Abstract
The present study was undertaken to characterize the consequences of RLIP76-loss with respect to drug-resistance, transport, radiation-resistance, and alternative transport mechanisms in mouse embryonic fibroblasts. MEFs were derived from RLIP76+/+, RLIP76+/− and RLIP76−/− mice. The transport of doxorubicin, colchicine, leukotriene C4 and dinitrophenyl S-glutathione was analyzed in inside-out vesicles (IOVs) prepared from MEFs. We used immuno-titration of transport activity to determine the contribution of RLIP76, MRP1, and Pgp towards total transport activity. Loss of RLIP76 alleles resulted in significant sensitization to radiation, DOX, cisplatin, and vinorelbine. In IOVs prepared from MEFs, we observed a stepwise loss of transport activity. Loss of RLIP76 confers sensitivity to xenobiotics and radiation due to the loss of a common transport mechanism for glutathione-electrophile-conjugates and xenobiotics.
Keywords: RLIP76, drug-resistance, glutathione-conjugate, transport, embryonic fibroblasts
1. Introduction
Chain reaction of lipid-peroxidation is an obligate common chemical reaction triggered by both oxidant and radiant stress [1–3]. These stressors can directly generate the OH. radical which initiate lipid-peroxidation and formation of lipid-peroxy radicals, which have sufficiently long half-lives to allow diffusion to the nucleus, and sufficiently reactive to cause direct DNA scission [4]. Electrophilic alkenals, such as 4HNE, represent down-stream lower energy by-products of lipid-peroxidation which are known to induce stress-resistance mechanisms at low concentrations, and apoptosis and necrosis at higher concentrations [2]. These α-β-unsaturated alkenals react with GSH to form Michael-adducts, a reversible reaction in presence of the catalyst glutathione S-transferases (GST). The further biotransformation of the thioether conjugate to mercapturate requires efflux from cells to be further metabolized by γ-glutamyl transpeptidase which is present on the outer leaflet of plasma membranes. Because GS-E is potent product inhibitors of GST, the removal of these products is also necessary to maintain GST-activity defending against foreign electrophilic toxins (xenobiotic) [5]. This model predicts that depletion of GS-E efflux capacity should inhibit phase II biotransformation. Our earlier descriptions of the existence of a single transporter protein which could mediate competing transport of acidic GS-E (products of phase II biotransformation) and weakly basic amphiphilic drugs such as DOX (substrates of phase I biotransformation) suggested the intriguing possibility that such a mechanism would be in the unique position of regulating biotransformation at both ends-regulating levels of substrates of phase I biotransformation, and products of phase II biotransformation. We have subsequently shown that RLIP76 is the predominant mechanism mediating the shared transport of xenobiotic as well as GS-E [6–13].
RLIP76, the first known effector of Ral, is a 76-kDa multi-functional ATPase that couples the movement of substances with ATP-hydrolysis. It is found throughout the cell, in membrane, cytosol and the nucleus, and is known to shift between these compartments in response to stress. It is a component of important multi-functional protein complexes including the mitotic spindle, the receptor signaling complexes for EGF, TGF-β̃, insulin, and clathrin-dependent endocytosis [14–16]. We have shown that RLIP76 is a stress-protective multi-specific transporter of chemotherapeutic agents and GS-E [6–10]. MEFs were derived from RLIP76+/+ (wild-type), RLIP76+/− (hetero) and RLIP76−/− (homozygous) mice and to characterize the consequences of RLIP76 loss with respect to drug-resistance, transport mechanism and radiation-resistance in MEF model. This model predicted that sensitivity to stressor such as radiation, oxidants, and xenobiotic metabolized through glutathionylation should be increased in animals lacking RLIP76. The effect of RLIP76 loss on the transport of several model substrates including, physiologic and pharmacologic GS-E, and chemotherapeutic drugs such as DOX and COL, and the relative contributions of RLIP76 and other known efflux pumps (Pgp and MRP) in the transport of these substrates were also examined in an MEF model.
2. Materials and Methods
2.1. Animal studies
Animal studies were performed as approved by the IACUC in the institutional animal care facility. Colonies of RLIP76+/+, RLIP76+/− and RLIP76−/− animals have been bred according to IACUC protocol.
2.2. Materials
DOX and COL were obtained from Adria Laboratories (Columbus, OH) and Bedford Laboratories (Bedford, OH), respectively. Melphalan, busulfan and cyclophosphamide were procured from Sigma (St. Louis, MO). 14C-DOX (specific activity 54 mCi/mmol), 3H-LTC4 (specific activity 140 Ci/mmol) and 3H-COL (specific activity 70 Ci/mmol) were purchased from NEN Life Sciences (Boston, MA). Polyclonal rabbit-anti-human-RLIP76 IgG were prepared and purified as described earlier [6] whereas antibodies against MRP1 (N-19) and Pgp (C-19) were purchased from Santa-Cruz Biotechnology (Santa Cruz, CA). DNP-SG was synthesized from CDNB and GSH according to the method described by us [6].
2.3. Method for preparing mouse embryonic fibroblast cultures
Twelve weeks old C57BL/6 mice born of heterozygous×heterozygous mating were genotyped by PCR strategy on mouse tail DNA using forward, reverse and long terminal region primers. These mice were commissioned from Lexicon Genetics. Embryo fibroblast lines were prepared from RLIP76+/+, RLIP76+/−, RLIP76−/− mice on the 13th or 14th day of pregnancy [17].
2.4. Preparation of RLIP76-liposomes
Recombinant human RLIP76 was purified as previously described. Purity was checked by Western-blot analysis, and MALDI-MS. Purified protein was reconstituted into artificial asolectincholesterol liposomes [6].
2.5. Transport of 14C-DOX, 3H-COL, 3H-DNP-SG and 3H-LTC4
In-side-out vesicles (IOV) were prepared from RLIP76+/+, RLIP76+/−, RLIP76−/− MEFs and transport studies in IOV were performed as described previously [18]. ATP-dependent uptake of 14C-DOX (specific activity 8.5 × 104 cpm/nmol), 3H-COL (specific activity 1.4 × 104 cpm/nmol), 3H-DNPSG (specific activity 3.6 × 103 cpm/nmol), 3H-LTC4 (specific activity 1.6 × 103 cpm/pmol), were determined by subtracting the radio-activity (cpm) of the control without ATP from that of the experimental containing ATP, and the transport rate was calculated in terms of pmol/min/mg protein. In one of the controls, IOV was excluded while the other control was incubated with an equal amount of heat-inactivated IOV. Each determination was performed in triplicate.
2.6. Transport in vesicles coated with antibodies
IOV (20 µg protein / 30 µl reaction mixture) were incubated separately with 1 µg of each anti-RLIP76, anti-MRP1, and anti-Pgp antibodies for 30 min at room temperature. In one of the controls, IgG was excluded while the other control was treated with an equal amount of pre-immune IgG. After incubation, the ATP-dependent transport of 14C-DOX, 3H- COL, 3H- DNPSG, and 3H-LTC4 in IOV coated with different antibodies were measured [19].
2.7. Drug-sensitivity assay
Cell density measurements were performed using a hemocytometer to count reproductive cells resistant to staining with trypan blue. Approximately 20,000 cells were seeded into each well of 96-well plates containing 160 μl medium. Post 24 h incubation, 40 μl aliquots of drug concentrations ranging from 0.1nm to 10,000 nm was then added to eight replicate wells to assess the IC50 of drug. After 96 h incubation, 20 μl of 5 mg/ml MTT was introduced to each well and incubated for 2 h. The plates were centrifuged and cells were subsequently dissolved in 100 μl DMSO with gentle shaking for 2 h at room temperature, followed by measurement of OD at 570 nm [8].
2.8. Effect of RLIP76-liposomes on 14C-DOX accumulation and efflux
Drug-accumulation and efflux studies were performed in RLIP76+/+, RLIP76−/− and RLIP76-liposomes (40 μg/ml) treated RLIP76−/− MEFs, according to the protocol described by us [8].
2.9. Colony forming assay
MEFs (0.1 × 106 cells / 500 μl) were irradiated at 0 (no radiation), 100, 200, 500 and 1000 cGY (6 × 106 volt-photon/min) for 1.25 min at the Texas Cancer Center, Arlington, TX, then aliquots of 50 and 100 μl in 60-mm Petri dishes, separately, in a total volume of 4 ml by adding medium. After 10 days, un-irradiated and irradiated cells were stained with methylene blue for 30 min, and colonies were counted using an Innotech Alpha-Imager HP [13].
2.10. Effect of oxidative-stress on RLIP76 expression
MEFs were treated with either 50 μM H2O2 or 25 mM glucose for 20 min at 37 °C followed by washing with PBS, resuspended in the medium and was allowed to recover for 2 h. Cells were solubilized in lysis buffer and centrifuged at 105,000g for 1 h at 4 °C and the supernatant was collected. 100 μg proteins were subjected to SDS-PAGE followed by western-blot analyses using anti-RLIP76 IgG.
2.11. Statistical Methods
The Kaplan Meier method was used to create the survival curves for chemotherapy. All data were evaluated with a two-tailed unpaired student’s t test or compared by one-way ANOVA and are expressed as the mean ± SD. A value of P < 0.05 was considered statistically significant
3. Results and discussion
3.1. MEF model system
Western-blot analyses of crude membrane extracts from RLIP76+/+, RLIP76+/− and RLIP76−/− MEFs were performed against anti-RLIP76 IgG (Fig. 1A). A single protein band was seen at 95 kDa in homogenate of RLIP76+/+ and RLIP76+/− MEF, with the amount of protein in the heterozygous knockout approximately half of that in wild-type. No RLIP76-cross-reactive protein was found in RLIP76−/− MEFs, consistent with their homozygous gene disruption (Fig. 1A, lane 4). Western-blot analyses of RLIP76−/− MEFs were also performed after RLIP76-liposomal delivery by incubating the cells with 40 μg/ml RLIP76-liposomes, indicating detectable level of RLIP76 (Fig. 1A, lane 5). These results confirm that the MEF model system has the expected 3 levels of RLIP76 protein, and no other immuno-logically related proteins, and also proof that RLIP76-liposomes can be used to replenish RLIP76 in RLIP76−/− MEFs.
Figure 1. RLIP76-expression in MEF.
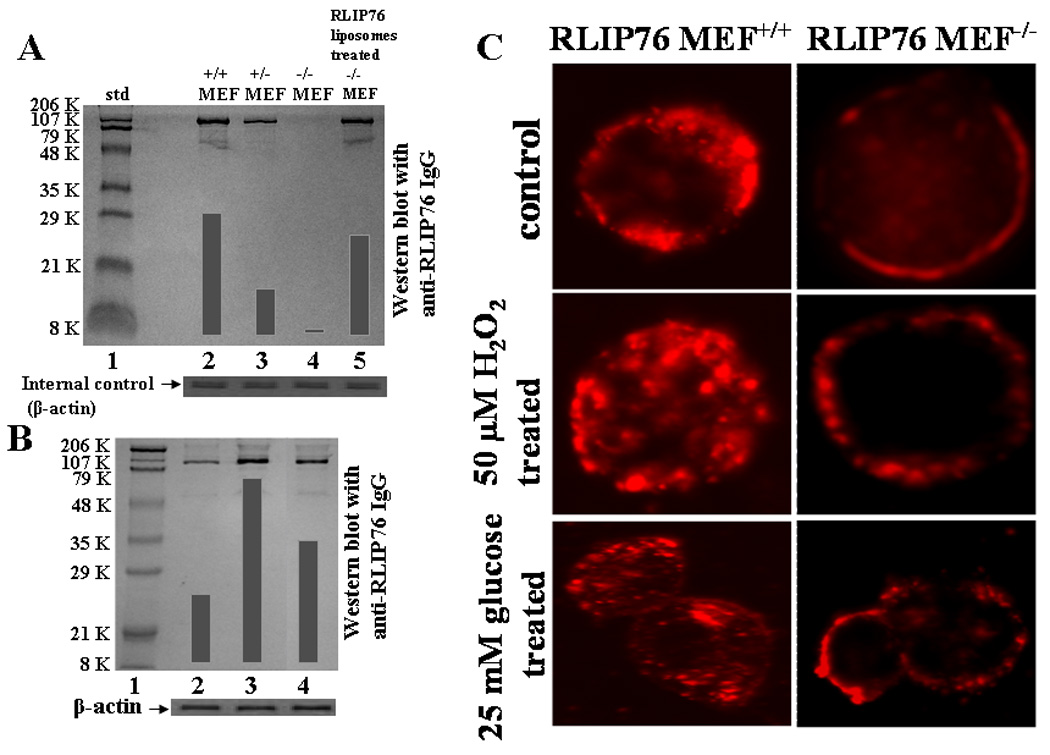
Aliquots of crude membrane fraction from RLIP76+/+, RLIP76+/−, RLIP76−/− and RLIP76−/− MEFs treated with RLIP76-liposomes (lanes 2–5, respectively), containing 200 μg protein were applied in Western-blot analyses against anti-RLIP76 IgG as the primary antibody followed by peroxidase-conjugated goat-anti-rabbit-IgG as a secondary antibody (panel A). In panel B, Western-blot for RLIP76 were performed on RLIP76+/+ MEFs (lane 2), treated with 50 μM H2O2 (lane 3) and 25 mM glucose (lane 4). Aliquots of 100 μg crude membrane fraction were loaded in SDS-PAGE, transblotted, and probed using anti-RLIP76 IgG as a primary and peroxidase-conjugated goat-anti-rabbit IgG as a secondary antibody (panel B). The blots were developed with 4-chloro-1-napthol as chromogenic substrate and developed bands were quantified by scanning densitometry. β-actin expression was used as loading control. Insulin-rhodamine Quantum dots (QD) using Confocal laser microscopy RLIP76+/+ and RLIP76−/− MEFs (0.1 × 106 cells/ml) were grown on cover slips, followed by incubation with either 50 μM H2O2 or 25 mM glucose for 20 min at 37 °C, and allowed to recover for 2 h. Cells were treated with 10% goat serum for 30 min. and labeled with molar ratio 6:1 of insulin:QD on ice for 45 min, washed and incubated for 10 min at 37 °C and fixed in cold 4% paraformaldehyde. Slides were analyzed using confocal laser-scanning microscopy (panel C).
3.2. Transient oxidative-stress causes increased insulin-rhodamine Quantum- dots endocytosis
We have previously shown that a rapid induction of RLIP76 occurs in cells exposed to mild transient oxidative-stress and these cells transport GS-E at a several-fold faster rate in order to protect themselves from the toxicity of the electrophilic products of lipid-peroxidation [20]. Reasoning that transient oxidative-stress could also affect RLIP76-mediated endocytosis, we examined the insulin: Quantum dots endocytosis in RLIP76+/+ MEF subjected to mild oxidative-stress. Results of these experiments clearly demonstrate that after a brief exposure to 50 μM H2O2 (oxidant stress) or 25 mM glucose (glycemic stress), results in an increased endocytosis of insulin-receptor (Fig. 1C) along with marked induction of RLIP76-expression (~ 2.7 fold by H2O2 and ~ 1.9 fold by glucose; Fig. 1B, lanes 3 and 4, respectively). These findings are consistent with the hypothesis that RLIP76-mediated GS-E transport is crucial to the regulation of endocytosis and strong evidence for vital role of RLIP76 in receptor-ligand endocytosis and may explain the mechanism for transient glucose intolerance caused by stress. We also confirmed that RLIP76−/− mice are insulin-sensitive which are accounted for by both increased insulin-mediated suppression of HGO and increased peripheral glucose-uptake and the ratio of plasma insulin/glucose was found to be lowest in RLIP76−/− mice (Singhal, S.S., Unpublished observations).
3.3. RLIP76 loss increases toxicity of anthracycline and alkylating agents in mice
Recently, we have shown that RLIP76−/− mice were significantly more sensitive to radiation as compared with RLIP76+/+. The LD50 of RLIP76+/+ mice was between 200 and 300 cGy, and that for RLIP76−/− mice was between 50 and 100 cGy indicating a dose-modification factor between 3 and 4. Administration of 200 μg RLIP76-liposomes at a single dose was administered 14 h after radiation, we observed a remarkable improvement in survival of RLIP76−/− animals [9,21].
We have previously demonstrated that about 80 % activity for DOX-transport in the tissues of RLIP76−/− mice was abrogated, suggesting RLIP76 to be the major transporter of this non-alkylating drug also [16]. Present studies showed that the RLIP76−/− mice had significantly shorter survival as compared with RLIP76+/+ mice when treated with identical doses of DOX (p<0.01). A similar pattern was observed when the mice were treated with the classical alkylating agents, melphalan (p<0.05) and busulfan (p<0.05). For cyclophosphamide (p<0.07), the difference did not reach statistical significance (Fig. 2). The difference in the sensitivity of drugs between RLIP76−/− and RLIP76+/+ mice was more prominent with DOX, in which all animals died within 13 days in RLIP76−/− group as compared to 35 days for RLIP76+/+ group. Taken together, these studies demonstrate a significantly greater sensitivity to the toxic effects of radiation and alkylating agents as well as anthracycline chemotherapy drugs caused by loss of RLIP76, and indicate that the impairment of RLIP76-mediated transport of GS-E and DOX in RLIP76−/− mice leads to increased sensitivity not only to radiation but also to the widely used chemotherapeutic agents.
Figure 2. RLIP76 loss sensitizes to chemotherapy toxicity in mice.
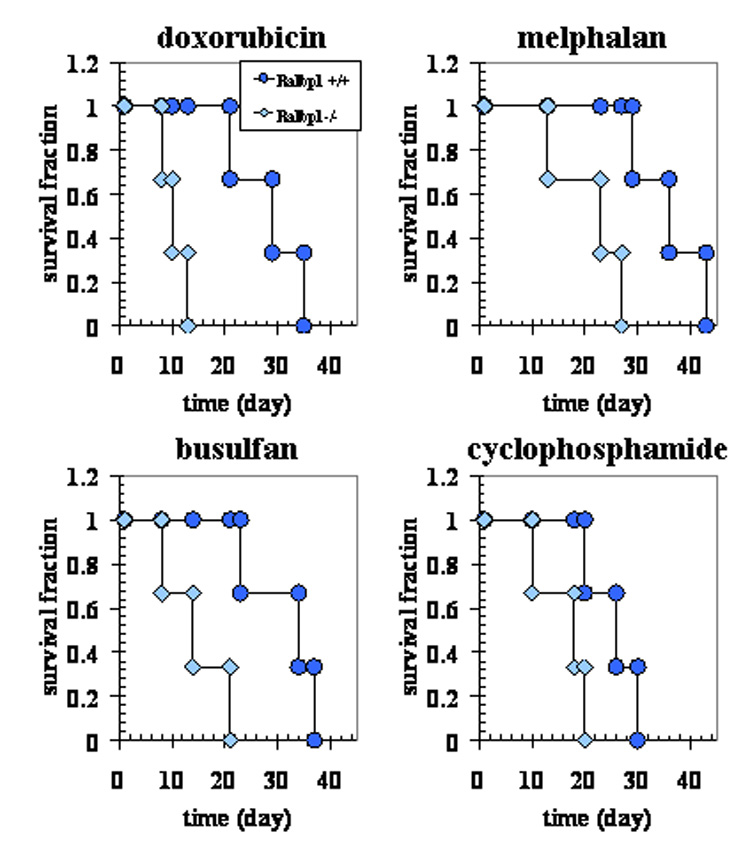
The experiment had a 2 × 4 factorial design (genotype × drugs) with 3 animals per group. C57 black mice, confirmed as having either RLIP76+/+ (circles) or RLIP76−/− (diamonds) genotyped as previously described [9], were weighed and randomized to treatment with a single i.p. injection of PBS containing either DOX (11.1 mg/Kg b.w.), p < 0.01, when compared RLIP76+/+ vs. RLIP76−/−; melphalan (6 mg/Kg b.w.), p < 0.05, when compared RLIP76+/+ vs. RLIP76−/−; busulfan (86 mg/Kg b.w.), p < 0.05, when compared RLIP76+/+ vs. RLIP76−/− or cyclophosphamide (159 mg/Kg b.w.) p < 0.07, when compared RLIP76+/+ vs. RLIP76−/−. Animals were monitored twice daily for survival.
3.4. Effect of RLIP76 loss on Radiation- sensitivity
X-irradiation sensitivity of the MEFs was tested in dose-response studies utilizing 100–1000 cGy single dose X-irradiation, followed by colony-forming assays. The colony-forming activity of the RLIP76+/− and RLIP76−/− was lower than RLIP76+/+ (84 and 73% respectively). Normalized to respective un-irradiated controls, at all radiation levels, the RLIP76+/+ MEFs were least sensitive to radiation (Fig. 3). Results shown in Fig. 3 showed that RLIP76+/+ were the most radio-resistant, followed by RLIP76+/−, and then RLIP76−/−.
Figure 3. Radiation-sensitization by loss of RLIP76.
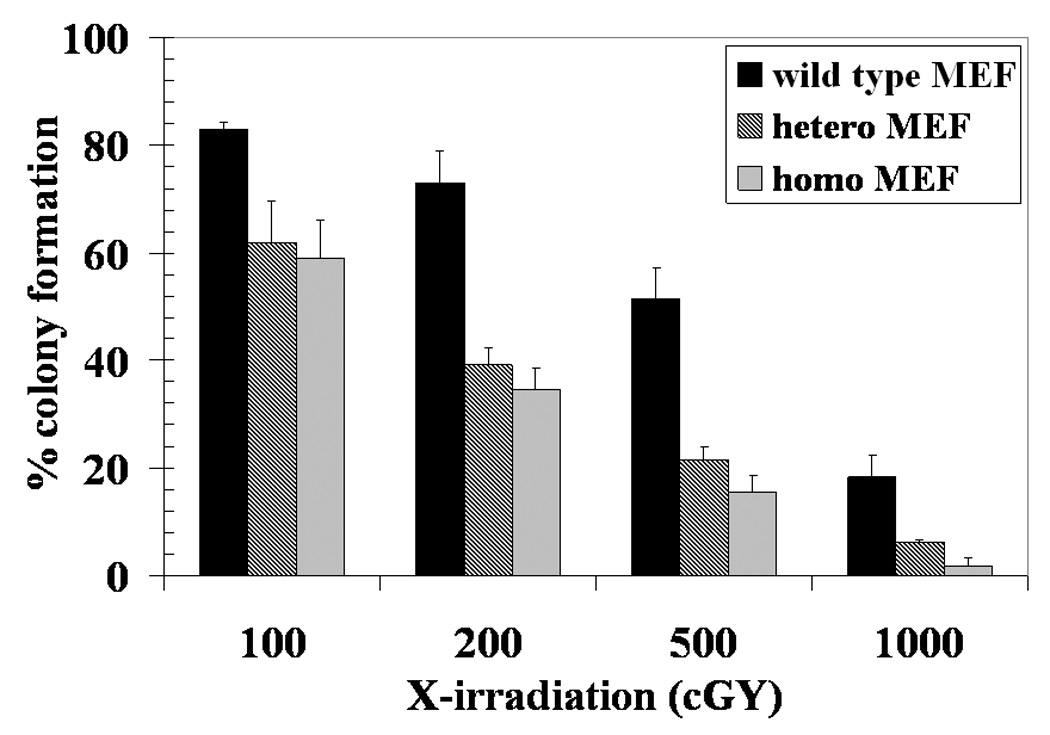
RLIP76+/+ (black bar), RLIP76+/− (crosshatched bar) and RLIP76−/− (gray bar) MEFs (0.1 × 106 cells / 500 μl in triplicates) were irradiated at 0 (no radiation), 100, 200, 500 and 1000 cGY (6 × 106 volt-photon/min) for 1.25 min then aliquots 50 and 100 μl in 60 mm Petri-dishes, separately, in a total volume of 4 ml medium. After 10 days, un-irradiated and irradiated cells were stained with methylene blue for 30 min. than colonies were counted using an Innotech Alpha-Imager HP [13]. The results of colony-forming assays of radiated cells are shown normalized to the respective un-irradiated controls. Plating efficiency of RLIP76+/− and RLIP76−/− MEFs was 84% and 73%, respectively, as compared to RLIP76+/+ MEF. Values are means ± S.D. of three experiments. Statistical analyses by ANOVA were significant at p< 0.05 for RLIP76+/+ vs. RLIP76+/−, RLIP76+/+ vs. RLIP76−/−, and RLIP76+/− vs. RLIP76−/− at 200, 500 and 1000 cGY.
3.5. Effect of RLIP76 loss on sensitivity to chemotherapy
Since RLIP76 is a very broad-specificity transporter that in addition to GS-E, is an efficient transporter of chemotherapy drugs, and because RLIP76−/− mice are more sensitive to chemotherapy agents such as the GS-E forming alkylating agents (cyclophosphamide, busulfan and melphalan) as well as DOX, we expected that MEFs with loss of RLIP76 should also be sensitized to chemotherapy drugs.
Therefore, the sensitivity of RLIP76+/+, RLIP76+/−, and RLIP76−/− MEFs towards the anthracycline drug (DOX), the vinca alkaloid (VRL) and the platinum coordinate cisplatin (a GS-E forming drug) were compared using an MTT cytotoxicity assay [8]. Results of these studies clearly demonstrated a stepwise loss in drug-resistance to all three classes of chemotherapy drugs with loss or RLIP76 alleles (Table 1) confirming the result of whole-animals studies [9,21].
Table 1.
IC50 values in MEFs
| MEFs | IC50 (nM) | ||
|---|---|---|---|
| vinorelbine | cisplatin | doxorubicin | |
| RLIP76+/+ | 2.2 ± 0.31 | 1520 ± 180 | 110 ± 20 |
| RLIP76+/− | 1.3 ± 0.15 | 830 ± 110 | 42 ± 8 |
| RLIP76−/− | 0.4 ± 0.03 | 360 ± 50 | 23 ± 3 |
| RLIP76-liposomes treated RLIP76−/− | 1.8 ± 0.22 | 1270 ± 185 | 86 ± 11 |
Drug sensitivity assays were performed using MTT (at 96 h) to determine IC50 values [23]. The values are presented as mean ± SD from three separate determinations with eight replicates each (n =24). Statistical analyses by ANOVA were significant at p< 0.01 for RLIP76+/+ vs. RLIP76+/−, RLIP76+/+ vs. RLIP76−/−, RLIP76+/− vs. RLIP76−/− and RLIP76−/− vs. RLIP76-proteoliposomes treated RLIP76−/−,
3.6. Effect of RLIP76 loss on transport activity for xenobiotic and GS-E
Anthracyclines and vinca-alkaloids (VRL and COL) have been shown previously to be transported by RLIP76 [8,22]. To determine whether increased drug-sensitivity of RLIP76−/− was indeed due to loss of transport function, we compared ATP-dependent transport of DOX, COL and two representative GS-E (LTC4, and DNP-SG) in IOVs prepared from MEFs. A stepwise loss, more prominent when compared between 2 vs. 1 allele, was seen in transport activity of all three drugs (Fig. 4 and Table II). Remarkably, over 80% of the transport activity for all these compounds was absent in RLIP76−/− MEFs, indicating that RLIP76 is the major transporter of anthracyclines, vinca alkaloids and GS-Es. Transport activity in IOVs prepared from RLIP76−/− MEFs were also performed after RLIP76-liposomal delivery by incubating RLIP76−/− MEFs with 40 μg/ml RLIP76-liposomes, showed transport activity with all the substrates were comparable to RLIP76+/+ (Table II).
Figure 4. Loss of transport of anthracycline, vinca-alkaloid, and GS-E with loss of RLIP76.
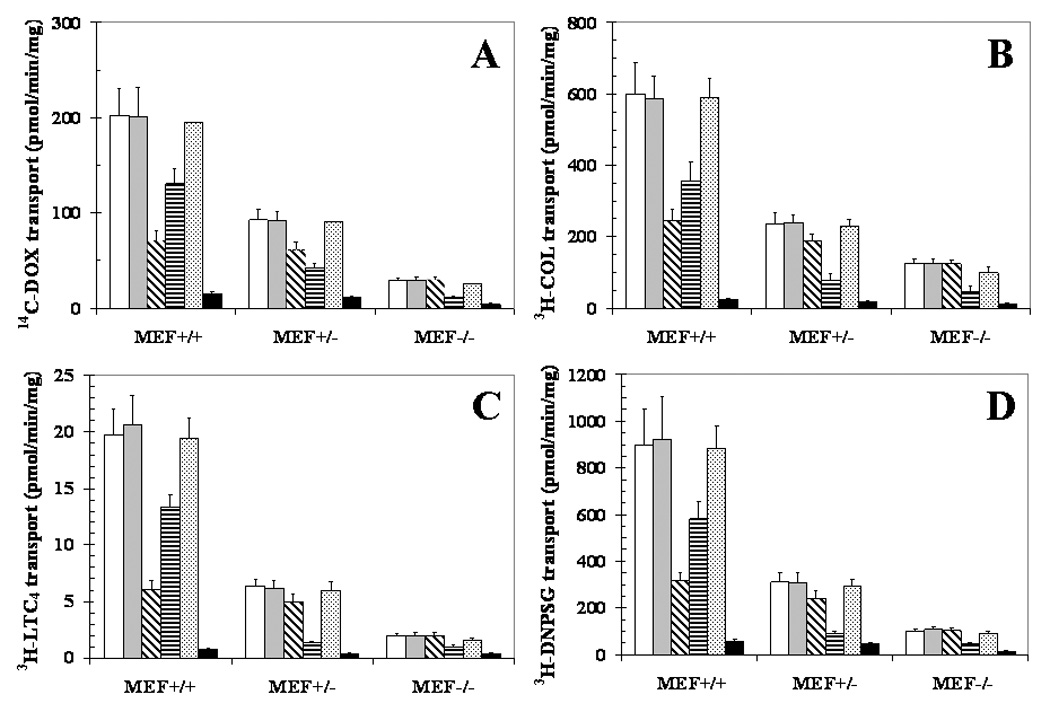
IOVs from the membranes of RLIP76+/+, RLIP76+/−, RLIP76−/− MEFs were prepared [18]. IOVs protein from each MEF (20 μg) was coated separately with buffer alone (white bar), pre-immune-serum (gray bar), anti-RLIP76 IgG (cross-hatched bar), anti-MRP1 IgG (horizontal line bar) and anti-Pgp IgG (dotted bar) and incubated for 30 min at room temperature. Uptake of 14C-DOX (panel A), 3H-COL (panel B), 3H-LTC4 (panel C) or 3H-DNP-SG (panel D) in IOVs coated separately with different antibodies was measured [19]. Heat-inactivated IOVs protein (70 °C × 5 min) was also used for additional control (black bar). For all panels, data represent mean ± SD of three separate experiments done in triplicate. Statistical analyses by ANOVA were significant at p< 0.01 for RLIP76+/+ vs. RLIP76+/−, RLIP76+/+ vs. RLIP76−/−, and RLIP76+/− vs. RLIP76−/−.
Table II.
Xenobiotic and GS-E transport activity in MEFs
| MEFs | Transport activity (pmol/min/mg protein) | |||
|---|---|---|---|---|
| 3H-COL | 14C-DOX | 3H-DNP-SG | 3H-LTC4 | |
| RLIP76+/+ | 587 ± 52 | 196 ± 23 | 1058 ± 87 | 19.1 ± 2.2 |
| RLIP76+/− | 238 ± 34 | 71 ± 9 | 361 ± 28 | 7.2 ± 1 |
| RLIP76−/− | 91 ± 14 | 25 ± 3 | 129 ± 15 | 2.1 ± 0.3 |
| RLIP76-liposomes treated RLIP76−/− | 488 ± 38 | 170 ± 12 | 861 ± 66 | 17.7 ± 1.5 |
Effect of RLIP76 genotype on 14C-DOX, 3H-COL, 3H-DNP-SG and 3H-LTC4 transport activity in IOVs prepared from each MEFs. Statistical analyses by ANOVA were significant at p< 0.01 for RLIP76+/+ vs. RLIP76+/−, RLIP76+/+ vs. RLIP76−/−, RLIP76+/− vs. RLIP76−/− and RLIP76−/− vs. RLIP76-proteoliposomes treated RLIP76−/−,
3.7. Relative contributions to transport activity
To evaluate the relative role of various transporters for these substrates, we performed immunotitration studies using anti-RLIP76, anti-Pgp and anti-MRP antibodies. In previous studies, we have demonstrated stringently the lack of cross-reactivity of these antibodies and have shown the ability of all three antibodies to precipitate out the respective antigens to inhibit transport activity in IOVs [19]. In wild-type MEFs, whereas anti-RLIP76 IgG precipitated out over 2/3 of total transport activity for all four agents, anti-Pgp IgG did not affect transport significantly. Anti-MRP IgG precipitated out about 1/3 of transport activity towards all four substrates in wild-type MEFs, but over half of the residual activity in the RLIP76+/− MEFs was precipitated out by these antibodies. These findings suggested that MRP functions as a backup mechanism upon RLIP76 loss. As expected, in the RLIP76−/− MEFs, anti-RLIP76 IgG had no effect on transport, whereas anti-MRP IgG reduced the residual transport to about half. The lack of effect of anti-RLIP76 IgG in the homozygous knockout MEFs is indicative of the specificity of the antibodies. Heat-inactivated vesicles had near complete loss of transport, confirming that the transport observed in present conditions is due to a biological transporter rather than any non-specific effect of the assay (Fig. 4).
3.8. Effect of RLIP76-liposomes on 14C-DOX accumulation and efflux
The effect of augmentation of RLIP76 on drug uptake/efflux, transport and resistance were studied in MEFs. Cellular RLIP76 was augmented by liposomal-delivery of purified RLIP76, were analyzed for RLIP76 protein, cellular DOX uptake/efflux, transport in IOV and DOX cytotoxicity. These results showed RLIP76-liposomes treated RLIP76−/− MEF had lower uptake and higher IC50 for DOX and greater transport rate for DOX as well as GS-E. These findings clearly demonstrate that augmentation of cellular RLIP76 confers a drug-accumulation deficient phenotype due to increased efflux (Fig. 5).
Figure 5. Effect of RLIP76-liposomes on 14C-DOX accumulation and efflux in MEFs.
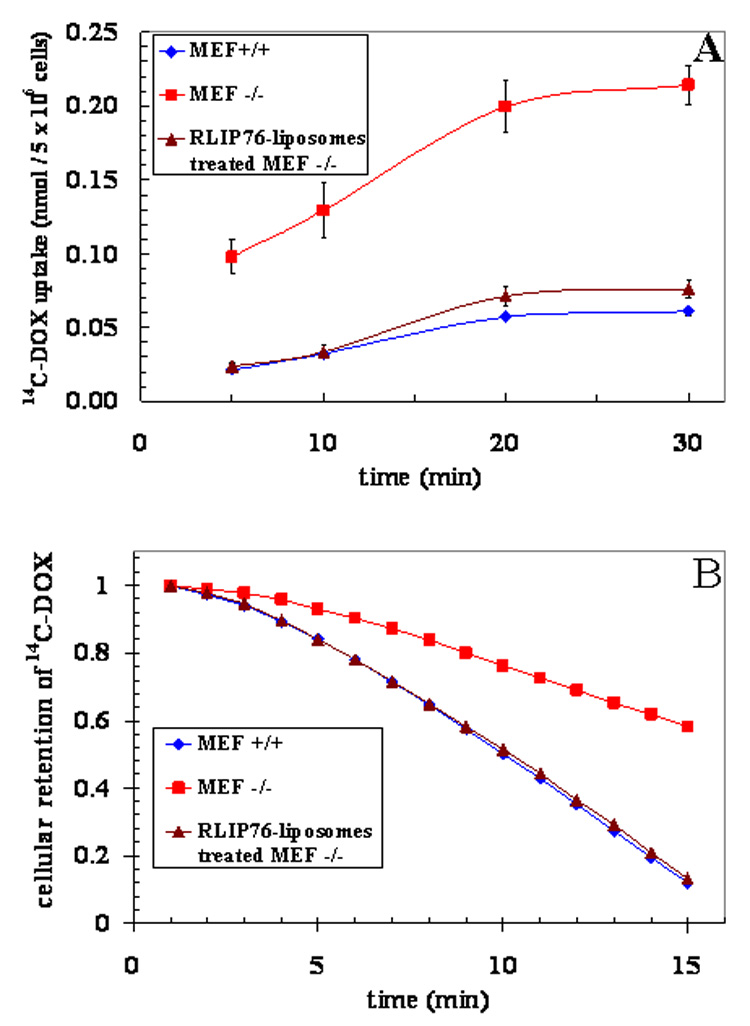
The augmentation of RLIP76 by RLIP76-liposomes in RLIP76−/− MEFs was confirmed by Western-blot using anti-RLIP76 IgG (see Fig. 1A). The rate of cellular accumulation and efflux of 14C-DOX was evaluated using established methods [8]. All studies were performed in triplicates, with means and ± SD presented. RLIP76+/+ MEFs (♦); RLIP76−/− MEFs (■); RLIP76-liposomes-treated RLIP76−/− MEFs (▲). Statistical analyses by ANOVA were significant at p< 0.01 for RLIP76−/− vs. RLIP76+/+ and RLIP76−/− vs. RLIP76-proteoliposomes treated RLIP76−/−,
RLIP76 is a fundamental link between biochemical pathways of GSH-linked metabolism of xenobiotic and stress-defense signaling pathways. It plays a central role in radiation and chemotherapy resistance through its activity as a multi-specific ATP-dependent transporter. Taken together, present studies in MEFs, as well as previous studies in whole animals [9,21] and cultured cells [17,21], have established a new paradigm for oxidant/radiant stress protection mechanism by RLIP76-liposomes delivery and have provided a sound mechanistic rationale for placing RLIP76 at a crucial position among these defenses.
4. Conclusions
Studies presented in this communication demonstrate for the first time that loss of RLIP76 confers sensitivity to xenobiotic and radiation due to the loss of a common transport mechanism for both xenobiotic and glutathione-conjugates (GS-E). These finding are of fundamental significance because they provide evidence for a single target for enhancing chemotherapy and radiation.
Acknowledgements
This work was supported in part by National Institutes of Health Grants CA 77495 and CA 104661, Cancer Research Foundation of North Texas, Institute for Cancer Research and the Joe & Jessie Crump Fund for Medical Education.
The abbreviations used are
- RLIP76 (RalBP1)
Ral-interacting protein
- GSH
glutathione
- GS-E
glutathione-electrophile-conjugates
- DNP-SG
dinitrophenyl S-glutathione
- POB1
partner of RLIP76
- COL
colchicine
- DOX
doxorubicin
- 4-HNE
4-hydroxynonenal
- IOVs
inside-out vesicles
- LTC4
leukotriene C4
- MEFs
mouse embryonic fibroblasts
- MRP
multi-drug resistance associated protein
- Pgp
p-glycoprotein
- VRL
vinorelbine
- RLIP76+/+
wild-type
- RLIP76+/−
heterozygous
- RLIP76−/−
homozygous
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bickers DR. Photoradiation diagnosis and therapy: Dermatologic and photobiologic aspects. Invest. Radiol. 1986;21:885–890. doi: 10.1097/00004424-198611000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Awasthi YC, Yang Y, Tiwari NK, Patrick B, Sharma A, Awasthi S. Regulation of 4-HNE mediated signaling by glutathione S-transferases. Free Radic. Biol. Med. 2004;37:607–619. doi: 10.1016/j.freeradbiomed.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 3.Warner DS, Sheng H, Batinic-Haberle I. Oxidants, antioxidants and the ischemic brain. J. Exp. Biol. 2004;207:3221–3231. doi: 10.1242/jeb.01022. [DOI] [PubMed] [Google Scholar]
- 4.Flowers L, Ohnishi ST, Penning TM. DNA strand scission by polycyclic aromatic hydrocarbon o-quinones: role of reactive-oxygen species, Cu(II)/Cu(I) redox cycling, and o-semiquinone anion radicals. Biochemistry. 1997;36:8640–8648. doi: 10.1021/bi970367p. [DOI] [PubMed] [Google Scholar]
- 5.Jakoby WB. The glutathione S-transferases: A group of multifunctional detoxification proteins. Adv. Enzymol. Mol. Biol. 1978;46:383–414. doi: 10.1002/9780470122914.ch6. [DOI] [PubMed] [Google Scholar]
- 6.Awasthi S, Cheng J, Singhal SS, Saini MK, Pandya U, Pikula S, Singh SV, Zimniak P, Awasthi YC. Novel function of human RLIP76: ATP-dependent transport of glutathione-conjugates and doxorubicin. Biochemistry. 2000;39:9327–9334. doi: 10.1021/bi992964c. [DOI] [PubMed] [Google Scholar]
- 7.Sharma R, Singhal SS, Wickramarachchi D, Awasthi YC, Awasthi S. RLIP76-mediated transport of leukotriene C4 in cancer cells: implications in drug-resistance. Int. J. Cancer. 2004;112:934–942. doi: 10.1002/ijc.20516. [DOI] [PubMed] [Google Scholar]
- 8.Stuckler D, Singhal J, Singhal SS, Yadav S, Awasthi YC, Awasthi S. RLIP76 transports vinorelbine and mediates drug-resistance in non-small cell lung cancer. Cancer Res. 2005;65:991–998. [PubMed] [Google Scholar]
- 9.Awasthi S, Singhal SS, Yadav S, Singhal J, Drake K, Nadkar A, Zajac E, Rowe N, Yacoub A, Boor P, Dwivedi S, Dent P, Jarman W, John B, Awasthi YC. RLIP76 is a major determinant of radiation-sensitivity. Cancer Res. 2005;65:6022–6028. doi: 10.1158/0008-5472.CAN-05-0968. [DOI] [PubMed] [Google Scholar]
- 10.Singhal SS, Wickramarachchi D, Singhal J, Yadav S, Awasthi YC, Awasthi S. Determinants of differential DOX-sensitivity between SCLC and NSCLC. FEBS Lett. 2006;580:2258–2264. doi: 10.1016/j.febslet.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 11.Singhal SS, Awasthi YC, Awasthi S. Regression of melanoma in a murine model by RLIP76 depletion. Cancer Res. 2006;66:2354–2360. doi: 10.1158/0008-5472.CAN-05-3534. [DOI] [PubMed] [Google Scholar]
- 12.Singhal SS, Singhal J, Yadav S, Dwivedi S, Boor PJ, Awasthi YC, Awasthi S. Regression of lung and colon cancer xenografts by depleting or inhibiting RLIP76. Cancer Res. 2007;67:4382–4389. doi: 10.1158/0008-5472.CAN-06-4124. [DOI] [PubMed] [Google Scholar]
- 13.Singhal SS, Yadav S, Drake K, Singhal J, Awasthi S. Hsf-1 and POB1 induce drug-sensitivity and apoptosis by inhibiting Ralbp1. J. Biol. Chem. 2008;283:19714–19729. doi: 10.1074/jbc.M708703200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quaroni A, Paul EC. Cytocentrin is a Ral-binding protein involved in the assembly and function of the mitotic-apparatus. J. Cell Sci. 1999;112:707–718. doi: 10.1242/jcs.112.5.707. [DOI] [PubMed] [Google Scholar]
- 15.Jullien-Flores V, Mahe Y, Mirey G, Leprince C, Meunier B, Sorkin A, Camonis JH. Ralbp1, an effector of the GTPase Ral, interacts with the AP2 complex: involvement of the Ral-pathway in receptor-endocytosis. J. Cell Sci. 2003;113:2837–2844. doi: 10.1242/jcs.113.16.2837. [DOI] [PubMed] [Google Scholar]
- 16.Awasthi S, Singhal SS, Sharma R, Zimniak P, Awasthi YC. Transport of glutathione-conjugates and chemotherapeutic drugs by RLIP76: a novel link between G-protein and tyrosine-kinase signaling and drug-resistance. Int. J. cancer. 2003;106:635–646. doi: 10.1002/ijc.11260. [DOI] [PubMed] [Google Scholar]
- 17.Singhal SS, Yadav S, Singhal J, Awasthi YC, Awasthi S. Mitogenic and drug-resistance mediating effects of PKCα require RLIP76. Biochem. Biophys. Res. Commun. 2006;348:722–727. doi: 10.1016/j.bbrc.2006.07.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Awasthi S, Singhal SS, Srivastava SK, Zimniak P, Bajpai KK, Saxena M, Sharma R, Ziller SA, III, Frenkel E, Singh SV, Awasthi YC. ATP-dependent transport of doxorubicin, daunomyicn, and vinblastine in human tissues by a mechanism distinct from the P-glycoprotein. J. Clin. Invest. 1994;93:958–965. doi: 10.1172/JCI117102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Awasthi S, Singhal SS, Singhal J, Cheng J, Zimniak P, Awasthi YC. Role of RLIP76 in lung cancer doxorubicin-resistance: Doxorubicin-transport in lung cancer by Ralbp1. Int. J. Oncol. 2003;22:713–720. [PubMed] [Google Scholar]
- 20.Cheng J, Sharma R, Yang Y, Singhal SS, Sharma A, Saini MK, Singh SV, Zimniak P, Awasthi S, Awasthi YC. Accelerated metabolism and exclusion of 4-HNE through induction of RLIP76 and hGST5.8 is an early adaptive response of cells to heat and oxidative-stress. J. Biol. Chem. 2001;276:41213–41223. doi: 10.1074/jbc.M106838200. [DOI] [PubMed] [Google Scholar]
- 21.Singhal J, Singhal SS, Yadav S, Suzuki S, Warnke M, Yacoub A, Dent P, Bae S, Sharma R, Awasthi YC, Armstrong DW, Awasthi S. RLIP76 in defense of radiation poisoning. Int. J. Rad. Oncol. Biol. Phys. 2008;72:553–561. doi: 10.1016/j.ijrobp.2008.06.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Awasthi S, Singhal SS, Pandya U, Singh SV, Awasthi YC. ATP-dependent colchicine transport by human erythrocyte glutathione-conjugate transporter. Toxicol. Appl. Pharmocol. 1999;155:215–226. doi: 10.1006/taap.1998.8617. [DOI] [PubMed] [Google Scholar]
- 23.Singhal SS, Yadav S, Singhal J, Zajac E, Drake K, Awasthi YC, Awasthi S. Depletion of RLIP76 sensitizes lung cancer cells to DOX. Biochem. Pharmacol. 2005;70:481–488. doi: 10.1016/j.bcp.2005.05.005. [DOI] [PubMed] [Google Scholar]


