Abstract
Objective
Perform a systematic review of studies reporting on the association between maternal prenatal cigarette smoking and child overweight.
Design
Meta-analysis of observational studies.
Data sources
Medline search and review of reference lists among studies published through June 2006.
Review methods
Included studies reported an association between maternal smoking during pregnancy and risk of overweight among children at least 2 years of age. We did not include in the meta-analysis studies that provided only a continuous measure of adiposity, although those studies are discussed separately.
Results
Based on results of 84 563 children reported in 14 observational studies, children whose mothers smoked during pregnancy were at elevated risk for overweight (pooled adjusted odds ratio (OR) 1.50, 95% CI: 1.36, 1.65) at ages 3–33 years, compared with children whose mothers did not smoke during pregnancy. The pooled estimate from unadjusted odds ratios (OR 1.52, 95% CI: 1.36, 1.69) was similar to the adjusted estimate, suggesting that sociodemographic and behavioral differences between smokers and nonsmokers did not explain the observed association. Although we observed evidence for publication bias, simulating a symmetric set of studies yielded a similar estimate (OR 1.40, 95% CI: 1.26, 1.55).
Conclusions
Prenatal smoking exposure appears to increase rates of overweight in childhood. In parts of the world undergoing the epidemiologic transition, the continuing increase in smoking among young women could contribute to spiraling increases in rates of obesity-related health outcomes in the 21st century.
Keywords: smoking, pregnancy, overweight, meta-analysis, systematic review
Introduction
Despite decades of research, press, counter-advertising, and litigation regarding its adverse effects, tobacco use remains a major cause of preventable morbidity and mortality world-wide.1 Although fewer women in the US and Britain now smoke than in past decades, an increasing number of teenage girls are initiating smoking, and smoking rates are declining less rapidly among women than among men, so cigarette smoking remains common among women who are of childbearing age, pregnant, or breastfeeding.1–4 In the developing world, a small but rapidly expanding proportion of women smoke.5
Exposure to cigarette smoke in utero puts a fetus at increased risk for a number of adverse health outcomes, including growth restriction.2,6 Whereas smaller size at birth is generally associated with reduced later risk for overweight,7 recent research suggests that mothers who smoke during pregnancy have children at increased risk for later obesity. The combination of small size at birth and overweight in later life is not only characteristic of the epidemiologic transition from acute to chronic disease, but also confers a high risk of cardiovascular outcomes in adulthood.7–9
The magnitude of the association between prenatal smoking and childhood overweight and independence from social influences are not clear. In this paper, we review the evidence from both human and animal studies linking prenatal nicotine or tobacco exposure with offspring adiposity. We hypothesized that exposure prenatal smoking would predispose offspring to elevated risk for overweight. We performed a meta-analysis of published observational cohort studies to obtain a pooled estimate of the risk for overweight associated with maternal smoking during pregnancy, using unadjusted estimates and estimates adjusted for factors that may confound the association. We chose to evaluate a dichotomous outcome for weight because overweight is a routinely used measure and has established public health impact. We discuss possible mechanisms by which intrauterine tobacco exposure might program child weight. Finally, we discuss the potential of increasing rates of smoking during pregnancy to fuel the epidemiologic transition to obesity-related adverse health outcomes in the developing world.
Search strategy
To find studies of prenatal smoking and risk for offspring overweight, we performed online searches of the published literature using Pub Med online (1966 to June 2006) with the keywords ‘smoking’, ‘pregnancy’, and ‘overweight or obesity’, yielding 416 references. We also searched using Ovid, with the same terms, each exploded and searched both as a keyword and as a subject heading, yielding 308 references, all of which were also obtained via Pub Med. One of us (EO) reviewed all titles for studies with potential information about smoking during pregnancy and child overweight. We then obtained the full text of candidate articles, and reviewed reference lists from all relevant papers and reviews to identify additional candidates. We performed a full text review on 69 papers. We excluded 13 studies that did not have information on prenatal smoking exposure, and 38 without information on overweight as an outcome, or with outcome prior to age 3 years (Figure 1). Of the 18 remaining studies, 2 did not provide data regarding the multivariable association between prenatal smoking and child overweight, and we were unable to obtain these data by contacting the authors.10,11 We also excluded two studies that presented data wholly presented in another publication.12,13 We did not contact authors or otherwise attempt to obtain unpublished data. None of the candidates were published in a language other than English, and we identified no data that were published only in abstract form. We thus included in our meta-analysis the 14 studies that met our a priori inclusion criteria. We obtained unadjusted estimates from authors of four studies (Table 1).
Figure 1.
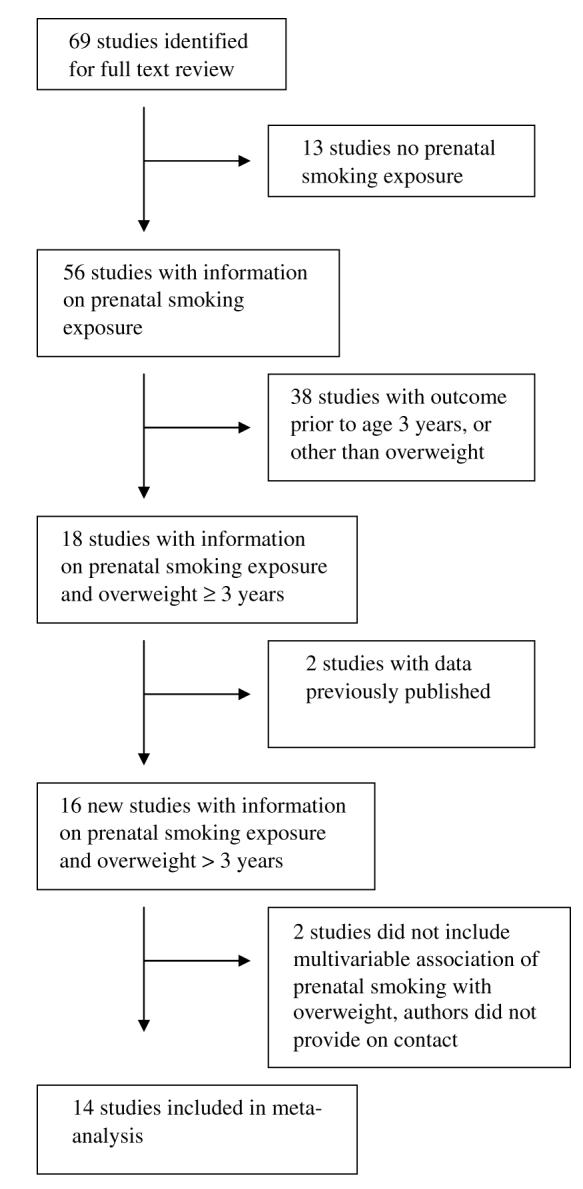
Flow diagram showing the number of studies included in and excluded from the meta-analysis.
Table 1.
Studies included in meta-analysis of maternal smoking during pregnancy and child overweight
|
Study, 1st author (year), population |
N in analysis (cohort) |
Child age in years at outcome |
Smoking exposure (prevalence) |
Covariates | Outcome (prevalence) |
Unadjusted OR (95% CI) |
Adjusted OR (95% CI) |
|---|---|---|---|---|---|---|---|
| Adams (2005)14 | 252 | 3 | At initial WIC | Maternal: age, BMI, education, income |
BMI≥85th percentile (40.9%) | 1.74 (1.05, 2.90)a | 2.16 (1.05, 4.47) |
| American Indians in Wisconsin enrolled in the Supplemental Program for Women, Infants, and Children (WIC) |
(3015) | visit—‘most 1st or 2nd trimester’ (42.5%) Maternal report at prenatal WIC visit |
Child: birthweight, ever breastfed | Height and weight measured at WIC visit |
|||
| Al Mamun (2006)15 | 3253 (7223) |
14 | During pregnancy (36.6%) |
Maternal: age, marital status, income |
85th ≤BMI <95th percentile (19.3%) |
1.30 (1.07, 1.58) | 1.30 (1.05, 1.60) |
| Mater University Study of Pregnancy and its Outcomes, Australia |
Maternal report during pregnancy and at birth |
Child: age, sex, breastfeeding, consumption of fast food, salad, soft drinks, red meat, TV viewing, participation in sports and exercise |
BMI≥95th percentile (6.2%) Research measurement of height and weight |
1.41 (1.04, 1.91) | 1.40 (1.01, 1.94) | ||
| Bergmann (2003)16 German Multicenter Atopy Study |
480 (1314) |
6 | During pregnancy (19.6%) Not reported how assessed |
Maternal: social status, overweight in pregnancy Child: breastfeeding |
BMI≥90th percentile (9.5%) BMI≥97th percentile (9.5%) Research measurement of height and weight |
1.78 (1.21, 2.64) | 2.08 (1.19, 3.63) 2.30 (1.15, 4.60) |
| Chen (2006)17 | 34 866 (44 213) |
8 | During pregnancy (51.4%) |
Maternal: age, race, pre- pregnancy BMI, marital status, education, socioeconomic index, gestational age at recruitment, recruitment site |
BMI≥85th percentile (11%) | 1.23 (1.08, 1.39) Ma 1.29 (1.14, 1.47) Faa |
1.21 (1.05, 1.39) M 1.37 (1.19, 1.58) F |
| Collaborative Perinatal Project | Maternal report during pregnancy |
Child: age, birth order, breastfeeding |
BMI≥95th percentile (4%) Research measurement of height and weight |
1.17 (0.96, 1.43) Ma 1.20 (0.99, 1.44) F |
1.21 (0.96, 1.51) M 1.31 (1.06, 1.61) F |
||
| Dubois (2006)18 Quebec Longitudinal Study Child Development, Canada |
1550 (2103) |
4.5 | During pregnancy (25.2%) Maternal report at 5 months postpartum |
Maternal: overweight, Child: birth weight, gestational age, weight gain birth to 5 months Other: paternal overweight, household income |
BMI≥95th percentile (8.5%) Not reported how height and weight measured |
1.6 (1.1, 2.4) | 1.8 (1.2, 2.8) |
| Oken (2005)19 Project Viva, US |
746 (2128) |
3 | Early pregnancy (10%) Maternal report during pregnancy |
Maternal: pre-pregnancy BMI, gestational weight gain, education, race/ethnicity, parity Child: age, sex, fetal growth, gestation length Other: Paternal BMI, household income |
BMI≥85th percentile (27%) Research measurement of height and weight |
2.5 (1.5, 4.1)a | 2.2 (1.2, 3.9) |
| Power (2002)20 National Child and Development Study, UK |
5839 (17 414) |
33 | After the 4th month of pregnancy (33.5%) Maternal report at birth |
Maternal: BMI, Child: social class at birth, age 7, and age 33, birthweight, infant feeding, physical inactivity age 23, diet age 33 (fried foods, chips, biscuits, sweets, chocolates), attained education age 33 |
BMI≥30 Research measurement of height and weight |
1.56 (1.22, 2.00) M 1.41 (1.12, 1.79) F |
1.55 (1.19, 2.00) M 1.45 (1.13, 1.87) F |
| Reilly (2005)21 Avon Longitudinal Study of Parents and Children, UK |
5493 (13 971) |
7 | During pregnancy 1-9 cigs/day vs 0 (5.5%) (in total 14% smoked) Maternal report during pregnancy |
Maternal: age, parity, obesity Child: birth weight, sex, season at birth, gestational age, plurality, breast feeding, introduction of solids, number of siblings, ethnicity, in childhood time spent watching TV, in car, sleeping. Dietary pattern in childhood. Other: paternal obesity |
BMI≥95th percentile (8.6%) Research measurement of height and weight |
1.60 (1.17, 2.18) | 1.76 (1.21, 2.52) |
| Salsberry (2005)22 National Longitudinal Survey of Youth, US |
3022 (6615) |
6–7 | During pregnancy (29%) Maternal report -not reported when assessed |
Maternal: Age, parity, race/ ethnicity, pre-pregnancy BMI, education, marital status Child: sex, age, birth year, breast feeding, height and weight measured |
BMI≥95th percentile (12%) Not reported how height and weight measured |
1.43 | 1.74 (1.32, 2.29) |
| Toschke (2002)23 Bavarian schoolchildren, Germany |
8765 (13 345) |
5–6.9 | Throughout pregnancy (7.5%) Maternal report at outcome assessment |
Child: low birth weight, prematurity, breastfeeding Other: parental education |
BMI≥90th percentile (10%) BMI≥97th percentile (3%) Clinical measurement of height and weight |
1.85 (1.47, 2.33) 2.32 (1.63, 3.30) |
1.58 (1.23, 2.04) 1.92 (1.29, 2.86) |
| Toschke (2003)24 Bavarian schoolchildren, Germany |
4974 (7026) |
5–6.9 | Early pregnancy (10.9%) Maternal report at outcome assessment |
Maternal: education, obesity Child: breastfeeding, TV viewing, video games, physical activity, high infant weight gain Other: paternal obesity |
‘Overweight’ (10.4%) ‘Obesity’ (2.7%) Clinical measurement of height and weight |
1.66 (1.27, 2.18) 2.41 (1.41, 3.91) |
1.52 (1.14, 2.01) 2.22 (1.33, 3.69) |
| Von Kries (2002)25 Bavarian schoolchildren, Germany |
6483 (9731) |
5–6.9 | During pregnancy (9.8%) Maternal report at outcome assessment |
Maternal: education, overweight Child: birth weight > 90th percentile, any breastfeeding, TV/video game > 1 h daily, eating snacks in front of TV Other: paternal education overweight |
BMI≥90th percentile (10%) BMI≥97th percentile (3%) Clinical measurement of height and weight |
1.97 (1.52, 2.56) 2.96 (1.97, 4.46) |
1.43 (1.07, 1.90) 2.06 (1.31, 3.23) |
| Whitaker (2004)26 Ohio children enrolled in WIC, US |
8494 | 2–4 | During pregnancy (32.9%) Birth certificate data |
Maternal: age, BMI, race/ethnicity, parity, pregnancy weight gain, education, marital status Child: fetal growth, sex, year of birth |
BMI≥95th percentile age 2 (9.5%) BMI≥95th percentile age 3 (12.5%) BMI≥95th percentile age 4 (14.8%) Height and weight measured at WIC visit |
1.20 (1.02, 1.42)a 1.09 (0.93, 1.28)a 1.06 (0.90, 1.24)a |
1.43 (1.19, 1.72) 1.25 (1.05, 1.49) 1.21 (1.01, 1.45) |
| Wideroe (2003)27 Population based pregnancy cohort in Norway and Sweden |
346 (5722) |
5 | At week 17 (31.3%) Maternal report during pregnancy |
Maternal: age, skinfold thickness, education, total energy intake week 17, fat and carbohydrate intake week 33 Child: birth weight, breastfeeding duration Other: paternal education |
BMI≥85th percentile (15%) Research measurement of height and weight |
3.0 (1.6, 5.6) | 3.8 (2.0, 7.2) |
Unadjusted estimate provided by personal communication from study authors.
Methods
Two authors (EO and EL) performed independent data extractions of the eligible studies, in accordance with the ‘MOOSE’ guidelines.28 We used random-effects models weighted by the inverse of the variance of each study29 to calculate pooled odds ratios across studies. We tested for heterogeneity between studies and calculated the proportion of the total variability attributable to between-study heterogeneity (I2).30 To assess potential publication bias, we plotted the natural logarithm of the published odds ratio versus its standard error, and performed a rank correlation test.31 When we observed evidence for a publication bias, we used the trim and fill method of Duval and Tweedie32 to impute ‘missing’ studies and simulate an unbiased dataset. With this method, we removed the most extreme studies one by one, and tested the remaining studies for symmetry. Once the group was no longer asymmetrical, we mirrored the studies that were removed, and included both the published studies and the simulated mirrored studies in the analysis.
We included studies that presented results as an odds ratio for overweight or obesity associated with smoking at some point during pregnancy. We used the final fully adjusted model presented by study authors, although the covariates they included differed across studies. For studies with outcome assessment at multiple ages, we chose the oldest age. When study authors presented multiple outcomes, we chose for the primary analysis overweight, which we defined as BMI≥85th percentile or ≥90th percentile for age and sex. When study authors reported multiple exposures, we selected the lowest smoking dose, and smoking in early pregnancy as opposed to later in pregnancy or throughout pregnancy. We performed secondary analyses (1) using unadjusted estimates, (2) excluding studies in which exposure and outcome were assessed simultaneously, and (3) including obesity (BMI≥95th percentile or ≥97th percentile) as the outcome instead of overweight. We additionally examined the effect of replacing endpoints measured at age 33 years in the 1958 British birth cohort20 with those measured at age 7 years, closer to endpoints for the other studies.
Results
Prenatal smoking and child overweight
Our literature review yielded 14 studies eligible for inclusion in the meta-analysis of smoking and overweight risk14–27 (Table 1). These studies included 84 563 children, and represent pregnancies that occurred from 1958 to 2002 in low and non-low income populations in Australia, North America, and Europe. The prevalence of smoking during pregnancy in the studied populations ranged from 7.5 to 51%. We did not include in the meta-analysis studies that presented results as a continuous measure of adiposity or as height and weight individually, although we discuss them below.
Based on our meta-analysis, children whose mothers smoked during pregnancy were at elevated risk for overweight (pooled adjusted odds ratio (OR) 1.50, 95% CI: 1.36, 1.65, P for heterogeneity = 0.02, I2 = 49%, 95% CI: 8, 71%) compared with children whose mothers did not smoke during pregnancy (Figure 2). The study by Wideroe et al.27 had the largest effect on the pooled estimate, but excluding that study only slightly influenced the pooled odds ratio (OR 1.44, 95% CI: 1.33, 1.56, P for heterogeneity = 0.13, I2=30%, 95% CI: 0, 62%). Excluding studies in which mothers were asked about prenatal smoking at the same time outcomes were assessed23–25 did not change the pooled estimate (OR 1.51, 95% CI: 1.35, 1.70, P for heterogeneity=0.005, I2 = 57%, 95% CI: 21, 77%). Including the age 7 endpoint, rather than that at age 33, from the paper by Powers et al.,20 resulted in a somewhat lower pooled odds ratio (OR 1.44, 95% CI: 1.29, 1.60, P for heterogeneity <0.001, I2=63, 37, 78%). Using obesity cut points, where available, the pooled estimate (OR 1.52, 95% CI: 1.36, 1.69, P for heterogeneity = 0.07, I2 = 40%, 95% CI: 0, 69%) was similar to that for overweight cut points.
Figure 2.
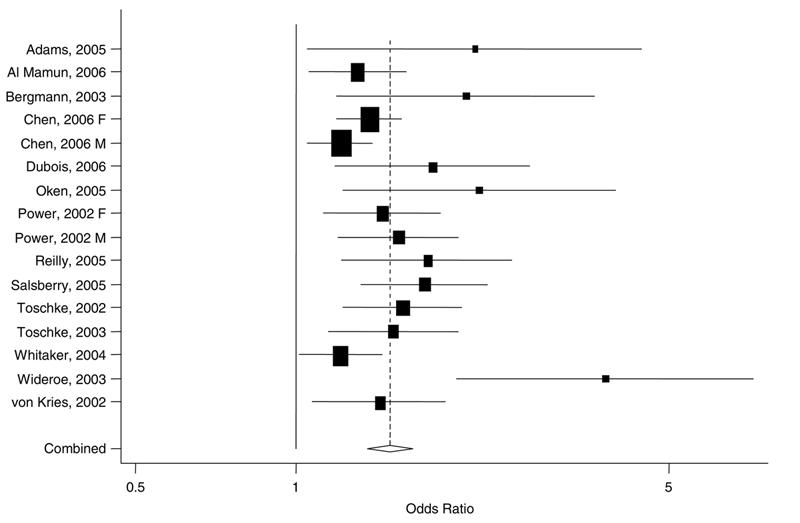
Results of meta-analysis of maternal smoking during pregnancy and child overweight. Individual study estimates are indicated by boxes with 95% CIs indicated by lines. The size of the box is inversely proportional to the variance. The pooled estimate is represented by the diamond, with the width of the diamond representing the pooled CI (pooled OR 1.50, 95% CI: 1.36, 1.65).
Mothers who smoked during pregnancy tended to be substantially different from nonsmokers in ways that also predict child obesity risk. In general, smokers had lower income, were less educated, heavier, and less likely to breastfeed, and their children had more rapid infancy weight gain and were more inactive.19,24,25 However, in older US cohorts and in European populations in which smoking was more common, smokers differed from nonsmokers less than among populations in which smoking was more rare. The published studies evaluated and included different covariates, although most accounted for maternal weight, fetal growth, and some measure of socioeconomic status (Table 1). In most studies the association between prenatal smoking exposure and child overweight was either unaffected or slightly attenuated by confounder adjustment (Table 1), with a few exceptions16,21,26 though authors generally did not report which factors accounted for the change in risk. In one study, adjustment for breastfeeding substantially strengthened the risk of overweight associated with maternal smoking.27 The pooled estimate from the unadjusted odds ratios (OR 1.52, 95% CI: 1.36, 1.69, P for heterogeneity <0.001, I2=67, 45, 81%) was similar to the adjusted estimate.
We observed evidence for a small study effect suggestive of publication bias, based on asymmetry of the funnel plot (Figure 3) and by rank correlation testing (P<0.001). We then imputed ‘missing’ studies to simulate a dataset without publication bias (Figure 3). The resulting pooled odds ratio (OR 1.40, 95% CI: 1.26, 1.55) was somewhat lower, but still indicated a substantial detrimental effect of prenatal smoking.
Figure 3.
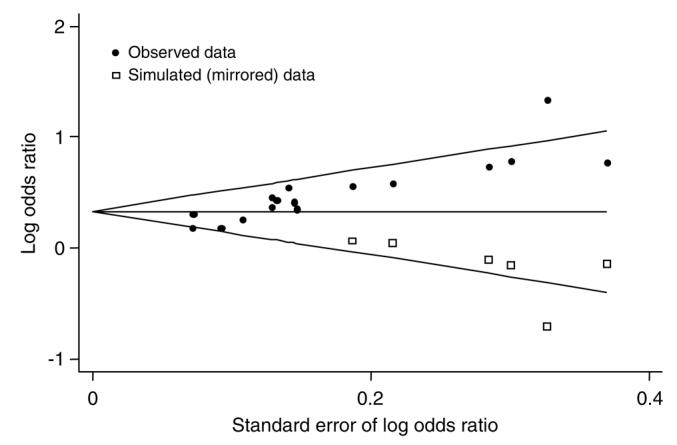
Begg’s funnel plot with pseudo 95% CIs. Filled circles indicate observed data, and open squares indicate simulated data.
Several studies reported associations of prenatal smoking with continuous measures of adiposity. As these studies used a number of different outcome measures, including BMI, BMI z-score, weight-for-height z-score, ponderal index, and skinfold thicknesses, assessed at different ages, we did not pool the continuous outcomes. Consistent with the dichotomous overweight outcome, most estimated greater adiposity in offspring of mothers who had smoked during pregnancy.14,15,17,19,20,27,33–36 Two studies reported associations that were not statistically significant, although they did not provide adjusted estimates.37,38 No study reported less adiposity. A recent study evaluated children at age 7 with DXA scanning, and reported substantially higher over all body fat, but not truncal distribution of fat, and to a lesser extent higher lean body mass, in children exposed to prenatal smoking.39 Several other publications reporting continuous measures of adiposity were interim analyses of longitudinal cohort studies, including the National Child and Development Study,40–42 the Collaborative Perinatal Project,43,44 and the ALSPAC cohort45,46 for which later publications included a measure of overweight and thus were included in our meta-analysis.17,20,21
A few studies provided information on weight and height individually. Three recent studies reported higher weight but no difference in height in children exposed to prenatal smoking.14,19,33 Others have found that prenatally exposed children were both shorter and heavier17,34,35,44,47 with shorter leg length.48 Additional older studies with no information on weight found that children of smokers were shorter.40–42,49,50
Despite considerable differences in the study populations and exposure metrics, some general patterns were evident that add further detail to the association between prenatal smoking and child overweight. Among studies with information on the amount of maternal smoking, all found evidence for a dose-response.19–21,25,27,33,39 Two studies with repeated outcome measurements found that obesity risk increased with increasing child age.17,20 Associations between smoking and child overweight were present in studies using never smokers as a reference group and in those that used women not smoking during the index pregnancy, that is, including former smokers, as a reference group.
The timing of smoking exposure was important. In general, smoking throughout pregnancy was associated with a greater risk for child overweight than was smoking in early pregnancy only17,20,23 although other studies reported similar estimates for smoking regardless of time during pregnancy.24,39 One study found an increased risk of overweight among children whose mothers reported having quit smoking prior to pregnancy,23 although this finding was not supported elsewhere.15,17,19 Maternal smoking limited to after pregnancy did not appear to increase risk for offspring overweight.23
Two publications report follow-up of children whose mothers participated in a randomized controlled trial of smoking cessation during pregnancy. MacArthur et al.51 reported on 9-year follow-up of an antismoking intervention consisting of an educational leaflet and a one-time clinician-led discussion and advice to quit, compared with usual care. The intervention resulted in reduced maternal smoking and higher birth weight.52 At age 9 years, children whose mothers were randomized to usual care were slightly shorter (0.3 cm) and lighter (0.3 kg), although differences were not statistically significant. When the authors analyzed data according to whether mothers quit smoking or continued smoking, regardless of group assignment, children of persistent smokers were significantly shorter (1.0 cm) but ‘not different’ in weight (0.6 kg lighter).51 Fox et al.53 did not provide results according to randomization, but similarly found that 3-year-old children of persistent smokers were significantly shorter (1.04 cm) but not different in weight (0.32 kg lighter) compared with children of women who quit during pregnancy. Neither study reported weight-for-height or BMI.
Discussion
Our systematic review suggests that exposure to prenatal smoking increases risk for overweight in childhood. The association was unaffected by adjustment for parental sociodemographic factors and body size, gestational weight gain, infant feeding, and child behaviors, indicating that social and behavioral differences between smokers and nonsmokers are not likely to account for the observed differences in overweight risk. In all of the studies, smoking during pregnancy was associated with increased overweight in offspring, despite a wide range of populations, smoking habits, birth years, and overweight prevalence. Compared with children of nonsmokers, children exposed to prenatal smoking may be heavier, shorter or both.
Publication bias likely exists, as smaller studies reported stronger effects than larger ones, and no published studies were null or reported an inverse association. The data in our review come from observational studies rather than registered clinical trials, so we cannot determine the number of unpublished null studies. We did not contact authors to obtain unpublished effect estimates. However, our efforts to impute the unpublished data suggest that the observed association does not entirely derive from a publication bias.
Other studies have evaluated additional endpoints related to overweight. Single studies have suggested that individuals exposed to prenatal smoking may have greater weight gain from birth to age 2 years,45 earlier puberty,33 elevated risk for diabetes mellitus,54 and exhibit a high-risk cluster of low birth weight and higher attained BMI,55 or of higher BMI, blood pressure, lipids, and glucose levels.56 Several investigators have studied associations between prenatal smoking exposure and blood pressure in childhood. In the studies that accounted for sociodemographics and other characteristics including child size, systolic blood pressure was consistently about 1 mm Hg higher among children who were exposed to prenatal maternal smoking.19,36,57,58
A growing body of epidemiologic and experimental evidence indicates that the prenatal period is a sensitive period for later health outcomes.59 Early research in the field considered birth weight as a marker for intrauterine nutrition. The example of maternal smoking highlights the importance of looking at more refined measures, such as body composition, rather than birth weight alone in studying prenatal influences on later size and disease risk. Maternal smoking may result in lower fetal growth but more rapid postnatal weight gain, yet both fetal growth and weight gain in early infancy are directly associated with weight in later life.7 In our meta-analysis, associations of smoking with child overweight were independent of birth weight or fetal growth and postnatal weight gain. These apparently incongruous relationships may be explained by reports that even at birth, infants born to mothers who smoked in pregnancy have a preserved ponderal index60 (a measure of weight for length) compared with infants of nonsmokers, and have relatively more body fat but less lean body mass.61,62 These findings also reinforce the need for research aimed at understanding pathways independent of early growth by which early life experiences may influence later health and disease risk.59
Mechanisms by which maternal smoking may program child weight are not yet well characterized. Likely culprits for the physiologic effects of cigarette smoking during pregnancy are nicotine, which is transported across the placenta, and carbon monoxide, which may influence placental vascular function and cause fetal hypoxia. In both humans and animals, nicotine acts both centrally and peripherally to reduce appetite and body weight, and nicotine withdrawal results in hyperphagia and weight gain.63–65 Children of smokers tend to be less physically active and have poorer diet quality.66–68 In one study, adults with prenatal smoking exposure were more likely to report having a ‘poor appetite’, although dietary intake was not measured; since they were also heavier, appetite perception might have been influenced rather than appetite itself.20,69
In animal studies, administration of nicotine to pregnant mothers resulted in offspring that were smaller at birth but had increased body fat.70–73 In other studies, rats prenatally exposed to low doses of nicotine were not smaller at birth but became heavier by 5–10 weeks of age74,75 and had higher overall body fat as well as increased perivascular adipose tissue, resulting in decreased vascular relaxation.75 In another protocol, prenatal nicotine exposure did not influence body weight, but caused higher blood pressure only among genetically susceptible rats.76 Among humans, some researchers have studied whether hormones such as leptin may mediate associations of smoking with offspring size. Maternal smoking throughout pregnancy may be associated with lower cord blood leptin77,78 though other studies have not suggested an association.79,80 Investigations of other possible mediators, including growth hormone and IGF-1, have not been as well studied,80 and no data exist regarding levels of any of these hormones beyond the newborn period.
A recent systematic review of prenatal programming of childhood overweight and obesity also considered prenatal smoking, concluding that strong evidence linked maternal smoking with childhood obesity.81 However, those authors included only 8 studies of the 14 that we evaluated, primarily those with simultaneous collection of exposure and outcome data and with clinical height and weight measurement, did not calculate a pooled effect size, and did not consider publication bias. This present study overcomes these limitations and allows for an estimation of the public health impact on overweight risk resulting from maternal prenatal smoking.
All of the studies included in the present meta-analysis relied upon maternal self-report of smoking behaviors without biochemical validation. However, self-report of smoking during pregnancy has reasonable validity,82 and any under-reporting would likely bias results towards the null. Although some studies collected information on prenatal smoking at the same time that child outcomes were assessed, we doubt recall bias as exclusion of these studies did not substantially change estimates. All studies used measured height and weight as the endpoint, none relied on self-report. Sufficient data do not exist to determine whether quitting smoking would lower the risk of child overweight to the same degree as never having smoked.
In addition to demographic differences such as education and income, smokers and their children also tend to have less healthy lifestyle habits, including diet and physical activity.66–68,83 Residual confounding may explain the observed higher risk for overweight in exposed children. The similar pooled estimates for unadjusted and adjusted associations suggest that sociodemographic, behavioral, and dietary differences between smokers and nonsmokers did not explain the observed association. A recent publication found that associations of maternal prenatal smoking with child body fat were only slightly stronger than associations of paternal prenatal smoking, and concluded that residual confounding may explain the observed association.39 However, the evidence for dose-effect, the lack of association with smoking before or after pregnancy, the support from animal studies, and the consistency of results across populations with markedly different demographic profiles and confounding structures make it more likely that a causal association exists. Future studies following children whose mothers participated in randomized trials of smoking cessation during pregnancy might help further elucidate the role of confounding.
In conclusion, available evidence suggests that maternal smoking during pregnancy increases risk for overweight in childhood. These findings add to the recognized health burden from tobacco, already estimated at almost 5 million deaths per year.5 The overall population impact of a 50% increased risk of overweight is likely to be large. In the US, where 11% of pregnant women currently smoke4 and child overweight is highly prevalent,84 about 715 000 US children may be overweight because their mothers smoked. Individuals who are born small and later become obese are at highest risk for developing cardiovascular disease.7 This phenotype, typical of exposure to maternal prenatal smoking, is also characteristic of parts of the world undergoing the nutritional transition to high fat diets and sedentary lifestyle and the accompanying epidemiologic transition to chronic diseases associated with obesity.8 In such populations, the combination of poor maternal nutrition, lower birth weight, and increasing adoption of a western lifestyle heralds an explosion in obesity and cardiovascular disease that may well intensify if young women respond to tobacco companies’ advertisements targeting them.8,85
Acknowledgements
This project was supported by grants from the Robert Wood Johnson Foundation, the National Institutes of Health (HD 34568, HL 64925, HL 68041, HD 44807, HL 07374), and by Harvard Medical School and the Harvard Pilgrim Health Care Foundation. We appreciate the assistance of Trish Elliott with database development.
References
- 1.Department of Health and Human Services CfDCaP . Tobacco Control State Highlights 2002: Impact and Opportunity. Office on Smoking and Health; Atlanta, GA: 2002. [Google Scholar]
- 2.Orleans CT, Barker DC, Kaufman NJ, Marx JF. Helping pregnant smokers quit: meeting the challenge in the next decade. Tob Control. 2000;9(Suppl 3):III6–III11. doi: 10.1136/tc.9.suppl_3.iii6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor TLD, Bryant A, Keyse L, McDuff TJ. Smoking-Related Behavior and Attitudes, 2005. Office for National Statistics; London: 2006. [Google Scholar]
- 4.Hamilton BE, Martin JA, Sutton PD. Births: Preliminary data for 2003. National Vital Statistics Reports. 2004;53:1–18. [PubMed] [Google Scholar]
- 5.Samet J, Young SY. Women and the Tobacco Epidemic: Challenges for the 21st Century. World Health Organization; Geneva: 2001. [Google Scholar]
- 6.Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ. 1987;65:663–737. [PMC free article] [PubMed] [Google Scholar]
- 7.Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;11:496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 8.Popkin BM. The nutrition transition and obesity in the developing world. J Nutr. 2001;131:871S–873S. doi: 10.1093/jn/131.3.871S. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson JG, Forsen T, Tuomilehto J, Winter PD, Osmond C, Barker DJ. Catch-up growth in childhood and death from coronary heart disease. BMJ. 1999;318:427–431. doi: 10.1136/bmj.318.7181.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bogen DL, Hanusa BH, Whitaker RC. The effect of breast-feeding with and without formula use on the risk of obesity at 4 years of age. Obes Res. 2004;12:1527–1535. doi: 10.1038/oby.2004.190. [DOI] [PubMed] [Google Scholar]
- 11.Hediger ML, Overpeck MD, Kuczmarski RJ, Ruan WJ. Association between infant breastfeeding and overweight in young children. JAMA. 2001;285:2453–2460. doi: 10.1001/jama.285.19.2453. [DOI] [PubMed] [Google Scholar]
- 12.Toschke AM, Beyerlein A, von Kries R. Children at high risk for overweight: a classification and regression trees analysis approach. Obes Res. 2005;13:1270–1274. doi: 10.1038/oby.2005.151. [DOI] [PubMed] [Google Scholar]
- 13.von Kries R. Obesity among Bavarian children—experiences from school admittance examinations. Gesundheitswesen. 2004;66(Suppl 1):S80–S85. doi: 10.1055/s-2004-812770. [DOI] [PubMed] [Google Scholar]
- 14.Adams AK, Harvey HE, Prince RJ. Association of maternal smoking with overweight at age 3 y in American Indian children. Am J Clin Nutr. 2005;82:393–398. doi: 10.1093/ajcn.82.2.393. [DOI] [PubMed] [Google Scholar]
- 15.Al Mamun A, O’Callaghan FV, Alati R, O’Callaghan M, Najman JM, Williams GM, et al. Does maternal smoking during pregnancy predict the smoking patterns of young adult offspring? A birth cohort study. Tob Control. 2006;15:452–457. doi: 10.1136/tc.2006.016790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergmann KE, Bergmann RL, Von Kries R, Bohm O, Richter R, Dudenhausen JW, et al. Early determinants of childhood overweight and adiposity in a birth cohort study: role of breast-feeding. Int J Obes Relat Metab Disord. 2003;27:162–172. doi: 10.1038/sj.ijo.802200. [DOI] [PubMed] [Google Scholar]
- 17.Chen A, Pennell ML, Klebanoff MA, Rogan WJ, Longnecker MP. Maternal smoking during pregnancy in relation to child overweight: follow-up to age 8 years. Int J Epidemiol. 2006;35:121–130. doi: 10.1093/ije/dyi218. [DOI] [PubMed] [Google Scholar]
- 18.Dubois L, Girard M. Early determinants of overweight at 4.5 years in a population-based longitudinal study. Int J Obes (Lond) 2006;30:610–617. doi: 10.1038/sj.ijo.0803141. [DOI] [PubMed] [Google Scholar]
- 19.Oken E, Huh SY, Taveras EM, Rich-Edwards JW, Gillman MW. Associations of maternal prenatal smoking with child adiposity and blood pressure. Obes Res. 2005;13:2021–2028. doi: 10.1038/oby.2005.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Power C, Jefferis BJ. Fetal environment and subsequent obesity: a study of maternal smoking. Int J Epidemiol. 2002;31:413–419. [PubMed] [Google Scholar]
- 21.Reilly JJ, Armstrong J, Dorosty AR, Emmett PM, Ness A, Rogers I, et al. Early life risk factors for obesity in childhood: cohort study. BMJ. 2005;330:1357. doi: 10.1136/bmj.38470.670903.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salsberry PJ, Reagan PB. Dynamics of early childhood overweight. Pediatrics. 2005;116:1329–1338. doi: 10.1542/peds.2004-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toschke AM, Koletzko B, Slikker W, Jr, Hermann M, von Kries R. Childhood obesity is associated with maternal smoking in pregnancy. Eur J Pediatr. 2002;161:445–448. doi: 10.1007/s00431-002-0983-z. [DOI] [PubMed] [Google Scholar]
- 24.Toschke AM, Montgomery SM, Pfeiffer U, von Kries R. Early intrauterine exposure to tobacco-inhaled products and obesity. Am J Epidemiol. 2003;158:1068–1074. doi: 10.1093/aje/kwg258. [DOI] [PubMed] [Google Scholar]
- 25.von Kries R, Toschke AM, Koletzko B, Slikker W., Jr Maternal smoking during pregnancy and childhood obesity. Am J Epidemiol. 2002;156:954–961. doi: 10.1093/aje/kwf128. [DOI] [PubMed] [Google Scholar]
- 26.Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics. 2004;114:e29–e36. doi: 10.1542/peds.114.1.e29. [DOI] [PubMed] [Google Scholar]
- 27.Wideroe M, Vik T, Jacobsen G, Bakketeig LS. Does maternal smoking during pregnancy cause childhood overweight? Paediatr Perinat Epidemiol. 2003;17:171–179. doi: 10.1046/j.1365-3016.2003.00481.x. [DOI] [PubMed] [Google Scholar]
- 28.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 29.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 30.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 31.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 32.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 33.Fried PA, James DS, Watkinson B. Growth and pubertal milestones during adolescence in offspring prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 2001;23:431–436. doi: 10.1016/s0892-0362(01)00161-1. [DOI] [PubMed] [Google Scholar]
- 34.Vik T, Jacobsen G, Vatten L, Bakketeig LS. Pre- and post-natal growth in children of women who smoked in pregnancy. Early Hum Dev. 1996;45:245–255. doi: 10.1016/0378-3782(96)01735-5. [DOI] [PubMed] [Google Scholar]
- 35.Blake KV, Gurrin LC, Evans SF, Beilin LJ, Landau LI, Stanley FJ, et al. Maternal cigarette smoking during pregnancy, low birth weight and subsequent blood pressure in early childhood. Early Hum Dev. 2000;57:137–147. doi: 10.1016/s0378-3782(99)00064-x. [DOI] [PubMed] [Google Scholar]
- 36.Williams S, Poulton R. Twins and maternal smoking: ordeals for the fetal origins hypothesis? BMJ. 1999;318:897–900. doi: 10.1136/bmj.318.7188.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka T, Matsuzaki A, Kuromaru R, Kinukawa N, Nose Y, Matsumoto T, et al. Association between birthweight and body mass index at 3 years of age. Pediatr Int. 2001;43:641–646. doi: 10.1046/j.1442-200x.2001.01468.x. [DOI] [PubMed] [Google Scholar]
- 38.Day N, Cornelius M, Goldschmidt L, Richardson G, Robles N, Taylor P. The effects of prenatal tobacco and marijuana use on offspring growth from birth through 3 years of age. Neurotoxicol Teratol. 1992;14:407–414. doi: 10.1016/0892-0362(92)90051-b. [DOI] [PubMed] [Google Scholar]
- 39.Leary SD, Smith GD, Rogers IS, Reilly JJ, Wells JC, Ness AR. Smoking during pregnancy and offspring fat and lean mass in childhood. Obesity (Silver Spring) 2006;14:2284–2293. doi: 10.1038/oby.2006.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldstein H. Factors influencing the height of seven year old children—results from the National Child Development Study. Hum Biol. 1971;43:92–111. [PubMed] [Google Scholar]
- 41.Butler NR, Goldstein H. Smoking in pregnancy and subsequent child development. Br Med J. 1973;4:573–575. doi: 10.1136/bmj.4.5892.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fogelman KR, Manor O. Smoking in pregnancy and development into early adulthood. BMJ. 1988;297:1233–1236. doi: 10.1136/bmj.297.6658.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fisch RO, Bilek MK, Ulstrom R. Obesity and leanness at birth and their relationship to body habitus in later childhood. Pediatrics. 1975;56:521–528. [PubMed] [Google Scholar]
- 44.Naeye RL. Influence of maternal cigarette smoking during pregnancy on fetal and childhood growth. Obstet Gynecol. 1981;57:18–21. [PubMed] [Google Scholar]
- 45.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood. BMJ. 2000;320:967–971. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ong KK, Preece MA, Emmett PM, Ahmed ML, Dunger DB. Size at birth and early childhood growth in relation to maternal smoking, parity and infant breast-feeding: longitudinal birth cohort study and analysis. Pediatr Res. 2002;52:863–867. doi: 10.1203/00006450-200212000-00009. [DOI] [PubMed] [Google Scholar]
- 47.Jones G, Riley M, Dwyer T. Maternal smoking during pregnancy, growth, and bone mass in prepubertal children. J Bone Miner Res. 1999;14:146–151. doi: 10.1359/jbmr.1999.14.1.146. [DOI] [PubMed] [Google Scholar]
- 48.Leary S, Smith G Davey, Ness A. Smoking during pregnancy and components of stature in offspring. Am J Hum Biol. 2006;18:502–512. doi: 10.1002/ajhb.20518. [DOI] [PubMed] [Google Scholar]
- 49.Rantakallio P. A follow-up study up to the age of 14 of children whose mothers smoked during pregnancy. Acta Paediatr Scand. 1983;72:747–753. doi: 10.1111/j.1651-2227.1983.tb09805.x. [DOI] [PubMed] [Google Scholar]
- 50.Wingerd J, Schoen EJ. Factors influencing length at birth and height at five years. Pediatrics. 1974;53:737–741. [PubMed] [Google Scholar]
- 51.MacArthur C, Knox EG, Lancashire RJ. Effects at age nine of maternal smoking in pregnancy: experimental and observational findings. BJOG. 2001;108:67–73. doi: 10.1111/j.1471-0528.2001.00006.x. [DOI] [PubMed] [Google Scholar]
- 52.MacArthur C, Newton JR, Knox EG. Effect of anti-smoking health education on infant size at birth: a randomized controlled trial. Br J Obstet Gynaecol. 1987;94:295–300. doi: 10.1111/j.1471-0528.1987.tb03094.x. [DOI] [PubMed] [Google Scholar]
- 53.Fox NL, Sexton M, Hebel JR. Prenatal exposure to tobacco: I. Effects on physical growth at age three. Int J Epidemiol. 1990;19:66–71. doi: 10.1093/ije/19.1.66. [DOI] [PubMed] [Google Scholar]
- 54.Montgomery SM, Ekbom A. Smoking during pregnancy and diabetes mellitus in a British longitudinal birth cohort. BMJ. 2002;324:26–27. doi: 10.1136/bmj.324.7328.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Power C, Li L, Manor O, Smith G Davey. Combination of low birth weight and high adult body mass index: at what age is it established and what are its determinants? J Epidemiol Community Health. 2003;57:969–973. doi: 10.1136/jech.57.12.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang RC, Burke V, Newnham JP, Stanley FJ, Kendall GE, Landau LI, et al. Perinatal and childhood origins of cardiovascular disease. Int J Obes (Lond) 2007;31:236–244. doi: 10.1038/sj.ijo.0803394. [DOI] [PubMed] [Google Scholar]
- 57.Law CM, Barker DJ, Bull AR, Osmond C. Maternal and fetal influences on blood pressure. Arch Dis Child. 1991;66:1291–1295. doi: 10.1136/adc.66.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lawlor DA, Najman JM, Sterne J, Williams GM, Ebrahim S, Smith G Davey. Associations of parental, birth, and early life characteristics with systolic blood pressure at 5 years of age: findings from the Mater-University study of pregnancy and its outcomes. Circulation. 2004;110:2417–2423. doi: 10.1161/01.CIR.0000145165.80130.B5. [DOI] [PubMed] [Google Scholar]
- 59.Gillman MW. Developmental origins of health and disease. N Engl J Med. 2005;353:1848–1850. doi: 10.1056/NEJMe058187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller HC, Hassanein K, Hensleigh PA. Fetal growth retardation in relation to maternal smoking and weight gain in pregnancy. Am J Obstet Gynecol. 1976;125:55–60. doi: 10.1016/0002-9378(76)90891-7. [DOI] [PubMed] [Google Scholar]
- 61.Cliver SP, Goldenberg RL, Cutter GR, Hoffman HJ, Davis RO, Nelson KG. The effect of cigarette smoking on neonatal anthropometric measurements. Obstet Gynecol. 1995;85:625–630. doi: 10.1016/0029-7844(94)00437-I. [DOI] [PubMed] [Google Scholar]
- 62.Harvey NC, Poole JR, Javaid MK, Dennison EM, Robinson S, Inskip HM, et al. Parental determinants of neonatal body composition. J Clin Endocrinol Metab. 2007;92:523–526. doi: 10.1210/jc.2006-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williamson DF, Madans J, Anda RF, Kleinman JC, Giovino GA, Byers T. Smoking cessation and severity of weight gain in a national cohort. N Engl J Med. 1991;324:739–745. doi: 10.1056/NEJM199103143241106. [DOI] [PubMed] [Google Scholar]
- 64.Li MD, Parker SL, Kane JK. Regulation of feeding-associated peptides and receptors by nicotine. Mol Neurobiol. 2000;22:143–165. doi: 10.1385/MN:22:1-3:143. [DOI] [PubMed] [Google Scholar]
- 65.Jo YH, Talmage DA, Role LW. Nicotinic receptor-mediated effects on appetite and food intake. J Neurobiol. 2002;53:618–632. doi: 10.1002/neu.10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burke V, Gracey MP, Milligan RA, Thompson C, Taggart AC, Beilin LJ. Parental smoking and risk factors for cardiovascular disease in 10- to 12-year-old children. J Pediatr. 1998;133:206–213. doi: 10.1016/s0022-3476(98)70221-5. [DOI] [PubMed] [Google Scholar]
- 67.Johnson RK, Wang MQ, Smith MJ, Connolly G. The association between parental smoking and the diet quality of low-income children. Pediatrics. 1996;97:312–317. [PubMed] [Google Scholar]
- 68.Crawley HF, While D. Parental smoking and the nutrient intake and food choice of British teenagers aged 16–17 years. J Epidemiol Community Health. 1996;50:306–312. doi: 10.1136/jech.50.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Toschke AM, Ehlin AG, von Kries R, Ekbom A, Montgomery SM. Maternal smoking during pregnancy and appetite control in offspring. J Perinat Med. 2003;31:251–256. doi: 10.1515/JPM.2003.034. [DOI] [PubMed] [Google Scholar]
- 70.Williams CM, Kanagasabai T. Maternal adipose tissue response to nicotine administration in the pregnant rat: effects on fetal body fat and cellularity. Br J Nutr. 1984;51:7–13. doi: 10.1079/bjn19840004. [DOI] [PubMed] [Google Scholar]
- 71.Newman MB, Shytle RD, Sanberg PR. Locomotor behavioral effects of prenatal and postnatal nicotine exposure in rat offspring. Behav Pharmacol. 1999;10:699–706. doi: 10.1097/00008877-199911000-00017. [DOI] [PubMed] [Google Scholar]
- 72.Holloway AC, Lim GE, Petrik JJ, Foster WG, Morrison KM, Gerstein HC. Fetal and neonatal exposure to nicotine in Wistar rats results in increased beta cell apoptosis at birth and postnatal endocrine and metabolic changes associated with type 2 diabetes. Diabetologia. 2005;48:2661–2666. doi: 10.1007/s00125-005-0022-5. [DOI] [PubMed] [Google Scholar]
- 73.Grove KL, Sekhon HS, Brogan RS, Keller JA, Smith MS, Spindel ER. Chronic maternal nicotine exposure alters neuronal systems in the arcuate nucleus that regulate feeding behavior in the newborn rhesus macaque. J Clin Endocrinol Metab. 2001;86:5420–5426. doi: 10.1210/jcem.86.11.8033. [DOI] [PubMed] [Google Scholar]
- 74.Chen WJ, Kelly RB. Effect of prenatal or perinatal nicotine exposure on neonatal thyroid status and offspring growth in rats. Life Sci. 2005;76:1249–1258. doi: 10.1016/j.lfs.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 75.Gao Y-J, Holloway AC, Zeng Z, Lim GE, Petrik JJ, Foster WG, et al. Prenatal exposure to nicotine causes postnatal obesity and altered perivascular adipose tissue function. Obes Res. 2005;13:1–6. doi: 10.1038/oby.2005.77. [DOI] [PubMed] [Google Scholar]
- 76.Pausova Z, Paus T, Sedova L, Berube J. Prenatal exposure to nicotine modifies kidney weight and blood pressure in genetically susceptible rats: a case of gene-environment interaction. Kidney Int. 2003;64:829–835. doi: 10.1046/j.1523-1755.2003.00172.x. [DOI] [PubMed] [Google Scholar]
- 77.Mantzoros CS, Varvarigou A, Kaklamani VG, Beratis NG, Flier JS. Effect of birth weight and maternal smoking on cord blood leptin concentrations of full-term and preterm newborns. J Clin Endocrinol Metab. 1997;82:2856–2861. doi: 10.1210/jcem.82.9.4248. [DOI] [PubMed] [Google Scholar]
- 78.Ozkan B, Ermis B, Tastekin A, Doneray H, Yildirim A, Ors R. Effect of smoking on neonatal and maternal serum and breast milk leptin levels. Endocr Res. 2005;31:177–183. doi: 10.1080/07435800500371748. [DOI] [PubMed] [Google Scholar]
- 79.Helland IB, Reseland JE, Saugstad OD, Drevon CA. Smoking related to plasma leptin concentration in pregnant women and their newborn infants. Acta Paediatr. 2001;90:282–287. [PubMed] [Google Scholar]
- 80.Coutant R, de Casson F Boux, Douay O, Mathieu E, Rouleau S, Beringue F, et al. Relationships between placental GH concentration and maternal smoking, newborn gender, and maternal leptin: possible implications for birth weight. J Clin Endocrinol Metab. 2001;86:4854–4859. doi: 10.1210/jcem.86.10.7971. [DOI] [PubMed] [Google Scholar]
- 81.Huang JS, Lee TA, Lu MC. Prenatal programming of childhood overweight and obesity. Matern Child Health J. 2007;11:461–473. doi: 10.1007/s10995-006-0141-8. [DOI] [PubMed] [Google Scholar]
- 82.Parazzini F, Davoli E, Rabaiotti M, Restelli S, Stramare L, Dindelli M, et al. Validity of self-reported smoking habits in pregnancy: a saliva cotinine analysis. Acta Obstet Gynecol Scand. 1996;75:352–354. doi: 10.3109/00016349609033330. [DOI] [PubMed] [Google Scholar]
- 83.Dallongeville J, Marecaux N, Richard F, Bonte D, Zylberberg G, Fantino M, et al. Cigarette smoking is associated with differences in nutritional habits and related to lipoprotein alterations independently of food and alcohol intake. Eur J Clin Nutr. 1996;50:647–654. [PubMed] [Google Scholar]
- 84.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 85.Jha P, Chaloupka F. Tobacco Control in Developing Countries. Oxford University Press; Gary, NC: 2000. p. 512. [Google Scholar]


