Abstract
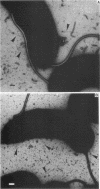
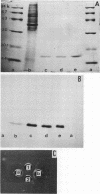
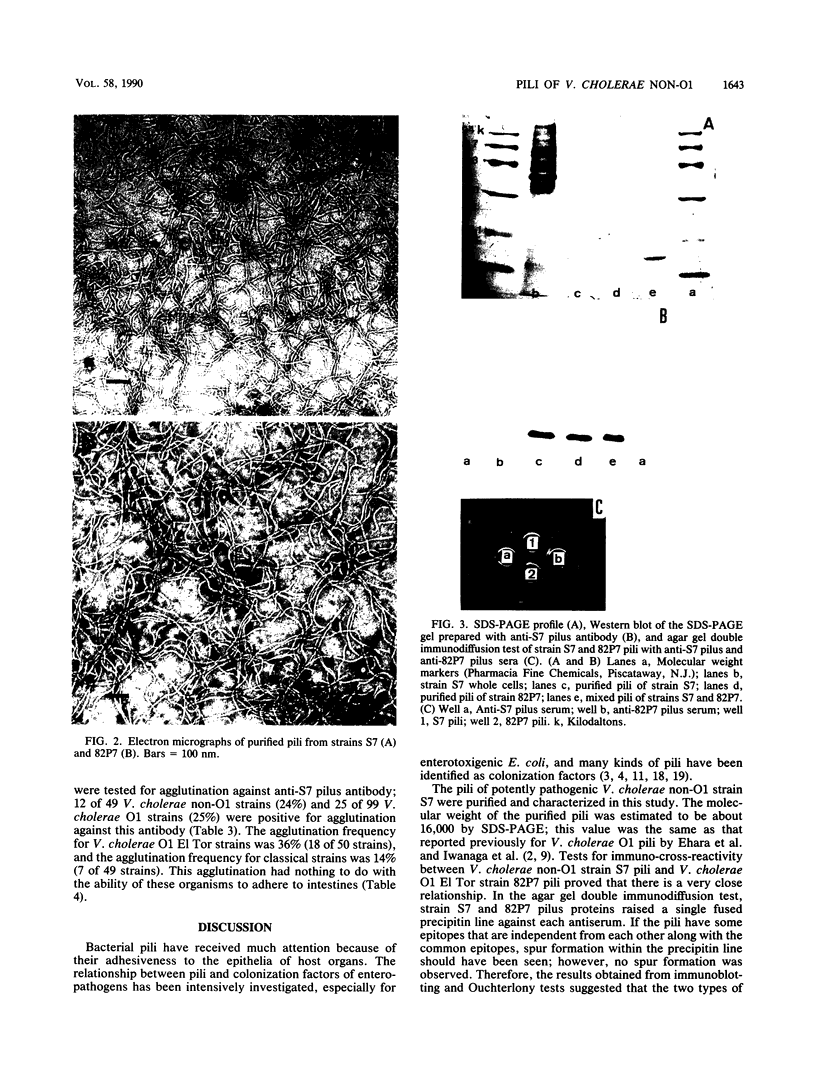
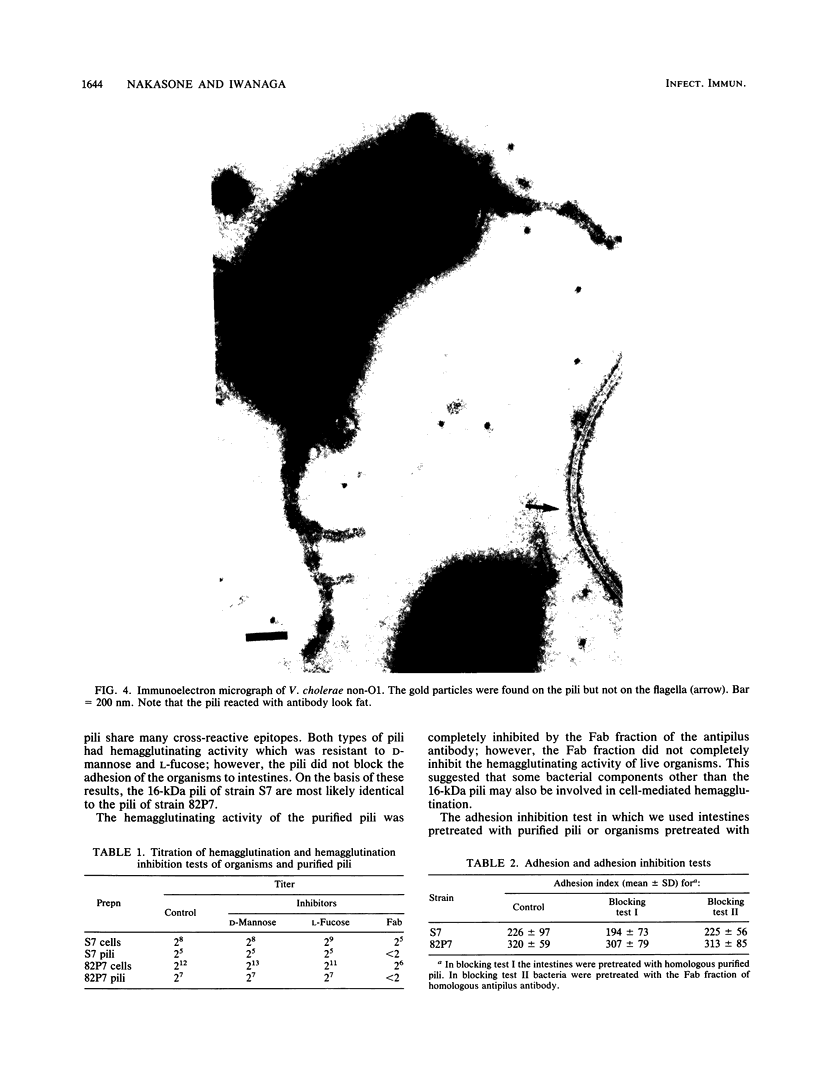
Pili of Vibrio cholerae non-O1 strain S7 were purified and characterized. The pili of S7 were morphologically, electrophoretically, and immunologically (as far as polyclonal antibody was used) indistinguishable from the 16-kilodalton pili of V. cholerae O1 strain 82P7. The purified pili and organisms had D-mannose- and L-fucose-resistant hemagglutinin. The hemagglutinating activity of the purified pili was inhibited by the Fab fraction of antipilus antibody, but the hemagglutinating activity of live organisms was not inhibited completely. The purified pili or Fab fraction of antipilus antibody did not inhibit the adhesion of V. cholerae non-O1 to rabbit intestines. Therefore, the pili were not regarded as a colonization factor of V. cholerae non-O1. A total of 148 V. cholerae non-O1 and O1 clinical isolates were screened for the presence of S7 pili by using an agglutination test with anti-S7 pilus serum; 12 of 49 V. cholerae non-O1 strains and 25 of 99 V. cholerae O1 strains were positive for agglutination. These agglutination reactions were not correlated with adhesion of the organisms to intestines.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Ehara M., Ishibashi M., Ichinose Y., Iwanaga M., Shimotori S., Naito T. Purification and partial characterization of fimbriae of Vibrio cholerae O1. Vaccine. 1987 Dec;5(4):283–288. doi: 10.1016/0264-410x(87)90153-8. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr New surface-associated heat-labile colonization factor antigen (CFA/II) produced by enterotoxigenic Escherichia coli of serogroups O6 and O8. Infect Immun. 1978 Aug;21(2):638–647. doi: 10.1128/iai.21.2.638-647.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Silver R. P., Evans D. J., Jr, Chase D. G., Gorbach S. L. Plasmid-controlled colonization factor associated with virulence in Esherichia coli enterotoxigenic for humans. Infect Immun. 1975 Sep;12(3):656–667. doi: 10.1128/iai.12.3.656-667.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Hanne L. F. Purification and characterization of the soluble hemagglutinin (cholera lectin)( produced by Vibrio cholerae. Infect Immun. 1982 Jun;36(3):1199–1208. doi: 10.1128/iai.36.3.1199-1208.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Arita M., Takeda T., Yoh M., Miwatani T. Non-O1 Vibrio cholerae produces two newly identified toxins related to Vibrio parahaemolyticus haemolysin and Escherichia coli heat-stable enterotoxin. Lancet. 1985 Jul 20;2(8447):163–164. doi: 10.1016/s0140-6736(85)90277-6. [DOI] [PubMed] [Google Scholar]
- Honda T., Kasemsuksakul K., Oguchi T., Kohda M., Miwatani T. Production and partial characterization of pili on non-O1 Vibrio cholerae. J Infect Dis. 1988 Jan;157(1):217–218. doi: 10.1093/infdis/157.1.217. [DOI] [PubMed] [Google Scholar]
- Iwanaga M., Nakasone N., Ehara M. Pili of Vibrio cholerae O1 biotype E1 Tor: a comparative study on adhesive and non-adhesive strains. Microbiol Immunol. 1989;33(1):1–9. doi: 10.1111/j.1348-0421.1989.tb01492.x. [DOI] [PubMed] [Google Scholar]
- Jones G. W., Abrams G. D., Freter R. Adhesive properties of Vibrio cholerae: adhesion to isolated rabbit brush border membranes and hemagglutinating activity. Infect Immun. 1976 Jul;14(1):232–239. doi: 10.1128/iai.14.1.232-239.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. The association of K88 antigen with haemagglutinating activity in porcine strains of Escherichia coli. J Gen Microbiol. 1974 Sep;84(1):135–144. doi: 10.1099/00221287-84-1-135. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morris J. G., Jr, Black R. E. Cholera and other vibrioses in the United States. N Engl J Med. 1985 Feb 7;312(6):343–350. doi: 10.1056/NEJM198502073120604. [DOI] [PubMed] [Google Scholar]
- Nakasone N., Iwanaga M. Pili of a Vibrio parahaemolyticus strain as a possible colonization factor. Infect Immun. 1990 Jan;58(1):61–69. doi: 10.1128/iai.58.1.61-69.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakasone N., Iwanaga M. Quantitative evaluation of colonizing ability of Vibrio cholerae O1. Microbiol Immunol. 1987;31(8):753–761. doi: 10.1111/j.1348-0421.1987.tb03137.x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Punsalang A. P., Jr, Sawyer W. D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973 Aug;8(2):255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. K., Miller V. L., Furlong D. B., Mekalanos J. J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci U S A. 1987 May;84(9):2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Al-Omani M., Honda T., Takeda Y., Miwatani T. Non-O1 Vibrio cholerae hemolysin: purification, partial characterization, and immunological relatedness to El Tor hemolysin. Infect Immun. 1984 Jul;45(1):192–196. doi: 10.1128/iai.45.1.192-196.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Takeda Y., Miwatani T., Craig J. P. Evidence that a non-O1 Vibrio cholerae produces enterotoxin that is similar but not identical to cholera enterotoxin. Infect Immun. 1983 Sep;41(3):896–901. doi: 10.1128/iai.41.3.896-901.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Yokota T. Vibrio cholerae non-O1: production of cell-associated hemagglutinins and in vitro adherence to mucus coat and epithelial surfaces of the villi and lymphoid follicles of human small intestines treated with formalin. J Clin Microbiol. 1988 Oct;26(10):2018–2024. doi: 10.1128/jcm.26.10.2018-2024.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]