Abstract
Background
Cyclooxygenase-2 (COX-2) is upregulated in pulmonary artery smooth muscle cells (PASMC) during hypoxia and may play a protective role in the lung’s response to hypoxia. Selective COX-2 inhibition may have detrimental pulmonary vascular consequences during hypoxia.
Methods and Results
To investigate the role of COX-2 in the pulmonary vascular response to hypoxia, we subjected wild-type and COX-2 deficient mice to a model of chronic normobaric hypoxia. COX-2 null mice developed severe pulmonary hypertension with exaggerated elevation of right ventricular systolic pressure, significant right ventricular hypertrophy, and striking vascular remodeling following hypoxia. Pulmonary vascular remodeling in COX-2 deficient mice was characterized by PASMC hypertrophy, but not increased proliferation. Furthermore, COX-2 deficient mice had significant upregulation of the ET-1 receptor (ETAR) in the lung following hypoxia. Similarly, selective pharmacologic inhibition of COX-2 in wild-type mice exacerbated hypoxia-induced pulmonary hypertension and resulted in PASMC hypertrophy and increased ETAR expression in pulmonary arterioles. Absence of COX-2 in vascular smooth muscle cells during hypoxia in vitro augmented traction forces and enhanced contractility of an extracellular matrix. Treatment of COX-2 deficient PASMC with iloprost, a prostaglandin (PG) I2 analog, as well as PGE2, abrogated the potent contractile response to hypoxia and restored the wild-type phenotype.
Conclusions
Our findings reveal that hypoxia-induced pulmonary hypertension and vascular remodeling is exacerbated in the absence of COX-2 with enhanced ETA receptor expression and increased PASMC hypertrophy. COX-2 deficient PASMC have a maladaptive response to hypoxia manifested by exaggerated contractility which may be rescued by either COX-2-derived PGI2 or PGE2.
Keywords: hypertension, pulmonary, hypertrophy, hypoxia, prostaglandins, remodeling, vasculature
Introduction
Pulmonary hypertension is a severe and frequently fatal disease commonly associated with chronic hypoxemia in disorders such as chronic obstructive pulmonary disease and interstitial lung disease.1,2 The hallmark of pulmonary hypertension is the development of elevated pulmonary vascular resistance leading to increased right ventricular afterload and ultimately progression to right heart failure and death. The mechanisms by which low resistance arterioles in the pulmonary circulation narrow include pulmonary vasoconstriction, in situ thrombosis, and pulmonary vascular remodeling.1 Vascular remodeling involves pathological changes in all three layers of the pulmonary arteries including endothelial dysfunction, smooth muscle cell hyperplasia and hypertrophy, as well as adventitial fibroblast proliferation, myofibroblast differentiation, and extracellular matrix deposition.3 Endothelial injury leads to release of potent vasoconstrictors including thromboxane A2 (TXA2) and endothelin-1 (ET-1) which can overwhelm the effects of endothelial-derived vasodilators such as prostacyclin (PGI2) and nitric oxide (NO), thereby promoting remodeling of the arteriolar wall.1–3
While current state-of-the art therapy with vasodilators, endothelin receptor antagonists, and phosphodiesterase inhibitors may stabilize disease and improve quality of life in patients with pulmonary hypertension,1,2 these agents do not reverse the underlying vascular remodeling process. There is therefore a need to identify novel pathways and potential therapeutic targets that target vascular remodeling to halt or reverse progression of this devastating disease.
The cyclooxygenase enzymes (COX-1 and COX-2), which catalyze conversion of arachidonic acid to a series of prostanoids, may play a key role in the development of pulmonary vascular remodeling in response to hypoxia. COX-2, the inducible isoform of cyclooxygenase, is upregulated by hypoxia in pulmonary artery smooth muscle cells (PASMC) and both elevated TXA2 levels and reduced PGI2 levels have been demonstrated in patients with idiopathic and secondary forms of pulmonary hypertension.4,5 Overexpression of PGI2 synthase in the lung protects against the development of hypoxia-induced pulmonary hypertension in mice6 and continuous administration of prostacyclin to patients with pulmonary arterial hypertension improves mortality and quality of life.7 Furthermore, deletion of the PGI2 receptor exacerbates vascular remodeling in a mouse model of hypobaric hypoxia-induced pulmonary hypertension.8 However, the role of COX-2 in hypoxia-induced pulmonary vascular remodeling has not yet been elucidated.
Recent studies have demonstrated accelerated atherosclerosis9,10 and vascular remodeling in mice lacking the PGI2 receptor.11 Deletion of the PGI2 receptor or selective COX-2 inhibition enhances vascular hyperplasia and remodeling of the systemic vasculature in murine models of transplant arteriosclerosis and flow-dependent vascular remodeling.11 As well, recent work suggests that COX-2 inhibition enhances platelet deposition and intravascular thrombosis in a rat model of hypobaric hypoxia-induced pulmonary hypertension.12 In addition, selective inhibition of COX-2 is associated with an increased incidence of adverse cardiovascular events.13–15 These potential vascular sequelae associated with pharmacologic COX-2 inhibition appear to arise from alterations in multiple vascular effectors, including PGI2 and PGE2, which may directly or indirectly modulate platelet function, vascular tone, and remodeling.15 Selective COX-2 inhibition may thus perturb the complex balance of vascular mediators and promote vascular remodeling and/or a pro-thrombotic state in susceptible patients.13,15
Given the potential consequences of COX-2 inhibition on the systemic vasculature, we examined the effect of COX-2 deficiency on the development of pulmonary hypertension and vascular remodeling in a mouse model of chronic hypoxia. Mice deficient in COX-2 developed an exaggerated response to hypoxia with elevated right ventricular systolic pressure (RVSP), striking pulmonary vascular remodeling, and severe right ventricular hypertrophy (RVH). Interestingly, absence of COX-2 during hypoxia led to increased PASMC hypertrophy, but did not affect smooth muscle cell proliferation under hypoxic conditions either in vivo or in vitro. In addition, deficiency of COX-2 during hypoxia resulted in significant upregulation of the ET-1 receptor (ETA), increased traction forces, and augmented contractility of PASMC on collagen gel matrices. This enhanced contractility was attenuated by both exogenous iloprost, a PGI2 analog, as well as PGE2. Our findings suggest that COX-2 plays a critical protective role in the pulmonary vasculature under hypoxic conditions and that selective COX-2 inhibition may be hazardous to patients with pulmonary hypertension, particularly under conditions of hypoxemia.
Methods
Detailed methods are described in the Expanded Methods in the Data Supplement, available online at http://circ.ahajournals.org.
Animals
Mice that were wild-type (+/+, WT) or homozygous null (−/−) for targeted disruption of COX-2 (B6:129S7-Ptgs2tm1Jed, Jackson Laboratories, Bar Harbor, ME) were studied.
Hypoxic Exposure and Hemodynamic Measurements
Eight- to 10-week old COX-2−/− and COX-2+/+ littermates were exposed to normobaric hypoxia (10% O2, OxyCycler chamber, Biospherix Ltd, Redfield, NY)16,17 or normoxia (21% O2) for 2 weeks. Eight- to 10-week old C57BL/6 WT mice were treated with vehicle or nimesulide (40 mg/L)11,18 in the drinking water during a 2 week exposure to hypoxia or normoxia. Following exposure, mice were anesthetized with sodium pentobarbital (60 mg/kg) and hemodynamic measurements were performed.17,19 The hearts were excised and the ventricles dissected and weighed. RVH was assessed by normalizing RV weight to total body weight (TBW) (RV weight/TBW).16,17
Histologic Analysis and Morphometry
Lungs were inflated, harvested, fixed in Methyl Carnoy’s solution, and embedded in paraffin. Sections were stained with hematoxylin and eosin (H&E)20 and immunostained for α-smooth muscle actin (α-SMA, 1:50).21,22 Remodeling was quantitated as described previously.16,17,19 Percent wall thickness was calculated as: wall thickness (%) = (areaext – areaint) ÷ areaext × 100, where areaext represents the external diameter and areaint represents the internal diameter of each vessel respectively.16,17,19 PASMC hypertrophy was calculated as: vessel wall area ÷ # nuclei per vessel and reported as area per cell.
Western Blot Analysis
Protein extracts from lungs exposed to hypoxia or normoxia were analyzed by Western blot analysis22,23 with a monoclonal α-SMA antibody (1:2000) and a monoclonal endothelin-1 receptor (ETA) antibody (1:500, BD Biosciences). Equal loading was confirmed with an anti-tubulin antibody (1:8000).
Cell culture
Primary aortic smooth muscle cells (VSMC) were isolated from COX-2−/− and COX-2+/+ embryos at 18.5 dpc as described.22,24 Primary PASMC were isolated from adult (8–10 week old) COX-2−/− and COX-2+/+ mice as described with modification.25 Hypoxia experiments were performed in an Invivo2 400 Hypoxia Workstation (Biotrace International BioProducts, Bothell, WA).26
Traction force microscopy
Contractile forces exerted by COX-2−/− and COX-2+/+ VSMC were assessed by traction force microscopy as described.27–29 Cells were exposed to hypoxia (1%) or normoxia for 24 h and in certain experiments, cells were treated with ET-1 (20 nM). Traction forces exerted by individual cells before and after ET-1 treatment were determined.
Collagen matrix contraction assay
COX-2−/− and COX-2+/+ PASMC and VSMC were plated on type I collagen gel matrices and exposed to hypoxia or normoxia for 24 h. Gel size was defined as the sum of the two longest gel diameters and gel contraction expressed as a percentage of the original gel size.30 In certain experiments, cells were treated with either PGE2 (1 μM), iloprost (1 μM), or vehicle (30% ethanol in PBS) during hypoxic exposure. In other experiments, cells were treated with either forskolin (10 μM) or vehicle (4% ethanol in DMEM) during hypoxic exposure. RPASMC were treated with NS-398 (5 μM) or vehicle (25% DMSO in PBS).
Statistical analysis
Data are presented as mean ± SEM. Statistical significance was determined by the Student’s t-test for comparisons between 2 groups, and analysis of variance (ANOVA) for comparisons between more than 2 groups or for multiple comparisons. Statistical significance was accepted at p<0.05.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Absence of COX-2 leads to exaggerated elevation of RVSP and severe RVH following chronic hypoxia
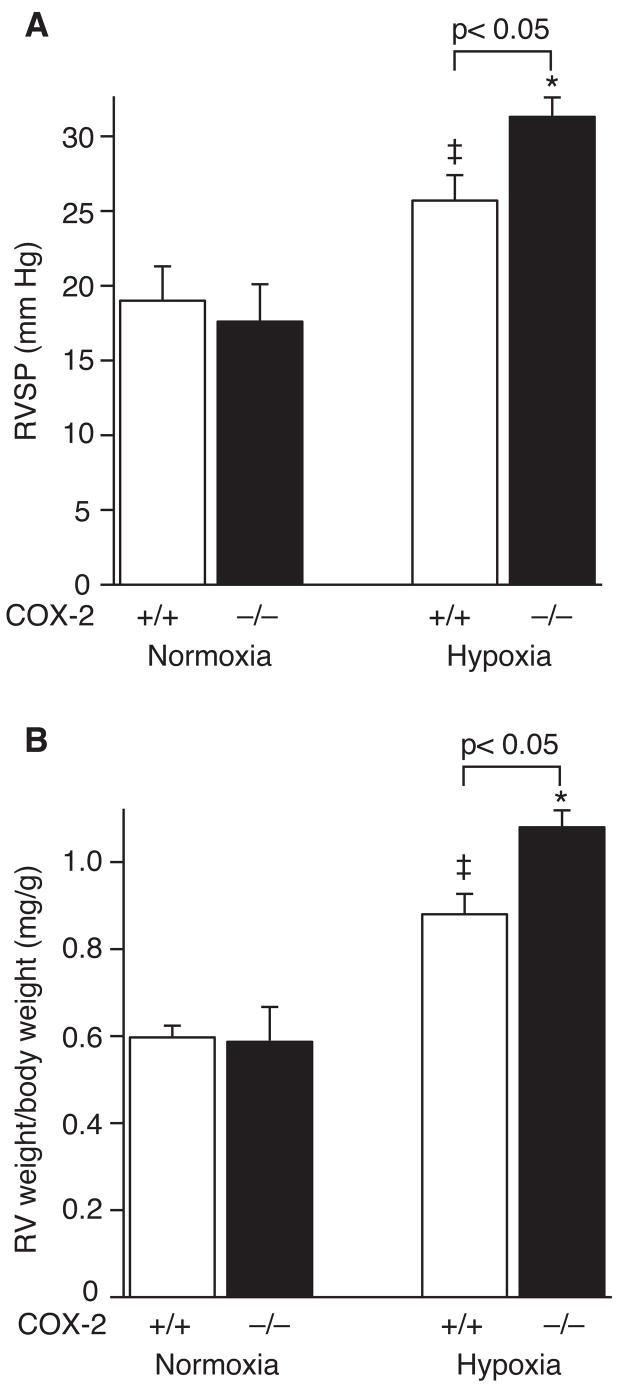
To determine the role of COX-2 in the pulmonary vascular response to hypoxia, COX-2−/− and COX-2+/+ mice were exposed to 2 weeks of normobaric hypoxia or normoxia. COX-2 was induced 5-fold in the lungs of wild-type (WT) mice following hypoxia with no difference in COX-1 expression between COX-2−/− and COX-2+/+ mice either at baseline or following exposure to hypoxia (Supplemental Figure 1). COX-2−/−mice developed significant elevation in RVSP (31.3 ± 1.3 mm Hg) compared with COX-2+/+ mice (25.7 ± 1.5 mm Hg; p<0.05) and normoxic controls (Figure 1A). In addition, COX-2−/− mice developed significant RVH (RV weight/body weight 1.08 ± 0.04 mg/g) compared with COX-2+/+ mice (0.88 ± 0.05 mg/g; p<0.05) and normoxic controls (Figure 1B). Total body weight was not different between COX-2−/− (24.1 ± 0.6 g) and COX-2+/+ (23.5 ± 0.45 g) mice.
Figure 1. COX-2−/− mice have exaggerated elevation of RVSP and severe RVH following chronic hypoxia.
A) RVSP in COX-2+/+ (□) and COX-2−/− (■) mice following hypoxia (n=18 per group) and normoxia (n=8 per group). Error bars represent SE (p<0.05 for hypoxic COX-2−/− mice vs. hypoxic COX-2+/+ mice, *p<0.05 for hypoxic COX-2−/− mice vs. normoxic controls, and ‡p<0.05 for hypoxic COX-2+/+ mice vs. normoxic controls). B) RV weight (mg) normalized for body weight (g) in COX-2+/+ (□) and COX-2−/− (■) mice following hypoxia (n=18 per group) and normoxia (n=8 per group). Error bars represent SE (p<0.05 for hypoxic COX-2−/− mice vs. hypoxic COX-2+/+ mice, *p<0.05 for hypoxic COX-2−/− mice vs. normoxic controls, and ‡p<0.05 for hypoxic COX-2+/+ mice vs. normoxic controls).
In addition, WT mice were treated with nimesulide, a selective COX-2 inhibitor, during exposure to hypoxia versus normoxia. Similar to COX-2−/− mice, nimesulide-treated WT mice developed more severe pulmonary hypertension following exposure to hypoxia with a significant increase in RVSP (26 ± 1 mm Hg) compared with vehicle-treated WT mice (22 ± 1.3 mm Hg; p<0.05) and normoxic controls. As well, nimesulide-treated WT mice developed exaggerated RVH in response to hypoxia with a significant percentage increase in RV weight (33%, p<0.05) compared with vehicle-treated WT mice.
Absence of COX-2 leads to enhanced pulmonary vascular remodeling following chronic hypoxia
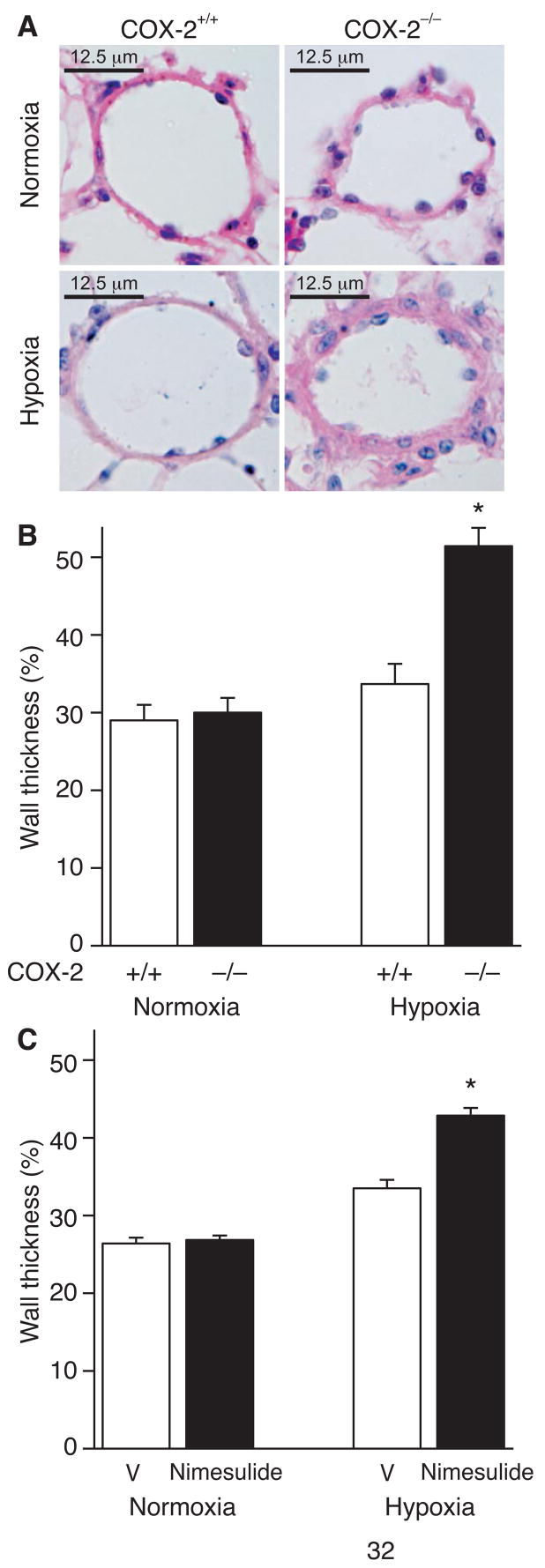
H&E staining revealed enhanced vascular remodeling in COX-2−/− mice following hypoxia compared with COX-2+/+ mice (Figure 2A). COX-2−/− mice developed exaggerated vascular remodeling with a significant increase in wall thickness of pulmonary arterioles (51 ± 2.4%) following hypoxia compared with COX-2+/+ mice (33.7 ± 2.6%; p<0.05) and normoxic controls (Figure 2B). Similarly, nimesulide-treated WT mice developed enhanced vascular remodeling following exposure to hypoxia with a significant increase in pulmonary arteriolar wall thickness (43 ± 1%) compared with vehicle-treated WT mice (33.5 ± 1.1%; p<0.05) and normoxic controls (Figure 2C).
Figure 2. Absence of COX-2 results in enhanced pulmonary vascular remodeling following chronic hypoxia.
A) Representative 5 μm H&E-stained sections from COX-2+/+ (left) and COX-2−/− (right) mice following normoxia (top) and hypoxia (bottom). Quantitation of percent wall thickness of pulmonary arterioles in the lungs of (B) COX-2+/+ (□) and COX-2−/− (■) mice following normoxia (n=5 per group) and hypoxia (n=8 per group) and (C) vehicle (V) and nimesulide-treated WT mice following normoxia (n=6 per group) and hypoxia (n=10 per group). Ten vessels were analyzed per mouse. Data are expressed as mean ± SE (*p<0.05 for hypoxic COX-2−/− mice vs. hypoxic COX-2+/+ mice and normoxic controls (B); *p<0.05 for nimesulide vs. vehicle-treated hypoxic WT mice and normoxic controls (C)).
Neither proliferation nor migration differ between COX-2−/− and COX-2+/+ VSMC exposed to hypoxia
To elucidate the mechanisms by which COX-2 modulates pulmonary vascular remodeling, we investigated the effect of COX-2 deficiency on VSMC proliferation and migration. As shown in Supplemental Figure 2, during exposure to hypoxia, neither proliferation nor migration differed between COX-2−/− and COX-2+/+ VSMC in response to PDGF. In addition, BrdU staining demonstrated no difference in proliferation in vivo between COX-2−/− and COX-2+/+ mice following hypoxia (data not shown), however there was a clear increase in the number of BrdU-positive cells in the pulmonary vessels of both hypoxic groups compared with baseline. These results demonstrate that neither enhanced VSMC proliferation nor migration accounts for the hypoxic vascular remodeling in COX-2−/− mice.
COX-2−/− mice have enhanced PASMC hypertrophy following hypoxia
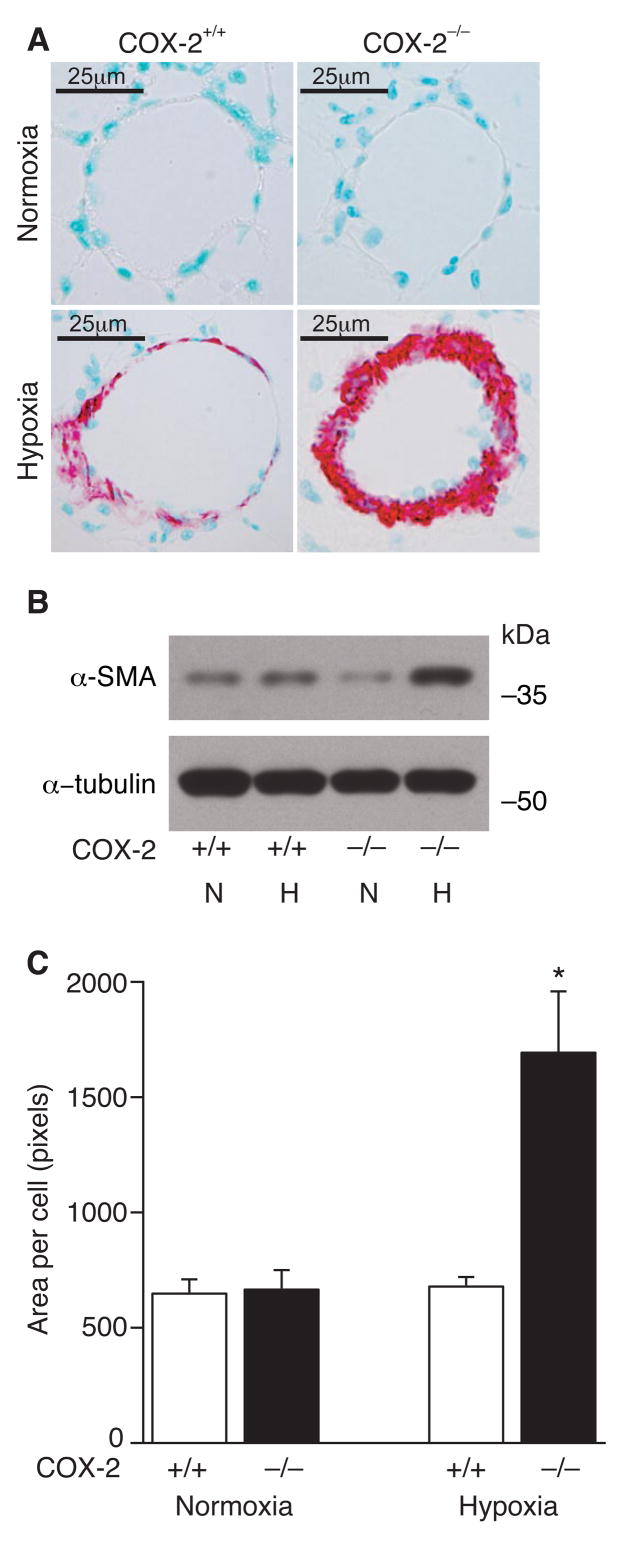
Trichrome staining revealed minimal collagen deposition in the distal remodeled vessels with no difference between COX-2−/− and COX-2+/+ mice (data not shown). However, immunostaining for α-SMA demonstrated that, following hypoxia, COX-2−/− mice developed striking vascular remodeling with neomuscularization of distal pulmonary arterioles, characterized by large neointimas containing α-SMA positive cells (Figure 3A). In contrast, COX-2+/+ mice developed significantly less remodeling with few α-SMA positive cells in remodeled vessels. To quantitate α-SMA in the lungs of COX-2−/− and COX-2+/+ mice following hypoxia and normoxia, Western blot analysis was performed for α-SMA. When corrected for loading, there was no difference in α-SMA protein expression between COX-2−/− and COX-2+/+ mice at baseline. However following hypoxia, lungs of COX-2−/− mice demonstrated a nearly 3-fold increase in α-SMA protein expression compared with only a 1.3-fold increase in lungs of COX-2+/+ mice (Figure 3B).
Figure 3. COX-2−/− mice have enhanced PASMC hypertrophy following hypoxia.
A) Immunostaining of lungs from COX-2+/+ (left) and COX-2−/− (right) mice following normoxia (top) and hypoxia (bottom) for α-SMA. B) Western blot analysis for α-SMA on lungs from COX-2+/+ and COX-2−/− mice following hypoxia and normoxia. C) Quantitation of PASMC size in COX-2+/+ (□) and COX-2−/− (■) mice. Ten vessels were analyzed per mouse following normoxia (n=5 per group) and hypoxia (n=8 per group). Data are expressed as mean ± SE (*p<0.05 for hypoxic COX-2−/− mice vs. hypoxic COX-2+/+ mice and normoxic controls).
These findings, in addition to our proliferation and migration results, suggested that smooth muscle cell hypertrophy may be the predominant mechanism driving hypoxic vascular remodeling in lungs of COX-2−/− mice. Indeed, morphometric analysis demonstrated that COX-2−/− mice developed significant PASMC hypertrophy following hypoxia with a significant increase in area per cell (1693 ± 266 pixels) compared with COX-2+/+ mice (678 ± 41 pixels; p<0.05) (Figure 3C). Similarly, nimesulide-treated WT mice developed exaggerated PASMC hypertrophy following exposure to hypoxia with a significant increase in area per cell (1405 ± 62 pixels) compared with vehicle-treated WT mice (686 ± 30 pixels; p<0.05) and normoxic controls (Supplemental Figure 3).
COX-2−/− VSMC have enhanced traction forces following hypoxia
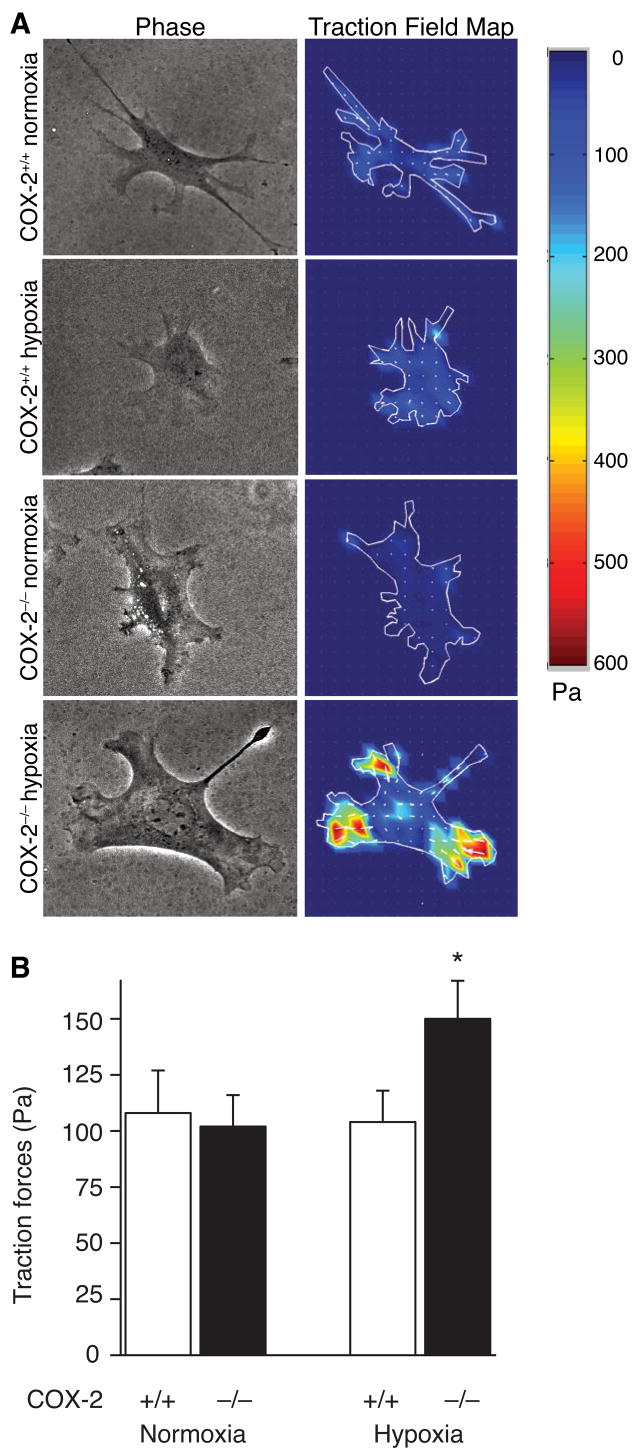
Previous studies with cultured pulmonary VSMC have shown that myosin light chain phosphorylation and cell contractility increase in parallel with cell area as the cells spread on the extracellular matrix.29 Thus, to determine whether a difference in VSMC contractility may contribute to the exaggerated pulmonary hypertension and vascular remodeling in COX-2−/− mice, we used traction force microscopy (TFM) to measure the traction forces exerted by individual VSMC following exposure to hypoxia in vitro (Figure 4A). COX-2+/+ and COX-2−/− VSMC had no difference in traction forces under normoxic conditions (COX-2−/− 102 ± 14 Pa; COX-2+/+ 108 ± 19 Pa), however following hypoxia, COX-2−/− VSMC developed a significant increase in traction forces (150 ± 18 Pa) compared with COX-2+/+ VSMC (104 ± 14 Pa; p<0.05) (Figure 4B). These data suggest that deficiency of COX-2 during hypoxia dramatically alters the contractile response of individual VSMC.
Figure 4. COX-2−/− VSMC have enhanced traction forces following hypoxia.
A) Representative phase contrast views (left) and traction field maps (right) in COX-2+/+ (top) and COX-2−/− (bottom) VSMC following normoxia (COX-2+/+, n=16 cells; COX-2−/−, n=23 cells) and hypoxia (COX-2+/+, n=27 cells; COX-2−/−, n=19 cells). Color scale indicates magnitude of traction in Pascals (Pa). B) Traction forces (Pa) in COX-2+/+ and COX-2−/− VSMC following normoxia and hypoxia. Data are expressed as mean ± SE (*p<0.05 for hypoxic COX-2−/− VSMC vs. COX-2+/+ VSMC and normoxic controls).
Absence of COX-2 during hypoxia leads to enhanced ETA receptor expression and exaggerated traction forces in response to ET-1
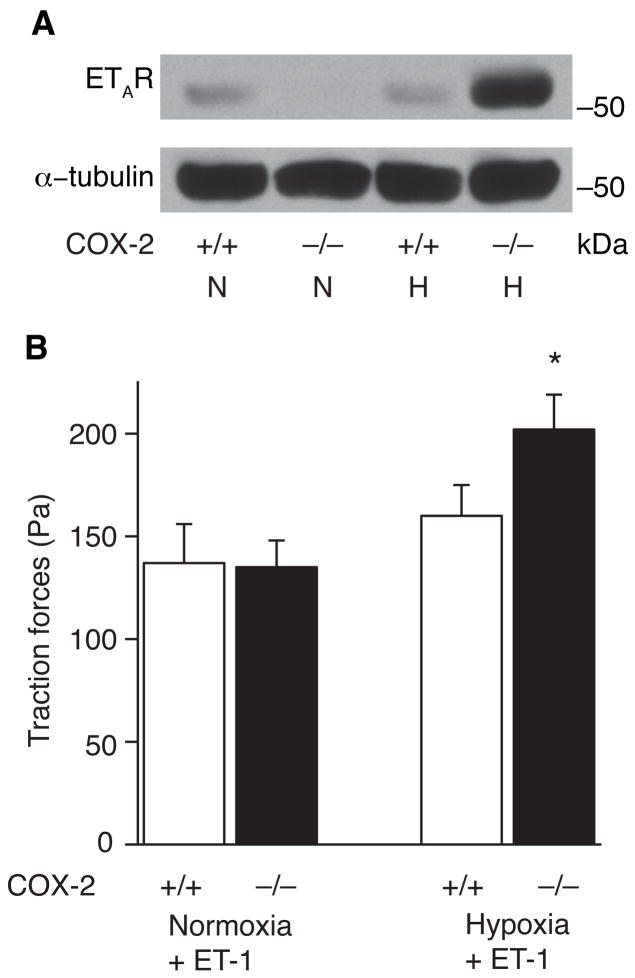
To investigate the mechanism by which deficiency of COX-2 augments contractility of vascular smooth muscle cells during hypoxia, we harvested protein from lungs of COX-2−/− and COX-2+/+ mice and performed Western blot analysis for the ETA receptor (ETAR). COX-2−/− mice had dramatic induction of the ETA receptor following hypoxia with a 5-fold induction in ETAR protein expression compared with only a 5% increase in COX-2+/+ mice (Figure 5A). In addition, following exposure to hypoxia, nimesulide-treated WT mice demonstrated a greater than 30-fold increase in ETAR expression in pulmonary arterioles by immunohistochemistry compared with only a 3-fold increase in vehicle-treated WT mice (Supplemental Figure 4). Furthermore, when COX-2+/+ and COX-2−/− VSMC were treated with ET-1 following hypoxia, COX-2−/− VSMC developed a significant increase in traction forces (202 ± 17 Pa) compared with COX-2+/+ VSMC (160 ± 15 Pa; p<0.05) and normoxic controls (Figure 5B).
Figure 5. Absence of COX-2 during hypoxia leads to enhanced ETA receptor expression in lungs and enhanced traction forces in response to ET-1.
A) Total protein was isolated from lungs of COX-2+/+ and COX-2−/− mice following hypoxia and normoxia and Western blot analysis performed for the ETA receptor. Loading was quantitated with an anti-tubulin antibody. A representative of three experiments is shown. B) Traction forces of COX-2+/+ and COX-2−/− VSMC following normoxia (COX-2+/+, n=16 cells; COX-2−/−, n=23 cells) and hypoxia (COX-2+/+, n=27 cells; COX-2−/−, n=19 cells) following stimulation with ET-1. Data are expressed as mean ± SE (*p<0.05 for hypoxic COX-2−/− VSMC vs. COX-2+/+ VSMC and normoxic controls).
COX-2−/− PASMC have enhanced contractility on collagen gels following hypoxia
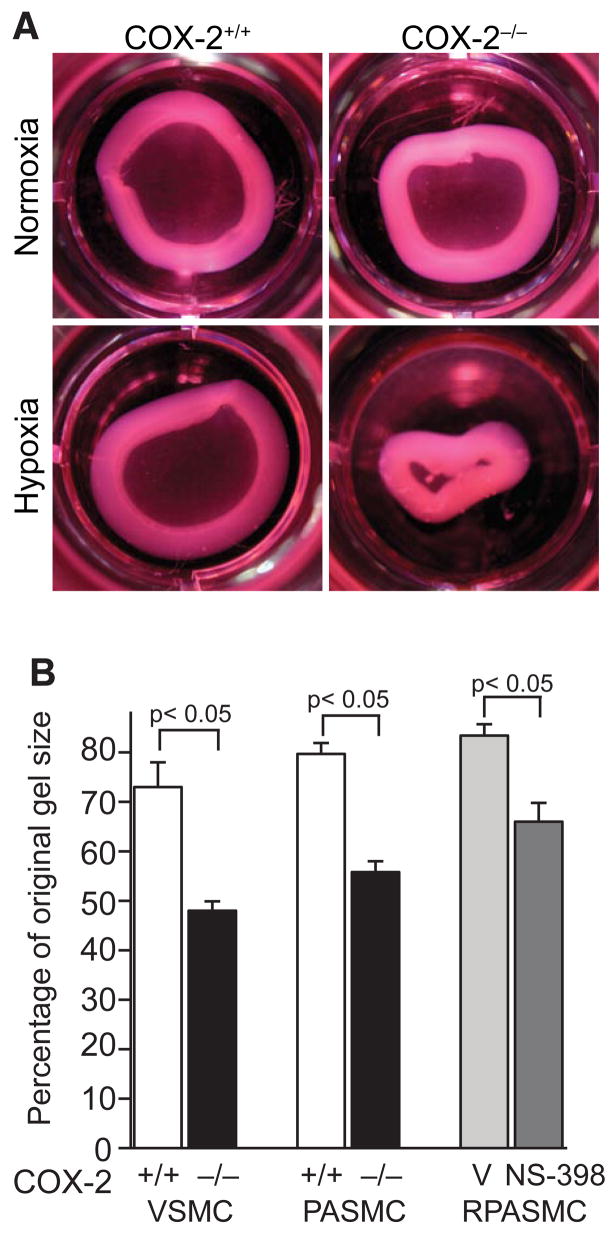
Given these findings, we investigated whether COX-2−/− PASMC would demonstrate enhanced contractility of a 3-dimensional collagen matrix. Consistent with our TFM results, COX-2−/− PASMC demonstrated enhanced contraction of collagen matrices following hypoxia (Figure 6A) compared with COX-2+/+ PASMC. At 4 h following matrix release, hypoxic COX-2−/− PASMC exhibited exaggerated gel contraction (55 ± 2.2% original gel size) compared with hypoxic COX-2+/+ PASMC (80 ± 2.3% original gel size; p<0.05) (Figure 6B). Similarly, COX-2−/− VSMC demonstrated exaggerated gel contraction (48 ± 1.9% original gel size) compared with COX-2+/+ VSMC (73 ± 5% original gel size; p<0.05) following hypoxic exposure (Figure 6B). In addition, pharmacologic inhibition of COX-2 in a rat pulmonary artery smooth muscle (RPASMC) cell line resulted in increased contraction (67 ± 3.4% original gel size) during hypoxia compared with vehicle control (83 ± 1.6% original gel size; p<0.05) (Figure 6B).
Figure 6. COX-2−/− PASMC have enhanced contractility on collagen gels following hypoxia.
A) Representative photographs of collagen gels from COX-2+/+ (left) and COX-2−/− (right) PASMC under normoxic (top) and hypoxic (bottom) conditions. B) Gel contraction following matrix release. Data are presented as the percentage of the original collagen gel size for VSMC, PASMC (COX-2+/+ □ and COX-2−/− ■), and RPASMC (vehicle
 and NS-398
and NS-398
 ) exposed to hypoxia. Data are expressed as mean ± SE (p<0.05 for COX-2−/− VSMC vs. COX-2+/+ VSMC; p<0.05 for COX-2−/− PASMC vs. COX-2+/+ PASMC; p<0.05 for NS-398-treated vs. vehicle-treated RPASMC).
) exposed to hypoxia. Data are expressed as mean ± SE (p<0.05 for COX-2−/− VSMC vs. COX-2+/+ VSMC; p<0.05 for COX-2−/− PASMC vs. COX-2+/+ PASMC; p<0.05 for NS-398-treated vs. vehicle-treated RPASMC).
Iloprost and PGE2 attenuate enhanced contractility of COX-2−/− PASMC on collagen gels following hypoxia
To determine if the administration of prostanoids could rescue COX-2−/− PASMC from this enhanced contractile response during hypoxia, we first analyzed the abundance and relative contribution of COX-2-derived prostanoids in the pulmonary versus the systemic circulation following hypoxia. COX-2−/− and COX-2+/+ PASMC and VSMC were exposed to hypoxia for 24 h and supernatants analyzed for PGE2 and 6-keto-PGF1α, a stable PGI2 metabolite. Levels of PGE2 were significantly higher in WT VSMC compared with WT PASMC following hypoxia. In addition, 6-keto-PGF1α was equally as abundant as PGE2 in WT VSMC following hypoxia, while almost 8-fold more abundant than PGE2 in hypoxia-exposed WT PASMC (Supplemental Figure 5). As expected, PGE2 and 6-keto-PGF1α levels were markedly lower in COX-2−/− PASMC and VSMC.
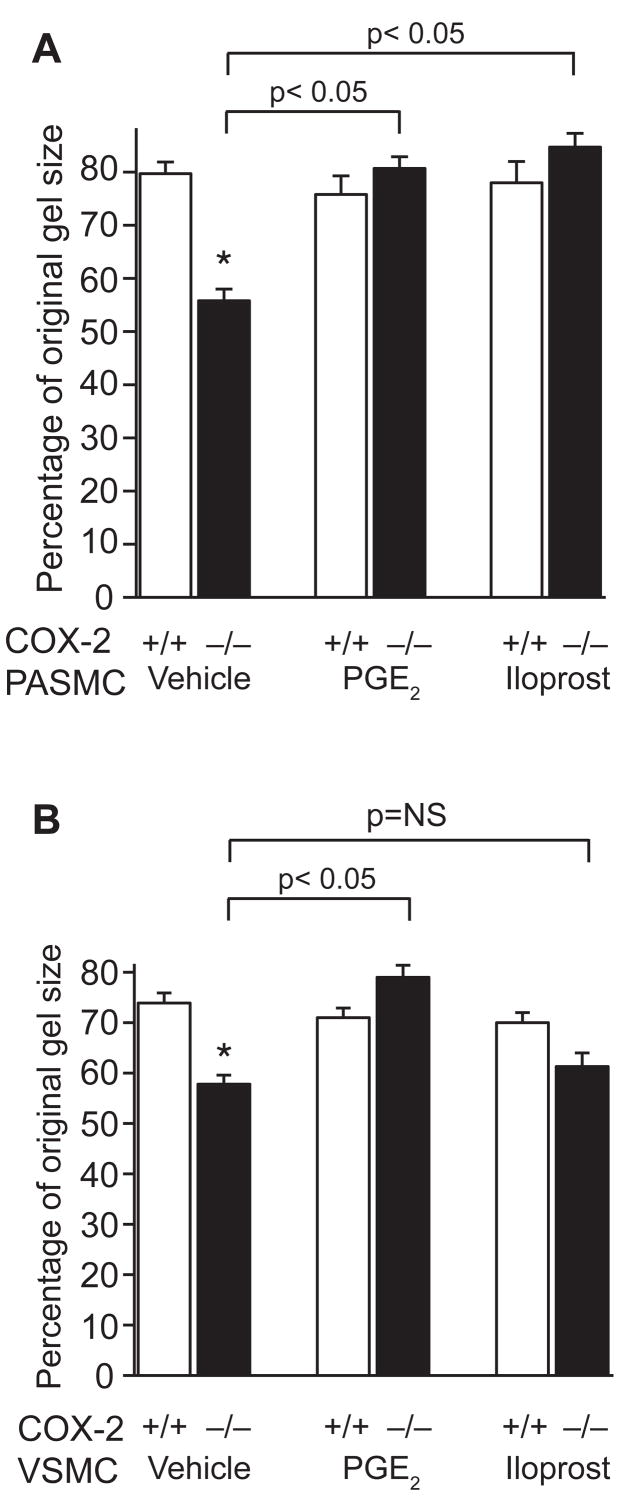
To investigate whether repletion of these COX-2-derived prostanoids would alter the contractile phenotype of COX-2−/− PASMC and VSMC during hypoxia, we performed collagen matrix contraction assays in the presence of exogenous PGE2 or iloprost, a PGI2 analog. Vehicle-treated COX-2−/− PASMC and VSMC both demonstrated exaggerated gel contraction following hypoxia compared with WT controls. Contraction by hypoxic COX-2−/− PASMC was significantly attenuated by either iloprost (84 ± 2.6% original gel size; p<0.05) or PGE2 (81 ± 2.3% original gel size; p<0.05) (Figure 7A). However, exaggerated contraction by COX-2−/− VSMC was attenuated only by PGE2 (79 ± 2.4% original gel size; p<0.05), not iloprost (61 ± 2.7% original gel size) (Figure 7B). In addition, to determine whether rescue of this phenotype is cAMP-mediated, we performed collagen contraction assays in the presence of forskolin, an activator of adenylate cyclase. Similar to PGE2, forskolin attenuated contraction of both COX-2−/− PASMC and VSMC following exposure to hypoxia (Supplemental Figure 6).
Figure 7. Iloprost and PGE2 attenuate contractility of COX-2−/− PASMC on collagen gels following hypoxia.
Gel contraction by COX-2+/+ and COX-2−/− PASMC (A) and VSMC (B) following treatment with prostanoid analogs (PGE2 1 μM, iloprost 1 μM) under hypoxic conditions. Data are presented as the percentage of the original collagen gel size for hypoxic PASMC and VSMC (COX-2+/+ □ and COX-2−/− ■) treated with PGE2, iloprost, or vehicle. Data are expressed as mean ± SE (*p<0.05 for vehicle-treated COX-2−/− PASMC vs. vehicle-treated COX-2+/+ PASMC, p<0.05 for PGE2-treated and iloprost-treated COX-2−/− PASMC vs. vehicle (A); *p<0.05 for vehicle-treated COX-2−/− VSMC vs. vehicle-treated COX-2+/+ VSMC, p<0.05 for PGE2-treated COX-2−/− VSMC vs. vehicle (B)).
Discussion
This study highlights three important new concepts. First, deficiency or pharmacologic inhibition of COX-2 is detrimental during exposure to hypoxia leading to exacerbation of pulmonary hypertension, accelerated vascular remodeling characterized by PASMC hypertrophy, and significant upregulation of the ETA receptor. Second, deficiency of COX-2 in vascular smooth muscle cells during hypoxia enhances contractile forces both at a cellular level and in their interactions with the extracellular matrix. Third, repletion of either COX-2-derived PGI2 or PGE2 to COX-2−/− PASMC attenuates their potent contractility in response to hypoxia, thus restoring the wild-type phenotype.
In this study, we examined the role of COX-2 in pulmonary vascular remodeling using a murine model of chronic hypoxia-induced pulmonary hypertension. COX-2 is upregulated in PASMC under hypoxic conditions12,31 and our data provide evidence that it plays a protective role in response to hypoxia. Pharmacologic inhibition of COX-2 is associated with remodeling of the systemic vasculature in murine models,11 however the effect of COX-2 deficiency on the pulmonary vasculature, particularly under conditions of hypoxemia, has not been fully defined. Recent work by Pidgeon et al. suggests that pharmacologic inhibition of COX-2 in a rat model of hypobaric hypoxia enhances platelet activation and intravascular thrombosis that was partially attenuated by a TX receptor antagonist.12 However, the effect of COX-2 deficiency on remodeling and contractility of PASMC in response to hypoxia has not yet been elucidated.
Our findings illustrate that COX-2 deficient mice develop severe pulmonary hypertension characterized by exaggerated elevation of RVSP, significant RVH, and striking vascular remodeling following only two weeks of hypoxia. In contrast, WT mice develop less severe pulmonary hypertension and minimal vascular remodeling in response to two weeks of hypoxia. In addition, selective pharmacologic COX-2 inhibition during exposure to chronic hypoxia led to an exaggerated response to hypoxia, similar to COX-2 null mice, with severe pulmonary hypertension and profound pulmonary vascular remodeling compared with vehicle-treated controls. We observed the same extent of cellular proliferation in COX-2−/− and COX-2+/+ mice following hypoxia, but COX-2−/− mice developed significant PASMC hypertrophy accounting for the dramatic vascular remodeling. Our findings suggest that this enhanced hypertrophic response of the pulmonary vasculature to hypoxia in COX-2 null mice may, in part, be due to enhanced expression of the ETA receptor during hypoxia, as ET-1 has been linked to VSMC hypertrophy.32,33 Interestingly, while PGI2 has been shown to have inhibitory effects on proliferation of human PASMC,31,34 our data demonstrate that genetic deficiency of COX-2 does not alter the proliferative response of the pulmonary arteriolar vasculature to hypoxia, but rather promotes a hypertrophic remodeling response.
In addition to vascular remodeling, pulmonary vascular resistance may also increase as a result of intravascular thrombosis following chronic hypoxia.1 We did not observe significant intravascular thrombosis in our mouse model as had been previously observed in a rat model of hypobaric hypoxia-induced pulmonary hypertension.12 Our findings now provide evidence that, in addition to vascular thrombosis, 12 COX-2 deficiency results in enhanced vascular remodeling which exacerbates the rise in pulmonary vascular resistance in response to hypoxia.
The present study also extends our understanding of how chronic hypoxia alters PASMC contractility at a cellular level. We have demonstrated that absence of COX-2 during hypoxia enhances traction forces generated in individual vascular smooth muscle cells and augments contractility of PASMC on an extracellular matrix. Previous work has illustrated a direct correlation between cellular traction forces and myosin light chain (MLC) phosphorylation in PASMC under normoxic conditions.29 As MLC phosphorylation and cell contractility have been shown to increase as cells enlarge by spreading on an extracellular matrix,29 our findings suggest that hypertrophy may explain the increased contractility of COX-2 deficient PASMC under hypoxic conditions. Upregulation of the ETA receptor in COX-2 null mice during hypoxia likely accounts for this enhanced contractile phenotype during hypoxia, as we found an exaggerated contractile response to ET-1 in COX-2 deficient VSMC. These findings expand upon prior work demonstrating that prostacyclin analogs can inhibit ET-1 release in human PASMC35 and intravenous prostacyclin may either increase ET-1 clearance or decrease its release in patients with idiopathic pulmonary hypertension.36 Furthermore, we have shown that this phenotype can be reversed with exogenous iloprost and, interestingly, PGE2 treatment. While attenuation of contractility with PGI2 was selective for PASMC, both PGE2 and forskolin, an activator of adenylate cyclase, rescued the contractile phenotype in both COX-2 null PASMC and VSMC, suggesting a cAMP-dependent mechanism.
Taken together, our results demonstrate that under hypoxic conditions, COX-2 deficient PASMC have an enhanced hypertrophic and contractile response to ET-1 due, in part, to upregulation of the ETA receptor. Our findings suggest that COX-2 induction during hypoxia attenuates expression of the ETA receptor via a cAMP-dependent signaling pathway, thereby modulating the contractile and growth-promoting effects of ET-1. We cannot, however, exclude other potential mechanisms of enhanced contractility that COX-2 may modulate. For example, both acute and chronic hypoxia may regulate activity or expression of voltage-gated potassium (Kv) channels, which could alter Ca2+ influx and activate MLC kinase.37,38 Potential downstream signaling mechanisms by which COX-2 may modulate ETAR expression and mediate protection against hypoxia-induced pulmonary vascular remodeling include the protein kinase A 33 and Epac (exchange protein directly activated by cAMP) 39 signaling pathways and will be the subject of future investigations.
In summary, our findings have revealed a novel role for COX-2 in mediating protection against hypoxia-induced pulmonary hypertension and vascular remodeling, as well as modulating PASMC contractility. Pharmacologic inhibition of COX-2 with selective COX-2 inhibitors has received significant attention in the literature recently. We now report that, in addition to well-recognized pro-thrombotic cardiovascular risks, selective COX-2 inhibition may have detrimental pulmonary vascular consequences. These findings may have significant clinical implications in patients with hypoxemic lung diseases or pre-existing pulmonary hypertension.
Supplementary Material
Acknowledgments
The authors thank Dr. Rebecca M. Baron, Dr. Matthew Layne, Dr. Shaw-Fang Yet, and Dr. Bruce D. Levy for helpful comments and suggestions, Dr. Jiao Wei and Bonna Ith for technical assistance, and Dr. Stephen R. Walsh for careful review of the manuscript.
Sources of Funding This work was supported by a Parker B. Francis Fellowship (LEF) and an American Lung Association Biomedical Research Grant (LEF), as well as grants from the National Heart, Lung, and Blood Institute including T32 HL0007633 (LEF), P50 HL67669 (DEI, SAM, & SK), R01 HL55454 (SK), R01 HL60788 (MAP), and R01 GM053249 (MAP).
Footnotes
Disclosures The authors report no conflicts.
References
- 1.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351:1655–1665. doi: 10.1056/NEJMra035488. [DOI] [PubMed] [Google Scholar]
- 2.Zaiman A, Fijalkowska I, Hassoun PM, Tuder RM. One hundred years of research in the pathogenesis of pulmonary hypertension. Am J Respir Cell Mol Biol. 2005;33:425–431. doi: 10.1165/rcmb.F307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006;99:675–691. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 4.Tuder RM, Cool CD, Geraci MW, Wang J, Abman SH, Wright L, Badesch D, Voelkel NF. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med. 1999;159:1925–1932. doi: 10.1164/ajrccm.159.6.9804054. [DOI] [PubMed] [Google Scholar]
- 5.Christman BW, McPherson CD, Newman JH, King GA, Bernard GR, Groves BM, Loyd JE. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med. 1992;327:70–75. doi: 10.1056/NEJM199207093270202. [DOI] [PubMed] [Google Scholar]
- 6.Geraci MW, Gao B, Shepherd DC, Moore MD, Westcott JY, Fagan KA, Alger LA, Tuder RM, Voelkel NF. Pulmonary prostacyclin synthase overexpression in transgenic mice protects against development of hypoxic pulmonary hypertension. J Clin Invest. 1999;103:1509–1515. doi: 10.1172/JCI5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubin LJ, Mendoza J, Hood M, McGoon M, Barst R, Williams WB, Diehl JH, Crow J, Long W. Treatment of primary pulmonary hypertension with continuous intravenous prostacyclin (epoprostenol). Results of a randomized trial. Ann Intern Med. 1990;112:485–491. doi: 10.7326/0003-4819-112-7-485. [DOI] [PubMed] [Google Scholar]
- 8.Hoshikawa Y, Voelkel NF, Gesell TL, Moore MD, Morris KG, Alger LA, Narumiya S, Geraci MW. Prostacyclin receptor-dependent modulation of pulmonary vascular remodeling. Am J Respir Crit Care Med. 2001;164:314–318. doi: 10.1164/ajrccm.164.2.2010150. [DOI] [PubMed] [Google Scholar]
- 9.Cheng Y, Austin SC, Rocca B, Koller BH, Coffman TM, Grosser T, Lawson JA, FitzGerald GA. Role of prostacyclin in the cardiovascular response to thromboxane A2. Science. 2002;296:539–541. doi: 10.1126/science.1068711. [DOI] [PubMed] [Google Scholar]
- 10.Egan KM, Lawson JA, Fries S, Koller B, Rader DJ, Smyth EM, Fitzgerald GA. COX-2-derived prostacyclin confers atheroprotection on female mice. Science. 2004;306:1954–1957. doi: 10.1126/science.1103333. [DOI] [PubMed] [Google Scholar]
- 11.Rudic RD, Brinster D, Cheng Y, Fries S, Song WL, Austin S, Coffman TM, FitzGerald GA. COX-2-derived prostacyclin modulates vascular remodeling. Circ Res. 2005;96:1240–1247. doi: 10.1161/01.RES.0000170888.11669.28. [DOI] [PubMed] [Google Scholar]
- 12.Pidgeon GP, Tamosiuniene R, Chen G, Leonard I, Belton O, Bradford A, Fitzgerald DJ. Intravascular thrombosis after hypoxia-induced pulmonary hypertension: regulation by cyclooxygenase-2. Circulation. 2004;110:2701–2707. doi: 10.1161/01.CIR.0000145613.01188.0B. [DOI] [PubMed] [Google Scholar]
- 13.Antman EM, DeMets D, Loscalzo J. Cyclooxygenase inhibition and cardiovascular risk. Circulation. 2005;112:759–770. doi: 10.1161/CIRCULATIONAHA.105.568451. [DOI] [PubMed] [Google Scholar]
- 14.Kearney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ. 2006;332:1302–1308. doi: 10.1136/bmj.332.7553.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grosser T, Fries S, FitzGerald GA. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J Clin Invest. 2006;116:4–15. doi: 10.1172/JCI27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christou H, Morita T, Hsieh CM, Koike H, Arkonac B, Perrella MA, Kourembanas S. Prevention of hypoxia-induced pulmonary hypertension by enhancement of endogenous heme oxygenase-1 in the rat. Circ Res. 2000;86:1224–1229. doi: 10.1161/01.res.86.12.1224. [DOI] [PubMed] [Google Scholar]
- 17.Minamino T, Christou H, Hsieh CM, Liu Y, Dhawan V, Abraham NG, Perrella MA, Mitsialis SA, Kourembanas S. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc Natl Acad Sci. 2001;98:8798–8803. doi: 10.1073/pnas.161272598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pratico D, Tillmann C, Zhang ZB, Li H, FitzGerald GA. Acceleration of atherogenesis by COX-1-dependent prostanoid formation in low density lipoprotein receptor knockout mice. Proc Natl Acad Sci. 2001;98:3358–3363. doi: 10.1073/pnas.061607398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yet SF, Perrella MA, Layne MD, Hsieh CM, Maemura K, Kobzik L, Wiesel P, Christou H, Kourembanas S, Lee ME. Hypoxia induces severe right ventricular dilatation and infarction in heme oxygenase-1 null mice. J Clin Invest. 1999;103:R23–29. doi: 10.1172/JCI6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fredenburgh LE, Baron RM, Carvajal IM, Mouded M, Macias AA, Ith B, Perrella MA. Absence of heme oxygenase-1 expression in the lung parenchyma exacerbates endotoxin-induced acute lung injury and decreases surfactant protein-B levels. Cell Mol Biol. 2005;51:513–520. [PubMed] [Google Scholar]
- 21.Yet SF, Layne MD, Liu X, Chen YH, Ith B, Sibinga NE, Perrella MA. Absence of heme oxygenase-1 exacerbates atherosclerotic lesion formation and vascular remodeling. FASEB J. 2003;17:1759–1761. doi: 10.1096/fj.03-0187fje. [DOI] [PubMed] [Google Scholar]
- 22.Wei J, Gorman TE, Liu X, Ith B, Tseng A, Chen Z, Simon DI, Layne MD, Yet SF. Increased neointima formation in cysteine-rich protein 2-deficient mice in response to vascular injury. Circ Res. 2005;97:1323–1331. doi: 10.1161/01.RES.0000194331.76925.5c. [DOI] [PubMed] [Google Scholar]
- 23.Chung SW, Chen YH, Yet SF, Layne MD, Perrella MA. Endotoxin-induced down-regulation of Elk-3 facilitates heme oxygenase-1 induction in macrophages. J Immunol. 2006;176:2414–2420. doi: 10.4049/jimmunol.176.4.2414. [DOI] [PubMed] [Google Scholar]
- 24.Gunther S, Alexander RW, Atkinson WJ, Gimbrone MA., Jr Functional angiotensin II receptors in cultured vascular smooth muscle cells. J Cell Biol. 1982;92:289–298. doi: 10.1083/jcb.92.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fouty BW, Grimison B, Fagan KA, Le Cras TD, Harral JW, Hoedt-Miller M, Sclafani RA, Rodman DM. p27(Kip1) is important in modulating pulmonary artery smooth muscle cell proliferation. Am J Respir Cell Mol Biol. 2001;25:652–658. doi: 10.1165/ajrcmb.25.5.4592. [DOI] [PubMed] [Google Scholar]
- 26.Wu X, Chang MS, Mitsialis SA, Kourembanas S. Hypoxia regulates bone morphogenetic protein signaling through C-terminal-binding protein 1. Circ Res. 2006;99:240–247. doi: 10.1161/01.RES.0000237021.65103.24. [DOI] [PubMed] [Google Scholar]
- 27.Butler JP, Tolic-Norrelykke IM, Fabry B, Fredberg JJ. Traction fields, moments, and strain energy that cells exert on their surroundings. Am J Physiol Cell Physiol. 2002;282:C595–605. doi: 10.1152/ajpcell.00270.2001. [DOI] [PubMed] [Google Scholar]
- 28.Dembo M, Wang YL. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys J. 1999;76:2307–2316. doi: 10.1016/S0006-3495(99)77386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polte TR, Eichler GS, Wang N, Ingber DE. Extracellular matrix controls myosin light chain phosphorylation and cell contractility through modulation of cell shape and cytoskeletal prestress. Am J Physiol Cell Physiol. 2004;286:C518–528. doi: 10.1152/ajpcell.00280.2003. [DOI] [PubMed] [Google Scholar]
- 30.Grinnell F, Ho CH, Lin YC, Skuta G. Differences in the regulation of fibroblast contraction of floating versus stressed collagen matrices. J Biol Chem. 1999;274:918–923. doi: 10.1074/jbc.274.2.918. [DOI] [PubMed] [Google Scholar]
- 31.Yang X, Sheares KK, Davie N, Upton PD, Taylor GW, Horsley J, Wharton J, Morrell NW. Hypoxic induction of COX-2 regulates proliferation of human pulmonary artery smooth muscle cells. Am J Respir Cell Mol Biol. 2002;27:688–696. doi: 10.1165/rcmb.2002-0067OC. [DOI] [PubMed] [Google Scholar]
- 32.Chua BH, Krebs CJ, Chua CC, Diglio CA. Endothelin stimulates protein synthesis in smooth muscle cells. Am J Physiol. 1992;262:E412–416. doi: 10.1152/ajpendo.1992.262.4.E412. [DOI] [PubMed] [Google Scholar]
- 33.Taurin S, Hogarth K, Sandbo N, Yau DM, Dulin NO. Gβγ-mediated prostacyclin production and cAMP-dependent protein kinase activation by endothelin-1 promotes vascular smooth muscle cell hypertrophy through inhibition of glycogen synthase kinase-3. J Biol Chem. 2007;282:19518–19525. doi: 10.1074/jbc.M702655200. [DOI] [PubMed] [Google Scholar]
- 34.Wharton J, Davie N, Upton PD, Yacoub MH, Polak JM, Morrell NW. Prostacyclin analogues differentially inhibit growth of distal and proximal human pulmonary artery smooth muscle cells. Circulation. 2000;102:3130–3136. doi: 10.1161/01.cir.102.25.3130. [DOI] [PubMed] [Google Scholar]
- 35.Wort SJ, Woods M, Warner TD, Evans TW, Mitchell JA. Cyclooxygenase-2 acts as an endogenous brake on endothelin-1 release by human pulmonary artery smooth muscle cells: implications for pulmonary hypertension. Mol Pharmacol. 2002;62:1147–1153. doi: 10.1124/mol.62.5.1147. [DOI] [PubMed] [Google Scholar]
- 36.Langleben D, Barst RJ, Badesch D, Groves BM, Tapson VF, Murali S, Bourge RC, Ettinger N, Shalit E, Clayton LM, Jobsis MM, Blackburn SD, Crow JW, Stewart DJ, Long W. Continuous infusion of epoprostenol improves the net balance between pulmonary endothelin-1 clearance and release in primary pulmonary hypertension. Circulation. 1999;99:3266–3271. doi: 10.1161/01.cir.99.25.3266. [DOI] [PubMed] [Google Scholar]
- 37.Mauban JR, Remillard CV, Yuan JX. Hypoxic pulmonary vasoconstriction: role of ion channels. J Appl Physiol. 2005;98:415–420. doi: 10.1152/japplphysiol.00732.2004. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Juhaszova M, Rubin LJ, Yuan XJ. Hypoxia inhibits gene expression of voltage-gated K+ channel alpha subunits in pulmonary artery smooth muscle cells. J Clin Invest. 1997;100:2347–2353. doi: 10.1172/JCI119774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci. 2006;31:680–686. doi: 10.1016/j.tibs.2006.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.