Abstract
Many promising MRI approaches for research or clinical management of multiple sclerosis (MS) have recently emerged, or are under development or refinement. Advanced MRI methods need to be assessed to determine whether they allow earlier diagnosis or better identification of phenotypes. Improved post-processing should allow more efficient and complete extraction of information from images. Magnetic resonance spectroscopy should improve in sensitivity and specificity with higher field strengths and should enable the detection of a wider array of metabolites. Diffusion imaging is moving closer to the goal of defining structural connectivity and, thereby, determining the functional significance of lesions at specific locations. Cell-specific imaging now seems feasible with new magnetic resonance contrast agents. The imaging of myelin water fraction brings the hope of providing a specific measure of myelin content. Ultra-high-field MRI increases sensitivity, but also presents new technical challenges. Here, we review these recent developments in MRI for MS, and also look forward to refinements in spinal-cord imaging, optic-nerve imaging, perfusion MRI, and functional MRI. Advances in MRI should improve our ability to diagnose, monitor, and understand the pathophysiology of MS.
Introduction
MRI is playing an increasing role in the scientific investigation and clinical management of multiple sclerosis (MS). However, several limitations have emerged, such as the low sensitivity of conventional MRI to grey-matter involvement and to diffuse damage in white matter. In addition, conventional MRI shows only limited associations with clinical status. As new uses for conventional MRI are developed and non-conventional MRI methods continue to advance, we are gaining insight into the full extent of tissue damage in patients with MS. However, there is a need to refine the techniques and clinically validate the available tools so that they can be properly applied. Our aim is to review the most promising MRI approaches in MS that have recently emerged or are currently under development. We will explain how these new techniques will fill voids in our current understanding of MS and improve our ability to diagnose, monitor, and define the pathophysiology of the disease. The topics will include diagnosis and classification of patients, new uses of conventionally obtained MRI data, proton magnetic resonance spectroscopy (1H-MRS), magnetisation transfer imaging, diffusion imaging, functional MRI, optic-nerve imaging, spinal-cord imaging, myelin water fraction (MWF) imaging, perfusion MRI, and MRI at field strengths higher than 1·5 T. The sections begin with a brief summary of the current status, followed by a discussion of unmet needs and the new techniques or approaches to address those needs.
Current status and the near future
Diagnosis and classification
The past few years have seen an unprecedented number of publications that have addressed diagnostic criteria in MS, mainly as a consequence of the increased use of MRI, and the acceptance that MRI can provide evidence for dissemination in both space and time,1 in addition to its established role in the exclusion of conditions that are clinically similar to MS.2 With the advent of MRI, researchers have cautiously ensured that diagnostic criteria have a high specificity, often at the expense of sensitivity.3 The key change in recent years has been towards simplifying diagnostic criteria so that they become easier to use with increased levels of sensitivity while maintaining their specificity.4,5 Thus, the 2005 modified McDonald criteria1 have a simpler approach to dissemination in time, and more recently, a further simplification has been proposed in terms of dissemination in time and space.6,7 These more recent criteria have been found to be slightly more sensitive than the original 2001 McDonald criteria3 and the 2005 revised criteria,1 while maintaining high specificity.6,7 Thus, these criteria might allow a reliable diagnosis of MS to be made during the year after onset of a typical clinically isolated syndrome suggestive of MS. The main advantage of the newest criteria is that they do not require the use of contrast agents, thus saving both time and expense.6 The disadvantage is the slight loss of differential diagnostic information, and consequently they should be used with caution in older patients.
The challenges to be addressed include the provision of better evidence for determining the precise role of lesions seen on conventional MRI of the spinal cord and assessment of the value of these diagnostic criteria in prospective studies in non-specialist centres. Further contributions from MRI might come from the ability to measure the degree of tissue damage, including diffuse changes in the normal-appearing white matter, and the potential for the greater sensitivity of higher field systems to more subtle abnormalities.8 We also need to know whether advanced MRI methods will provide a better assessment of the risk of conversion from a clinically isolated syndrome to MS than can be obtained with conventional imaging approaches.
In patients with established MS, classification made on the basis of the magnetic resonance patterns of CNS involvement is difficult, and the differentiation between a clinically isolated syndrome, relapsing-remitting MS (RRMS), and secondary progressive MS is seen as little more than a gradual increase in lesion load, reduction in brain volume, and an increase in diffuse change in normal-appearing brain tissue. There is general agreement that patients with primary progressive MS have fewer lesions in the cerebrum and perhaps less enhancement in the CNS,9 although this is a relative rather than an absolute difference. As magnetic resonance techniques become more pathologically specific, there could be the potential to explore the pathological classification proposed by Lassmann and colleagues.10 Although MRI techniques are unlikely to advance in the foreseeable future to allow accurate and direct biological classification of MS, MRI provides various approaches to phenotype patients with MS for correlation with these biological classifications.
New uses of conventional MRI data
Lesion-based measures
Conventional MRI assessment of lesions on non-contrast T1-weighted and T2-weighted images, and on gadolinium-enhanced T1-weighted images, provides an important tool to monitor the disease course.11 However, the limitations of conventional MRI include the weak associations with clinical status and the lack of sensitivity to other clinically relevant findings, such as grey-matter disease and diffuse damage throughout the white matter.12,13
New approaches have emerged in the areas of data management and post-processing. One approach involves the serial analysis of images to study dynamic pixel-wise signal changes related to lesion evolution.14 Through this approach, changes in the progression pattern within individual lesions might indicate an overarching shift of the patient's disease from more inflammatory to more degenerative pathological processes, possibly heralding the advent of atrophy and clinical disability.14 Another related approach, known as subtraction imaging, displays changes over time between two scans in a single map.15 This provides increased sensitivity to lesion evolution compared with qualitative analysis (figure 1). Finally, lesion-based measures can be combined with advanced MRI measures of tissue integrity, such as 1H-MRS, diffusion imaging, and magnetisation transfer imaging, using voxel-wise probability maps and spatial distribution approaches. These advanced MRI techniques are discussed in more detail below.
Figure 1. Lesion change in MS over time by use of a subtraction method involving image normalisation, inhomogeneity correction, and co-registration.
A new juxtacortical lesion (arrow) in a 44-year-old woman with relapsing-remitting MS who was scanned at baseline and after 3 years. The juxtacortical lesion is difficult to appreciate on the native spin-echo proton-density images, comparing the baseline (A) to follow-up scan (B), but is clearly visible on the subtraction image (C). In all images, the skull has been removed. Subtle artefacts are seen on the outer edge of the brain surface due to slight misregistration. Adapted with permission from the American Society of Neuroradiology.
Atrophy-based measures
Over the past few years, we have witnessed a rapidly growing interest in the measurement of CNS atrophy in MS. Developments have been fuelled by the use of MRI techniques to determine the topography and rate of atrophy, with the possibility of visualising this process with sub-voxel accuracy (figure 2).16 By use of MRI-based approaches, cerebral volume changes can be measured within a relatively short period of time, which correlate well with cognitive impairment.17 An intriguing development is the exploration of associations between regional patterns of atrophy and specific functional impairment using voxel-based morphometry.18,19 To move beyond simple volumes of damaged tissue, we need to understand, for instance, the relations between regional atrophy and white-matter tract damage, and their clinical impact. If detailed mapping of the white-matter architecture is possible from diffusion tensor MRI,20 then it should be feasible to integrate quantitative measures of tissue damage along tracts of known functional significance to form a more clinically relevant assessment of the burden of disease.
Figure 2. Gain or loss in brain volume, as determined from serial MRI scans.

Using registration-based software, brain volume gain (red) or loss (blue) can be determined with sub-voxel accuracy from serial MRI scans. Currently, it is difficult to predict why some patients (left) have little atrophy (0·29% brain volume loss per year), whereas others (right) have a high atrophy rate (2·2% brain volume loss per year).
The topographic distribution of brain volume loss has emerged as a key challenge to be addressed. Tissue loss seems to affect grey matter more than white matter in patients with MS; furthermore, among grey-matter structures, the basal ganglia and thalamus are most susceptible to atrophy.16 The best methods for the segmentation and characterisation of grey-matter atrophy remain to be determined. One of the pitfalls is the misclassification of white-matter lesions as grey matter, which requires manual correction. Another variable is the range of methods available to determine regional grey-matter atrophy, such as fully automated (eg, voxel-based morphometry)18,19 and semi-automated (atlas-based) segmentation.21,22
Clinical trials are now incorporating cerebral volume measurement to determine the efficacy of experimental treatments.16,23 However, although cerebral volume changes are thought to indicate cerebral atrophy, confounding factors include the effects of osmotic agents (eg, alcohol) and modifiers of the natural ageing process, such as APOE status.16 Recent treatment trials have shown a decrease in cerebral volume after the initiation of immunomodulatory treatment with corticosteroids, natalizumab, interferon beta, or immunoablation followed by stem-cell transplantation.16,23-25 Such short-term changes might be driven in part by osmotic and anti-inflammatory effects, referred to as pseudo-atrophy. More work is required to help separate true atrophy from pseudo-atrophy, perhaps with the use of advanced magnetic resonance sequences that can distinguish axonal loss from transient changes in water content.
A more fundamental question is, “what drives cerebral atrophy?”.26 The number and volume of focal lesions visible on T2-weighted scans bear some relation to the degree of atrophy, but more importantly, cerebral volume loss seems to be driven by changes occurring in normal-appearing white matter and grey matter.27,28 More work is needed to understand the interrelation between demyelination, neuronal or axonal loss, neurodegeneration, and cerebral volume (changes) before we can reliably use this MRI measure to make management or treatment decisions.
New contrast agents
On conventional MRI scans, the enhancement of lesions by gadolinium injection indicates the accumulation of the contrast agent in the interstitial space due to increased blood–brain barrier permeability. Currently, there is a major effort to find biological markers of MS, especially cell subsets and molecules that are important to the pathophysiology of MS. New MRI contrast agents composed of iron particles, ultra-small particles of iron oxide, or super-paramagnetic iron particles of oxide have been used in patients with MS to track macrophages (figure 3).29,30 Two MRI studies of patients with RRMS that used ultra-small particles of iron oxide and gadolinium have confirmed a mismatch of enhancement, indicating heterogeneity of the underlying pathology.29,30 The complementary information provided by tracking macrophages with iron particles might play a unique part in the monitoring of the efficacy of drugs targeting the cellular components of inflammation.
Figure 3. Mismatch between gadolinium and ultra-small particles of iron oxide (USPIO) contrast agents in an acute lesion in a patient with MS.

The lesion is hyperintense on the spin-echo T2-weighted image (A), but does not enhance with gadolinium on the T1-weighted image (B). On the post-USPIO T2-weighted image (C), the USPIO enhancement leads to a decrease in signal intensity (T2 shortening) due to iron. However, the lesion is enhanced after administration of USPIO on the T1-weighted image (D). Reproduced with permission from the American Society of Neuroradiology.
Other makers of inflammation or neuronal dysfunction might be targeted by new contrast agents.31 Myeloperoxidase activity in inflamed tissues can be detected and labelled in vivo by a myeloperoxidase-sensitive “smart” molecular imaging probe.32 Another novel gadolinium-based MRI contrast agent, Gadofluorine M, selectively accumulates in nerve fibres undergoing Wallerian degeneration and appears bright on T1-weighted images.33 These new contrast agents need proper validation and safety assessment before their role in disease monitoring can be determined.
Non-conventional MRI
1H-MRS
1H-MRS can be used to measure metabolites such as N-acetyl-aspartate. A decrease in N-acetyl-aspartate is associated with axonal/neuronal damage or dysfunction. Choline-containing compounds are often increased during myelin breakdown, remyelination, and inflammation. Creatine concentration increases with cell density. Mainly found in glial cells, increased myoinositol is suggestive of glial proliferation and astrogliosis. Amino acids acting as neurotransmitters, such as glutamate, glutamine, and GABA (γ-aminobutyric acid), can also be measured.
In active enhancing MS lesions, increases in creatine, choline, myoinositol, and glutamate are seen, whereas N-acetyl-aspartate might be low or only slightly decreased. In normal-appearing white matter, a similar pattern of abnormal metabolites can be detected. In typical chronic non-enhancing lesions, N-acetyl-aspartate is greatly reduced, myoinositol is increased, and glutamate concentrations are normal.34,35
Current opportunities for advances in 1H-MRS include the following: (1) short echo-time spectroscopy, which should allow the detection of more metabolites; (2) field strengths higher than 1·5 T to increase the resolution and signal-to-noise ratio; (3) improvement in absolute metabolite quantification methods to avoid the confounding effects of ratio analysis; and (4) standardisation of acquisition, calibration, reconstruction, and quantification methods across different scanner types for multicentre clinical trials.36
Additional metabolites relevant to MS are under active investigation, such as glutathione, GABA, ascorbic acid (vitamin C), as well as the macromolecular (background) signal. Such macromolecules, which can be better appreciated with the use of inversion recovery sequences,37 include valine, alanine, leucine, isoleucine, and threonine, which together account for up to 60% of myelin content. The quantification of such a broad neurochemical profile in vivo by use of a single method should provide insights into the roles of neurodegeneration, tissue repair, antioxidant therapy, and oxidative stress in MS. Hyperpolarised 13C-MRS, which can enhance the magnetic resonance signal by up to 100 000 times, is also under development for studying metabolism.38
Magnetisation transfer MRI
Magnetisation transfer MRI measures the interactions between protons in free fluids and protons bound to macromolecules by use of the magnetisation transfer ratio (MTR), whereby a low MTR is an indicator of damage to myelin and axonal membranes.39 Post-mortem studies have shown unambiguously that MTR is strongly associated with the percentage of residual axons and the degree of demyelination in T2-visible lesions and normal-appearing brain tissues of patients with MS.40,41 Various degrees of MTR reduction have been shown in acute and chronic MS lesions.42 These changes are more pronounced in lesions that appear as hypointense on T1-weighted images, and can precede T2-visible lesion formation.42 Decreased MTR has also been detected in the normal-appearing white matter and grey matter of patients with MS,43,44 and these abnormalities are more pronounced in patients with the progressive forms of MS and tend to worsen over time.42
Although significant efforts have been made to standardise magnetisation transfer data acquisition across different scanners,45 magnetisation transfer MRI has been used in only a few trials and in selected groups of patients.42 Preliminary studies have shown that magnetisation transfer MRI has prognostic value for subsequent disease evolution,42 and thus shows promise as an adjunctive paraclinical tool in large-scale longitudinal studies.
Several voxel-based approaches have been developed that allow the anatomical location of MTR decreases to be assessed. More recently, these approaches have also been applied to the tracking of demyelination and remyelination in individual MS lesions,46 and now need to be applied in cross-sectional and longitudinal studies in patients with heterogeneous clinical characteristics to improve our understanding of the changes that underlie the accumulation of irreversible disability. Atlas-based approaches have also been used in longitudinal studies to monitor the evolution of MTR changes within T2-visible lesions.47 This should allow lesions to be classified chronologically by generating maps of new, stable, and resolved lesions, thus potentially improving correlations with clinical manifestations of the disease and allowing the monitoring of the effect of treatment on myelin repair and neuroprotection. To overcome some of the limitations of simple MTR measurements, which are pulse sequence and hardware dependent, a more complete characterisation of the magnetisation transfer phenomenon has been proposed, which can be done by acquiring a larger dataset and extracting data related to the magnetic resonance properties of the protons and their local chemical environment. The method has been termed “quantitative magnetisation transfer imaging”,48,49 and has been applied in a few preliminary studies in patients with MS.49-51
Diffusion MRI
Diffusion weighting sensitises MRI scans to the microscopic Brownian motion of water molecules. This motion is hindered by cellular structures, such as cell membranes and axonal cytoskeletons. Abnormalities in diffusivity patterns have been seen in both focal MS lesions and in normal-appearing white matter. By applying diffusion-weighting magnetic field gradients in many directions, one can infer the orientation of the axons, and reconstruct the pathways of the major white-matter bundles by diffusion tensor MRI and so-called fibre tracking.52,53
Tracking through MS lesions is difficult because of tissue disruption (figure 4), but atlas-based approaches could overcome this problem.54 Grey-matter-to-grey-matter connections have direct functional significance, and white matter provides the wiring. However, we are still some way from determining connectivity,55 a term that is sometimes loosely used to mean the degree of confidence with which two regions of grey matter are said to be linked. Following the tracts in the subcortical region is difficult because of the complex connections; significant improvements in image resolution that are realised at much higher field strengths should help, particularly in resolving subcortical connections, an area that has been neglected in MRI assessments of MS. There is also scope for integrating measures of connectivity with functional MRI (fMRI) and magnetoencephalography data to better understand how different pathological changes within MS lesions can affect nerve transmission rates, functional reserve, and brain plasticity.56 Examining connectivity could also lead to a new type of image segmentation based on functional significance rather than on tissue type and signal intensity, thus improving our understanding of the pathology and reorganisation that underpin clinical deficits.
Figure 4. Composite image showing information from several sequential MRI scans of a patient with MS.

The transparent brain surface shows the location of the lesions (red) determined from a T2-weighted image. Diffusion tensor fibre tracking was initiated in the right internal capsule, and the presence of the lesions has caused the tracts to deviate from the motor tract across the corpus callosum. Different approaches to tractography might allow tracking even in areas of severe axonal damage.
Several groups are working on the direct MRI detection of neuronal activation, either by diffusion-weighted imaging, or by the effect that neuronal currents have on the local externally applied magnetic field (B0) or on the force on the neuron.57,58 This research could provide fascinating insights into neuronal health, the time course of inflammation, demyelination and remyelination, and the impact that these have on cognitive and motor function.
fMRI
fMRI depends on the blood-oxygenation-level-dependent (BOLD) contrast mechanism, which is secondary to differences in deoxyhaemoglobin concentration in the blood in activated areas as a consequence of variations in neuronal activity.59 fMRI investigations of the visual, cognitive, and motor networks in patients with MS have shown an altered recruitment of regions normally devoted to the performance of a given task and/or the recruitment of additional areas in comparison to healthy individuals.60 The correlations found between measures of abnormal activation and MRI measures of structural damage suggest that brain plasticity might help to limit the clinical consequences of widespread tissue damage. These functional cortical changes vary across patients at different stages of the disease, after an acute relapse, and in clinically stable patients.60,61 An abnormal pattern of brain activation has also been related to fatigue.60
Several studies have attempted to develop sophisticated statistical approaches to establish strength of activation and synchrony between specific brain areas by analysis of functional and effective connectivity.62 The optimisation of analysis methods, as well as the comparison of models of activation between patients with MS and controls, might help to explain abnormalities of function of specific brain networks and their relation to clinical symptoms. The combination of measures of functional connectivity with measures of structural damage within specific white-matter fibre bundles is likely to improve our understanding of the relation between structural and functional abnormalities, as suggested by two studies in patients with RRMS and benign MS (figure 5).63,64 The role of longitudinal and multi-site fMRI studies in MS has yet to be fully explored. Previous findings support the use of fMRI in large-scale longitudinal trials to monitor the effect of motor and cognitive rehabilitation or pharmacological therapies on the enhancement of any beneficial effect of cortical adaptive plasticity.60,65 Other aspects that should be considered are the development of fMRI paradigms unbiased by differences in task performance between patients with MS and controls, which could make the assessment of more disabled patients feasible, and the development of acquisition protocols specifically tailored to the imaging of function and structure of relatively small regions, now that high-field scanners are increasingly available to provide improved spatial resolution.
Figure 5. Areas of increased activation in patients with benign MS compared with healthy controls during the analysis of the Stroop interference condition.
(A,B) Patients with benign MS had increased activity in several areas located in the frontal and parietal lobes, bilaterally, including the anterior cingulate cortex, superior frontal sulcus, inferior frontal gyrus, precuneus, secondary sensorimotor cortex, visual cortex, and cerebellum. (C) The analysis of functional connectivity, by use of dynamic causal modelling, showed different connectivity strengths between patients with benign MS and controls: within-group connections that were significant with a one-sample t-test are shown as black arrows in healthy controls and as dashed arrows in patients with MS. The arrows and p values resulting from the between-group t-test comparisons are shown in red in cases of increased strength of connection in patients versus controls, and in blue in cases of reduced strength of connection in patients versus controls (two p values are shown for all bi-directional associations). Reproduced with permission from John Wiley and Sons.64
Beyond brain imaging
Optic-nerve imaging
Imaging of the optic nerves is challenging because they are so small and prone to motion artefacts. Structural differentiation from surrounding tissues and magnetic susceptibility effects are problematic. However, optic neuritis is an excellent model with which to study the pathophysiology of damage and repair in MS.
Optic-nerve cross-sectional area and lesion length can be accurately quantified, allowing clinical and electrophysiological correlations, and have linked acute inflammation to conduction block in optic neuritis.66 More recently, dynamic MTR changes have indicated myelin damage and repair,67 and diffusion tensor MRI has shown reduced structural integrity of the nerves68 due to axonal degeneration and demyelination. Finally, the new technique of optical coherence tomography shows promise as a non-invasive surrogate marker of axonal loss.69
Important questions remain to be addressed about the pathophysiology of optic neuritis and the role of imaging in longitudinal monitoring. The relative contributions of inflammation, oedema, gliosis, and myelin and axonal changes to the processes of damage and repair in optic neuritis are incompletely understood. 1H-MRS has the potential to show the time course of inflammatory changes, gliosis, and axonal loss. Diffusion tensor imaging and magnetisation transfer imaging have the potential to show axonal integrity and myelin content of the optic nerve, but there are still technical barriers to be overcome. In addition, correlations between MRI and histology are needed to identify how directly each MRI measure relates to a specific tissue change.
Spinal-cord imaging
In patients with clinically isolated syndrome, the value of conventional MRI of the spinal cord in showing lesion dissemination in space and its contribution to excluding other conditions that can mimic MS have been formally recognised in internationally accepted diagnostic criteria.1 Conventional MRI sequences sensitive to MS-related damage have been developed and applied in patients with different disease phenotypes.70 However, technical developments have mainly been focused on imaging of the cervical portion of the cord, and more effort should be devoted to improving MRI of the entire cord.
The development of sophisticated magnetic resonance receiver coils and fast imaging techniques has led to more reliable imaging of the spinal cord, including the use of quantitative techniques. Reduced MTR and abnormal diffusion tensor MRI metrics have been shown in the cervical cord of patients with MS, and the abnormalities are more pronounced in patients with progressive disease phenotypes.71 However, the prognostic value of magnetisation transfer and diffusion tensor MRI measures in longitudinal studies with long follow-up periods remains to be established. Recent developments, including quantitative magnetisation transfer and diffusion tractography, as well as other methods that are widely used for imaging brain damage in these patients, such as 1H-MRS and fMRI, have been applied in preliminary studies of the cervical cord in small groups of patients.72-74 Reduced N-acetyl-aspartate, lower structural connectivity, and lower diffusion anisotropy in the spinal cords of patients with a cervical cord relapse have been shown when compared with controls. These measures were found to correlate with disability.74 This suggests that the assessment of regional damage in the cervical cord can be used to clarify which factors are associated with the development of disability in these patients, as has been shown by a recent magnetisation transfer MRI study of damage to the cord grey matter, which reported a correlation between cervical cord grey-matter MTR and the degree of disability in patients with RRMS.75
The more distant future
Myelin imaging
MWF, derived from precise measurements of transverse relaxation time, shows specificity for myelin content and integrity. Studies have demonstrated multi-component T2 relaxation in biological tissue and have shown it to be due to compartmentation.76 The water signal can be separated into three components: (1) a long T2 component (>1·5 s) due to CSF; (2) an intermediate component (about 100 ms) arising from intracellular and extracellular water; and (3) a short T2 component (20–50 ms) due to water enclosed between the myelin bilayers.77 The sum of the three T2 components is the total MRI-visible water content. The ratio of the myelin water (short T2) to the total signal gives the MWF. Moore and colleagues78 showed that MWF relates closely to the distribution of brain myelin and noticed the diminution of the short T2 component in chronic MS plaques in a formalin-fixed MS brain.
Brain studies in vivo have shown that a large MWF is seen in white matter, whereas a very small fraction can be detected in grey matter. A 30–50% decrease in MWF is seen in MS lesions and a 7–15% decrease is seen in normal-appearing white matter in MS.79,80 MWF imaging in supratentorial normal-appearing white matter regions was sensitive enough to detect changes in patients within 5 years of disease onset. The reduction of the MWF in normal-appearing white matter in MS is dominated by the loss of myelin integrity rather than increased oedema or inflammation.80
However, MWF imaging remains a challenging technique. T2 decay has traditionally been measured using a multi-echo readout, such as a single-slice 32-echo-spin-echo sequence. Long scan times and limited brain coverage make this approach less practical clinically. Recently, two-dimensional multi-slice non-linearly spaced 12-echo datasets provided MWF estimates in white and grey matter of a quality similar to those from 32-echo datasets.81 Use of phased array coils gives more uniform and smoother MWF maps than those from standard head coils, especially at 3 T. The development of three-dimensional volumetric acquisition is actively being researched, which would allow whole brain coverage. Despite the longer acquisition time, additional longer echo times (over 1 s) with an echo train of up to 48 echoes were shown to be useful for characterising MS lesions and normal-appearing white matter while assessing the pool of free intracellular and extracellular water protons.82 Finally, multi-component T2 also has the potential to assess myelin integrity in the spinal cord.
Perfusion MRI
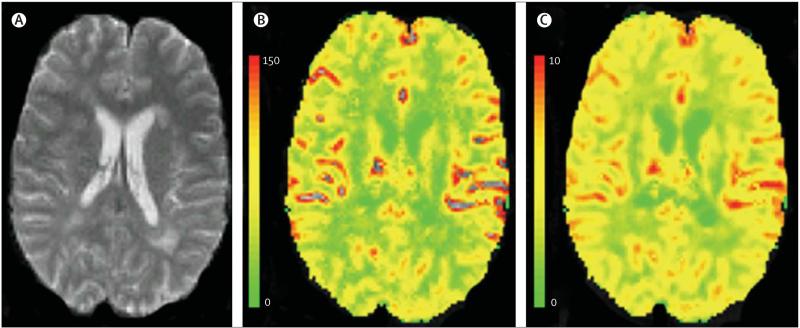
By use of MRI, assessment of brain tissue perfusion in vivo is now possible. Acute MS lesions are characterised by increased perfusion, whereas normal-appearing white and grey matter are characterised by reduced perfusion.83-85 Hypoperfusion in the brain can indicate various underlying processes, such as hypometabolism, ischaemia, tissue injury, or tissue destruction. Perfusion MRI techniques use either exogenous tracers, such as gadolinium chelates (bolus tracking), or endogenous arterial water (arterial spin labelling). These techniques have the following advantages over PET and single-photon emission CT, which were initially used to assess brain perfusion: higher signal-to-noise ratio and contrast-to-noise ratio, better spatial and anatomical resolution, shorter acquisition times, and the avoidance of radioactive material.84 In addition, the sensitivity and resolution of both gadolinium bolus-tracking MRI (figure 6) and arterial spin labelling methods are boosted by using multi-channel receiver coils and high field strength,85,86 thus allowing white matter and smaller regions, such as lesions, to be analysed.
Figure 6. Axial gradient-echo echo-planar MRI showing cerebral blood flow and volume in a patient with MS.
(A) Axial gradient-echo echo-planar MRI, (B) colour-coded cerebral blood flow map, and (C) colour-coded cerebral blood volume map from a patient with MS. The colour bars indicate the cerebral blood flow (mL/100 g/min) and the cerebral blood volume (mL/100 g).
Both bolus-tracking and arterial spin labelling need further development to improve quantification of cerebral blood flow and volume. The reliability and reproducibility of absolute measures of brain perfusion in general warrant further investigation. In addition, the sensitivity and clinical relevance of perfusion MRI scans in detecting longitudinal, MS-related changes also need to be established before the technique can be used for monitoring MS evolution and treatment efficacy in multicentre clinical studies.
Field strengths higher than 1·5 T
High-field (typically 3·0 T) and ultra-high field (≥7·0 T) MRI scanners have the potential to revolutionise research in MS. The data available show that magnets operating at 3·0–4·0 T detect a greater number and volume of T2 hyperintense (figure 7) and gadolinium-enhancing brain lesions than those operating at 1·5 T.11,87 In addition, their use might improve the early diagnosis of MS.8 Both high-field and ultra-high field MRI have particular value for the detection of cortical MS lesions,88 and can improve 1H-MRS, magnetisation transfer MRI, diffusion tensor MRI, perfusion imaging, fMRI, and relaxometry studies in MS.11,35,79,85,86,89,90 Because of the increased magnetic field gradients caused by magnetic susceptibility, use of high and ultra-high field results in a new image contrast mechanism becoming apparent, which has been termed “phase susceptibility imaging”. The contrast is related to blood oxygenation, vascularity, and macrophage activity, and has already shown interesting heterogeneity in what would otherwise appear as diffuse MS lesions.91 Sodium imaging represents another appealing development at high field strength,92 which might provide important information for the understanding of disease pathophysiology. Visualisation of axonal health might also be possible by use of MRI methods to monitor the sodium concentration gradient that exists across the intracellular/extracellular space in healthy axons.92
Figure 7. Comparison of 1·5 T and 3·0 T MRI in two patients with MS.

(A) 1·5 T and (B) 3·0 T MRI scans from a 48-year-old woman with secondary progressive MS, and (C) 1·5 T and (D) 3·0 T MRI scans from a 21 year-old man with relapsing-remitting MS are shown. (A) 1·5 T axial fast fluid inversion recovery (FLAIR) and (C) coronal spoiled gradient images of the brain, and 3·0 T images (B, D) of the same regions with equivalent pulse sequences on each patient display the improved sensitivity in lesion-detecting capabilities (arrows) and tissue resolution (tissue-CSF and grey-white matter differentiation) of the 3·0 T scanner. Reproduced with permission from Elsevier.
Higher field strength MRI might be important for studying iron deposition in the grey matter of patients with MS. As recently reviewed,93 many grey-matter areas, including the thalamus, dentate nucleus, basal ganglia, and rolandic cortex, commonly show hypointensity on T2-weighted images in patients with MS. Iron deposition has been postulated to be a cause of this hypointensity, since it reduces T2 relaxation times and is found in pathological excess in the MS brain.93 Grey-matter T2 hypointensity is related to clinical impairment in patients with MS, which shows stronger correlation at 3 T than at 1·5 T.94,95 Whether iron deposition contributes to neurotoxicity in grey matter or is purely an epiphenomenon remains unclear. Other methods used at 3·0 T and higher field strengths, such as T2, T2*, T2′, or T2-rho relaxometry, and magnetic field correlation imaging, should boost the sensitivity and specificity in detecting iron-related neurodegeneration in grey matter, and increase our understanding of the role that iron has in the pathophysiology of MS.96
In the context of high-field imaging, it is worth remembering that magnetic resonance images have an arbitrary brightness scale and are prone to many artefacts. The challenge for MRI is in characterising and quantifying the pathological processes that occur in the degenerating brain. For this, we need more accurate and reliable image acquisition methods so that images can be converted to physically meaningful values, such as absolute T1 and T2.11,97 The advent of high-field scanners with array receiver coils makes this even more of an imperative, because of poorer image uniformity. Consensus and standardisation of acquisition methods between scanner manufacturers would be a substantial boost for quantitative imaging.
Several additional challenges remain for use of these new technologies.98 These include the high costs (installation and maintenance), large footprint of the hardware, challenges in gradient and radio frequency coil design, poorer field homogeneity, worsening susceptibility and chemical shift artefacts, and dielectric effects. The full range of pulse sequences available at 1·5 T might not run well at 3·0 T and above. Patient safety and comfort issues, such as radiofrequency energy deposition, compatibility with metallic implants, and sensory symptoms experienced during scanning, must be addressed. For example, the doubling of field strength from 1·5 T to 3·0 T quadruples the specific absorption rate, all other things being equal. This can lead to increases in scan time, the need to pause to allow cooling of the patient, and a reduction in the number of slices obtained per repetition time.
Conclusions
The extensive application of conventional and modern magnetic-resonance-based techniques to the study of MS has undoubtedly improved our ability to diagnose and monitor the disease, as well as our understanding of disease pathophysiology. Nevertheless, many challenges remain. New techniques need to be refined and validated before they can be properly integrated into clinical research and practice. New acquisition schemes and analysis procedures require standardisation and optimisation so that they can be used in multi-site settings, both in natural history studies and treatment trials. From the data available, it is evident that the combining of different magnetic resonance methods, which are sensitive to different aspects of MS pathology, is a promising way to increase further our understanding of the mechanisms underlying the accumulation of irreversible disability. Finally, the increasing availability of high field strength MRI (≥3·0 T) presents new technical challenges that will require extensive refinement during the next few years. One of the most important tasks for the future is to establish how these advances in MRI technology might contribute to a better correlation between clinical and MRI findings, and thus provide relevant information to improve prognosis and predict therapeutic response.
Search strategy and selection criteria.
References for this Review were identified by searches of PubMed from January, 1985, to April, 2008, by use of the terms “MRI” or “imaging” and “multiple sclerosis”. Articles resulting from that search and references cited in those articles were considered for this Review. The articles chosen focus on the latest and most promising advances in the field. Articles were also identified through searches of the authors' own files. Only papers published in English were reviewed.
Acknowledgments
RB acknowledges support of research grants from the US National Institutes of Health (NIH; 1R01NS055083-01) and National Multiple Sclerosis Society (RG3705A1; RG3798A2). MI acknowledges support of a research grant from the NIH (5R01NS051623-03). CRGG acknowledges support of research grants from the NIH (P41RR13218-01) and National Multiple Sclerosis Society (RG3574A1). The images in figure 2 were kindly provided by Bas Jasperse, VU University Medical Centre, Amsterdam. We are grateful to Sophie Tamm for assistance with manuscript preparation.
Footnotes
Conflicts of interest
RB has received honoraria for lectures and travel expenses, and consulting fees as an investigator in previous and current treatment trials from Biogen Idec, Genentech, Merck-Serono, Teva Neuroscience, and Pepgen. AJT serves on advisory boards for Novartis and Genentech, chairs Teva's data, safety, and monitoring committee for the GA for ALS trial, and has received honoraria for lecturing from Bayer-Schering and Merck-Serono. MAR has received personal compensation for speaking activities from Merck-Serono and Biogen-Dompè. DP has received personal compensation for speaking activities and consulting services from Biogen Idec, Teva Neuroscience, Synar Inc., and Genentech. VD has received honoraria from Biogen and Guerbet for lectures and travel expenses. FB has received personal compensation for consulting services from Merck-Serono, Bayer-Schering, Biogen Idec, Novartis, Aventis, Wyeth, and Teva. MI has received honoraria for lecturing from Teva Neuroscience. CRGG has received honoraria for lectures and travel expenses, and consulting fees as an investigator in previous and current treatment trials from Biogen Idec, Merck-Serono, Teva Neuroscience, and Pepgen. MAH has received consulting fees for work in previous and current treatment trials from Teva, Merck-Serono, and Bayer-Schering. MF has received honoraria for lectures and travel expenses, and consulting fees as an investigator in previous and current treatment trials from Teva, Merck-Serono, Bayer-Schering, Biogen-Dompè, Genmab, and Pepgen.
References
- 1.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58:840–46. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 2.Charil A, Yousry TA, Rovaris M, et al. MRI and the diagnosis of multiple sclerosis: expanding the concept of “no better explanation”. Lancet Neurol. 2006;5:841–52. doi: 10.1016/S1474-4422(06)70572-5. [DOI] [PubMed] [Google Scholar]
- 3.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol. 2001;50:121–27. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 4.Dalton CM, Brex PA, Miszkiel KA, et al. New T2 lesions enable an earlier diagnosis of multiple sclerosis in clinically isolated syndromes. Ann Neurol. 2003;53:673–76. doi: 10.1002/ana.10580. [DOI] [PubMed] [Google Scholar]
- 5.Tintore M, Rovira A, Rio J, et al. New diagnostic criteria for multiple sclerosis. Application in first demyelinating episode. Neurology. 2003;60:27–30. doi: 10.1212/wnl.60.1.27. [DOI] [PubMed] [Google Scholar]
- 6.Swanton JK, Fernando K, Dalton CM, et al. Modification of MRI criteria for multiple sclerosis in patients with clinically isolated syndromes. J Neurol Neurosurg Psychiat. 2006;77:830–33. doi: 10.1136/jnnp.2005.073247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swanton JK, Rovira A, Tintore M, et al. MRI criteria for multiple sclerosis in patients presenting with clinically isolated syndromes: a retrospective study. Lancet Neurol. 2007;6:677–86. doi: 10.1016/S1474-4422(07)70176-X. [DOI] [PubMed] [Google Scholar]
- 8.Wattjes MP, Harzheim M, Kuhl CK, et al. Does high-field MR imaging have an influence on the classification of patients with clinically isolated syndromes according to current diagnostic MR imaging criteria for multiple sclerosis? AJNR Am J Neuroradiol. 2006;27:1794–98. [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson AJ, Montalban X, Barkhof F, et al. Diagnostic criteria for primary progressive multiple sclerosis: a position paper. Ann Neurol. 2000;47:831–35. [PubMed] [Google Scholar]
- 10.Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210–18. doi: 10.1111/j.1750-3639.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neema M, Stankiewicz J, Arora A, Guss ZD, Bakshi R. MRI in multiple sclerosis: what's inside the toolbox? Neurotherapeutics. 2007;4:602–17. doi: 10.1016/j.nurt.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pirko I, Lucchinetti CF, Sriram S, Bakshi R. Gray matter involvement in multiple sclerosis. Neurology. 2007;68:634–42. doi: 10.1212/01.wnl.0000250267.85698.7a. [DOI] [PubMed] [Google Scholar]
- 13.Miller DH, Thompson AJ, Filippi M. Magnetic resonance studies of abnormalities in the normal appearing white matter and grey matter in multiple sclerosis. J Neurol. 2003;250:1407–19. doi: 10.1007/s00415-003-0243-9. [DOI] [PubMed] [Google Scholar]
- 14.Meier D, Weiner HL, Guttmann CRG. MR imaging intensity modeling of damage and repair in multiple sclerosis: relationship of short-term lesion recovery to progression and disability. AJNR Am J Neuroradiol. 2007;28:1956–63. doi: 10.3174/ajnr.A0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan Y, Hildenbrand PG, Sampat MP, et al. Segmentation of subtraction images for measurement of lesion change in multiple sclerosis. AJNR Am J Neuroradiol. 2008;29:340–46. doi: 10.3174/ajnr.A0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bermel RA, Bakshi R. The measurement and clinical relevance of brain atrophy in multiple sclerosis. Lancet Neurol. 2006;5:158–70. doi: 10.1016/S1474-4422(06)70349-0. [DOI] [PubMed] [Google Scholar]
- 17.Lazeron RH, de Sonneville LM, Scheltens P, Polman CH, Barkhof F. Cognitive slowing in multiple sclerosis is strongly associated with brain volume reduction. Mult Scler. 2006;12:760–68. doi: 10.1177/1352458506070924. [DOI] [PubMed] [Google Scholar]
- 18.Prinster A, Quarantelli M, Orefice G, et al. Grey matter loss in relapsing-remitting multiple sclerosis: a voxel-based morphometry study. NeuroImage. 2006;29:859–67. doi: 10.1016/j.neuroimage.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 19.Morgen K, Sammer G, Courtney SM, et al. Evidence for a direct association between cortical atrophy and cognitive impairment in relapsing-remitting MS. NeuroImage. 2006;30:891–98. doi: 10.1016/j.neuroimage.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 20.Nucifora PG, Verma R, Lee SK, Melhem ER. Diffusion-tensor MR imaging and tractography: exploring brain microstructure and connectivity. Radiology. 2007;245:367–84. doi: 10.1148/radiol.2452060445. [DOI] [PubMed] [Google Scholar]
- 21.Carone DA, Benedict RH, Dwyer MG, et al. Semi-automatic brain region extraction (SABRE) reveals superior cortical and deep gray matter atrophy in MS. NeuroImage. 2006;29:505–14. doi: 10.1016/j.neuroimage.2005.07.053. [DOI] [PubMed] [Google Scholar]
- 22.Sicotte NL, Kern KC, Giesser BS, et al. Regional hippocampal atrophy in multiple sclerosis. Brain. 2008;131:1134–41. doi: 10.1093/brain/awn030. [DOI] [PubMed] [Google Scholar]
- 23.Rudick RA. Impact of disease-modifying therapies on brain and spinal cord atrophy in multiple sclerosis. J Neuroimaging. 2004;14:54S–64S. doi: 10.1177/1051228404266269. [DOI] [PubMed] [Google Scholar]
- 24.Miller DH, Soon D, Fernando KT, et al. MRI outcomes in a placebo-controlled trial of natalizumab in relapsing MS. Neurology. 2007;68:1390–401. doi: 10.1212/01.wnl.0000260064.77700.fd. [DOI] [PubMed] [Google Scholar]
- 25.Chen JT, Collins DL, Atkins HL, et al. Brain atrophy after immunoablation and stem cell transplantation in multiple sclerosis. Neurology. 2006;66:1935–37. doi: 10.1212/01.wnl.0000219816.44094.f8. [DOI] [PubMed] [Google Scholar]
- 26.Jasperse B, Minneboo A, de Groot V, et al. Determinants of cerebral atrophy rate at the time of diagnosis of multiple sclerosis. Arch Neurol. 2007;64:190–94. doi: 10.1001/archneur.64.2.190. [DOI] [PubMed] [Google Scholar]
- 27.Kalkers NF, Vrenken H, Uitdehaag BM, Polman CH, Barkhof F. Brain atrophy in multiple sclerosis: impact of lesions and of damage of whole brain tissue. Mult Scler. 2002;8:410–14. doi: 10.1191/1352458502ms833oa. [DOI] [PubMed] [Google Scholar]
- 28.Bermel RA, Puli SR, Rudick RA, et al. Prediction of longitudinal brain atrophy in multiple sclerosis by gray matter magnetic resonance imaging T2 hypointensity. Arch Neurol. 2005;62:1371–76. doi: 10.1001/archneur.62.9.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dousset V, Brochet B, Deloire MS, et al. MR imaging of relapsing multiple sclerosis patients using ultra-small-particle iron oxide and compared with gadolinium. AJNR Am J Neuroradiol. 2006;27:1000–05. [PMC free article] [PubMed] [Google Scholar]
- 30.Vellinga MM, Oude Engberink RD, Seewann A, et al. Pluriformity of inflammation in multiple sclerosis shown by ultra-small iron oxide particle enhancement. Brain. 2008;131:800–07. doi: 10.1093/brain/awn009. [DOI] [PubMed] [Google Scholar]
- 31.Strijkers GJ, Mulder WJ, van Tilborg GA, Nicolay K. MRI contrast agents: current status and future perspectives. Anticancer Agents Med Chem. 2007;7:291–305. doi: 10.2174/187152007780618135. [DOI] [PubMed] [Google Scholar]
- 32.Chen JW, Sans MQ, Bogdanov A, Jr, Weissleder R. Imaging of myeloperoxidase in mice by using novel amplifiable paramagnetic substrates. Radiology. 2006;240:473–81. doi: 10.1148/radiol.2402050994. [DOI] [PubMed] [Google Scholar]
- 33.Wessig C, Bendszus M, Stoll G. In vivo visualization of focal demyelination in peripheral nerves by gadofluorine M-enhanced magnetic resonance imaging. Exp Neurol. 2007;204:14–19. doi: 10.1016/j.expneurol.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 34.Helms G. Volume correction for edema in single-volume proton MR spectroscopy of contrast-enhancing multiple sclerosis lesions. Magn Reson Med. 2001;46:256–63. doi: 10.1002/mrm.1186. [DOI] [PubMed] [Google Scholar]
- 35.Srinivasan R, Sailasuta N, Hurd R, Nelson S, Pelletier D. Evidence of elevated glutamate in multiple sclerosis using magnetic resonance spectroscopy at 3 T. Brain. 2005;128:1016–25. doi: 10.1093/brain/awh467. [DOI] [PubMed] [Google Scholar]
- 36.De Stefano N, Filippi M, Miller D, et al. Guidelines for using proton MR spectroscopy in multicenter clinical MS studies. Neurology. 2007;69:1942–52. doi: 10.1212/01.wnl.0000291557.62706.d3. [DOI] [PubMed] [Google Scholar]
- 37.Mader I, Seeger U, Weissert R, et al. Proton MR spectroscopy with metabolite-nulling reveals elevated macromolecules in acute multiple sclerosis. Brain. 2001;124:953–61. doi: 10.1093/brain/124.5.953. [DOI] [PubMed] [Google Scholar]
- 38.Kohler SJ, Yen Y, Wolber J, et al. In vivo 13 carbon metabolic imaging at 3T with hyperpolarized 13C-1-pyruvate. Magn Reson Med. 2007;58:65–69. doi: 10.1002/mrm.21253. [DOI] [PubMed] [Google Scholar]
- 39.Wolff SD, Balaban RS. Magnetization transfer imaging: practical aspects and clinical applications. Radiology. 1994;192:593–99. doi: 10.1148/radiology.192.3.8058919. [DOI] [PubMed] [Google Scholar]
- 40.van Waesberghe JH, Kamphorst W, De Groot C, et al. Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability. Ann Neurol. 1999;46:747–54. doi: 10.1002/1531-8249(199911)46:5<747::aid-ana10>3.3.co;2-w. [DOI] [PubMed] [Google Scholar]
- 41.Schmierer K, Scaravilli F, Altmann DR, Barker GJ, Miller DH. Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann Neurol. 2004;56:407–15. doi: 10.1002/ana.20202. [DOI] [PubMed] [Google Scholar]
- 42.Filippi M, Rocca MA. Magnetization transfer magnetic resonance imaging of the brain, spinal cord, and optic nerve. Neurotherapeutics. 2007;4:401–13. doi: 10.1016/j.nurt.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Filippi M, Iannucci G, Tortorella C, et al. Comparison of MS clinical phenotypes using conventional and magnetization transfer MRI. Neurology. 1999;52:588–94. doi: 10.1212/wnl.52.3.588. [DOI] [PubMed] [Google Scholar]
- 44.Ge Y, Grossman RI, Udupa JK, Babb JS, Mannon LJ, McGowan JC. Magnetization transfer ratio histogram analysis of normal-appearing gray matter and normal-appearing white matter in multiple sclerosis. J Comput Assist Tomogr. 2002;26:62–68. doi: 10.1097/00004728-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Horsfield MA, Barker GJ, Barkhof F, Miller DH, Thompson AJ, Filippi M. Guidelines for using quantitative magnetization transfer magnetic resonance imaging for monitoring treatment of multiple sclerosis. J Magn Reson Imaging. 2003;17:389–97. doi: 10.1002/jmri.10266. [DOI] [PubMed] [Google Scholar]
- 46.Chen JT, Kuhlmann T, Jansen GH, et al. Voxel-based analysis of the evolution of magnetization transfer ratio to quantify remyelination and demyelination with histopathological validation in a multiple sclerosis lesion. NeuroImage. 2007;36:1152–58. doi: 10.1016/j.neuroimage.2007.03.073. [DOI] [PubMed] [Google Scholar]
- 47.Chen JT, Collins DL, Freedman MS, Atkins HL, Arnold DL, Canadian MS/BMT Study Group Local magnetization transfer ratio signal inhomogeneity is related to subsequent change in MTR in lesions and normal-appearing white-matter of multiple sclerosis patients. NeuroImage. 2005;25:1272–78. doi: 10.1016/j.neuroimage.2004.12.046. [DOI] [PubMed] [Google Scholar]
- 48.Sled JG, Pike GB. Quantitative interpretation of magnetization transfer in spoiled gradient echo MRI sequences. J Magn Reson. 2000;145:24–36. doi: 10.1006/jmre.2000.2059. [DOI] [PubMed] [Google Scholar]
- 49.Ropele S, Seifert T, Enzinger C, Fazekas F. Method for quantitative imaging of the macromolecular H fraction in tissues. Magn Reson Med. 2003;49:864–71. doi: 10.1002/mrm.10427. [DOI] [PubMed] [Google Scholar]
- 50.Tozer D, Ramani A, Barker GJ, Davies GR, Miller DH, Tofts PS. Quantitative magnetization transfer mapping of bound protons in multiple sclerosis. Magn Reson Med. 2003;50:83–91. doi: 10.1002/mrm.10514. [DOI] [PubMed] [Google Scholar]
- 51.Narayanan S, Francis SJ, Sled JG, et al. Axonal injury in the cerebral normal-appearing white matter of patients with multiple sclerosis is related to concurrent demyelination in lesions but not to concurrent demyelination in normal-appearing white matter. NeuroImage. 2006;29:637–42. doi: 10.1016/j.neuroimage.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 52.Behrens TEJ, Johansen-Berg H, Woolrich MW, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–57. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- 53.Audoin B, Guye M, Reuter F, et al. Structure of WM bundles constituting the working memory system in early multiple sclerosis: a quantitative DTI tractography study. NeuroImage. 2007;36:1324–30. doi: 10.1016/j.neuroimage.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 54.Pagani E, Filippi M, Rocca MA, Horsfield MA. A method for obtaining tract-specific diffusion tensor MRI measurements in the presence of disease: application to patients with clinically isolated syndromes suggestive of multiple sclerosis. NeuroImage. 2005;26:258–65. doi: 10.1016/j.neuroimage.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 55.Jbabdi S, Woolrich MW, Andersson JLR, Behrens TE. A Bayesian framework for global tractography. NeuroImage. 2007;37:116–29. doi: 10.1016/j.neuroimage.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 56.Kamada K, Houkin K, Takeuchi F, et al. Visualization of the eloquent motor system by integration of MEG, functional, and anisotropic diffusion-weighted MRI in functional neuronavigation. Surg Neurol. 2003;59:353–62. doi: 10.1016/s0090-3019(03)00018-1. [DOI] [PubMed] [Google Scholar]
- 57.Bodurka J, Bandettini PA. Toward direct mapping of neuronal activity: MRI detection of ultraweak, transient magnetic fields changes. Magn Reson Med. 2002;47:1052–58. doi: 10.1002/mrm.10159. [DOI] [PubMed] [Google Scholar]
- 58.Truong TK, Song AW. Finding neuroelectric activity under magnetic-field oscillations (NAMO) with magnetic resonance imaging in vivo. Proc Natl Acad Sci U S A. 2006;103:12598–601. doi: 10.1073/pnas.0605486103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87:9868–72. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rocca MA, Filippi M. Functional MRI in multiple sclerosis. J Neuroimaging. 2007;1:36S–41S. doi: 10.1111/j.1552-6569.2007.00135.x. [DOI] [PubMed] [Google Scholar]
- 61.Rocca MA, Colombo B, Falini A, et al. Cortical adaptation in patients with MS: a cross-sectional functional MRI study of disease phenotypes. Lancet Neurol. 2005;4:618–26. doi: 10.1016/S1474-4422(05)70171-X. [DOI] [PubMed] [Google Scholar]
- 62.Stephan KE, Harrison LM, Kiebel SJ, David O, Penny WD, Friston KJ. Dynamic causal models of neural system dynamics:current state and future extensions. J Biosci. 2007;32:129–44. doi: 10.1007/s12038-007-0012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rocca MA, Pagani E, Absinta M, et al. Altered functional and structural connectivities in patients with MS: a 3-T study. Neurology. 2007;69:2136–45. doi: 10.1212/01.wnl.0000295504.92020.ca. [DOI] [PubMed] [Google Scholar]
- 64.Rocca MA, Valsasina P, Ceccarelli A, et al. Structural and functional MRI correlates of Stroop control in benign MS. Hum Brain Mapp. 2007 doi: 10.1002/hbm.20504. published online Nov 27. DOI: 10.1002/ hbm.20504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wegner C, Filippi M, Korteweg T, et al. Relating functional changes during hand movement to clinical parameters in patients with multiple sclerosis in a multi-centre fMRI study. Eur J Neurol. 2008;15:113–22. doi: 10.1111/j.1468-1331.2007.02027.x. [DOI] [PubMed] [Google Scholar]
- 66.Youl BD, Turano G, Miller DH, et al. The pathophysiology of acute optic neuritis. An association of gadolinium leakage with clinical and electrophysiological deficits. Brain. 1991;114:2437–50. doi: 10.1093/brain/114.6.2437. [DOI] [PubMed] [Google Scholar]
- 67.Hickman SJ, Toosy AT, Jones SJ, et al. Serial magnetization transfer imaging in acute optic neuritis. Brain. 2004;127:692–700. doi: 10.1093/brain/awh076. [DOI] [PubMed] [Google Scholar]
- 68.Trip SA, Wheeler-Kingshott C, Jones SJ, et al. Optic nerve diffusion tensor imaging in optic neuritis. NeuroImage. 2006;30:498–505. doi: 10.1016/j.neuroimage.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 69.Frohman E, Costello F, Zivadinov R, et al. Optical coherence tomography in multiple sclerosis. Lancet Neurol. 2006;5:853–63. doi: 10.1016/S1474-4422(06)70573-7. [DOI] [PubMed] [Google Scholar]
- 70.Lycklama à Nijeholt G, Thompson A, Filippi M, et al. Spinal-cord MRI in multiple sclerosis. Lancet Neurol. 2003;2:555–62. doi: 10.1016/s1474-4422(03)00504-0. [DOI] [PubMed] [Google Scholar]
- 71.Agosta F, Filippi M. MRI of spinal cord in multiple sclerosis. J Neuroimaging. 2007;1:46S–49S. doi: 10.1111/j.1552-6569.2007.00137.x. [DOI] [PubMed] [Google Scholar]
- 72.Agosta F, Valsasina P, Caputo D, Stroman PW, Filippi M. Tactile-associated recruitment of cervical cord is altered in patients with multiple sclerosis. NeuroImage. 2008;39:1542–48. doi: 10.1016/j.neuroimage.2007.10.048. [DOI] [PubMed] [Google Scholar]
- 73.Kendi AT, Tan FU, Kendi M, Yilmaz S, Huvaj S, Tellioğlu S. MR spectroscopy of cervical spinal cord in patients with multiple sclerosis. Neuroradiology. 2004;46:764–69. doi: 10.1007/s00234-004-1231-1. [DOI] [PubMed] [Google Scholar]
- 74.Ciccarelli O, Wheeler-Kingshott CA, McLean MA, et al. Spinal cord spectroscopy and diffusion-based tractography to assess acute disability in multiple sclerosis. Brain. 2007;130:2220–31. doi: 10.1093/brain/awm152. [DOI] [PubMed] [Google Scholar]
- 75.Agosta F, Pagani E, Caputo D, Filippi M. Associations between cervical cord gray matter damage and disability in patients with multiple sclerosis. Arch Neurol. 2007;64:1302–05. doi: 10.1001/archneur.64.9.1302. [DOI] [PubMed] [Google Scholar]
- 76.Whittall KP, MacKay AL, Graeb DA, Nugent RA, Li DK, Paty DW. In vivo measurement of T2 distributions and water contents in normal human brain. Magn Reson Med. 1997;37:34–43. doi: 10.1002/mrm.1910370107. [DOI] [PubMed] [Google Scholar]
- 77.Beaulieu C, Fenrich FR, Allen PS. Multicomponent water proton transverse relaxation and T2-discriminated water diffusion in myelinated and nonmyelinated nerve. Magn Reson Imaging. 1998;16:1201–10. doi: 10.1016/s0730-725x(98)00151-9. [DOI] [PubMed] [Google Scholar]
- 78.Moore GR, Leung E, MacKay AL, et al. A pathology-MRI study of the short-T2 component in formalin-fixed multiple sclerosis brain. Neurology. 2000;55:1506–10. doi: 10.1212/wnl.55.10.1506. [DOI] [PubMed] [Google Scholar]
- 79.Oh J, Han ET, Lee MC, Nelson SJ, Pelletier D. Multislice brain myelin water fractions at 3T in multiple sclerosis. J Neuroimaging. 2007;17:156–63. doi: 10.1111/j.1552-6569.2007.00098.x. [DOI] [PubMed] [Google Scholar]
- 80.Laule C, Vavasour IM, Moore GR, et al. Water content and myelin water fraction in multiple sclerosis. A T2 relaxation study. J Neurol. 2004;251:284–93. doi: 10.1007/s00415-004-0306-6. [DOI] [PubMed] [Google Scholar]
- 81.Oh J, Han ET, Pelletier D, Nelson SJ. Measurement of in vivo multi-component T2 relaxation times for brain tissue using multi-slice T2 prep at 1·5 and 3 T. Magn Reson Imaging. 2006;24:33–43. doi: 10.1016/j.mri.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 82.Laule C, Vavasour IM, Kolind SH, et al. Long T(2) water in multiple sclerosis: what else can we learn from multi-echo T(2) relaxation? J Neurol. 2007;254:1579–87. doi: 10.1007/s00415-007-0595-7. [DOI] [PubMed] [Google Scholar]
- 83.Ge Y, Law M, Johnson G, et al. Dynamic susceptibility contrast perfusion MR imaging of multiple sclerosis lesions: characterizing hemodynamic impairment and inflammatory activity. AJNR Am J Neuroradiol. 2005;26:1539–47. [PMC free article] [PubMed] [Google Scholar]
- 84.Ge Y, Law M, Inglese M, Grossman RI. Perfusion MRI. In: Filippi M, Rovaris M, Comi G, editors. Neurodegeneration in multiple sclerosis. Springer-Verlag; Milan: 2007. pp. 55–63. [Google Scholar]
- 85.Adhya S, Johnson G, Herbert J, et al. Pattern of hemodynamic impairment in multiple sclerosis: dynamic susceptibility contrast perfusion MR imaging at 3·0 T. NeuroImage. 2006;33:1029–35. doi: 10.1016/j.neuroimage.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang J, Alsop DC, Li L, et al. Comparison of quantitative perfusion imaging using arterial spin labeling at 1·5 and 4·0 Tesla. Magn Reson Med. 2002;48:242–54. doi: 10.1002/mrm.10211. [DOI] [PubMed] [Google Scholar]
- 87.Sicotte NL, Voskuhl RR, Bouvier S, Klutch R, Cohen MS, Mazziotta JC. Comparison of multiple sclerosis lesions at 1·5 and 3·0 Tesla. Invest Radiol. 2003;38:423–27. doi: 10.1097/01.RLI.0000065426.07178.f1. [DOI] [PubMed] [Google Scholar]
- 88.Kangarlu A, Bourekas EC, Ray-Chaudhury A, Rammohan KW. Cerebral cortical lesions in multiple sclerosis detected by MR imaging at 8 Tesla. AJNR Am J Neuroradiol. 2007;28:262–66. [PMC free article] [PubMed] [Google Scholar]
- 89.Smith SA, Farrell JA, Jones CK, Reich DS, Calabresi PA, van Zijl PC. Pulsed magnetization transfer imaging with body coil transmission at 3 Tesla: feasibility and application. Magn Reson Med. 2006;56:866–75. doi: 10.1002/mrm.21035. [DOI] [PubMed] [Google Scholar]
- 90.Neema M, Stankiewicz J, Arora A, et al. T1 and T2 based MRI measures of diffuse gray matter and white matter damage in patients with multiple sclerosis. J Neuroimaging. 2007;17:16S–21S. doi: 10.1111/j.1552-6569.2007.00131.x. [DOI] [PubMed] [Google Scholar]
- 91.Hammond KE, Lupo JM, Xu D, et al. Development of a robust method for generating 7·0 T multichannel phase images of the brain with application to normal volunteers and patients with neurological diseases. NeuroImage. 2008;39:1682–92. doi: 10.1016/j.neuroimage.2007.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thulborn KR, Davis D, Snyder J, Yonas H, Kassam A. Sodium MR imaging of acute and subacute stroke for assessment of tissue viability. Neuroimaging Clin N Am. 2005;15:639–53. doi: 10.1016/j.nic.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 93.Stankiewicz J, Panter SS, Neema M, Arora A, Batt CE, Bakshi R. Iron in chronic brain disorders: imaging and neurotherapeutic implications. Neurotherapeutics. 2007;4:371–86. doi: 10.1016/j.nurt.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brass SD, Benedict RHB, Weinstock-Guttman B, Munschauer FE, Bakshi R. Cognitive impairment is associated with subcortical MRI gray matter T2 hypointensity in multiple sclerosis. Mult Scler. 2006;12:437–44. doi: 10.1191/135248506ms1301oa. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Y, Zabad RK, Wei X, Metz LM, Hill MD, Mitchell JR. Deep gray matter “black T2” on 3 tesla magnetic resonance image correlates with disability in multiple sclerosis. Mult Scler. 2007;13:880–83. doi: 10.1177/1352458507076411. [DOI] [PubMed] [Google Scholar]
- 96.Ge Y, Jensen JH, Lu H, et al. Quantitative assessment of iron accumulation in the deep gray matter of multiple sclerosis by magnetic field correlation imaging. AJNR Am J Neuroradiol. 2007;28:1639–44. doi: 10.3174/ajnr.A0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vrenken H, Geurts JJG, Knol DL, et al. Whole-brain T1 mapping in multiple sclerosis: global changes of normal-appearing gray and white matter. Radiology. 2006;240:811–20. doi: 10.1148/radiol.2403050569. [DOI] [PubMed] [Google Scholar]
- 98.Tanenbaum LN. Clinical 3T MR imaging: mastering the challenges. Magn Reson Imaging Clin N Am. 2006;14:1–15. doi: 10.1016/j.mric.2005.12.004. [DOI] [PubMed] [Google Scholar]