Abstract
The authors examined the interaction between the development of postural control and the development of the executive function of attention in 13 children and 6 adults in dual-task conditions. Participants performed an attentionally demanding cognitive task and a postural task simultaneously. The authors equalized the attentional load of the cognitive task across age groups. Comparative changes in the center of pressure in dual- and single-task conditions indicated that dual tasks interfered with postural performance in the wide stance (WS) and the modified Romberg stance (RS). Children at 4–6 years of age (but not children at ages 7–12 years of age or adults) experienced postural control interference in both stance positions, but interference was greater in the RS (p = .018). For all participants, cognitive task performance in RS was unchanged from that in WS. The knowledge gained from the results of this study will contribute to the design and implementation of academic and preacademic programming for young children. Their performance of an intentionally demanding cognitive task would be enhanced by the provision of appropriately sized desks and chairs or their use of an alternate, less demanding position.
Keywords: development, dual-task, executive function of attention, postural control
In academic settings, younger children have often been found to have difficulties sustaining attention to the subjects being taught. Educators commonly see frequent readjustment of posture and fidgeting while sitting at a desk or standing in line in children with attentional problems. According to the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 1994), a manual used by physicians and mental health providers for the diagnosis of psychological disorders, those behaviors could be a sign of deficits in attention. However, multiple factors may contribute to attentional performance in the academic setting. For example, when children are performing a motor task (standing, walking, or reaching for an object) while also performing a cognitive task, the attentional requirements of the motor task may compete for processing resources within the limited attentional capacity of the child, causing the child to perform less efficiently on both the motor and cognitive tasks. Therefore, it is important to understand the factors competing for attentional resources during children’s performance of the different tasks so that educators can create an age-appropriate academic environment that is most conducive to learning.
For our purpose in this study, we define the term attention as a reservoir of processing resources on the basis of the limited capacity model of Kahneman (1973). When an individual performs two attentionally demanding tasks simultaneously, competition for processing resources may occur. The interference may lead to deterioration in the performance of one or both tasks (Neumann, 1984; Wickens, 1989).
Postural control was traditionally considered to be automatic (i.e., reflexively controlled and requiring minimal conscious processing of information), but recent studies with adults and children have shown that the process of maintaining or regaining stability requires attentional resources (Beauchet, Dubost, Gonthier, & Kressig, 2005; Blanchard et al., 2005; Brauer, Woollacott, & Shumway-Cook, 2001, 2002; Brown, McKenzie, & Doan, 2005; Pellecchia, 2003; Rankin, Woollacott, Shumway-Cook, & Brown, 2000; Schrodt, Mercer, Giuliani, & Hartman, 2004; Shumway-Cook & Woollacott, 2000; Silsupadol, Siu, Shumway-Cook, & Woollacott, 2006). In research on attention and postural control in adults, investigators have used dual-task paradigms in which participants performed a postural control task and a cognitive task together. The researchers have used the degree to which participants’ performance on either one or both tasks declined as an index of the extent of sharing of attentional resources.
Results of experiments in which dual-task designs were used have led researchers to propose a hierarchy of postural control tasks on the basis of attentional-processing requirements. Less resources are required for relatively undemanding postural tasks such as sitting or standing with feet shoulder-width apart, whereas attentional demands are increased when individuals stand in the more demanding Romberg or narrow stance positions (Teasdale, Bard, LaRue, & Fleury, 1993), walking (Teasdale et al.), recovering from external perturbations (Brown, Shumway-Cook, & Woollacott, 1999; Rankin et al., 2000), and responding to change in the sensory environment (Shumway-Cook & Woollacott, 2000).
In addition, the attentional load of the cognitive task has been found to affect the degree of postural control interference in reactive and static postural control (Maylor & Wing, 1995; Pellecchia, 2003; Rankin et al., 2000; Shumway-Cook, Woollacott, Kerns, & Baldwin, 1997). Pellecchia investigated postural control interference in a dual-task condition by using information-reduction tasks (digit reversal, digit classification, and counting backward by threes) to manipulate the attentional load of the cognitive tasks. Difficulty of the task was related to the degree to which participants mentally reduced the information of the stimulus to process a response. As the cognitive task increased in the amount of information that was reduced from stimulus to response, so too did the degree of postural control interference.
There has been one other recent study in which dual-task effects on postural control in children were investigated. Blanchard et al. (2005) examined postural control in quiet stance in children at ages 8–9 years who performed either a counting-backward or a reading task. Postural sway increased with simultaneous performance of either cognitive task.
One neural component of attentional processing has been labeled executive function of attention. That component has a role in resolving conflicts among competing sensory stimuli, detecting errors, and planning new actions (Posner & Rothbart, 1998). In addition, the executive network of attention has a role in the encoding and maintaining of items in visual short-term memory (Awh & Jonides, 2001; Awh, Vogel, & Oh, 2006). Kane, Bleckley, Conway, and Engle (2001; Kane & Engle, 2003) demonstrated a strong link between the capacity of short-term memory and the efficiency of the executive function of attention. For our purpose in this study, we used a short-term memory task to probe the efficiency of the executive function of attention.
To assess developmental trends in the capacity of the executive function of attention networks, researchers use tasks designed to establish conflict between the processing of two competing stimuli and to determine concomitant motor responses that require suppression of the prepotent response in the conflicting condition. It appears from those developmental studies that executive attention matures significantly between the ages of 5 and 10 years, reaching near-maturity at 10 years (Ridderinkhof, van der Molen, Band, & Bashore, 1997; Rueda et al., 2004). In several studies, investigators have shown that the greatest development of executive attention occurs between the ages of 6 and 8 years (Diamond & Taylor, 1996; Ridderinkhof et al.; Rueda et al.). That finding implies that by 8 years of age, children’s and adults’ ability to attend to tasks should differ only minimally.
Previous researchers have shown that attainment of motor skills in sitting, standing, and walking depends on the development of the posture control system (Burtner, Qualls, & Woollacott, 1998; Hadders-Algra, Brogen, & Forssberg, 1996; Sakaguchi, Taguchi, Miyashita, & Katsuno, 1994; Sundermier & Woollacott, 1985; Sveistrup & Woollacott, 1985). Later in childhood, as children mature, their quiet stance posture stability improves to adult-like levels (Cherng, Lee, & Su, 2003; Riach & Hayes, 1987; Roncesvalles, Woollacott, & Jensen, 2001; Shumway-Cook & Woollacott, 1985). Sakaguchi et al. showed that children younger than 12 years of age have greater total center of pressure (COP) displacement than do older children and adults. Shumway-Cook and Woollacott showed that children between the ages of 4 and 6 years have a greater area and variability of COP movement than do older children (7–10 years) and adults. Cherng et al., who examined the frequency of sway through spectral analysis, found that children between the ages of 7 and 10 years swayed with greater frequency than did adults.
When researchers combine the results of the developmental studies of executive attention and of postural control, it becomes apparent that children in the younger age groups (4–6 years) may have difficulty in simultaneously performing a postural task and an attentionally demanding cognitive task. Although researchers have independently shown the ages at which both attention and postural control mature, there are no reported investigations of the development of the interaction between these two processes.
Our goal in this study was to investigate that interaction by using a dual-task paradigm in which participants performed an attentionally demanding cognitive task and a postural control task simultaneously. We expected that young children’s level of performance in one or both tasks would be compromised in comparison with that of older children with increase in the attentional load of the postural control task.
When researchers assess dual-task interference in different participant groups, it is important for them to equate task demands to ensure that each group faces the same relative cognitive workload. They can accomplish the equalization by first determining the level of task difficulty at which each group’s performance is equivalent. If researchers do not equate secondary task difficulty, then there may be a confound between changes in task performance caused by the secondary task’s relative level of difficulty and the direct attentional interference that the secondary task induces (Woollacott & Shumway-Cook, 2002). In addition, investigators have shown that the motor output of articulation increases body sway, thus complicating their interpretation of dual-task effects on postural control (Yardley, Gardner, Leadbetter, & Lavie, 1999). For those reasons, we used a short-term working memory task (Vogel, Woodman, & Luck, 2001) that we could systematically manipulate in terms of difficulty and which did not require verbal output until after we measured the postural response. Moreover, we were not interested in the interactions between short-term working memory and postural control but in the interaction between the engagement of executive function of attention induced by participants’ performance of the working memory task and their ability to maintain posture.
We kept the level of difficulty of the cognitive task constant between trials and between the different groups of children by means of the following procedure: We performed titration trials of the visual short-term memory task before the experiment to determine the maximum number of colored items participants recalled (short-term memory capacity) with a 70% accuracy rate (defined as threshold). To remove the influential effects of articulation, we analyzed movement of the COP before the time of the verbal response.
We used accuracy of responses as an indicator of cognitive task performance. Changes in body sway as measured by COP displacement and velocity in anteroposterior (AP) and mediolateral (ML) directions determined the status of postural control between and within participant groups. Interference in postural control or the cognitive task, or both, were indicative of the shared attentional resources the two tasks required.
Because efficiency of the executive function of attention underlies individuals’ ability to simultaneously perform two tasks by allocating attentional resources to the processing of each task, we considered its efficiency to be a factor in the determination of attentional requirements of postural control and postural control interference in the dual-task condition. Because of the link between the constructs of executive function of attention and visual short-term memory, we inferred the efficacy of the executive component of attention from the capacity of the short-term memory system.
Method
Participants
Participants were thirteen 4- to 12-year-old children and six 20- to 26-year-old adults. We recruited the adults from the graduate and undergraduate programs at the University of Oregon. We recruited children by means of fliers given to faculty and staff at the University of Oregon and by public advertisements approved by the Committee for the Protection of Human Subjects at the University of Oregon. For data analysis, we divided participants into three age groups (see Table 1): Adults (A) were 20–26 years of age (M ± SD = 21.5 ± 1.5 years), older children (OC) were 7–12 years of age (M ± SD = 9 ± 2 years), and young children (YC) were 4–6 years of age (M ± SD = 5 ± 1 years).
TABLE 1.
Participants’ Demographics
|
n |
Age (years)
|
Ht. (m)
|
Wt. (kg)
|
FL (m)
|
WSBOS (m)
|
RSBOS (m)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | M | F | M | SD | M | SD | M | SD | PBS | CBC | M | SD | M | SD | M | SD |
| YC | 2 | 4 | 5.0 | 1.0 | 1.10 | 0.05 | 18.42 | 1.03 | 55.4 | 1.6 | 0.17 | 0.01 | 0.21 | 0.06 | 0.16 | 0.01 |
| OC | 4 | 3 | 9.0 | 2.0 | 1.36 | 0.13 | 33.68 | 11.2 | 56.0 | 2.4 | 0.21 | 0.02 | 0.23 | 0.03 | 0.20 | 0.20 |
| A | 2 | 4 | 21.5 | 1.5 | 1.64 | 0.16 | 64.87 | 13.5 | — | — | 0.24 | 0.02 | 0.27 | 0.02 | 0.19 | 0.01 |
Note. M = males, and F = females. Ht. = height. Wt. = weight. PBS = score on Pediatric Balance Scale (T. Achenbach, 2001). CBC = score on Child Behavior Checklist (M. R. Franjoine et al., 2003). FL = foot length. WSBOS = base of support width in wide stance. RSBOS = base of support in modified tandem Romberg stance. YC, OC, and A = young children, older children, and adults, respectively.
For the two groups of children, we assessed functional balance in sitting, standing, and reaching by using the Pediatric Balance Scale (PBS), which has been shown to have good test–retest reliability and interrater reliability (Franjoine, Gunther, & Taylor, 2003). The total score possible was 56, indicating good balance (see Table 1). Parents completed a Child Behavior Checklist (CBC), a criterion-based assessment that enables educators and health care professionals to determine disorders of attention in school-aged children at 6–18 years of age (Achenbach, 2001). For our purpose in this study, we considered children at 4–11 years of age to be within normal limits of attention if their criterion score was between 0 and 10, and children who were older than 11 years to be within those limits if their criterion scores were between 0 and 6. According to the attentional profiles, all children participating in this study scored in the normal range. Parents completed health questionnaires regarding the presence of any known impairments in the child’s musculoskeletal, cognitive, or neurological systems. After we explained the test procedures, the children and their parents signed consent forms approved by the Committee for the Protection of Human Subjects at the University of Oregon.
Cognitive Task
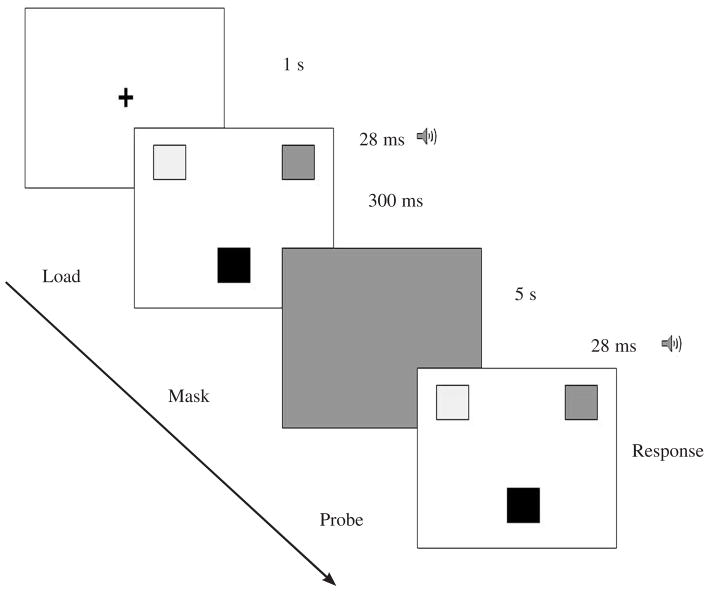
We modified the cognitive task from a visual working memory task that is used in studying adults’ short-term working memory capacity (Vogel et al., 2001) by manipulating the quantity of objects to be maintained and thus varying the attentional load (Posner & Rossman, 1965). The attentional load consisted of a variable number of colored shapes (squares, hearts, or stars) that we presented for 300 ms. Each trial consisted of three components. First, we presented a 28-ms auditory tone, which indicated the beginning of the load presentation. We subsequently presented a mask consisting of a gray screen for 5 s. After mask presentation, we presented a 28-ms auditory tone, which indicated the presentation of the probe. The probe consisted either of (a) the same presentation of colored shapes as the load or (b) a presentation in which the color of one shape had changed (see Figure 1). We asked participants to answer either yes if the shapes were similar in color to the load or no if one shape had changed color.
FIGURE 1.
Cognitive task presentation. Temporal sequencing of visual presentation of the short-term visual memory task is illustrated.
The titration task was the same aforementioned visual short-term memory task, but colored shapes varied in number between one and six from trial to trial. The initial trials had one or two colored shapes, and, depending on the participant’s accuracy, we increased the number of colored shapes in the following trials until the participant reached an accuracy of 70%. That procedure resulted in a threshold value for the number of shapes that participants could maintain in short-term memory. We used the threshold value (T), which varied across participants, during the dual-task trials.
Postural Control Task
The postural control tasks consisted of two stance positions: wide stance (WS) and modified tandem Romberg stance (RS). In the WS (i.e., easy) postural control task, participants stood barefoot on two force plates, with their feet side by side, shoulder width apart. For the more difficult, RS task, participants stood barefoot on two force plates, the left foot in front and medial to the right toe. In both stance postures, the arms were crossed over the chest. In separate blocks of trials, participants completed the postural tasks either in isolation or in combination with the secondary cognitive task.
During the dual-task trials, the number of shapes in the presentation was either T or T − 2 (threshold minus two colored shapes), as we had determined in the titration trials. We included the easier level of T − 2 in the presentation for comparison with T to ensure an appropriate degree of difficulty of the cognitive task at threshold levels. During the posture-only trials (single-task condition), we presented the same temporal sequence of the visual working-memory task. However, the screen was blank during the load and probe presentations. The auditory tone and mask were identical in sequence and timing to those we used for cognitive task presentation.
Apparatus
Kinetics
We collected vertical and horizontal ground reaction forces from a custom-built force platform located 35 cm above ground level and consisting of two force plates, each containing four strain gauge transducers. We collected data for a time period of 38 s at 360 Hz. We filtered the collected data to 5 Hz by using a low-pass fourth-order Butterworth filter. We performed the data collection and filtering during data analysis by using the MATLAB Version 6.50 (MathWorks Inc., Natick, MA) computer software program.
Computer system
A personal computer (PC) controlled by the first proctor triggered a digital–analog converter’s data acquisition for a block of four trials (38 s). A second PC collected analog data and converted them to digital data. We used a MATLAB computer software program to determine COP values. A second proctor, who controlled a third PC with SuperLab Pro Version 2.0 computer software (Cedrus, San Pedro, CA), presented visual targets for the experiment. A verbal signal between the first and second proctors synchronized the triggering of the data-acquisition computer and the visual presentation. The data-acquisition PC digitized and then collected the analog recordings of the auditory triggers generated by the visual presentation software, which were indicative of the start and end of each trial. The targets were back-projected onto a display screen positioned 10 feet in front of the participant, at eye level.
Headset with microphone
Participants wore a headset with microphone, which enabled us to record verbal responses. We used an analog–digital converter to collect and digitize analog data. The data were stored on a PC for later analysis.
Video recorder
We video-recorded the experimental session to determine trials in which participants lost balance, took a step, or moved their arms.
Protocol
We recorded anthropometric measurements of height, weight, and foot length and width for each participant in the PEAK software program (Peak Performance Technologies, Inc., Englewood, CO) before the experimental trials (see Table 1). Throughout the experiment, we gave participants rest breaks and refreshments.
We briefly explained the cognitive task, and participants performed a series of practice trials before the titration trials. We instructed participants to answer as accurately as possible. To enhance their viewing of the visual presentation and reduce visual distractions, we kept the room semidark.
We randomized the order in which participants performed the different stance configurations between participants. Children wore a safety harness (suspended from above the platform). A chalk outline of the feet on each of the force plates ensured consistent foot placement for every block of trials.
In the dual-task condition, we instructed participants at the beginning of each block of trials to stand as still as possible and to answer as quickly and accurately as possible. Participants stood barefoot on the force plates in one of the two foot configurations and responded to the cognitive task presentation. The presentation consisted of four blocks of four trials performed in 38 s. The instructions before the posture-only trials (single task) were to stand as still as possible and watch the screen. The single-task condition consisted of two blocks of four trials. All participants performed a total of 24 trials. Total time for all procedures was 1.5 hr.
Data Analysis
After viewing the video recordings, we eliminated approximately 2 trials from all the trials collected from the A, 3 from all the trials collected from the OC, and 10 from all the trials collected from the YC. Of the 10 trials eliminated, 8 were from the two 4-year-old children.
Cognitive task
We evaluated individual yes and no responses to the cognitive task (the composite of T and T − 2 trials) for accuracy by using SuperLab Pro computer software. Thus, we combined T and T − 2 trials for analysis. For later analysis, we used MATLAB computer software to digitize and then record on a PC the analog data collected from verbal responses and the auditory trigger cues generated by the presentation computer.
Posture task
For our purpose in this study, we defined postural stability in stance as maintenance of the center of gravity (COG) within the limits of stability as defined by the area of the base of support (Shumway-Cook & Woollacott, 2006). We considered an increase in amplitude or velocity of body sway, or both, to be a decrement in postural stability. Because of the relationship between COG movement and COP movement (Winter & Eng, 1995), we used COP displacement as a determinant of body sway.
We calculated COP variables by using MATLAB computer software. For each block of four trials (duration 38 s), we selected a consistent interval of time, 5,300 ms (load plus mask), for analyzing range and root-mean-squared (RMS) velocity of COP displacement in AP and ML directions. During that time interval, participants either (a) performed a posture-only task or (b) held the visual presentation in memory during the dual-task paradigm but before the probe test in which they articulated a response. We based the rationale for our selection of that interval on research by Yardley et al. (1999), who demonstrated the influence of articulation on the extent of body sway. We assessed interference in postural control in dual- versus single-task conditions by comparing the range and RMS velocity of COP displacement in AP and ML directions.
Statistical Analysis
Because limits of COP excursions in quiet stance depend on the boundaries of the base of support, we normalized the range of COP displacements before statistical analysis. In the AP direction, we normalized the range of COP displacements (max COPx – min COPx) by foot length; in the ML direction (max COPy – min COPy), we normalized the range by stance width.
Cognitive task
To ensure that the cognitive task had an appropriate degree of difficulty, we used a paired t test to evaluate significant differences in rate of accuracy between T and T − 2 for the total number of participants. We considered p = .05 to be an indication of a significant difference. We evaluated the accuracy of responses of a composite of T and T − 2 trials with a 3 × 2 statistical design consisting of one three-level between-participants factor (age group: YC, OC, A) and one two-level within-participant factor (stance position: WS, RS). We used logistic regression analysis to determine the odds ratios of giving correct versus incorrect responses for each age group and in each stance position.
Postural control task
To compare group differences in ranges and RMS velocities of COP displacement in AP and ML directions, we used a mixed linear model to evaluate the three-level between-participants factor (age group) and two two-level within-participants factors (dual vs. single task and WS vs. RS). We collected multiple observations on two types of stance and in two conditions from all participants. To determine the effects of condition, group, and stance, we computed F tests for specified contrasts of interest. We then extracted simple effects for each factor from those contrasts of group differences in dual- and single-task conditions in WS and RS and in dual- versus single-task conditions between groups in WS and RS.
The two- and three-factor interactions from a three-factor analysis of variance (ANOVA) are omnibus tests that refer to a set of very specific contrasts (actually, many independent sets of contrasts are inferred) that researchers usually test as if the contrasts were preplanned. For this study, we chose very specific and much simpler sets of pair-wise contrasts to evaluate with repeated measures ANOVA. Those contrasts are basically simple tests extracted from an overall set of contrasts that is simpler than those implied by the usual three-factor interaction. In that approach, power increases in accordance with a rationale set forth by Rosenthal, Rosnow, and Rubin (2000).
We performed post hoc analyses when the defined contrast for the between- and within-participants factors was significant at p = .05. We considered an omnibus F test for the defined contrast near significance if p = .05–.06. In the post hoc analyses for each defined contrast, we used Bonferroni correction to establish critical p values that would maintain an overall level of .05. For the defined contrast of dual tasks versus single task, we considered p < .0167(.05/3) to be significant and p = .0167–.0300 to be near significant.
To compare the attentional loads between the two stance conditions for each group, we used a paired t test to evaluate the dual-task effect (dual tasks minus single task) for the range of COP displacements in the AP direction in WS versus RS. We considered the comparison significant when p = .05 or less.
Executive component of attention
We used a single-factor ANOVA to test for significant differences in threshold (number of colored shapes recalled at 70% accuracy) between groups.
Results
Displacement of COP in Single-Task and Dual-Task Conditions
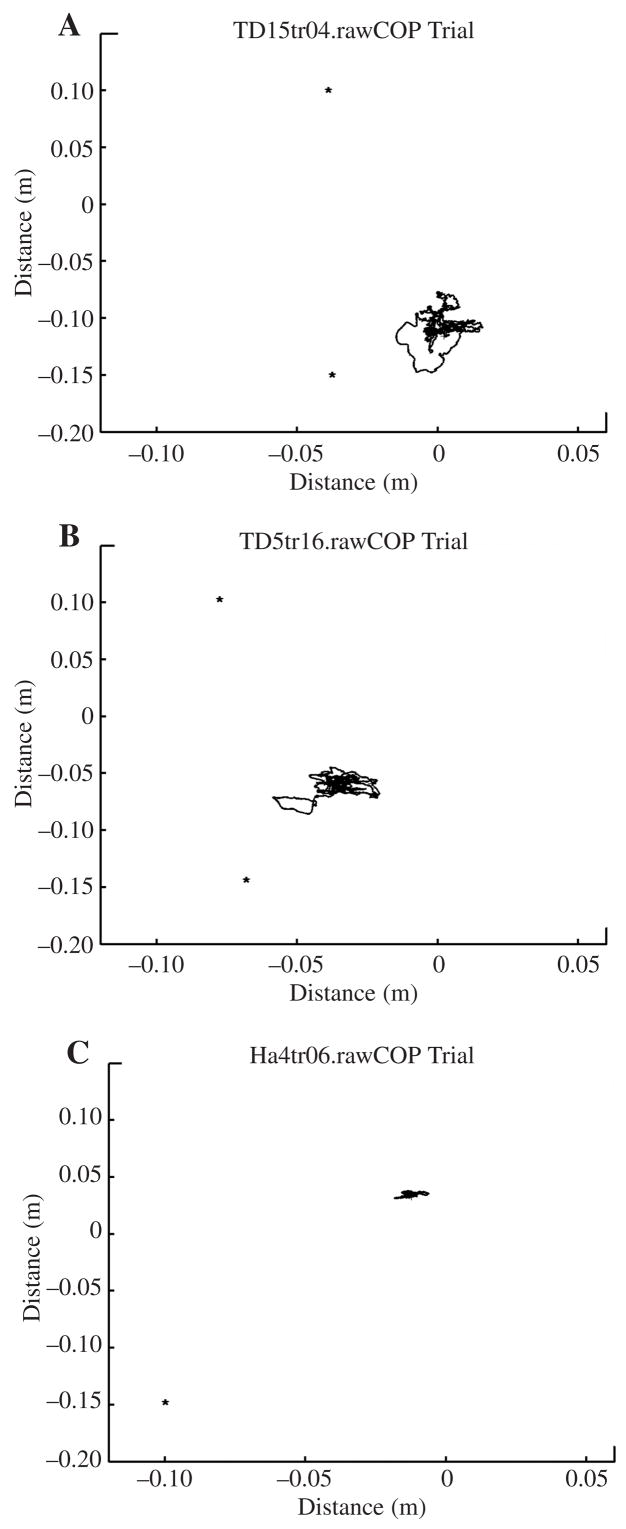
In the single-task conditions, we found developmental trends in postural stability that confirmed previous researchers’results. In general, YC were less stable than OC and A. Individual-participant COP tracings from a single block of four trials showed a developmental trend of total COP path from the young child’s (4 years) large excursions to the older child’s (10 years) and the adult’s smaller excursions (see Figure 2). That trend was apparent in both WS and RS conditions for most of the dependent variables (ML range of COP, RMS ML velocity, and RMS AP velocity of COP; see Table 2).
FIGURE 2.
Center of pressure (COP) tracings in the wide-stance single-task condition. Graphs show individual-participant COP tracings in anteroposterior direction (COPx AP) and mediolateral direction (COPy ML) of (A) young child, (B) older child (OC), and (C) adult in a 38-s block of four trials. Asterisks (°) mark locations of right and left lateral malleoli. Because of the restricted range of the COPy scale of measurement, only the adult’s right lateral mallelus is shown. The position of the adult’s left lateral malleolus was −0.09, 0.21 (x, y).
TABLE 2.
Single-Task Means and Standard Deviations of Normalized Range of Center of Pressure (COP) and Root Mean Squared (RMS) COP Velocity (vel.) in Anteroposterior (AP) and Mediolateral (ML) Directions in Wide Stance and Modified Romberg Stance
| Wide stance
|
Modified Romberg stance
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Measure | COP AP range (m/m) | COP AP RMS vel. (m/s) | COP ML range (m/m) | COP ML RMS vel. (m/s) | COPAP range (m/m) | COP AP RMS vel. (m/s) | COP ML range (m/m) | COP ML RMS vel. (m/s) |
| Young children | ||||||||
| M | 0.121 | .026 | 0.185** | 0.0488 | 0.142 | 0.036* | 0.294 | 0.063** |
| SD | 0.053 | 0.006 | 0.144 | 0.011 | 0.117 | 0.013 | 0.219 | 0.035 |
|
| ||||||||
| Older children | ||||||||
| M | 0.072 | 0.021 | 0.075 | 0.027* | 0.080 | 0.027 | 0.150 | 0.034* |
| SD | 0.030 | 0.006 | 0.035 | 0.009 | 0.027 | 0.006 | 0.050 | 0.009 |
|
| ||||||||
| Adults | ||||||||
| M | 0.035 | 0.009 | 0.022 | 0.012 | 0.042 | 0.015 | 0.102 | 0.022 |
| SD | 0.016 | 0.002 | 0.014 | 0.001 | 0.012 | 0.003 | 0.033 | 0.005 |
Significantly greater than adults (p ≤ .016).
Significantly greater than adults and older children.
WS
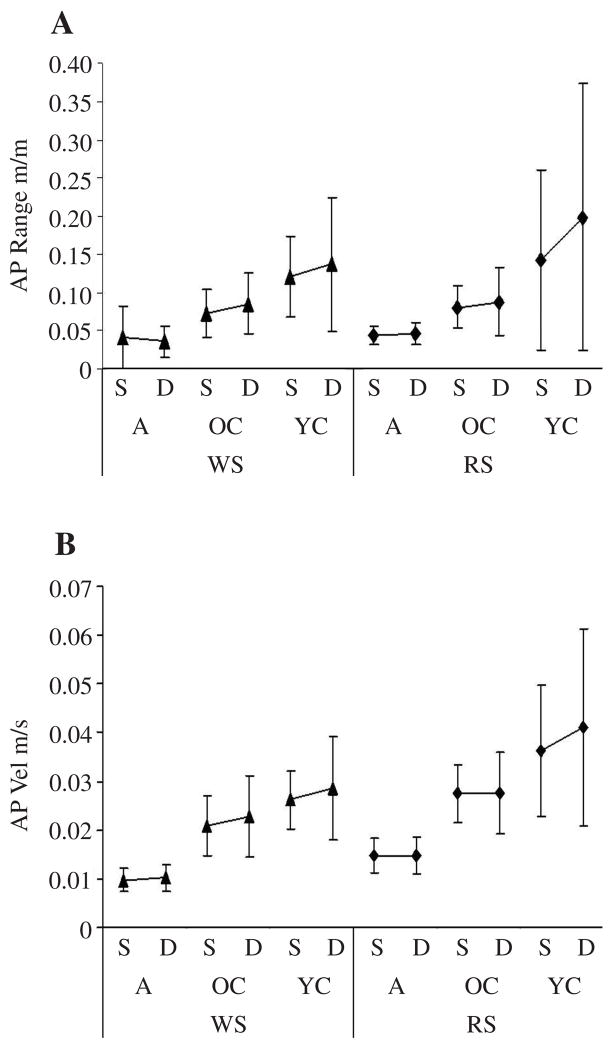
In the easy postural task (WS, with feet shoulder-width apart), the simple effect of YC versus A was significant in the AP range of COP displacement, F(2, 29) = 14.36, p < .0001, but only in the dual-task condition. Post hoc analysis showed that YC had a significantly greater range of COP displacements in the AP direction than did the A (p = .0023). The YC’s greater range of displacements resulted from interference YC experienced in controlling their range of body sway in the AP direction when they also performed the cognitive task. The simple effect of condition (dual vs. single task) was significant in the range of COP movements in the AP direction, F(3, 29) = 6.26, p = .0021, and in RMS AP velocity, F(3, 29) = 4.13, p = .0148. Post hoc analysis showed significance only for the YC in RMS AP velocity (p = .0043) and near significance in the AP range of COP movement (p = .030; see Figures 3A and 3B).
FIGURE 3.
Anteroposterior (AP) range and root mean squared (RMS) AP velocity. Graphs show group M ± SD of (A) AP range and (B) RMS AP velocity of center of pressure displacement in adults (A), older children (OC), and younger children (YC) in single (S) versus dual-task (D) conditions in wide stance (WS) and in modified tandem Romberg stance (RS).
Tandem RS
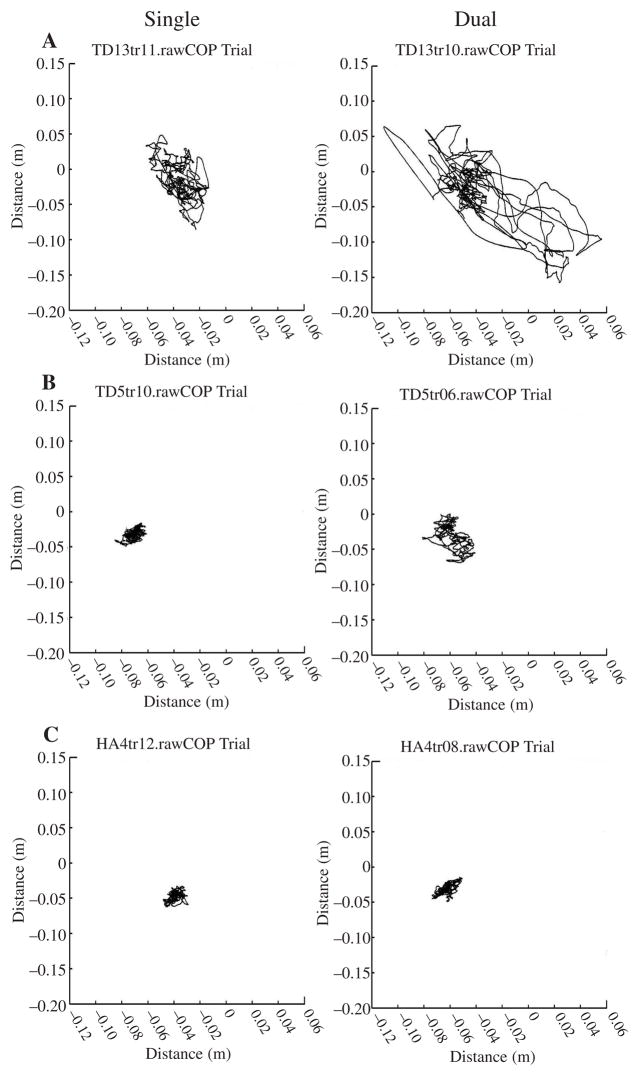
In the difficult RS postural task, the simple effect of YC versus OC was significant in the AP range of COP movement, F(2, 29) = 9.37, p = .0007. Post hoc analysis revealed that YC had a greater AP range, but only in the dual-task condition (p = .0002; see Figure 3A). The effect resulted from the near-significant dual-task effect that YC experienced but OC did not in the AP range of COP displacement, F(3, 29) = 2.75, p = .06. Post hoc tests of the simple effect of dual- versus single-task condition showed that the disruption in postural control in the AP range occurred only in the YC, p = .022. Individual-participant COP tracings in Figure 4 provide a clear comparison of postural control interference in the AP range experienced by a 4-year-old child, a 10-year-old child, and an adult. Other individual trials were less clear, although the overall effect of the secondary task was significant. For the YC, the dual-task effect on the range of COP displacements in the AP direction was significantly greater in RS (0.08 m/m) than in WS (0.02 m/m), p = .018 (see Figure 3A).
FIGURE 4.
Center of pressure (COP) tracings in tandem Romberg stance in single (left panels) vs. dual-task (right panels) condition. Graphs show individual-participant COP tracings in anteroposterior and mediolateral directions (COPx AP and COPy ML, respectively) of (A) young child, (B) older child, and (C) adult in a 38-s block of four trials.
Cognitive Task Performance
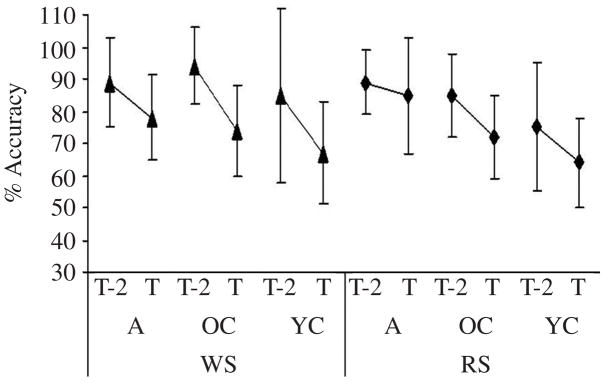
The comparison between the two degrees of cognitive task difficulty (T, T − 2) was significant for both WS, p = .003, and RS, p = .02, thus validating our use of a task with a comparable attentional load between age groups (see Figure 5).
FIGURE 5.
Group M and SD (bars) of rate of accuracy on the two degrees of cognitive task difficulty, threshold (T), and T − 2 shapes (T−2), in wide stance (WS) and (RS) for adults (A), older children (OC), and young children (YC).
Changes in proportions of correct responses and reaction times in WS versus RS determined the effect of stance condition on cognitive task performance. We determined developmental trends by comparing performances between the three age groups. There were no significant Group × Stance Configuration interaction effects and no main effect of group or stance configuration on reaction times or rate of accuracy in WS versus RS. Those findings demonstrate that the level of difficulty of the secondary task was equivalent across the three participant groups. Rate of accuracy was greater for the T − 2 trials than for the T trials for all age groups, confirming that participants performed the cognitive task at threshold levels (see Table 3).
Short-Term Memory Capacity
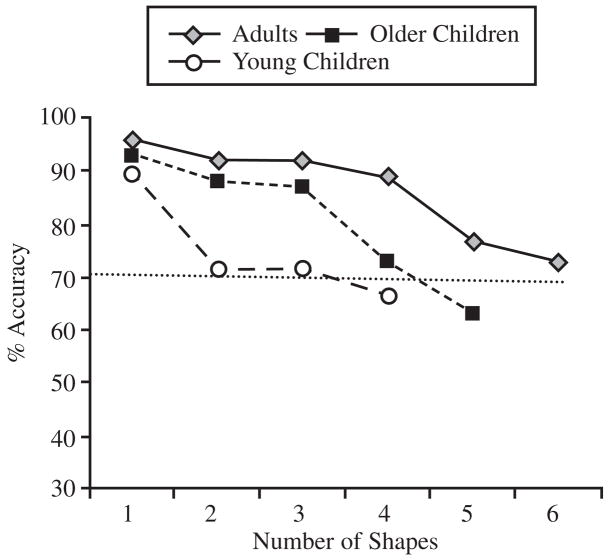
There was a significant developmental trend in short-term memory capacity, F(2, 9) = 23.57, p < .0001. The threshold for retention of colored shapes in short-term memory at the 70% accuracy rate was significantly less in YC (two to three shapes) than in OC (four to five shapes), p = .003, YC than in A (five to six shapes), p = .0002, and OC than in A, p = .025, as shown in the titration curve (see Figure 6).
FIGURE 6.
Titration curves for determining threshold of accuracy at 70% (T).
Discussion
Our main goal in this study was to examine the interaction between development of (a) postural control and (b) the executive function of attention in a dual-task condition. The accuracy of our interpretation of that interaction depended on our use of a research design that enabled us to control the attentional load of the cognitive task on the basis of the levels of the children. Previous researchers who investigated dual-task effects on postural control have not assessed the capacity of executive attention in the different participant groups and, at the same time, have not controlled for the attentional load of the cognitive task across groups (Blanchard et al., 2005; Brown et al., 1999; Kerr, Condon, & McDonald, 1985; LaJoie, Teasdale, Bard, & Fleury, 1993; Rankin et al., 2000; Shumway-Cook et al., 1997; Shumway-Cook & Woollacott, 2000; Teasdale et al., 1993). That failure has confounded their interpretation of the attentional requirement of postural control and their explanations for postural control interference in a dual-task condition. In our study, we resolved both issues by using a visual short-term memory task that we were able to manipulate to ensure a specific degree of difficulty (threshold). In addition, because of the link between the capacities of short-term memory and the efficiency of the executive function of attention, that task enabled us to obtain data concerning developmental trends in the executive function of the attentional system.
Because results of previous research (Cherng et al., 2003; Riach & Hayes, 1987; Ronncesvalles et al., 2001; Shumway-Cook & Woollacott, 1985) have shown an increase in both postural control and executive attentional function with age, we hypothesized that the younger children would experience the greatest interference in postural control in the dual-task conditions. First, we noted a developmental trend in the control of posture stability in both stance positions. In wide stance, children of both age groups (4–6 and 7–12 years) had a faster COP velocity in AP and ML directions than did adults. That finding supports the results of a similar study by Cherng et al. (2003), who demonstrated that children at 7–12 years of age have a greater frequency of body sway in the AP direction than do children older than 10 years of age. In the present study, children at 4–6 years of age swayed even faster and with greater distance in the ML direction than did the older children, supporting Shumway-Cook and Woollacott’s results. In the (modified tandem Romberg) single-task condition, we observed a similar developmental trend of postural control. Both age groups of children had significantly faster AP and ML body sway (COP velocity) than the adults did. However, it was the younger children who had the greatest range of sway in the ML direction in comparison with those of adults and older children, and the greater range of AP sway than that of adults.
Second, we noted a developmental trend in the capacity of the executive attentional system. We determined the trend from titration trials in which we assessed participants’ short-term memory (number of colored shapes accurately held in memory) at a 70% accuracy rate. The young children had the least developed memory capacity (two to three colored shapes) in comparison with those of the older children (four to five colored shapes) and the adults (five to six colored shapes).
In the dual-task condition, it was the younger children who experienced interference in postural control in the AP direction for both stance positions. In wide stance, interference in their control of velocity and range of body sway occurred. In the modified tandem Romberg stance, interference in their body sway range occurred. The 7- to 12-year-old children did not demonstrate postural control interference in the dual-task condition in either stance position. Those findings contradict the results of Blanchard et al. (2005), who examined dual-task effects on postural control in children at 8–10 years of age. The disparity between the two studies may have resulted from the level of difficulty of the different cognitive tasks or the level of difficulty of the postural control task. In the study by Blanchard et al., children stood in a narrow stance configuration (feet together). Another difference between the two studies is that in the dual-task condition, we eliminated the effect of articulation on body sway (Yardley et al., 1999) by selecting an interval of time between the presentation of the stimulus and participants’ motor response to measure COP, whereas Blanchard et al. did not.
Because development of the two processes of postural control and executive attention occur at similar rates, postural control interference in younger children could be the result of either the limited attentional resources available for performing both tasks simultaneously, the more unstable stance posture with its requirement of greater attentional resources for its control, or a combination of the two factors. The immaturity of plantar arch development in younger children, with the concomitant posterior location of the COP (Usui, Muekawa, & Hirasawa, 1995), could have reduced the posterior boundary of their stability limits, thus placing greater attentional demands on controlling body sway in the AP direction. In this study, there appeared to be a developmental trend in the location of the COP from a posterior position seen in younger children to a more anterior position that is observed in adults (see Figure 2).
Other researchers have shown that the sensory integrative function for postural control and executive attention develop at the same rate, reaching adult levels between the ages of 7–10 years (Forssberg & Nashner, 1982; Fourdriat, Di Fabio, & Anderson, 1993; Shumway-Cook & Woollacott, 1985), but we did not address that issue in this study. Postural control interference in younger children in the present study may also have resulted from immaturity of their sensory integrative function and a reduced ability to reweight the senses for postural control when the processing of visual stimuli relevant to maintaining balance competed with the processing of visual stimuli required for visual perception of colored objects. The older children and adults with the more developed sensory integrative function may have been able to reweight the control of posture stability through somatosensory and vestibular stimuli, thus avoiding the bottleneck of visual stimuli competing for processing.
We predicted that there would be a developmental trend in the attentional requirement for controlling posture stability in the more unstable position of the modified tandem Romberg stance. The differences in body sway between older children and adults increased in magnitude in the more difficult, modified tandem Romberg postural control task in comparison with that in shoulder-width stance, but it was the younger children who were the most unstable in the more difficult stance position. The rate of accuracy of all age groups on the cognitive task did not decline significantly when they performed the modified tandem Romberg stance. That finding indicates that when the older children and adults performed the attentionally demanding cognitive task in the modified tandem Romberg stance either they did not need more attentional resources than when they performed the same task in wide stance or their executive attention capacity was sufficiently developed for the simultaneous processing of the two attentionally demanding tasks. The finding that the younger children experienced dual-task interference in postural control in both stance positions, but an even greater interference in the modified tandem Romberg stance, suggests that the younger children had to allocate greater attentional resources to controlling posture stability in that position than in the easier postural control task of wide stance.
Limitations of the Study
Although our premise in this study was based on the limited capacity theory of attention (Kahneman, 1973), an alternate theory of selection for action may well explain the dual-task effects on postural control observed in the younger children. According to Neumann (1987), two tasks that are competing for attentional resources are not independent actions but instead are combined by the neuro-motor system into one skill that requires action planning. According to this model, the nervous system must integrate the two activities through action planning, and coordination of the performance of the two tasks improves with practice, with the result that the situation becomes a higher order single task. Pellecchia (2005) demonstrated that dual-task training can lessen dual-task effects on postural control, and concluded that that finding supports Neumann’s theory. Pellecchia stated that the fact that dual-task, but not single-task, training eliminated dual-task interference is counter to the prediction of limited capacity theories of attention. She believes that the dual-task training effect shows that individuals can best learn dual-task skills through dual-task practice rather than by practicing the tasks separately, thereby reducing the attentional resources required of each single task. Therefore, according to the model, the younger children may have had the greatest postural control interference in comparison with that experienced by the older children and adults because they had the least exposure to the dual-task settings.
In addition, the results of Stoffregen, Smart, Bardy, and Pagulayan (1999) suggest that postural tasks are not organized independently of other tasks in a dual-task context (called suprapostural tasks). Stoffregen et al. showed that when postural control is combined with certain visual tasks in adults, the visuomotor system may reduce postural sway to stabilize the visual image for performance of the second task. It is therefore possible that older children and adults have the ability to appropriately stabilize posture when performing a visually demanding task, whereas younger children do not. It is also possible that developmental differences in such visual functions as contrast sensitivity may influence the level of performance of different age groups when they perform a postural task and a secondary visual task (Kinsella-Shaw, Harrison, Colon-Semenza, & Turvey, 2006). However, we did not measure the different age groups’ visual contrast sensitivity.
Applications of This Research to the Academic Setting
Are the extraneous movements of younger children in the academic setting, especially those diagnosed with attention deficit disorders with hyperactivity, the result of a deficit of attention or are there other contributing factors? In this study, we provided evidence for an interaction between development of postural control and development of executive attention in dual-task situations. The older children with the more developed postural control and executive attention systems did not experience dual-task interference in either the postural or secondary task, even with an attentionally demanding cognitive task. The younger children, with less maturation of the two systems, experienced postural control interference that increased when the attentional load of the postural task increased. Therefore, early childhood educators should consider that interaction when planning an academic curriculum and creating an environment most conducive to learning. In younger children, an attentionally demanding cognitive task may overload the naturally limited attentional resources, resulting in postural control interference and manifesting as extraneous movements.
To reduce the attentional demands of postural control and optimize cognitive task performance in the dual-task condition, one must provide younger children with good postural support, especially when they use chairs, desks, or both. In this study, we showed that even when younger children were in the more unstable position (which could result in a loss of balance with increased instability), they compromised posture stability for the performance of the cognitive task. Because there may also be competition for visual resources between postural tasks and secondary tasks requiring visual processing, reducing extraneous visual stimuli in the classroom environment may decrease the degree of competition for those attentional resources.
Moreover, the results of this study support the need for educators to include in the curriculum for children at 4–6 years of age a program of perceptual-motor activities that emphasizes the development of postural control. The more automatic the postural control, the less attentional resources are required for postural control tasks and therefore the more attentional resources should be available for cognitive processing.
Acknowledgments
A grant from the National Institutes of Health to Marjorie H. Woollacott and Paul van Donkelaar supported this research. The authors thank Shirley Randolph for her clinical expertise and Cooper Boydston, Don Pate, and Jessie Chen for their technical support.
Biographies
Dinah S. Reilly is a school-based pediatric physical therapist. Her research insterests include normal and abnormal development of postural control.
Paul van Donkelaar teaches neurophysiology of concussion, systems neuroscience, human physiology, exercise as medicine and exercise and performance, and motor control. His current research interests are the neural mechanisms involved in attention, development of oculomotor control in children, and the influence of hand dominance on constraint-induced thereapy.
Sandy Saavedra is a pediatric physical therapist and a doctoral degree candidate. Her research interests include normal and abnormal development of motor control with emphasis on postural control, eye-hand coordination, and cerebral palsy.
Marjorie H. Woollacott teaches motor control and neural control of posture and locomotion. Her current research interests are motor control, balance and posture control, postural development across the lifespan, and rehabilitation and balance in patient populations.
References
- Achenbach T. Child Behavioral Checklist for ages 6–18. Burlington: University of Vermont Press; 2001. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Awh E, Jonides J. Overlapping mechanisms of attention and working memory. Trends in Cognitive Sciences. 2001;5:119–126. doi: 10.1016/s1364-6613(00)01593-x. [DOI] [PubMed] [Google Scholar]
- Awh E, Vogel EK, Oh SH. Interactions between attention and working memory. Neuroscience. 2006;139:201–208. doi: 10.1016/j.neuroscience.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Beauchet O, Dubost V, Gonthier R, Kressig RW. Dual-task-related gait changes in transitionally frail older adults: The type of the walking-associated cognitive task matters. Gerontology. 2005;51:48–52. doi: 10.1159/000081435. [DOI] [PubMed] [Google Scholar]
- Blanchard Y, Carey S, Coffey J, Cohen A, Harris T, Michlik S, et al. The influence of concurrent cognitive tasks on postural sway in children. Pediatric Physical Therapy. 2005;17:189–193. doi: 10.1097/01.pep.0000176578.57147.5d. [DOI] [PubMed] [Google Scholar]
- Brauer S, Woollacott MH, Shumway-Cook A. The interacting effects of cognitive demand and recovery of postural stability in balance-impaired elderly. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2001;56A:M489–M496. doi: 10.1093/gerona/56.8.m489. [DOI] [PubMed] [Google Scholar]
- Brauer S, Woollacott MH, Shumway-Cook A. The influence of a concurrent cognitive task on the compensatory stepping response to a stance perturbation in balance-impaired older adults. Gait & Posture. 2002;15:83–95. doi: 10.1016/s0966-6362(01)00163-1. [DOI] [PubMed] [Google Scholar]
- Brown LA, McKenzie NC, Doan JB. Age-dependent differences in the attentional demands of obstacle negotiation. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2005;60A:M924–M927. doi: 10.1093/gerona/60.7.924. [DOI] [PubMed] [Google Scholar]
- Brown LA, Shumway-Cook A, Woollacott M. Attentional demands and postural recovery: The effects of aging. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 1999;54A:M165–M171. doi: 10.1093/gerona/54.4.m165. [DOI] [PubMed] [Google Scholar]
- Burtner PA, Qualls C, Woollacott MH. Muscle activation characteristics of stance balance control in children with spastic cerebral palsy. Gait & Posture. 1998;8:163–174. doi: 10.1016/s0966-6362(98)00032-0. [DOI] [PubMed] [Google Scholar]
- Cherng RJ, Lee HY, Su FC. Frequency spectral characteristics of standing balance in children and young adults. Medical Engineering & Physics. 2003;25:509–515. doi: 10.1016/s1350-4533(03)00049-3. [DOI] [PubMed] [Google Scholar]
- Diamond A, Taylor C. Development of an aspect of executive control: Development of the abilities to remember what I said and “do as I say, not as I do. Developmental Psychobiology. 1996;29:315–334. doi: 10.1002/(SICI)1098-2302(199605)29:4<315::AID-DEV2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Nashner L. Ontogenetic development of postural control in man: Adaptation to altered support and visual conditions during stance. Journal of Neuroscience. 1982;2:545–552. doi: 10.1523/JNEUROSCI.02-05-00545.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourdriat BA, Di Fabio RP, Anderson JH. Sensory organization of balance responses in children 3–6 years of age: A normative study with diagnostic implications. International Journal of Pediatric Otorhinolaryngology. 1993;27:255–271. doi: 10.1016/0165-5876(93)90231-q. [DOI] [PubMed] [Google Scholar]
- Franjoine MR, Gunther JS, Taylor MJ. Pediatric Balance Scale. A modified version of the Berg Balance Scale for the school age child with mild to moderate motor impairment. Pediatric Physical Therapy. 2003;15:114–128. doi: 10.1097/01.PEP.0000068117.48023.18. [DOI] [PubMed] [Google Scholar]
- Hadders-Algra M, Brogen E, Forssberg H. Ontogeny of postural adjustments during sitting in infancy: Variation, selection, and modulation. Journal of Physiology. 1996;493:273–288. doi: 10.1113/jphysiol.1996.sp021382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D. Attention and effort. Upper Saddle River, NJ: Prentice Hall; 1973. [Google Scholar]
- Kane MJ, Bleckley MK, Conway ARA, Engle RW. A controlled-attention view of working memory capacity: Individual differences in memory span the control of visual orienting. Journal of Experimental Psychology: General. 2001;130:169–183. doi: 10.1037//0096-3445.130.2.169. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. Working memory capacity and the control of attention: The contributions of goal-neglect, response competition, and task set to Stroop interference. Journal of Experimental Psychology: General. 2003;132:47–70. doi: 10.1037/0096-3445.132.1.47. [DOI] [PubMed] [Google Scholar]
- Kerr B, Condon DM, McDonald LA. Cognitive spatial processing and the regulation of posture. Journal of Experimental Psychology. 1985;11:617–622. doi: 10.1037//0096-1523.11.5.617. [DOI] [PubMed] [Google Scholar]
- Kinsella-Shaw JM, Harrison SJ, Colon-Semenza C, Turvey MT. Effects of visual environment on quiet standing by young and old adults. Journal of Motor Behavior. 2006;38:251–264. doi: 10.3200/JMBR.38.4.251-264. [DOI] [PubMed] [Google Scholar]
- LaJoie Y, Teasdale N, Bard C, Fleury M. Attentional demands for static and dynamic equilibrium. Experimental Brain Research. 1993;97:139–144. doi: 10.1007/BF00228824. [DOI] [PubMed] [Google Scholar]
- Maylor EA, Wing AM. Age differences in postural stability are increased by additional cognitive demands. Journals of Gerontology Series B: Psycholgical Sciences and Social Sciences. 1995;51B:P143–P154. doi: 10.1093/geronb/51b.3.p143. [DOI] [PubMed] [Google Scholar]
- Neumann O. Automatic processing: A review of recent findings and a plea for an old theory. In: Prinz W, Sanders AF, editors. Cognition and motor processes. Berlin, Germany: Springer; 1984. pp. 255–293. [Google Scholar]
- Neumann O. Beyond capacity: A functional view of attention. In: Heuer H, Sanders AF, editors. Perspectives on perception and action. Mahwah, NJ: Erlbaum; 1987. pp. 361–394. [Google Scholar]
- Pellecchia G. Postural sway increases with attentional demands of concurrent cognitive task. Gait & Posture. 2003;18:29–34. doi: 10.1016/s0966-6362(02)00138-8. [DOI] [PubMed] [Google Scholar]
- Pellecchia G. Dual-task training reduces impact of cognitive task on postural sway. Journal of Motor Behavior. 2005;37:239–246. doi: 10.3200/JMBR.37.3.239-246. [DOI] [PubMed] [Google Scholar]
- Posner M, Rossman E. Effect of size and location of informational transforms upon short-term retention. Journal of Experimental Psychology. 1965;70:496–505. doi: 10.1037/h0022545. [DOI] [PubMed] [Google Scholar]
- Posner M, Rothbart M. Attention, self-regulation and consciousness. Philosophical Transactions of The Royal Society of London: Series B. Biological Sciences. 1998;353:1915–1927. doi: 10.1098/rstb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin J, Woollacott M, Shumway-Cook A, Brown L. Cognitive influence on postural stability: A neuromuscular analysis in young and older adults. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2000;55A:M112–M119. doi: 10.1093/gerona/55.3.m112. [DOI] [PubMed] [Google Scholar]
- Riach CL, Hayes KC. Maturation of postural sway in young children. Developmental Medicine and Child Neurology. 1987;29:650–658. doi: 10.1111/j.1469-8749.1987.tb08507.x. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van der Molen MW, Band GPH, Bashore TR. Sources of interference from irrelevant information: A developmental study. Journal of Experimental Child Psychology. 1997;65:315–341. doi: 10.1006/jecp.1997.2367. [DOI] [PubMed] [Google Scholar]
- Roncesvalles MN, Woollacott MH, Jensen JL. Development of lower extremity kinetics for balance control in infants and young children. Journal of Motor Behavior. 2001;33:180–192. doi: 10.1080/00222890109603149. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Rosnow RL, Rubin DB. Contrasts and effect sizes in behavioral research. Cambridge, England: Cambridge University Press; 2000. [Google Scholar]
- Rueda RM, Fan J, McCandliss BD, Halparin JD, Gruber DB, Lercari LP, et al. Development of attentional networks in childhood. Neuopsychologia. 2004;42:1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Sakaguchi M, Taguchi K, Miyashita Y, Katsuno S. Changes with aging in head and center of foot pressure sway in children. International Journal of Pediatric Otorhinolaryngology. 1994;29:101–109. doi: 10.1016/0165-5876(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Schrodt LA, Mercer VS, Giuliani CA, Hartman M. Characteristics of stepping over an obstacle in community dwelling older adults under dual-task conditions. Gait & Posture. 2004;79:279–287. doi: 10.1016/S0966-6362(03)00067-5. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, Woollacott M. The growth of stability: Postural control from a developmental perspective. Journal of Motor Behavior. 1985;17:131–147. doi: 10.1080/00222895.1985.10735341. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, Woollacott M. Attentional demands and postural control: The effect of sensory context. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2000;55A:M10–M16. doi: 10.1093/gerona/55.1.m10. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, Woollacott M. Motor control: Translating research into clinical practice. Philadelphia: Lippincott, Williams, and Wilkins; 2006. [Google Scholar]
- Shumway-Cook A, Woollacott M, Kerns KA, Baldwin M. The effects of two types of cognitive tasks on postural stability in older adults with and without a history of falls. Journals of Gerontology Series A: Medical Sciences and Biological Sciences. 1997;52A:M232–M240. doi: 10.1093/gerona/52a.4.m232. [DOI] [PubMed] [Google Scholar]
- Silsupadol P, Siu KC, Shumway-Cook A, Woollacott M. Training of balance under single- and dual-task conditions in older adults with balance impairment. Physical Therapy. 2006;86:269–281. [PubMed] [Google Scholar]
- Stoffregen TA, Smart LJ, Bardy BG, Pagulayan RJ. Postural stabilization of looking. Journal of Experimental Psychology. 1999;25:1641–1658. [Google Scholar]
- Sundermier L, Woollacott M. The development of balance control in children: Comparisons of EMG and kinetic variables and chronological and development groupings. Experimental Brain Research. 1985;136:340–350. doi: 10.1007/s002210000579. [DOI] [PubMed] [Google Scholar]
- Sveistrup H, Woollacott MH. Longitudinal development of the automatic postural response in infants. Journal of Motor Behavior. 1985;17:131–147. doi: 10.1080/00222895.1996.9941734. [DOI] [PubMed] [Google Scholar]
- Teasdale N, Bard C, LaRue J, Fleury M. On the cognitive penetrability of postural control. Experimental Aging Research. 1993;19:1–13. doi: 10.1080/03610739308253919. [DOI] [PubMed] [Google Scholar]
- Usui N, Muekawa K, Hirasawa Y. Development of the upright postural sway of children. Developmental Medicine and Child Neurology. 1995;37:985–996. doi: 10.1111/j.1469-8749.1995.tb11953.x. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Woodman GE, Luck SJ. Storage of features, conjunctions, and objects in visual working memory. Journal of Experimental Psychology: Human Perception and Performance. 2001;27:92–114. doi: 10.1037//0096-1523.27.1.92. [DOI] [PubMed] [Google Scholar]
- Wickens CD. Attention and skilled performance. In: Holding DH, editor. Human skills. New York: Wiley; 1989. pp. 71–105. [Google Scholar]
- Winter DA, Eng P. Kinetics: Our window into the goals and strategies of the central nervous system. Behavioral Brain Research. 1995;67:111–120. doi: 10.1016/0166-4328(94)00154-8. [DOI] [PubMed] [Google Scholar]
- Woollacott MH, Shumway-Cook A. Attention and the control of posture and gait: A review of an emerging area of research. Gait & Posture. 2002;16:1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- Yardley L, Gardner M, Leadbetter A, Lavie N. Effect of articulatory and mental tasks on postural control. NeuroReport. 1999;10:215–219. doi: 10.1097/00001756-199902050-00003. [DOI] [PubMed] [Google Scholar]