibodies produce destruction of rat cerebellar granule neurons in vitro. Neurology. 1993;43:2049–2054. doi: 10.1212/wnl.43.10.2049. [DOI] [PubMed] [Google Scholar]
- 6.Schäfer KH, Klotz M, Mergner M, Mestres P, Schimrigk K, Blaes F. IgG-mediated cytotoxicity to myenteric plexus cultures in patients with paraneoplastic neurological syndromes. J Autoimmun. 2000;15:479–484. doi: 10.1006/jaut.2000.0454. [DOI] [PubMed] [Google Scholar]
- 7.Sillevis Smitt PAE, Manley GT, Posner JB. Immunization with the paraneoplastic encephalomyelitis antigen HuD does not cause neurologic disease in mice. Neurology. 1995;45:1873–1878. doi: 10.1212/wnl.45.10.1873. [DOI] [PubMed] [Google Scholar]
- 8.Carpentier AF, Rosenfeld MR, Delattre JY, Whalen RG, Posner JB, Dalmau J. DNA vaccination with HuD inhibits growth of a neuroblastoma in mice. Clin Cancer Res. 1998;4:2819–2824. [PubMed] [Google Scholar]
- 9.Graus F, Ribalta T, Campo E, Monforte R, Urbano A, Rozman C. Immunohistochemical analysis of the immune reaction in the nervous system in paraneoplastic encephalomyelitis. Neurology. 1990;40:219–222. doi: 10.1212/wnl.40.2.219. [DOI] [PubMed] [Google Scholar]
- 10.Voltz R, Dalmau J, Posner JB, Rosenfeld MR. T-cell receptor analysis in anti-Hu associated paraneoplastic encephalomyelitis. Neurology. 1998;51:1146–1150. doi: 10.1212/wnl.51.4.1146. [DOI] [PubMed] [Google Scholar]
- 11.Wanschitz J, Hainfellner JA, Kristoferitsch W, Drlicek M, Budka H. Ganglionitis in paraneoplastic subacute sensory neuropathy: a morphologic study. Neurology. 1997;49:1156–1159. doi: 10.1212/wnl.49.4.1156. [DOI] [PubMed] [Google Scholar]
- 12.Plonquet A, Gherardi RK, Créange A, Antoine JC, Benyahia B, Grisold W, Drlicek M, Dreyfus P, Honnorat J, Khouatra C, Rouard H, Authier FJ, Farcet JP, Delattre JY, Delfau-Larue MH. Oligoclonal T-cells in blood and target tissues of patients with anti-Hu syndrome. J Neuroimmunol. 2002;122:100–105. doi: 10.1016/s0165-5728(01)00452-0. [DOI] [PubMed] [Google Scholar]
- 13.Benyahia B, Liblau R, Merle-Beral H, Tourani JM, Dalmau J, Delattre JY. Cell-mediated autoimmunity in paraneoplastic neurologic syndromes with anti-Hu antibodies. Ann Neurol. 1999;45:162–167. doi: 10.1002/1531-8249(199902)45:2<162::aid-ana5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka K, Tanaka M, Inuzuka T, Nakano R, Tsuji S. Cytotoxic T lymphocyte-mediated cell death in paraneoplastic sensory neuronopathy with anti-Hu antibody. J Neurol Sci. 1999;163(2):159–162. doi: 10.1016/s0022-510x(99)00029-5. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka M, Maruyama Y, Sugie M, Motizuki H, Kamakura K, Tanaka K. Cytotoxic T cell activity against peptides of Hu protein in anti-Hu syndrome. J Neurol Sci. 2002;201:9–12. doi: 10.1016/s0022-510x(02)00157-0. [DOI] [PubMed] [Google Scholar]
- 16.Plonquet A, Garcia-Pons F, Fernandez E, Philippe C, Marquet J, Rouard H, Delfau-Larue MH, Kosmatopoulos K, Lemonnier F, Farcet JP, Gherardi RK, Langlade-Demoyen P. Peptides derived from the onconeural HuD protein can elicit cytotoxic responses in HHD mouse and human. J Neuroimmunol. 2003;142:93–100. doi: 10.1016/s0165-5728(03)00269-8. [DOI] [PubMed] [Google Scholar]
- 17.Terasaki PI, McClelland JD. Microdroplet assay of human serum cytotoxins. Nature. 1964;204:998–1000. doi: 10.1038/204998b0. [DOI] [PubMed] [Google Scholar]
- 18.Choppin J, Martinon F, Gomard E, Barhaoui E, Connan F, Bouillot M, Lévy JP. Analysis of physical interaction between peptides and HLA molecules and application to the detection of HIV-1 antigenic peptides. J Exp Med. 1990;172:889–899. doi: 10.1084/jem.172.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connan F, Hlavac F, Hoebeke J, Guillet JG, Choppin J. A simple assay for detection of peptides promoting the assembly of HLA class I molecules. Eur J Immunol. 1994;24:777–780. doi: 10.1002/eji.1830240344. [DOI] [PubMed] [Google Scholar]
- 20.Subklewe M, Chahroudi A, Bickham K, Larsson M, Kurilla MG, Bhardwaj N, Steinman RM. Presentation of Epstein-Barr virus latency antigens to CD8+, interferon-γ-secreting, T lymphocytes. Eur J Immunol. 1999;29:3995–4001. doi: 10.1002/(SICI)1521-4141(199912)29:12<3995::AID-IMMU3995>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 21.Choppin J, Cohen W, Bianco A, Briand JP, Connan F, Dalod M, Guillet JG. Characteristics of HIV-1 Nef regions containing multiple CD8+ T cell epitopes: wealth of HLA-binding motifs and sensitivity to proteasome degradation. J Immunol. 2001;166:6164–6169. doi: 10.4049/jimmunol.166.10.6164. [DOI] [PubMed] [Google Scholar]
- 22.Lau LL, Jamieson BD, Somasundaram T, Ahmed R. Cytotoxic T-cell memory without antigen. Nature. 1994;369:648–652. doi: 10.1038/369648a0. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 24.Dalmau J, Graus F, Cheung NKV, Rosenblum MK, Ho A, Canete A, Delattre JY, Thompson SJ, Posner JB. Major histocompatibility proteins, anti-Hu antibodies, and paraneoplastic encephalomyelitis in neuroblastoma and small cell lung cancer. Cancer. 1995;75(1):99–109. doi: 10.1002/1097-0142(19950101)75:1<99::aid-cncr2820750117>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 25.Scheibenbogen S, Lee KH, Stevanovic S, Witzens M, Willhauck M, Waldmann V, Naeher H, Rammensee HG, Keilholz U. Analysis of the T cell response to tumor and viral peptide antigens by an IFN-γ-ELISPOT assay. Int J Cancer. 1997;71:932–936. doi: 10.1002/(sici)1097-0215(19970611)71:6<932::aid-ijc3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 26.Herr W, Schneider J, Lohse AW, Meyer zum Buschenfelde KH, Wolfel T. Detection and quantification of blood-derived CD8+ T lymphocytes secreting tumor necrosis factor alpha in response to HLA-A2.1-binding melanoma and viral peptide antigens. J Immunol Meth. 1996;191(2):131–142. doi: 10.1016/0022-1759(96)00007-5. [DOI] [PubMed] [Google Scholar]
- 27.Marincola FM, Rivoltini L, Salgaller ML, Player M, Rosenberg SA. Differential anti-MART-1/Melan-A CTL activity in peripheral blood of HLA-A2 melanoma patients in comparison to healthy donors: evidence of in vivo priming by tumor cells. J Immunother Emphasis Tumor Immunol. 1996;19(4):266–277. doi: 10.1097/00002371-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Loftus D, Castelli C, Clay TM, Squarcina P, Marincola FM, Nishimura MI, Parmiani G, Appella E, Rivoltini L. Identification of epitope mimics recognized by CTL reactive to the melanoma/melanocyte-derived peptide MART-1(27–35) J Exp Med. 1996;184(2):647–657. doi: 10.1084/jem.184.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bach JF, Koutoulov S, Van Endert PM. Are there unique autoantigens triggering autoimmune diseases ? Immunol Rev. 1998;164:139–155. doi: 10.1111/j.1600-065x.1998.tb01216.x. [DOI] [PubMed] [Google Scholar]
- 30.Rammensee HG, Friede T, Stevanovic S. MHC ligands and peptide motifs: first listing. Immunogenetics. 1995;50:201–212. [Google Scholar]
- 31.Scheibenbogen C, Lee KH, Mayer S, Stevanovic S, Moebius U, Herr W, Rammensee HG, Keilholz U. A sensitive ELISPOT assay for detection of CD8+ T lymphocytes specific for HLA class I-binding peptide epitopes derived from influenza proteins in the blood of healthy donors and melanoma patients. Clin Cancer Res. 1997;3:221–226. [PubMed] [Google Scholar]
- 32.Henson RA, Urich H. The Neurological Manifestations of Systemic Malignant Disease. Blackwell Scientific Publications; New York: 1982. Cancer and the Nervous System. [Google Scholar]
- 33.Lalvani A, Brookes R, Hambleton S, Britton WJ, Hill AVS, McMichael AJ. Rapid effector function in CD8+memory T cells. J Exp Med. 1997;186(6):859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]