Abstract
Objective
To report the definitive diagnosis of anti-NMDA receptor (NMDAR) encephalitis in four Japanese women previously diagnosed with “juvenile acute nonherpetic encephalitis” of unclear etiology, and to describe their long-term follow-up in the absence of tumor resection.
Methods
We extensively reviewed the case histories with current clinical and laboratory evaluations that include testing for antibodies to NR1/NR2 heteromers of the NMDAR in serum/CSF available from the time of symptom onset (4 to 7 years ago) and the present.
Results
All patients sequentially developed prodromal symptoms, psychosis, hypoventilation, severe orofacial dyskinesias, and bizarre immunotherapy-resistant involuntary movements that lasted 1 to 12 months. Two patients required mechanical ventilation for 6 and 9 months. Initial tests were normal or unrevealing, including the presence of nonspecific CSF pleocytosis, and normal or mild changes in brain MRI. Eventually, all patients had dramatic recovery of cognitive functions, although one had bilateral leg amputation due to systemic complications. Antibodies to NR1/NR2 heteromers were found in archived serum or CSF but not in long-term follow-up samples. An ovarian teratoma was subsequently demonstrated in three patients (all confirmed pathologically).
Conclusion
1) These findings indicate that “juvenile acute nonherpetic encephalitis” or a subset of this disorder is mediated by an antibody-associated immune response against NR1/NR2 heteromers of the NMDA receptor (NMDAR). 2) Our patients’ clinical features emphasize that anti-NMDAR encephalitis is severe but potentially reversible and may precede by years the detection of an ovarian teratoma. 3) Although recovery may occur without tumor removal, the severity and extended duration of symptoms support tumor removal.
In recent years, a severe but often reversible encephalitis of unknown etiology that predominantly affects young women has been increasingly recognized in Japan.1 The disorder has received several names, including acute diffuse lymphocytic meningoencephalitis,2,3 acute reversible limbic encephalitis,4 acute juvenile female nonherpetic encephalitis,1 or juvenile acute nonherpetic encephalitis.5 No association has yet been made with infections, cancer, or specific autoantibodies, but given that most patients develop a prodromic viral-like illness, a postinfectious immune-mediated etiology has been postulated.1 We were impressed by the phenotypic similarities between this disorder and the recently characterized ovarian teratoma associated encephalitis (OTE).6–12 This is a treatment-responsive paraneoplastic disorder that occurs in association with antibodies to NR1/NR2 heteromers of the NMDA receptor (NMDAR).11,12 Therefore, we reasoned that similar antibodies could be associated with juvenile acute nonherpetic encephalitis in Japan. To test this hypothesis, we reassessed four women who developed the disorder 4 to 7 years ago13 and since then have had regular clinical follow-up.
METHODS
Since 1999, we have identified at Kitasato University Hospital four young women with a clinical picture13 similar to the recently described OTE.6–12 Dyskinesias were carefully monitored with a digital video recorder with permission of the patients’ families.
Initial studies at symptom presentation
Initial studies at symptom presentation included cancer screening with serum tumor markers; CT of the chest, abdomen, and pelvis; and gallium citrate 67 scintigraphy. Other studies included brain MRI, cerebral blood flow SPECT, 2-[18F]fluoro-2-deoxy-d-glucose PET (FDG-PET), EEG monitoring, tests of thyroid function, antinuclear antibodies, antithyroglobulin, antimicrosomal, anti-DNA, antineutrophil cytoplasmic antibodies, Sjögren antibodies, anticardiolipin antibodies, angiotensin-converting enzyme, antibodies to herpes simplex virus (HSV), human herpes virus (HHV)-6 and HHV-7, CSF cell count, routine chemistry, PCR for HSV DNA, and cytology. Paraneoplastic antibodies (Hu, Yo, Ri, Ma1, Ma2, CV2/CRMP5, Tr, amphiphysin) and voltage-gated potassium channel antibodies14 were examined in sera.
Studies at reassessment
In January 2007, after informed consent, we performed reevaluation studies in which antibodies to NR1/NR2 heteromers of the NMDAR were determined in sera and CSF kept frozen since the time of symptom onset (4 to 7 years) and sera from the present. The techniques used for antibody analysis were identical to those previously reported11 and were performed in the same laboratory. In addition, all patients underwent a pelvic MRI to rule out the presence of an ovarian teratoma. A detailed clinical description of each patient is described in appendix e–1 on the Neurology® Web site at www.neurology.org.
RESULTS
Clinical findings
The mean age of the patients at time of symptom onset was 25.8 years (range 17 to 33 years). Clinical features and tests are summarized in tables 1 and 2. All indicated tests for infections, autoimmunity, and classic paraneoplastic antibodies were negative. In all four patients, the clinical course progressed through five phases: prodromal, psychotic, unresponsive, hyperkinetic, and gradual recovery (table 1).
Table 1.
Clinical features
| Patient no.; age, y/sex; date onset* | Prodrome (time to psychosis, d) | Psychiatric symptoms | Seizure after psychosis | Time from psychosis to unresponsive phase, d | Clinical features of unresponsive phase | Hypoventilation (duration of ventilatory support) | Clinical features of dyskinesias | Duration of dyskinesias |
|---|---|---|---|---|---|---|---|---|
| 1; 26/F; Nov 1999 | Fever, headache (5) | Apathy, fear, obsession, delusion of persecution, hallucinations | Convulsive seizure | 11 | Eyes remained open, mute, akinetic and nonresponsive to verbal commands. No withdrawal to noxious stimuli. | 10 weeks | Orofacial dyskinesia, sustained jaw movements, and athetoid dystonic movements | 2 mos. |
| 2; 27/F; Apr 2000 | General fatigue (3) | Depression, intolerable loneliness, confusion, agitation | Convulsive seizure | 8 | Eyes remained open, mute, akinetic and nonresponsive to verbal commands. No withdrawal to noxious stimuli, displayed echo phenomena (mimicking examiner’s movements). | No endotracheal intubation was required. | Orofacial dyskinesia, athetoid dystonic movements | 3 weeks |
| 3; 17/F; Mar 2001 | Cold (8) | Fear, confusion, obsession, delusion, agitation | Tonic seizure | 5 | Eyes remained open, mute, non-responsive to verbal commands. No withdrawal to noxious stimuli. | 9 mos | Orofacial dyskinesia, sustained jaw-opening, athetoid dystonic or limb-swinging movements | 12 mos. |
| 4; 33/F; Jan 2003 | Fever, vomiting (5) | Confusion, apprehension, obsession, anxiety, fear | Not observed | 3 | Eyes remained open, mute, nonresponsive to verbal commands. No withdrawal to noxious stimuli. Catalepsy-like symptoms. | 6 mos. | Orofacial dyskinesia, forceful eye closure, athetoid dystonic or limb-swinging movements | 6 mos. |
Date of symptom presentation.
Table 2.
Diagnostic tests and outcome
| Patient no. |
CSF (at symptom presentation) |
MRI* | EEG* | CBF SPECT* (tracer) |
Treatment | Outcome (duration follow-up) |
Hospital stay, mos. |
Time to tumor diagnosis |
Teratoma, side, size |
|---|---|---|---|---|---|---|---|---|---|
| 1 | WBC 17 (PMN 6%, mono 94%), protein 21 mg/dL, glucose 97 mg/dL, OCB negative | NR | Diffuse delta activity without PD | NR (HMPAO) | Acyclovir, AED, midazolam | Full recovery(7 y and 4 mos.) | 3 | 7 y 3 mos. | Right ovary, 60 mm |
| 2 | WBC 8, (mono 100%), protein 30 mg/dL, glucose 80 mg/dL, OCB negative | NR | Irregular slow activity without PD | NR (HMPAO) | Acyclovir, AED, midazolam | Full recovery (7 y) | 2 | 6 y 10 mos. | Right ovary, 66 mm |
| 3 | WBC 51 (PMN 10%, mono 90%), protein 40 mg/dL, glucose 66 mg/dL OCB positive | Mild FLAIR hyperintensity in medial temporal lobes | Diffuse delta activity without PD | NR (HMPAO) | Acyclovir, IVIg, IV corticosteroids, AED, midazolam, propofol, pentobarbital | Gradually improved over 4 y (6 y) | 14† | — | Not detected |
| 4 | WBC 32 (PMN 1%, mono 99%), protein 25 mg/dL, glucose 51 mg/dL, OCB negative | NR | Diffuse delta activity without PD | Hyperemia in F-T (IMP) | Acyclovir, IVIg, IV corticosteroids, AED, midazolam, propofol | Gradually improved over 3 y; went back to work (4 y and 3 mos.) | 9‡ | 4 y 1 mo. | Right ovary, 44 mm |
MRI, EEG, and SPECT findings obtained during active phase of hyperkinetic involuntary movements.
Patient 3 was discharged from the hospital to home nursing care in an apparent vegetative state with a gastrostomy and tracheostomy.
Patient 4 was transferred to a rehabilitation center 9 months after admission.
WBC = white blood cells; PMN = polymorphonuclear cells; mono = mononuclear cells; OCB = oligoclonal bands; NR = not revealing; FLAIR = fluid-attenuated inversion recovery; PD = paroxysmal discharges; HMPAO = 99mTc-d,l-hexamethyl-propyleneamine oxime; F-T = frontotemporal; IMP = N-isoprpyl-p-123I iodoamphetamine; AED = antiepileptic drugs; IVIg = intravenous immunoglobulin.
Prodromal phase
All patients presented with non-specific cold- or viral-like symptoms (fever, fatigue, or headache) and, after a mean period of 5 days, developed psychobehavioral symptoms.
Psychotic phase
At this stage, all four patients had emotional disturbances (e.g., apathy, lack of emotion, depression, loneliness, fear); cognitive decline (difficulty in using a cellular phone or passing through an automatic ticket gate); and prominent schizophrenia-like symptoms, including disorganized thinking, compulsive ideation, delusions, hallucinations, and loss of self-awareness. Amnesia was not prominent at onset. Strange behaviors were noted by the families, such as patients’ staring at their reflection in a mirror with an odd smile (Patients 2, 3, and 4). Consequently, all patients were initially diagnosed with a psychiatric disorder. However, within 2 weeks (mean 6.8 days) of developing these symptoms, all had lapsed into unresponsiveness and were transferred to our center. Three patients had seizures before being transferred.
Unresponsive phase
On admission, patients were mute, akinetic, and unresponsive to verbal commands while keeping their eyes open, resembling catatonia.15 Bizarre and inappropriate smiling was noted on admission in two patients. Athetoid dystonic postures (figure 1A), echo phenomenon (mimicking the examiner’s movements), and catalepsy-like symptoms (figure e-1A) were also noted. Brainstem reflexes were normal, but patients did not move the eyes spontaneously or close them to a visual threat. Paradoxical findings raised the possibility of a psychogenic reaction or malingering; e.g., some patients were unresponsive to painful stimuli but resisted passive opening of the eyes. However, diffuse delta slowing on EEG was not compatible with a psychogenic reaction or malingering.
Figure 1.
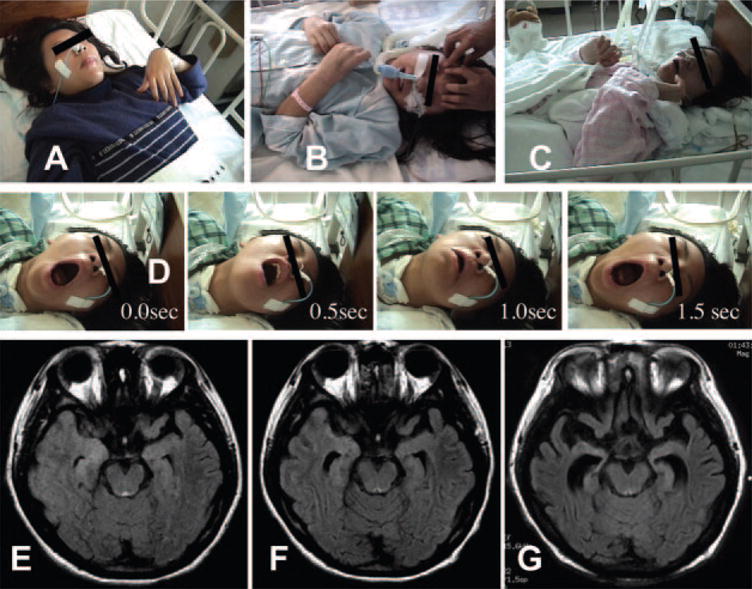
Static video clip images and of Patient 3
This patient gradually developed orolingual dyskinesia and athetoid dystonic postures (A), followed by bizarre involuntary movements including, clenching of the teeth (B), disconjugate ocular deviation (B, covered by black bar), rhythmic contractions of upper extremities synchronized with orofacial dyskinesias (B), unsynchronized arrhythmic wiggling of the hands (C), and forceful jaw opening (D). Panel D shows four sequential static video images taken every 0.5 seconds revealing continuous jaw opening movements mimicking jaw-opening dystonia. Video clips were recorded on the 3rd day of the hospital stay (A), the 23rd day (B), the 35th day (C), and the 32nd day (D). Serial fluid-attenuated inversion recovery MRI shows progressive brain atrophy in the mesiotemporal and frontal lobes (E, F, G). Brain MRIs were obtained on the 4th day (E), 48th day (F), and 11th month after admission (G).
Hyperkinetic phase
All patients gradually developed orolingual dyskinesias such as lip licking or chewing, and athetoid dystonic postures of the fingers (figures 1 and e-1). By the time the dyskinesias developed, none of the patients had received antipsychotic agents, but three had received IV phenytoin for convulsive seizures. Symptoms evolved to intractable bizarre orofacial-limb dyskinesias such as sustained jaw movements, forceful clenching of the teeth (figure e-1B), jaw-opening dystonia (figure 1D), grimacing (figure e-1B), intermittent ocular deviation or disconjugation, athetoid dystonic movements (figure 1, A and B, and figure e-1B), and dancing-like movements of the arms (figure e-1D). These dyskinesias varied in speed, distribution, and motor pattern, giving the impression of a psychogenic movement disorder (appendix e-1: features of dyskinesias). At this stage, all patients had symptoms of autonomic instability, including, among others, labile blood pressure, bradycardia or tachycardia, hyperthermia, and diaphoresis.
Other complications, treatment, and gradual recovery phase
All patients were initially treated with IV acyclovir (1,500 mg/day, 2 weeks), antiepileptic drugs, and anesthetics. Three patients developed central hypoventilation (unrelated to anesthetic agents) during the early stage of the hyperkinetic phase and required ventilatory support for 10 weeks to 9 months. In Patients 1 and 2, orofacial dyskinesias resolved within the first 2 months of the disease, and neuropsychiatric symptoms also improved. However, Patients 3 and 4 had persistent dyskinesias over 6 months, and multiple treatments were tried, including IV methylprednisolone and IV immunoglobulin, antiepileptic drugs (phenytoin, phenobarbital, zonisamide, clobazam, clonazepam, and valproate), quetiapine, trihexyphenidyl, and haloperidol. None of these drugs were effective, and the dyskinesias were eventually controlled with propofol and midazolam.
Overall, the duration of the hospital stay ranged from 2 to 14 months (mean 7 months). After discharge, all patients had spontaneous progressive improvement until recovery, which in two cases took more than 3 years. Patients 1, 2, and 4 made a full recovery both physically and cognitively; Patient 3 is cognitively intact (despite brain atrophy) but had amputation of the lower extremities due to deep vein thrombosis and infection. None of the patients has developed recurrent symptoms during a follow-up of 4 to 7 years.
MRI findings
In three patients, fluid-attenuated inversion recovery (FLAIR) and diffusion-weighted brain MRI images were unremarkable at symptom presentation. No abnormal gadolinium enhancement was seen. One patient had mild FLAIR hyperintensity in the medial temporal lobes (figure 1E). Frontotemporal atrophy was noted during the convalescent stage of two patients (figures 1G and e-3B).
SPECT and FDG-PET findings (appendix e-1)
SPECT studies showed no significant focal changes during the acute stage of the disease in three patients, but in Patient 4, three-dimensional stereotactic surface projection (SSP) maps showed frontotemporal hyperperfusion at the early stage (figure e-3A) and prefrontal hypoperfusion during convalescence (figure e-3D). In Patient 3, three-dimensional SSP maps showed hypoperfusion in the right inferior-frontal and temporal cortex during convalescence (not shown). In Patient 4, FDG-PET showed symmetric accumulation of the tracer in the primary motor, premotor, and supplementary motor areas, but not in the basal ganglia, during the time that the patient had severe orofacial dyskinesias (figure e-2C); however, no abnormal FDG uptake was seen during convalescence (figure e-2D).
Electrophysiologic findings
In all patients, scalp EEG monitoring during the unresponsive and hyperkinetic phases showed diffuse delta activity without paroxysmal discharges. In Patient 4, the slow EEG activity was replaced by drug-induced fast activity after increasing the dose of anesthetic agents (not shown). No giant somatosensory evoked potentials were demonstrated. Cortical responses were well preserved in two patients examined. Only Patient 4 developed EEG-confirmed convulsive status epilepticus 5 months after admission; however, her orofacial-limb dyskinesias did not have an epileptic correlate during extensive EEG monitoring.
Findings at reassessment: NMDAR antibodies and pelvic tumor
Archived sera and CSF from the time of symptom onset revealed in all patients the presence of antibodies to NR1/NR2B heteromers of the NMDAR (figure 2). In three patients with available serum and CSF, the presence of intrathecal synthesis of antibodies was confirmed (CSF antibody titers measured with HEK293 cells transfected with NR1/NR2B heteromers ranged from 1:500 to 1:2,000). These findings prompted a recall of the patients (year 2007) for serum antibody titers and tumor screening, including MRI of the pelvis. These studies showed that serum antibodies to NR1/NR2 heteromers were not longer detectable, but three of the patients had findings compatible with a teratoma of the ovary16 (figure 3). In retrospect, one of these patients had a small, 2-cm ovarian cyst detected by CT at the time of symptom presentation that on the current study had grown to 6 cm. Removal of the tumor in the three patients confirmed the diagnosis of mature cystic teratoma, which contained neural tissue in all three cases.
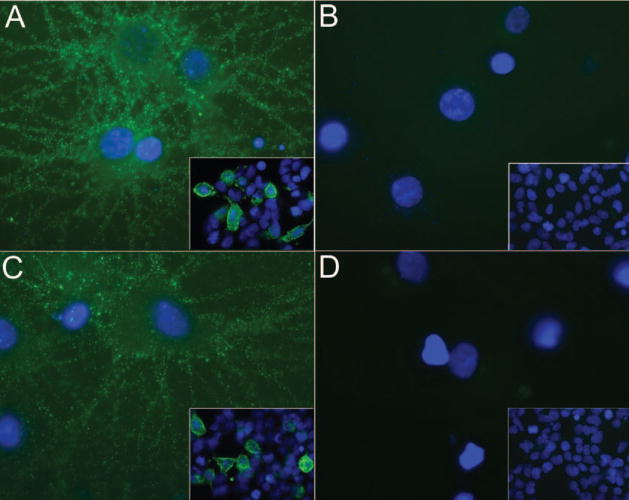
Figure 2.
Antibodies to NR1/NR2 heteromers of NMDA receptor
Panels A and C show nonpermeabilized cultures of hippocampal neurons incubated with sera of Patients 1 (A) and 3 (C) obtained at the time of symptom presentation. Panels B and D are similar cultures incubated with sera from the same patients obtained at follow-up 7 years 3 months and 6 years later. Note that sera obtained at the time of symptom presentation show intense immunolabeling of the cell surface of neurons and neuronal processes, whereas sera obtained at follow-up are not reactive. Insets correspond to sera from the same patients incubated with HEK293 cells expressing NR1/NR2B heteromers of the NMDA receptor. Sera from the time of symptom presentation (insets in A and C), but not from follow-up (insets in B and D), react with cells expressing NR1/NR2B heteromers (green cell membrane and cytoplasmic staining). The expression of NR1/NR2B heteromers was confirmed with double labeling with commercially available antibodies to NR1 and NR2B (not shown). Sera from controls (blood donors, lupus, and ovarian teratoma patients without encephalitis) did not show any reactivity with neurons or NR1/NR2B expressing cells (not shown). The methods used for these assays are identical to those previously reported.11 In all studies, sera were used at a dilution of 1:250. Immunofluorescence technique (A through D ×800, oil lens); all insets ×400. Nuclei of neurons and HEK293 cells visualized in blue with 4′,6-diamidino-2-phenylindole (DAPI).
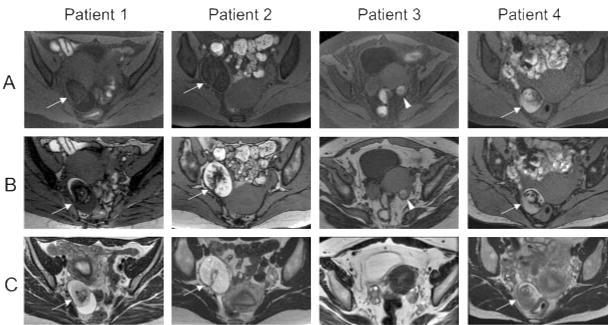
Figure 3.
Pelvic MRI of the patient obtained 4 to 7 years after symptom
An ovarian tumor is demonstrated in three patients (Patients 1, 2, and 4) on T1-weighted fat suppression images (FSIs, A), T1-weighted MRI (out-of-phase image, B), and T2-weighted MRI (C, arrows). Note that the T1-weighted hyperintensity in the tumors was suppressed on FSI, indicating the presence of fat, compatible with ovarian teratoma. In Patient 3, the T1-weighted hyperintensity in the left ovary was not suppressed on FSI (arrowhead), suggesting a follicular or corpus luteum hemorrhage; however, the possibility of bleeding from a cystic teratoma cannot be completely ruled out. No calcification was found on pelvic CT (not shown).
DISCUSSION
We report four Japanese women who were initially diagnosed with juvenile acute nonherpetic encephalitis,13 a disorder for which an etiology has remained unknown until now. Their clinical pictures had such similarity with the recently reported anti-NMDAR encephalitis in patients with ovarian teratoma11 that it led us to investigate this possible association. Hence, using sera and CSF archived since the time of symptom presentation, up to 7 years ago, we have now confirmed the presence of NMDAR antibodies in all patients. Analysis of the target antigens was performed as previously reported, demonstrating high expression in the hippocampus, detectable with immunohistochemical but not immunoblot techniques, expressed on the cell surface of neuronal cultures, and the identity unambiguously established with immunocytochemistry of HEK293 cells transfected to express functional NR1/NR2B heteromers of the NMDAR.11 In addition, the antibody binding required the presence of NR1 (NR1 dependent), differentiating these antibodies (NR1/NR2B) from the less-specific antibodies reported against linear epitopes of NR2B.11 These findings provide strong evidence of an immune mediated etiology for juvenile acute nonherpetic encephalitis.1–5 The detection of antibodies to NR1/NR2B also led to the subsequent demonstration of pelvic tumors in three patients, all with pathologically confirmed mature ovarian teratoma containing neural tissue. The experience gained from the close clinical monitoring and long-term follow-up of these cases has important implications.
The clinical features of these patients emphasize the concept that antibodies to NR1/NR2 heteromers of the NMDAR associate with a characteristic syndrome that develops in several predictable stages. The disorder usually presents with a prodromic episode of fever, headache, or malaise, followed a few days later by mood and behavioral changes (sometimes severe short-term memory loss), psychiatric symptoms suggesting schizophrenia or catatonia, decline of level of consciousness, hypoventilation, hyperkinesias, and eventual recovery or death. Compared with previously reported patients,11,12 whose tumors were removed at early stages of the disease, none of our patients had early tumor removal.
Recently, the “NMDAR hypofunction hypothesis” of schizophrenia has emerged primarily based on observations that NMDAR antagonists exacerbate psychiatric symptoms in schizophrenia and induce schizophrenic symptoms in healthy individuals whereas agents that enhance NMDAR function ameliorate schizophrenic symptoms.17–22 The psychomimetic effects of NMDAR antagonists have been attributed to the functional blocking of NMDAR in presynaptic γ-aminobutyric acid–mediated (GABAergic) interneurons of the thalamus and frontal cortex, causing a decrease of release of GABA. This results in disinhibition of postsynaptic glutamatergic transmission, excessive release of glutamate in the prefrontal cortex, and glutamate and dopamine dysregulation.22 Although NR1/NR2 heteromers are expressed in the entire nervous system, the main epitope targets of patients with NMDAR autoimmunity are contained in NR1/NR2B heteromers.11 These are preferentially expressed in the adult forebrain, including the prefrontal cortex, hippocampus, amygdala, and hypothalamus, all structures involved in our patients (figure e-3). Therefore, we postulate that NMDAR antibodies may cause inhibition, rather than stimulation, of the NMDAR, contributing to the development of schizophrenia-like symptoms.
Central hypoventilation is an important feature of anti-NMDAR encephalitis, having been reported in 14 of 17 cases (82%), including our 4 patients.11,12 When we temporarily turned off ventilatory support, spontaneous breathing did not return, but the orofacial-limb dyskinesias persisted, suggesting that the hypoventilation was independent of the dyskinesias. Of interest, NR1 knockout animals die of hypoventilation.23 Because reactivity of patients’ antibodies depends on the presence of NR1 subunits,11 the frequent hypoventilation of patients with this disorder can be explained by the immune response. In fact, recent studies demonstrate that patients’ antibodies react not only with NR2B, but also with NR1, and that the reactivity with NR2B largely depends of coexpression of functional NR1/NR2B heteromers (Dalmau et al., unpublished data).
The long-lasting dyskinesias of our patients posed a major clinical problem. They presented gradually as orolingual dyskinesias with athetoid postures, and evolved to intractable bizarre involuntary movements, some of which resembled complex partial seizure status epilepticus or a psychogenic disorder. Extensive monitoring with EEG showed absence of paroxysmal discharges, suggesting that the movements were not epileptic. After unsuccessful trials with multiple antiepileptics and sedatives, the dyskinesias responded to propofol and midazolam. The mechanism of these dyskinesias remains unclear. Although one may consider the possibility of a side effect of the antipsychotic medication, many patients with anti-NMDAR encephalitis (even those who do not receive antipsychotics as our patients) develop dyskinesias, suggesting that they are part of the immune disorder.11 We do not have neuropathologic studies in the present patients, but other Japanese patients with similar encephalitis2,3 had pathologic findings closely resembling those of patients with anti-NMDAR encephalitis.11 These studies emphasized the paucity of inflammatory infiltrates relative to the severity of the neurologic deficits.1,3 These findings, the normal or mild changes in the initial MRI of our patients, and preservation of drug-induced fast EEG activity (implying that cortical oscillations mediated by GABAergic inhibitory interneurons are preserved)24,25 all concur with the potential reversibility of anti-NMDAR encephalitis.
Our study indicates that anti-NMDAR encephalitis and juvenile acute nonherpetic encephalitis or a subgroup of this disorder are the same. The reasons why this disorder is more frequent (or has received more attention) in Japan are unclear. The 4 patients reported here were seen in the past 8 years at a single institution. Another 11 patients with a similar syndrome were reported from another institution (antibodies to NR1/NR2 heteromers were not studied).1 These experiences, as well as the fact that some patients survive without recognizing the tumor and others may die without a diagnosis (i.e., some cases11 were diagnosed postmortem), suggest that the frequency of anti-NMDAR encephalitis is underestimated.
Our findings raise two questions. First, how often is anti-NMDAR encephalitis paraneoplastic? After a rapid increase of the number of identified cases, we have found that 26 of 33 patients (79%), including 3 reported here, had tumors or cysts usually in the pelvic region (20 pathologically confirmed teratomas of the ovary, 1 in the mediastinum, and 5 ovarian cysts under study). No tumor has been identified in the other 7 cases (3 of them men), who are being closely followed up for a possible occult neoplasm (Dalmau et al., unpublished data).
Second, is it necessary to remove the tumor or use immunotherapy to treat this disorder? A previously reported patient (Case 8 of reference 11) and the current four patients demonstrate that the disorder may resolve without tumor removal. However, the duration of intensive care and ventilatory support in two of these patients (6 to 9 months) was significantly longer than that of all known cases that had tumor resection and immunotherapy (median 12 weeks, longest time of ventilation 4 months). Furthermore, the severity of this disorder, which may result in death or severe complications, and the reported responses to tumor resection and immunotherapy strongly support the use of these treatments.
Anti-NMDAR encephalitis is different from other types of paraneoplastic encephalitis in several ways: it results in a highly characteristic syndrome, usually affects young women, is treatment responsive, and associates with tumors that can be benign. Another difference shown here is that despite the presence of the tumor, the immune response is not maintained. This brings into consideration a contributory role of the prodromal “viral-like” disorder, which by itself or in combination with a teratoma sets off or enhances the autoimmune response. The answers to these questions will come from more experience and work in progress to model the disorder.
Supplementary Material
Acknowledgments
The authors thank Dr. Kimiyoshi Arimura and Dr. Osamu Watanabe (Department of Neurology and Geriatrics, Kagoshima University Graduate School of Medical and Dental Sciences), Dr. Yukitoshi Takahashi (National Epilepsy Center, Shizuoka MIND), and Dr. Keiko Tanaka (Department of Neurology, Brain Research Institute, Niigata University) for measuring antineuronal antibodies. The authors thank Dr. Atsuko Hara (School of Medicine, Kitasato University) for her pathologic assistance. They also thank Dr. Erdem Tüzün and Myrna R. Rosenfeld for their critical review of the manuscript and Mr. Jeffrey Rossi for his excellent technical assistance (University of Pennsylvania).
Supported in part by RO1 NS45986 (D.R.L.) and RO1CA89054, RO1CA107192 (J.D.).
GLOSSARY
- AED
antiepileptic drugs
- FDG-PET
[F]fluoro-2-deoxy-d-glucose PET
- FLAIR
fluid-attenuated inversion recovery
- F-T
frontotemporal
- GABA
γ-aminobutyric acid
- HHV
human herpes virus
- HMPAO
99mTc-d,l-hexamethyl-propyleneamine oxime
- HSV
herpes simplex virus
- IMP
N-isoprpyl-p-123I iodoamphetamine
- mono
mononuclear cells
- NMDAR
NMDA receptor
- OCB
oligoclonal bands
- OTE
ovarian teratoma associated encephalitis
- PD
paroxysmal discharges
- PMN
polymorphonuclear cells
- IVIg
intravenous immunoglobulin
- SSP
stereotactic surface projection
- WBC
white blood cells
Footnotes
Disclosure: The authors report no conflicts of interest.
This study was originally presented at the scientific session of the 57th Annual Meeting of American Academy of Neurology on April 13, 2005.
References
- 1.Kamei S. Acute juvenile female non-herpetic encephalitis: AJFNHE [in Japanese] Adv Neurol Sci. 2004;48:827–836. doi: 10.5692/clinicalneurol.48.916. [DOI] [PubMed] [Google Scholar]
- 2.Iizuka R. Contribution to acute diffuse lymphocytic meningoencephalitis and encephalopathy: clinical aspects and neuropathology of a non-specific form of reaction of the central nervous system. Arch Psychiatr Nervenkr. 1965;28:705–717. doi: 10.1007/BF00343107. [DOI] [PubMed] [Google Scholar]
- 3.Kurokawa Y, Kon Y, Asano Y, et al. A case of acute diffuse lymphocytic meningoencephalitis. Hokkaido Igaku Zasshi. 1987;62:859–865. [PubMed] [Google Scholar]
- 4.Yuasa T, Nemoto H, Kimura A. Four cases of acute reversible limbic encephalitis predominantly affecting juvenile female and presenting with psychosis with minimal changes on MRI [in Japanese] Neurol Med. 2003;59:45–50. [Google Scholar]
- 5.Kataoka H, Kohara N, Sato W, Sakaguchi M, Kawamoto M, Takano S. Acute non-herpetic viral encephalitis of juvenile onset: analysis of 11 cases based on initial clinical symptoms [in Japanese] No To Shinkei. 2005;57:599–606. [PubMed] [Google Scholar]
- 6.Vitaliani R, Mason W, Ances B, Zwerdling T, Jiang Z, Dalmau J. Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Ann Neurol. 2005;58:594–604. doi: 10.1002/ana.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ances BM, Vitaliani R, Taylor RA, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain. 2005;128:1764–1777. doi: 10.1093/brain/awh526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stein-Wexler R, Wootton Gorges SL, Greco CM, Brunberg JA. Paraneoplastic limbic encephalitis in a teen age girl with an immature ovarian teratoma. Pediatr Radiol. 2005;35:694–697. doi: 10.1007/s00247-005-1402-1. [DOI] [PubMed] [Google Scholar]
- 9.Bataller L, Kleopa KA, Wu GF, Rossi JE, Rosenfeld MR, Dalmau J. Autoimmune limbic encephalitis in 39 patients: immunophenotypes and outcomes. J Neurol Neurosurg Psychiatry. 2007;78:381–385. doi: 10.1136/jnnp.2006.100644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koide R, Shimizu T, Koike K, Dalmau J. EFA6A-like antibodies in paraneoplastic encephalitis associated with immature ovarian teratoma: a case report. J Neurooncol. 2007;81:71–74. doi: 10.1007/s11060-006-9200-7. [DOI] [PubMed] [Google Scholar]
- 11.Dalmau J, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sansing HL, Tüzün E, Ko WM, Baccon J, Lynch RD, Dalmau J. A patient with encephalitis associated with NMDA receptor antibodies. Nat Clin Pract Neurol. 2007;3:291–296. doi: 10.1038/ncpneuro0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iizuka T, Yoshii S, Sakai F, et al. A distinct syndrome of encephalitis presenting as acute onset of psychosis followed by unresponsiveness, hypoventilation, and intractable orofacial-limb hyperkinetic movements. Neurology. 2005;64:A249. Abstract. [Google Scholar]
- 14.Shillito P, Molenaar PC, Vincent A, et al. Acquired neuromyotonia: evidence for autoantibodies directed against K+ channels of peripheral nerves. Ann Neurol. 1995;38:714–722. doi: 10.1002/ana.410380505. [DOI] [PubMed] [Google Scholar]
- 15.Pommepuy N, Januel D. Catatonia: resurgence of a concept. A review of the international literature. Encephale. 2002;28:481–492. [PubMed] [Google Scholar]
- 16.Rha SE, Byun JY, Jung SE, et al. Atypical CT and MRI manifestations of mature ovarian cystic teratomas. AJR Am J Roentgenol. 2004;183:743–750. doi: 10.2214/ajr.183.3.1830743. [DOI] [PubMed] [Google Scholar]
- 17.Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33:523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- 18.Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- 19.Coyle JT, Tsai G, Goff D. Converging evidence of NMDA receptor hypofunction in the pathophysiology of schizophrenia. Ann NY Acad Sci. 2003;1003:318–327. doi: 10.1196/annals.1300.020. [DOI] [PubMed] [Google Scholar]
- 20.Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26:365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahata R, Moghaddam B. Activation of glutamate neurotransmission in the prefrontal cortex sustains the motoric and dopaminergic effects of phencyclidine. Neuropsychopharmacology. 2003;28:1117–1124. doi: 10.1038/sj.npp.1300127. [DOI] [PubMed] [Google Scholar]
- 22.Stone JM, Morrison P, Pilowsky LS. Glutamate and dopamine dysregulation in schizophrenia: a synthesis and selective review. J Psychopharmacol. 2007 Jan 26; doi: 10.1177/0269881106073126. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Forrest D, Yuzaki M, Soares HD, et al. Targeted disruption of NMDA receptor 1 gene abolishes NMDA response and results in neonatal death. Neuron. 1994;13:325–338. doi: 10.1016/0896-6273(94)90350-6. [DOI] [PubMed] [Google Scholar]
- 24.Baker MR, Baker SN. The effect of diazepam on motor cortical oscillations and corticomuscular coherence studied in man. J Physiol. 2003;546:931–942. doi: 10.1113/jphysiol.2002.029553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ploner M, Gross J, Timmermann L, Pollok B, Schnitzler A. Oscillatory activity reflects the excitability of the human somatosensory system. Neuroimage. 2006;32:1231–1236. doi: 10.1016/j.neuroimage.2006.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.