Abstract
C-telopeptides and related pyridinoline cross-links of bone Type I collagen are sensitive markers of bone resorption in osteolytic diseases such as osteoporosis and osteoarthritis. We have studied the release of C-telopeptide pyridinoline crosslinks of Type I collagen as measures of bone destruction in periodontal disease. Studies in preclinical animal models and humans have demonstrated the relationship between radiographic bone loss and crevicular fluid C-telopeptide levels. We have recently found that C-telopeptide levels correlate strongly with microbial pathogens associated with periodontitis and around endosseous dental implants. Host-modulation of bone-related collagen breakdown has been shown by studies in humans demonstrating that MMP inhibition blocks tissue destruction and release of C-telopeptides in patients with active periodontal disease.
Periodontal disease is initiated by microbial pathogens that elicit a host immune response with subsequent tissue destruction of the periodontal structures. 1 Patients afflicted with periodontitis experience breakdown of alveolar bone, periodontal ligament, and tooth root cementum. Uncontrolled periodontal tissue destruction eventually leads to tooth loss. A challenge in periodontology is the accurate and early assessment of tissue breakdown in patients with periodontal disease. Current methods of disease detection lack diagnostic sensitivity and specificity. Periodontal diagnostic procedures address several important functions, which include: screening; diagnosis of specific periodontal diseases; identification of sites or subjects at increased risk of experiencing progression of periodontal destruction; treatment planning; and monitoring of therapy.2
This paper focuses on the ability of the class of molecules known as the pyridinoline cross-links to identify tooth sites or subjects at an increased risk of experiencing periodontal tissue destruction. Research will be reviewed in preclinical and clinical investigations that illustrate the relationship among pyridinoline cross-links, periodontal pathogens, and alveolar bone loss. The paper concludes with recent developments in the inhibition of periodontal tissue destruction by matrix metalloproteinase (MMP) inhibitors such as doxycycline.
PYRIDINOLINE CROSS-LINKS:BIOCHEMISTRY AND CLINICAL CORRELATIONS
Pyridinoline cross-links have emerged as very promising biomarkers of bone resorption in an array of osteolytic diseases. Pyridinoline (hydroxylysl pyridinoline or Pyr) and deoxypyridinoline (lysyl pyridinoline or Dpy), N-telopeptides, and C-telopeptides have been the best studied members of this class of collagen-degradative molecules. 3 Newly formed collagen fibrils deposited in the extracellular matrix of bone are stabilized by mature cross-links formed by lysyl oxidase on lysine and hydroxylysine residues in the N-and C-terminal regions of collagen chains. This process results in the formation of divalent collagen cross-links that by further condensation yield trivalent Pyr and Dpy.
Osteoclastic bone resorption initiates the release of cross-linked immunoreactive telopeptides Pyr and Dpy. Urinary levels of Pyr and Dpy correlate with histological parameters of bone turnover from bone biopsies 4 as well as by radiopharmaceutical uptake at sites of bone resorption. 5 In bone turnover diseases such as osteoporosis, 6 rheumatoid arthritis, 7 and Paget’s disease, 8 increased levels of Pyr and Dpy are found in the circulation. In patients with metastatic bone disease, bisphosphanate therapy potently decreased circulating levels of Pyr and Dpy. 9 Furthermore, post-menopausal osteoporotic subjects experienced significant decreases in Pyr and Dpy after bisphosphanate 10 or estrogen 11 therapy, coincident with increases in spinal bone mineral density.
PYRIDINOLINE CROSS-LINKS AND BONE RESORPTION IN PERIODONTITIS
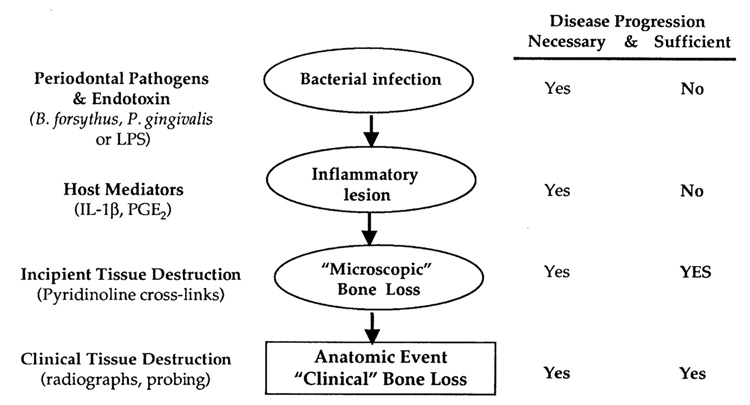
Given the important contribution of Pyr cross-links in systemic osteolytic diseases, over the past half of a decade researchers have begun to explore the ability of these molecules to detect bone resorption in the periodontium. The majority of diagnostic agents currently target the use of gingival crevicular fluid (GCF), which is an exudate that can be harvested from the gingival sulcus or periodontal pocket. As GCF traverses the inflamed tissue, it appears to carry molecules involved in the destructive process (FIG. 1). 12 Therefore, GCF offers great potential as a source for factors that may be associated with active tissue destruction. Several investigators have assessed the roles of various connective tissue breakdown products or enzymes involved in bone metabolism from GCF (reviewed in Refs. 13 and 14). However, most of these molecules are not bone-specific markers of tissue breakdown, and thus are simply mediators of combined soft and hard tissue inflammatory events (FIG. 2). Host factors in the GCF associated with the anatomic events of periodontitis and peri-implantitis may be useful as markers for identifying and predicting future disease progression. A biochemical marker specific for bone degradation may be useful to differentiate the presence of gingival inflammation from active periodontal and peri-implant bone destruction.14
FIGURE 1.
Release of pyridinoline cross-links during active alveolar bone resorption in periodontitis. Periodontitis is initiated by bacterial plaque and endotoxin, which cause a host immune response with recruitment of inflammatory cells such as neutrophils to the periodontal lesion. Neutrophils release MMP-8, which causes alveolar bone, periodontal ligament, and cementum destruction. Pyridinoline cross-links such as Dpy and C-telopeptide are released into the GCF soon after the initiation of osteoclastic bone resorption in periodontitis.
FIGURE 2.
Checkpoints of periodontal disease progression. This diagram demonstrates examples of pathogens, cytokines, tissue breakdown components, and clinical indices used to detect stages of active periodontitis. The pathway of pathogenic infection by bacteria and endotoxin leading to a host inflammatory response results in tissue destruction as measured by bone-degradative products (e.g., pyridinoline cross-links). The anatomic event of alveolar bone loss demonstrated on a radiograph or periodontal probing gives the patient’s diagnosis of periodontitis. Interventions identifying tissue destruction prior to significant anatomic events are goals of periodontal diagnostic tests.
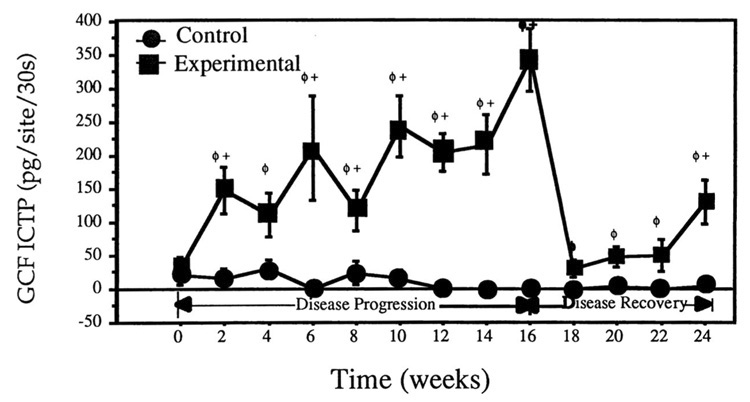
Recently, several studies have been conducted examining C-telopeptides in GCF. Talonpoika and Hämäläinen demonstrated strong correlations between C-telopeptide levels and clinical parameters of periodontal tissue destruction such as radiographic bone level and pocket depth. 15 Furthermore, they showed that in a small subset of patients provided periodontal therapy, dramatic reduction of GCF C-telopeptide levels resulted as soon as 2 days after treatment. In a ligature-induced experimental periodontitis study in dogs, the GCF levels of pyridinoline cross-links (C-telopeptide and deoxypyridinoline) significantly increased during the development of attachment loss and osteoclastic bone resorption 16,17 (FIG. 3). As early as 3 days after the initiation of experimental disease, tartrate-resistant acid phosphastase (TRAP+) mononuclear and TRAP+ multinucleated cells could be noted at the alveolar crest and gingival connective tissue. 17 The TRAP+ cells related strongly with elevated levels of Dpy in the serum, urine and GCF, suggesting that these markers are sensitive enough to be detected not only locally in the GCF but also systemically in the circulation. Moreover, C-telopeptide levels in GCF were highly sensitive and specific for predicting future alveolar bone loss as measured by computer-assisted digitizing radiography.16
FIGURE 3.
Crevicular fluid C-telopeptide levels in response to experimental periodontitis in beagles. Note significant increases in GCF C-telopeptide throughout the entire disease progression phase following the initiation of periodontal disease by silk ligatures. Once ligatures were removed, concomitant with scaling and root planing, GCF telopeptide levels decreased significantly compared to baseline for the first 6 weeks of the disease recovery phase followed by another significant increase in C-telopeptide at week 24. Error bars represent ± SE from a total of 36 samples/time point. +p < 0.05 compared to control; ϕp < 0.05 compared to baseline. (From Giannobile et al.16 Reproduced by permission.)
RELATIONSHIP BETWEEN PERIODONTAL PATHOGENS AND C-TELOPEPTIDES
Destructive periodontal diseases can be thought of as a series of infections that affect individual or multiple periodontal sites within an individual.1 A large body of literature supports an etiologic role of selected subgingival species such as Bacteroides forsythus, Porphyromonas gingivalis, and Treponema denticola in periodontal diseases. 18 These species may promote periodontal tissue destruction, including bone resorption, by the expression of a multitude of virulence factors.
Our group has recently demonstrated the relationship between GCF C-telopeptide levels and the associated subgingival microbiota in patients with periodontal disease. 19 Thirty-six periodontal subjects were evaluated for C-telopeptides in relation to a panel of subgingival species in subjects exhibiting various clinical presentations such as health, gingivitis, and periodontitis. Subgingival plaque and GCF samples were taken from tooth sites in each of 36 subjects. The presence and amounts of 40 subgingival taxa were determined in plaque samples using whole genomic DNA probes and checkerboard DNA-DNA hybridization, and GCF C-telopeptide levels were quantified by RIA. Clinical assessments made at the same sites included pocket depth and attachment level. Relationships between C-telopeptide levels and clinical parameters as well as subgingival species were determined by regression analysis. The results demonstrated significant differences among disease categories for GCF C-telopeptide levels for healthy persons (1.1±0.6 pg/site [mean ± SEM]) and patients with gingivitis (14.8 ± 6.6 pg/site) and periodontitis (30.3 ± 5.7 pg/site). C-telopeptide levels related modestly to several clinical parameters. Regression analysis indicated that C-telopeptide levels correlated strongly with mean subject levels of several periodontal pathogens including B. forsythus, P. gingivalis, P. intermedia, P. nigrescens, and T. denticola (p<0.01) (TABLE 1). The data indicate that there is a positive relationship between C-telopeptides and periodontal pathogens.
TABLE 1.
Relationship between prevealence of specific periodontal pathogens and mean subject C-telopeptide levels
| Species | r (s)* |
|---|---|
| C. rectus | 0.55 |
| B. forsythus | 0.54 |
| F. periodonticum | 0.53 |
| C. showae | 0.52 |
| P. intermedia | 0.49 |
| F. nucleatum ss nucleatum | 0.48 |
| P. nigrescens | 0.47 |
| E. nodatum | 0.46 |
| P. gingivalis | 0.42 |
| F. nucleatum ss polymorphum | 0.42 |
| T. denticola | 0.40 |
p< 0.01.
RELATIONSHIP BETWEEN PERIODONTAL PATHOGENS WITH C-TELOPEPTIDES AT ORAL IMPLANT FIXTURES
The treatment of edentulous and partially edentulous individuals with endosseous oral titanium implants is a predictable treatment option for tooth replacement. 20 However, a small portion of the population experiences peri-implantitis, an inflammatory condition characterized by both connective tissue and bone destruction that can result in dental implant failure. Current methods used to assess peri-implant and periodontal health include probing and radiography, which present a historical perspective, but give no information regarding current disease activity. Oringer and coworkers recently reported the application of the DNA-DNA hybridization technique on the enumeration of subgingival organisms associated with endosseous dental implant fixtures in 22 human subjects. 21 GCF and plaque samples were collected at implant and tooth sites. Radioimmunoassay techniques were utilized to determine GCF C-telopeptide levels, and plaque samples were analyzed utilizing checkerboard DNA-DNA hybridization. C-telopeptide levels and subgingival plaque composition were not significantly different between implants and teeth. Implant sites colonized by Prevotella intermedia, Capnocytophaga gingivalis, Fusobacterium nucleatum ss vincentii, and Streptococcus gordonii exhibited odds ratios of 12.4, 9.3, 8.1, and 6.7, respectively, of detecting C-telopeptide. These results suggest a relationship between elevated C-telopeptide levels at implant sites and some subgingival organisms associated with disease progression. Future studies should examine levels of C-telopeptide and other putative pyridinoline cross-links at implant fixtures exhibiting clinical signs of failure. Finally, longitudinal studies are necessary to examine the predictive ability of GCF C-telopeptide to identify the development of peri-implant bone loss.
BLOCKING OF PERIODONTAL TISSUE DESTRUCTION BY MATRIX METALLOPROTEINASES
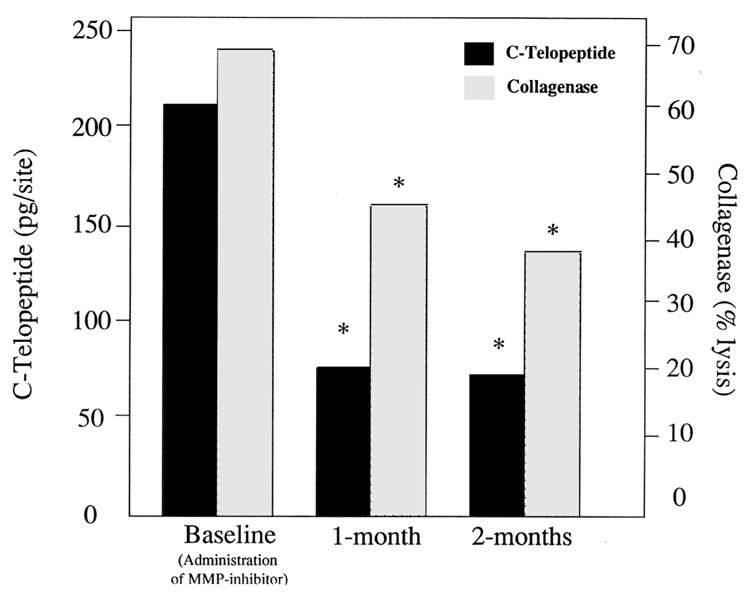
Studies performed by Golub and co-workers have helped to elucidate the ability of MMP inhibitors to block tissue destruction in patients with periodontal disease (reviewed in Refs. 22 and 23). We recently reported results from eighteen patients with moderate to severe periodontitis entered into an open-labeled study to examine the ability of systemically administered doxycycline (20 mg b.i.d.) on the reduction of tissue destruction. 24 At baseline, and at 1-and 2-month appointments, GCF samples were analyzed for C-telopeptide and osteocalcin by RIA, and collagenolytic enzyme activity and MMP species were studied by Western blotting. The results from this investigation revealed that C-telopeptide levels decreased nearly 70% after the first month of administration, while collagenase activity decreased more than 30% from baseline (FIG. 4). The reductions in GCF C-telopeptide and collagenase levels were sustained for an additional month. Interestingly, osteocalcin, a marker of bone turnover did not relate to C-telopeptides and did not predict response to therapy. Levels of matrix metalloproteinases (MMPs) −8 and −13 were decreased concomitant with C-telopeptide reductions during the 2-month observation period. These data suggest that MMP inhibitors, such as low-dose doxycycline, may block attachment and alveolar bone loss resulting from host-response modifiers such as MMPs.
FIGURE 4.
Doxycycline therapy inhibits collagenolytic breakdown of periodontal tissues. GCF C-telopeptide (by RIA) and collagenase (by % [3H-methyl] collagen α components degraded to αA fragments during incubation with 1.0 mM APMA at 22°C) in GCF of subjects with severe periodontitis before and during a 2-month regimen of 20 mg b.i.d. doxycycline. Each value represents the mean levels from 28 sites in 7 subjects. (*p < 0.05 as compared to baseline.) (From Weinberg and Bral.23 Reproduced by permission.)
CONCLUSIONS
Pyridinoline cross-links are important markers of bone resorption in a variety of bone metabolic diseases including periodontitis. These molecules may become important indicators of active tissue destruction in the accurate diagnosis of alveolar bone loss. Furthermore, the use of blockers of collagen breakdown such as matrix metalloproteinase inhibitors may prove useful in the control of periodontal tissue destruction as monitored by pyridinoline cross-links in periodontal patients.
ACKNOWLEDGMENTS
The collaborations and discussions with Drs. Sig Socransky, Anne Haffajee, Rich Oringer, Michael Palys, and Larry Golub are appreciated. These studies have been supported by grants from the NIH (Grants DE 04881 and DE 11814) and the Institute of Molecular Biology, Inc.
REFERENCES
- 1.Socransky SS, Haffajee AD. The nature of periodontal diseases. Ann.Periodontol. 1997;2:3–10. doi: 10.1902/annals.1997.2.1.3. [DOI] [PubMed] [Google Scholar]
- 2.Armitage GC. Periodontal diseases: diagnosis. Ann. Periodontol. 1996;1:37–215. doi: 10.1902/annals.1996.1.1.37. [DOI] [PubMed] [Google Scholar]
- 3.Calvo MS, Eyre DR, Gundberg CM. Molecular basis and clinical application of biologic markers of bone turnover. Endocrine Rev. 1996;17:333–368. doi: 10.1210/edrv-17-4-333. [DOI] [PubMed] [Google Scholar]
- 4.Delmas PD, Schlemmer A, Gineyts E, Riis B, Christiansen C. Urinary excretion of pyridinoline crosslinks correlates with bone turnover measured on iliac crest biopsy in patients with vertebral osteoporosis. J. Bone Mineral Res. 1991;6:639–644. doi: 10.1002/jbmr.5650060615. [DOI] [PubMed] [Google Scholar]
- 5.Eastell R, Hampton L, Colwell A, Green JR, Assiri AMA, Hesp R, Russell RGG. Urinary collagen crosslinks are highly correlated with radio-isotopic measurements of bone resorption. In: Christiansen C, Overgaard K, editors. Osteoporosis. Copenhagen: Osteopress ApS; 1990. pp. 469–470. [Google Scholar]
- 6.Eastell R, Robins SP, Colwell T, Assiri AMA, Riggs BL, Russell RGG. Evaluation of bone turnover in type I osteoporosis using biochemical markers specific for both bone formation and bone resorption. Osteoporosis Int. 1993;3:255–260. doi: 10.1007/BF01623829. [DOI] [PubMed] [Google Scholar]
- 7.Black D, Marabani M, Sturrock RD, Robins SP. Urinary excretion of the hydroxypyridinium cross links of collagen in patients with rheumatoid arthritis. Ann. Rheum. Dis. 1989;48:641–644. doi: 10.1136/ard.48.8.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uebelhart D, Gineyts E, Chapuy M-C, Delmas PD. Urinary excretion of pyridinium crosslinks: a new marker of bone resorption in metabolic bone disease. Bone Mineral. 1990;8:87–96. doi: 10.1016/0169-6009(91)90143-n. [DOI] [PubMed] [Google Scholar]
- 9.Kylmala T, Tammela TL, Risteli L, Risteli J, Kontturi M, Elomaa I. Type I collagen degradation product (ICTP) gives information about the nature of bone metastases and has prognostic value in prostate cancer. Br. J. Cancer. 1995;71:1061–1064. doi: 10.1038/bjc.1995.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garnero P, Shih WJ, Gineyts E, Karpf DB, Delmas PD. Comparison of new biochemical markers of bone turnover in late postmenopausal osteoporotic women in response to alendronate treatment. J. Endocrinol. Metab. 1994;79:1693–1700. doi: 10.1210/jcem.79.6.7989477. [DOI] [PubMed] [Google Scholar]
- 11.Yasumizu T, Hoshi K, Iijima S, Asaka A. Serum concentration of the pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (ICTP) is a useful indicator of decline and recovery of bone mineral density in lumbar spine: analysis in Japanese postmenopausal women with or without hormone replacement. Endocrine Journal. 1998;45:45–51. doi: 10.1507/endocrj.45.45. [DOI] [PubMed] [Google Scholar]
- 12.Cimasoni G. Crevicular Fluid Updated. Basel: Karger; 1983. [PubMed] [Google Scholar]
- 13.Lamster IB. Evaluation of components of gingival crevicular fluid as diagnostic tests. Ann. Periodontol. 1997;2:123–137. doi: 10.1902/annals.1997.2.1.123. [DOI] [PubMed] [Google Scholar]
- 14.Giannobile WV. Crevicular fluid biomarkers of oral bone loss. Curr. Opinion Periodontol. 1997;4:19–30. [PubMed] [Google Scholar]
- 15.Talonpoika JT, Hämäläinen MM. Type I carboxyterminal telopeptide in human gingival crevicular fluid in different clinical conditions and after periodontal treatment. J. Clin. Periodontol. 1994;21:21–34. doi: 10.1111/j.1600-051x.1994.tb00720.x. [DOI] [PubMed] [Google Scholar]
- 16.Giannobile WV, Lynch SE, Denmark RG, Paquette DW, Fiorellini JP, Williams RC. Crevicular fluid osteocalcin and pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (ICTP) as markers of rapid bone turn-over in periodontitis. J. Clin. Periodontol. 1995;22:904–910. doi: 10.1111/j.1600-051x.1995.tb01793.x. [DOI] [PubMed] [Google Scholar]
- 17.Shibutani T, Murahashi Y, Tsukada E, Iwayama Y, Heersche JNM. Experimentally induced periodontitis in beagle dogs causes rapid increases in osteoclastic resorption of alveolar bone. J. Periodontol. 1997;68:385–391. doi: 10.1902/jop.1997.68.4.385. [DOI] [PubMed] [Google Scholar]
- 18.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., JR Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 19.Palys MD, Haffajee AD, Socransky SS, Giannobile WV. Relationship between C-telopeptide pyridinoline cross-links (ICTP) and putative periodontal pathogens in periodontitis. J. Clin. Periodontol. 1998;25:865–871. doi: 10.1111/j.1600-051x.1998.tb02383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiorellini JP, Martuscelli G, Weber HP. Longitudinal studies on implant systems. Periodontology 2000. 1998;17:125–131. doi: 10.1111/j.1600-0757.1998.tb00130.x. [DOI] [PubMed] [Google Scholar]
- 21.Oringer RJ, Palys MD, Iranmanesh A, Fiorellini JP, Haffajee AD, Socransky SS, Giannobile WV. C-telopeptide pyridinoline cross-links (ICTP) and periodontal pathogens associated with endosseous oral implants. Clin.Oral Implants Res. 1998;9:365–373. doi: 10.1034/j.1600-0501.1996.090602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryan ME, Ramamurthy S, Golub LM. Matrix metalloproteinases and their inhibition in periodontal treatment. Curr. Opinion Periodontol. 1996;3:85–96. [PubMed] [Google Scholar]
- 23.Weinberg MA, Bral M. Tetracycline and its analogues: A therapeutic paradigm in periodontal diseases. Crit. Rev. Oral Biol. Med. 1998;9:322–332. doi: 10.1177/10454411980090030501. [DOI] [PubMed] [Google Scholar]
- 24.Golub LM, Lee HM, Greenwald RA, Ryan ME, Sorsa T, Salo T, Giannobile WV. A matrix metalloproteinase inhibitor reduces bone-type collagen degradation fragments and specific collagenases in gingival crevicular fluid during adult periodontitis. Inflamm. Res. 1997;46:310–319. doi: 10.1007/s000110050193. [DOI] [PubMed] [Google Scholar]