Summary
Borrelia burgdorferi, the causative agent of Lyme disease, activates multiple signaling pathways leading to induction of pro-inflammatory mediators at sites of inflammation. Binding of B. burgdorferi to integrin α3β1on human chondrocytes activates signaling leading to release of several pro-inflammatory mediators, but the B. burgdorferi protein that binds integrin α3β1and elicits this response has remained unknown. A search of the B. burgdorferi genome for a canonical integrin-binding motif, the RGD (Arg-Gly-Asp) tripeptide, revealed several candidate ligands for integrins. In this study we show that one of these candidates, BBB07, binds to integrin α3β1 and inhibits attachment of intact B. burgdorferi to the same integrin. BBB07 is expressed during murine infection as demonstrated by recognition by infected mouse sera. Recombinant purified BBB07 induces pro-inflammatory mediators in primary human chondrocyte cells by interaction with integrin α3β1. This interaction is specific, as P66, another integrin ligand of B. burgdorferi, does not activate signaling through α3β1. In summary, we have identified a B. burgdorferi protein, BBB07 that interacts with integrin α3β1 and stimulates production of pro-inflammatory mediators in primary human chondrocyte cells.
Introduction
Lyme borreliosis is the most common arthropod borne illness in the US, and is widespread in much of the rest of the northern hemisphere. The causative agent, Borrelia burgdorferi (sensu lato) is able to establish persistent infection in immunocompetent animals, and in humans often causes inflammatory arthritis as one of many possible disease manifestations. Lyme arthritis in human patients is associated with production of a limited set of matrix metalloproteases (MMPs), and human chondrocytes in culture produce the same MMPs after infection by B. burgdorferi (Hu et al., 2001). MMP-3 and MMP-19 are elevated in the joints of mice that develop arthritis in response to infection with B. burgdorferi, and levels of these MMPs correlate closely with severity of arthritis seen in different strains of mice in response to infection (Behera et al., 2005). We also showed that induction of these MMPs is through activation of p38 MAPK, JNK, JAK/STAT and PKC pathways (Shin et al., 2007; Behera et al., 2004).
Although B. burgdorferi cells and lipoproteins are able to activate the toll-like receptor (TLR) 2 pathway in human and murine cells (Sobek et al., 2004; Hirschfeld et al., 1999), the induction of MMPs in human chondrocytes is independent of this pathway (Behera et al., 2006). Instead, MMP induction is dependent on B. burgdorferi binding to integrin α3β1 (Behera et al., 2006). B. burgdorferi had previously been shown to bind to other integrins, particularly αIIbβ3, αvβ3, and α5β1 (Coburn et al., 1998; Coburn et al., 1993). P66, an outer membrane protein of B. burgdorferi, was shown to bind to both of the β3 integrins (Coburn and Cugini, 2003; Coburn et al., 1999). P66 also showed some ability to bind to α5β1, but was unable to compete with intact B. burgdorferi for binding to this receptor (Coburn et al., 1999), suggesting that this interaction is of lower affinity. The activation of signaling through integrin α3β1 by B. burgdorferi, as well as the apparent low affinity of P66 for integrin α5β1, led to a search for B. burgdorferi proteins that bind to either one or both of these integrins and are capable of initiating the signaling that results in induction of MMPs and proinflammatory cytokines.
Results
Identification of candidate integrin binding proteins of B. burgdorferi
Borrelia burgdorferi has been shown to bind to integrins αIIbβ3, α5β3, α3β1 and α5β1 (Behera et al., 2006; Coburn et al., 1998; Coburn et al., 1993), and the outer membrane protein P66 was previously identified as a ligand for the β3-chain integrins (Coburn and Cugini, 2003; Coburn et al., 1999). To identify additional potential integrin ligands expressed by B. burgdorferi, we took advantage of the fact that the sequences of the chromosome and all of the plasmids have been determined for B. burgdorferi strain B31 M1 (Casjens et al., 2000; Fraser et al., 1997). We searched every predicted open reading frame in the B. burgdorferi genome for the amino acid sequence XXXXRGDXXX (in which X is any amino acid). This search revealed a total of 30 known or predicted proteins; an additional 20 predicted proteins contain a single conservative substitution (e.g., R -> K) in the tripeptide. The RGD tripeptide was chosen because of its known role in integrin binding by several mammalian integrin ligands (Hynes, 2002; Hynes, 1992), and because RGD peptides compete with B. burgdorferi for integrin attachment. In addition, some, but not all, bacterial integrin ligands contain the RGD tripeptide. Examples of those that do contain the RGD sequence include the filamentous hemagglutinin and pertactin of Bordetella pertussis (Leininger et al., 1992; Leininger et al., 1991; Relman et al., 1990), and Asc 10 of Enterococcus faecalis (Vanek et al., 1999). The best-studied example of a non-RGD containing bacterial integrin ligand is invasin (Leong et al., 1995a; Isberg and Leong, 1990). However, a search of the B. burgdorferi genome for sequences homologous to the critical region of invasin (XXQGSDMSXX) yielded no matches. The RGD-containing proteins that were identified in the predicted proteome of B. burgdorferi were therefore evaluated for possible surface exposure.
In order for a protein to serve as an adhesin for any mammalian substrate, it must be located on the surface of the bacterial cell. B. burgdorferi encodes the components of the generalized secretion pathways for lipidated and non-lipidated exported proteins, and, by analogy to what is known for other bacteria (Pugsley, 1993), secretion signals can be found at the amino termini of only a few of the RGD-containing proteins. Table 1 shows the predicted proteins that contain RGD (or functionally similar) sequences and secretion signals, and some characteristics of each protein. In some cases, the RGD sequence is near the C-terminus of the protein, which could preclude selection of the protein through phage display, the approach that was used to identify P66 as an integrin ligand. In addition to these candidate adhesins, we examined BB0463, which is predicted to be the nucleoside diphosphate kinase (NDK) of B. burgdorferi and contains the RGD sequence, but is not thought to be exposed on the bacterial surface.
Table 1.
Candidate Integrin Ligands of Borrelia burgdorferi Identified on the Basis of Containing the RGD Tripeptide and a Predicted Secretion Signal Peptide
| gene designation | predicted protein characteristics |
|---|---|
| BB0058 | chromosomal gene encodes hypothetical protein of MW ~76,395 of unknown function with KGD (no RGD) and secretion signal |
| BB0108 | chromosomal gene encoding a hypothetical protein of MW ~37,995 with RGD and secretion signal of unknown function; is homologous to a putative membrane protein of T. pallidum |
| BB0463 | chromosomal gene encoding nucleoside diphosphate kinase (MW ~19,380); originally isolated in selection of filamentous phage that bind to integrin αIIbβ3, contains RGD, phage bind αIIbβ3 very efficiently, does not have an apparent secretion signal and is thought to be associated with the inner leaflet of the cytoplasmic membrane, but this has not been demonstrated in B. burgdorferi |
| BB0744 | chromosomal gene encodes antigen p83/100, AKA p93, contains RGD and secretion signal but is located in periplasm and has poor αIIbβ3 binding activity (Coburn et al., 1999) |
| BB0844 | chromosomal gene encoding a hypothetical protein of MW ~37,470 of unknown function with RGD and lipoprotein secretion signal |
| BBB07 | circular plasmid cp26 gene encoding a predicted outer surface protein of MW ~41,975 of unknown function with both RGD and KGD sequences |
| BBJ36 | linear plasmid Ip38 gene encoding a hypothetical protein of MW ~40,480 of unknown function with both RGD and KGD, and lipoprotein secretion signal |
| BBS42 | plasmid cp32-3 gene encodes BapA (MW ~19,435), localization unknown, contains RGD and secretion signal but has poor αIIbβ3 binding activity (Coburn et al., 1999) |
Integrin binding activities of the candidate ligands
Each of the genes encoding the candidate proteins was amplified by PCR and cloned in the pMalC2 vector to generate an in-frame fusion between the carboxyl terminus of MBP and the amino terminus at the predicted secretion signal cleavage site of the B. burgdorferi protein. We were unable to obtain intact clones of genes BBJ36 and BB0058, as every clone of each of these two genes contained a premature stop codon upstream of the RGD sequence. Because the same mutations were obtained from multiple PCR reactions we concluded that, in our clone of N40 (D10E9), these genes have been inactivated by mutation. The corresponding proteins therefore could not contribute to integrin binding by this strain. MBP fusion proteins corresponding to the remaining candidates were purified and tested for integrin binding activity.
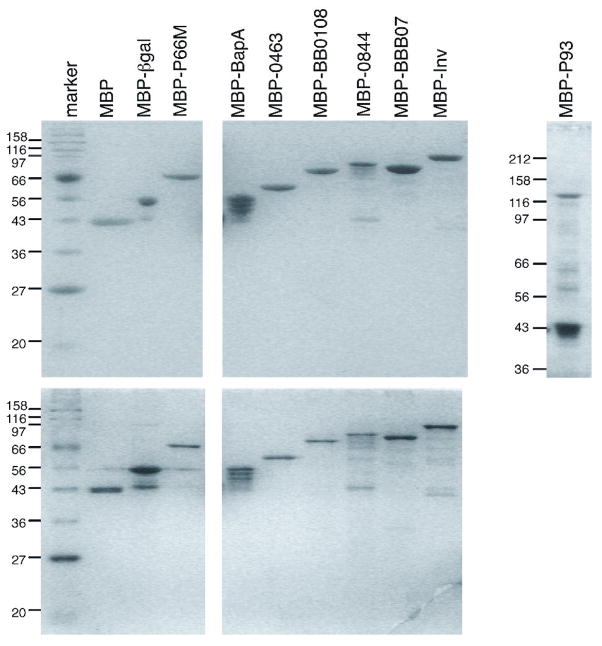
The recombinant proteins were first analyzed for purity and integrity by SDS-PAGE (Figure 1). In most of the preparations the full-length fusion protein was the predominant species by both Coomassie and silver staining. However, MBP-BapA contained significant levels of three additional bands that may be breakdown products, or the result of premature termination of protein synthesis. MBP-P93 contained two predominant bands, with the higher molecular weight band at the predicted fusion protein size constituting approximately one fifth of the total protein. The other band corresponds in size to MBP. In addition, bands corresponding in size to MBP were also present at lower levels in most of the protein preparations. Based on the protein contents as judged by SDS-PAGE and the concentrations measured by total protein determination, the “specific concentration” of each protein was adjusted so that the concentration tested for adhesion activity was that of the intact, full-length fusion protein.
Figure 1. Electrophoretic analysis of recombinant MBP fusions to candidate integrin ligands.
Top panel: Samples of approximately 1.5 μg by BCA determination were stained with Coomassie brilliant blue. Bottom panel: Samples of approximately 0.3 μg stained with silver. Positions and sizes in kilodaltons of molecular weight markers are shown on the left. P93 was fractionated on a 10% polyacrylamide gel, the other proteins were fractionated on 12% gels.
To test for integrin binding activity, integrins αIIbβ3, α5β1, and α3β1 were employed; the buffer in which the integrins were diluted served as the control for integrin-specific binding. The candidate integrin ligands fused to MBP were tested for specific binding in comparison to the control proteins MBP and MBP-βgal, which is produced from the plasmid in the absence of an insert. The positive controls were MBP-P66M and MBP-Invasin, which have previously been shown to bind to β3 and β1 chain integrins, respectively (Defoe and Coburn, 2001; Coburn et al., 1999; Leong et al., 1990).
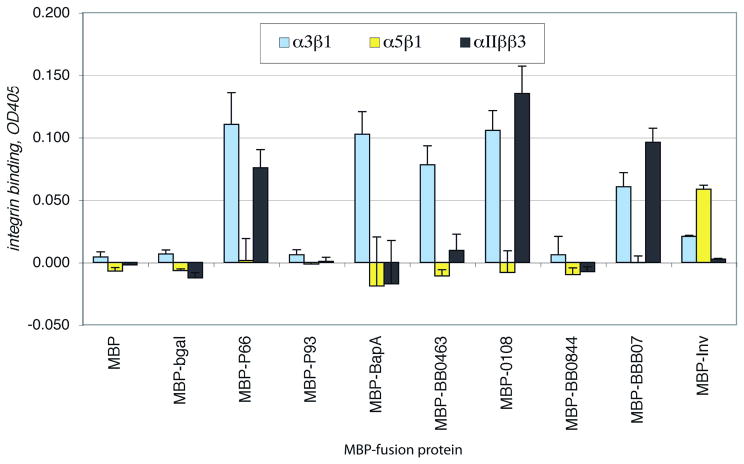
MBP-P66, MBP-BB0463, MBP-BB0108, and MBP-BBB07 all bound reproducibly to integrin α3β1 at significant levels, while none of these proteins bound to integrin α5β1 (Figure 2). The activity of α5β1 was confirmed, however, by the attachment of MBP-invasin to this integrin, while invasin showed relatively inefficient binding to α3β1 and no significant attachment to αIIbβ3. MBP-P66, MBP-BB0108, and MBP-BBB07 also attached efficiently to αIIbβ3, the latter two of which were somewhat surprising, as previous results had suggested that P66 is the major B. burgdorferi ligand for the other β3 integrin, αvβ3 (Coburn and Cugini, 2003). Both P93 (BB0744) and BB0844 were eliminated as candidate ligands for the β1 integrins that B. burgdorferi strain N40 D10E9 is known to recognize, as neither of these proteins showed any integrin binding activity in any of our experiments. While MBP-BapA showed significant attachment to α3β1, this protein also showed very high non-specific binding to the wells without integrin (not shown), and in some experiments, showed no specific binding to any integrin.
Figure 2. Binding of recombinant B. burgdorferi proteins to selected integrins.
Integrins at 3 μg/ml were immobilized in plastic 96 well plates. The buffer alone was also plated as a control. After blocking non-specific protein binding sites, the recombinant proteins at 10 μg/ml were added to the wells and incubated for 1.5 hours at ambient temperature. Unbound proteins were removed by washing and the plates were fixed, blocked, and protein binding was detected by ELISA using an anti-MBP antiserum. Shown are the means plus standard deviations of 4 replicates, after subtraction of binding to control wells with no integrin, from one representative of at least 3 independent experiments for each protein.
Expression of candidate integrin ligands during murine infection
For any bacterial integrin binding protein to have any possible significance in pathogenesis, it must be expressed during infection. P66 and P93 are expressed by B. burgdorferi during mammalian infection, as both proteins are antigens widely recognized by Lyme disease patient sera (Ntchobo et al., 2001; Dressler et al., 1993), and are considered part of the repertoire of diagnostic antigens as defined by the CDC (CDC, 1995). BapA is recognized by a low percentage of Lyme disease patient sera (Miller and Stevenson, 2003). This observation, together with the lack of reproducible integrin binding activity, MBP-BapA did not show reproducible binding to any of the integrins tested, led to the prioritization of other candidates. BB0463 is predicted to encode nucleoside diphosphate kinase, which in other bacteria is within the boundaries of the cytoplasmic membrane. BB0463 was therefore not considered to be a protein that would be likely to have a role in B. burgdorferi integrin binding activity, and so was not further studied.
To determine whether the remaining candidates, BB0108 and BBB07, are expressed during mammalian infection, the corresponding MBP fusion proteins and MBP were fractionated by SDS-PAGE and transferred to membranes for testing by immunoblot. Sera collected from mice infected by B. burgdorferi for 4 weeks, or uninfected controls, were tested for reactivity to the proteins. Two of the six mouse sera tested showed possible very weak reactivity to MBP-BB0108, suggesting either that the protein is not significantly expressed during infection, or that immunodominant epitopes of this protein might be conformation dependent and therefore would not be efficiently recognized in this approach. None of the mice showed any reactivity to MBP alone. BBB07, in contrast, was recognized by all 6 of the sera from infected mice, while none of the 3 uninfected mouse sera tested recognized this protein (data not shown). Due to its expression in infected mice and to its integrin binding activity in vitro, we have pursued further studies with BBB07.
Specificity of BBB07 attachment to integrin α3β1
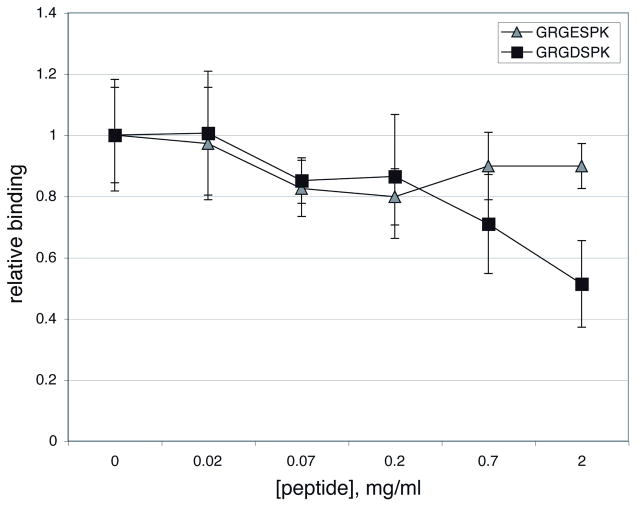
To establish whether binding of BBB07 to α3β1 is mediated by the ligand binding site of the integrin, or by another portion of the protein heterodimer, we tested the peptide GRGDSPK for the ability to inhibit binding of BBB07 to integrin α3β1. RGD containing peptides act as competitive inhibitors of ligand attachment to several integrins, i.e. integrins that recognize the canonical sequence RGD. Control peptides containing a D to E substitution are much less active as competitive inhibitors (D’Souza et al., 1991). The peptides GRGDSPK and GRGESPK were incubated at various concentrations with immobilized α3β1 prior to the addition of MBP-BBB07 or MBP. Neither peptide had a significant effect on binding of MBP (which was inefficient to begin with), but the RGD containing peptide did significantly inhibit BBB07 binding at the relatively high concentration of 2 mg/ml (Figure 3). The RGE peptide did not significantly inhibit binding, and neither peptide significantly inhibited attachment of MBP-BBB07 at lower concentrations. These results could suggest that BBB07 does not bind to the ligand-binding portion of the integrin, or more likely that the affinity of attachment of BBB07 is higher than that of the short peptide. In support of the latter interpretation, EDTA also inhibited binding of BBB07 to α3β1 (data not shown). EDTA chelates the divalent cations required for the interaction between the critical D residue of the ligand and the integrin.
Figure 3. RGD peptide Inhibition of MBP-BBB07 binding to α3β1.
Wells coated with the integrin at 3 μg/ml were incubated with varying concentrations of the peptides for 30 min. at ambient temperature, then MBP-BBB07 was added to 10 μg/ml. Binding was quantified by ELISA as described for Figure 2, then normalized to the level of binding in the absence of peptide. Shown are the means and standard deviations of 4 replicates from one representative experiment. In all experiments, binding of MBP was negligible and not affected by the peptides (not shown). Statistically significant (by the two tailed t test, p<0.05) inhibition of BBB07 binding in comparison to either the absence of peptide or GRGESPK was seen only with 2 mg/ml GRGDSPK.
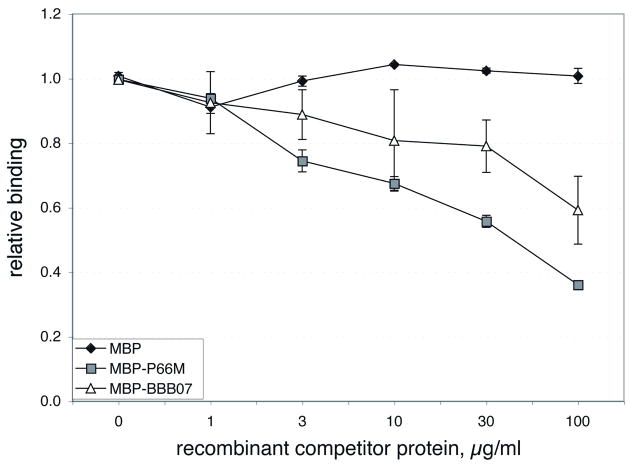
To determine whether B. burgdorferi and MBP-BBB07 are recognizing the same portion of integrin α3β1, we tested MBP-BBB07 for the ability to compete with intact B. burgdorferi for attachment to this integrin (Figure 4). MBP alone served as a negative control, and had no significant effect on B. burgdorferi binding as was seen previously for integrins αIIbβ3 and αvβ3 (Coburn et al., 1999). MBP-P66M did successfully compete with B. burgdorferi for attachment to α3β1, which is not surprising, as it binds to this integrin. MBP-P66 fusions also bind to both of the β3 integrins, and compete with B. burgdorferi to the same receptors (Coburn et al., 1999). MBP-BBB07 also competed with B. burgdorferi, suggesting that their recognition sites on the integrin at least overlap. MBP-BBB07 was less effective than MBP-P66 as a competitor of B. burgdorferi attachment, which is consistent with the relative binding of MBP-P66M and MBP-BBB07 shown in Figure 2.
Figure 4. Inhibition of B. burgdorferi attachment to integrin α3β1 by recombinant MBP-BBB07 and MBP-P66M.
Integrin α3β1 plated at 3 μg/ml was incubated for 30 min. with the recombinant proteins at the concentrations indicated prior to the addition of B. burgdorferi strain N40. Bacterial attachment was quantified by ELISA using an anti-Lyme spirochete rabbit antiserum followed by alkaline phosphatase-conjugated anti-rabbit IgG. Shown are the means and standard deviations of 4 replicates, representing two independent experiments for MBP and P66M, which previously showed similar curves with both β3 chain integrins (Coburn et al., 1999), and four independent curves for BBB07.
Activation of integrin α3β1-dependent signaling by BBB07 and P66
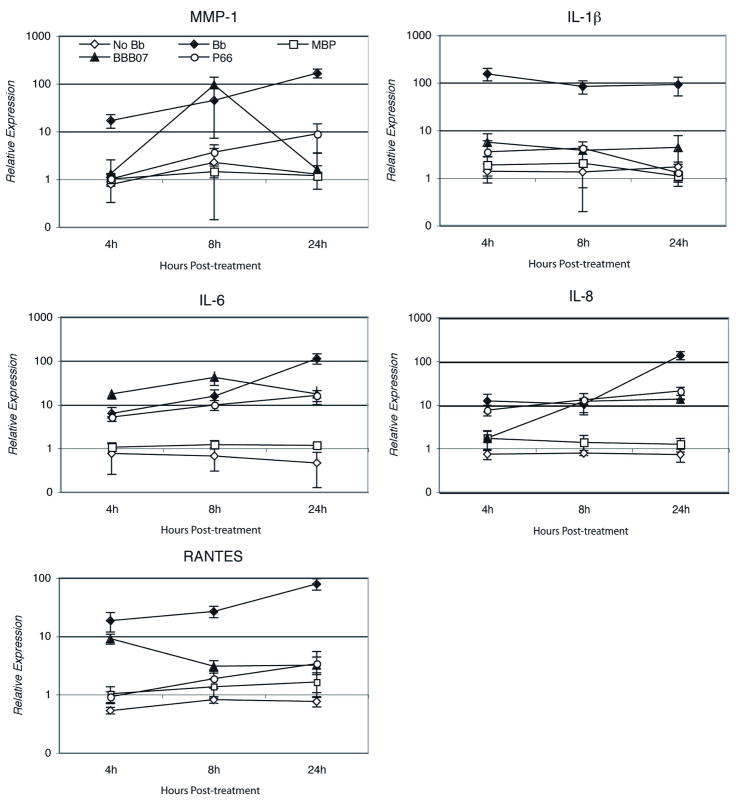
We have previously shown that B. burgdorferi induces expression of pro-inflammatory cytokines, chemokines and matrix metalloproteinases (MMPs) in human chondrocyte cells through binding to integrin α3β1 (Behera et al., 2006). Other integrins and TLRs did not appear to have significant roles in this induction. We therefore examined the roles of BBB07 and P66 in B. burgdorferi induced inflammatory processes by incubating primary human chondrocyte cells with either of the two proteins. Cells were harvested at different time points and total RNA was analyzed by real time RT-PCR. Transcription of a number of key pro-inflammatory cytokines, chemokines and MMPs was examined. Both BBB07 and P66 significantly induced expression of MMP-1, IL-1β, IL-6, IL-8 and RANTES, when compared to the expression by the control protein MBP-β-galactosidase (Figure 5). However, the kinetics of induction of these inflammatory molecules varied significantly following treatment with BBB07 vs. treatment with P66. Induction of MMP-1 and IL-6 peaked at 8h following treatment of cells with BBB07 and declined thereafter. Induction of IL-1β and RANTES peaked at 4h post-treatment with BBB07 and declined thereafter (Figure 5). Induction of IL-8 started at 8h post-BBB07 treatment and remained high through 24h. In contrast, P66-induced expression of MMP-1, IL-6, IL-8 and RANTES was greatest at 24h post-treatment, similar to the profile when intact bacteria were used. The magnitude of induction of MMP-1 and IL-6 was much lower by P66 when compared to that by BBB07. However, induction of IL-1β by P66 was very similar to that by BBB07, i.e., peaking at 4h and declining thereafter (Figure 5).
Figure 5. B. burgdorferi proteins BBB07 and P66 induce proinflammatory cytokines, chemokines and matrix metalloproteinases (MMPs) in primary human chondrocyte cells.
Human chondrocyte cells were treated with purified MBP-BBB07 (50pmol) for different time points as indicated and expression of proinflammatory cytokine, chemokines and MMPs was examined by real time RT-PCR. The Y-axis is shown in logarithmic scale. Purified MBP-LacZα at the same concentration was used as a control protein. All incubations included 50 μg/ml polymyxin B. The experiment was repeated three times with similar results and a representative experiment is shown. Error bars indicate standard deviations.
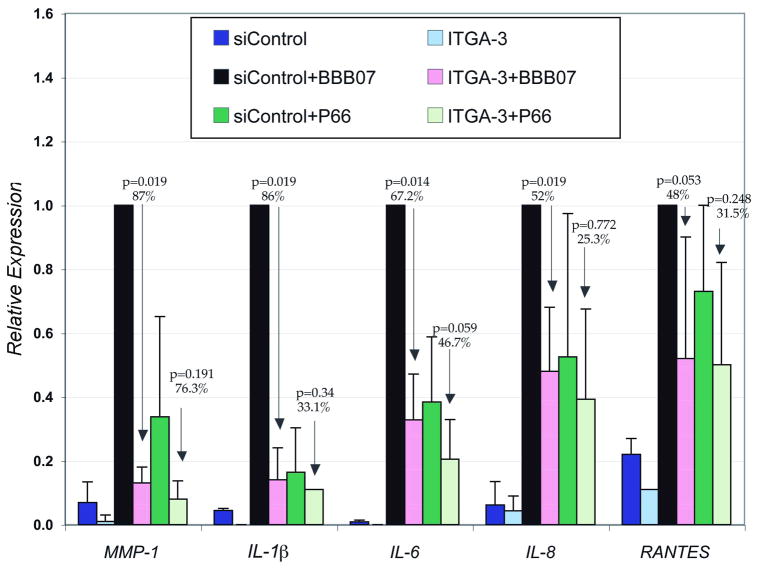
To determine whether the BBB07- or P66- mediated induction of pro-inflammatory molecules is mediated by integrin α3β1 we inhibited the expression of integrin α3 by specific siRNA (ITGA-3). Human chondrocyte cells were transfected with ITGA-3 or control siRNA (siControl), then treated with either BBB07 or P66 at 24h post-transfection. Since the induction of several of the pro-inflammatory molecules peaked at 8h following treatment with either BBB07 or P66, we harvested the cells at that time point. Expression of integrin α3 was silenced by 82% as determined by reduction of integrin α3 specific RNA transcripts (data not shown). Expression of MMP-1, IL-1β, IL-6, and IL-8 by BBB07 was significantly inhibited by 87, 86, 67.2 and 48%, respectively in cells transfected with α3 siRNA compared to cells transfected with control siRNA (Figure 6). BBB07-induced RANTES expression was inhibited by 48% in ITGA-3 transfected cells when compared to the siControl transfected cells, but this did not reach statistical significance. This is not unexpected as induction of RANTES peaked at 4h following treatment of cells with BBB07 and by 8h, RANTES expression had declined to close to the control level (Figure 5). While P66-induced expression of MMP-1, IL-1β, IL-6, IL-8, and RANTES was inhibited by 76.3, 33.1, 46.7, 25.3 and 31.5%, none of the changes were statistically significant when compared to the siControl treated group (Figure 6). These data suggest that BBB07 induces expression of pro-inflammatory molecules through integrin α3β1 in human chondrocyte cells. Although P66 also induced expression of these pro-inflammatory molecules in human chondrocyte cells, this induction appears not to be mediated by integrin α3β1.
Figure 6. B. burgdorferi protein BBB07 induced expression of proinflammatory cytokines, chemokines and matrix metalloproteinases (MMPs) is mediated by integrin α3 in human chondrocyte cells.
Primary HC cells were transfected with either human integrin α3 siRNA or control siRNA and at 24 h posttransfection treated with either MBP-BBB07 or MBP-P66 for 8h. All incubations included 50 μg/ml polymyxin B. Total cellular RNA was isolated, and q-RT-PCR was done. The relative expression of each treatment group is compared with siControl+BBB07 group, which is normalized to one. The experiment was repeated four times, and average of the all experiments is shown. Error bars represent standard deviations. The siControl had no effect on gene expression in response to either recombinant protein in comparison to either treatment with lipofectamine alone or no treatment with transfection reagents (data not shown).
Discussion
Interaction of B. burgdorferi with its host is complex and involves a number of spirochete and host factors. One such interaction, which has been characterized extensively, is binding of B. burgdorferi to integrins (Coburn et al., 1999; Coburn et al., 1998; Coburn et al., 1993). We have recently shown that B. burgdorferi binds to integrin α3β1 and that this interaction activates signaling events leading to induction of inflammatory mediators in human chondrocytes in culture (Behera et al., 2006). To identify bacterial ligands that bind to β1 integrins, we conducted a genome wide search for predicted proteins containing the integrin binding motif, RGD, and a predicted amino terminal secretion signal. One candidate, BBB07, fulfils several of the criteria that would be required of a B. burgdorferi ligand for α3β1. First, BBB07 is a predicted outer surface protein. Second, recombinant MBP-BBB07 expressed in E. coli binds to purified α3β1. This binding was specific, as the binding was inhibited by the competitive integrin antagonist RGD peptide. (Of note, although the RGD peptide is widely used for assessing integrin-ligand interaction, it is not suitable for assessing interactions using adherent cells in culture, as the peptide will lift the cells, which causes multiple changes in cellular physiology and gene expression, often leading to apoptosis). Third, B. burgdorferi and BBB07 recognize the same motif on integrin α3β1. Fourth, BBB07 is expressed during mammalian infection. Fifth, BBB07 induced expression of pro-inflammatory cytokines and chemokines in primary human chondrocyte cells, which was inhibited by silencing expression of integrin α3. Our previous results had demonstrated that either antibody-mediated blocking or siRNA silencing of the integrin α3 subunit, specifically, inhibited the proinflammatory response of human chondrocytes to B. burgdorferi, and that none of these reagents had any significant effect on the expression of inflammatory mediators in the absence of B. burgdorferi (Behera et al., 2006). Taken together, these characteristics make BBB07 an ideal candidate for B. burgdorferi ligand for integrin α3β1.
In addition, our data also demonstrate that the B. burgdorferi outer surface protein, P66, which has previously been shown to be a ligand for β3 chain integrins, also binds to α3β1. Neither BBB07 nor P66 bound efficiently to integrin α5β1. In addition, P66 was able to induce expression of proinflammatory mediators by human chondrocytes. However, there are distinct differences in the intensities and kinetics of induction of pro-inflammatory cytokines and chemokines by BBB07 and P66 in primary human articular chondrocyte cells. This could indicate that although both BBB07 and P66 bind to integrin α3β1, they may have distinct roles in B. burgdorferi infection and infection induced inflammation. This is likely to be a reflection of the particular ligand, integrin, and the cell type involved in the specificities of the down stream signaling resulting in expression of inflammatory mediators. Although P66 stimulated expression of pro-inflammatory mediators in primary human chondrocyte cells, this induction was not mediated by its interaction with integrin α3β1 to a statistically significant level. P66 has been shown to interact with integrins expressed by other cell types, and has a role in B. burgdorferi attachment to diverse cell types (Coburn and Cugini, 2003; Coburn et al., 1999; Coburn et al., 1998; Coburn et al., 1994; Coburn et al., 1993). The influence of P66 on gene expression by other human cell types is under investigation.
Integrin α3β1 is widely expressed, and mammalian ligands for integrin α3β1 are diverse. α3β1 can bind ligands with and without classical RGD integrin binding motifs (Elices et al., 1991; Hemler, 1990). Integrin α3β1 is a known receptor for various host proteins, including collagen (types I and VI), laminin-1 (α1β1γ1), laminin-5 (α3β3γ2), laminin-10 (α5β1γ1), laminin-11 (α5β2γ1), fibronectin, entactin (nidogen) and thrombospondin-1 (Guo et al., 2000; Kikkawa et al., 1998; Wu et al., 1995; Delwel et al., 1994; Weitzman et al., 1993; Dedhar et al., 1992; Carter et al., 1991; Elices et al., 1991). Thus binding of different B. burgdorferi ligands to integrin α3β1 may serve different functions during B. burgdorferi infection, depending on the particular site of the bacterium-host interaction.
The structure of mature BBB07 on the outer membrane of B. burgdorferi is not known. It has no significant sequence homology with any other characterized proteins of any origin. It does, however, contain the consensus integrin binding RGD motif, which is in the hydrophilic region of the protein (as determined by Kyte-Doolittle), and thus is likely to be exposed on the surface of the protein. The RGD sequences of other integrin ligands are on the exposed regions of the proteins as well. Detailed structural characterization of BBB07 and its interaction with integrin α3β1 will identify the exact domain that is crucial for recognition by integrin α3β1.
BBB07 and integrin α3β1 are the first specific B. burgdorferi ligand-human receptor pair that has been shown to be involved in B. burgdorferi induced inflammation. Generation of B. burgdorferi mutants deficient in expression of BBB07 will delineate the exact role of this protein in inflammatory signaling. Identification of the full receptor repertoire recognized by BBB07 will also aid in understanding the role of this protein in B. burgdorferi induced inflammation and pathogenesis.
Experimental Procedures
Bacterial Strains and Growth Conditions
The infectious Borrelia burgdorferi strain N40 clone D10E9 was described previously (Coburn et al., 1993). The bacteria were grown in MKP medium supplemented with human serum n place of rabbit serum (Leong et al., 1995b; Coburn et al., 1993; Preac-Mursic et al., 1986). For assays of attachment to integrins, stocks were thawed, washed in phosphate-buffered saline (PBS) supplemented with bovine serum albumin (BSA) to 0.2% w/v, and resuspended in 25 mM HEPES pH7.8, 150 mM NaCl, 1 mM MgCl2, 1 mM MnCl2, 0.25 mM CaCl2, (HBS) plus 1% BSA and 0.1% dextrose (HBSBD) at a concentration of 2.5 × 107/ml. Preparation of genomic DNA for PCR was performed as described previously (Sadziene et al., 1991). E. coli K-12 strains JM109 and KS330 (SR2) (Rankin et al., 1992) were grown in standard laboratory media (Maniatis et al., 1982). All molecular techniques were performed using standard protocols (Maniatis et al., 1982).
Reagents
Integrins were either purified from human placenta as previously described (Coburn et al., 1998), or were purchased from Chemicon International (Temecula, CA). Peptides GRGDSPK and GRGESPK were synthesized by the Tufts University Core Facility.
Generation of maltose binding protein fusions
The B. burgdorferi strain B31 M1 proteome posted on the TIGR website (http://cmr.tigr.org/tigr-scripts/CMR/GenomePage.cgi?database=gbb) was searched in the year 2000 for the amino acid sequence XXXXRGDXXX, in which X stands for any amino acid. This search string was used due to the minimum length requirements of the search engine. To refine the list of candidate integrin ligands and focus on those most likely to be expressed on the surface of B. burgdorferi, only candidates with predicted or known secretion signal peptides at the amino terminus were considered further. In addition, one protein that is not a predicted surface protein, BB0463, which is annotated as nucleoside diphosphate kinase, was included in our list as a positive control, as it contains the RGD tripeptide and was selected for binding to integrin αIIbβ3 (Coburn et al., 1999). The list of candidate integrin ligands identified by these criteria is presented in Table 1.
Candidate genes were amplified by PCR and cloned in plasmid pMalC2, which results in fusion of the maltose binding protein (MBP) of E. coli to the candidate integrin ligand. The vector was cleaved with Bam HI and Sal I to generate non-compatible cohesive overhangs that facilitate directional cloning. Insert DNA fragments were generated by PCR amplification of B. burgdorferi DNA using primers containing these restriction sites near the 5′ ends (Table 2). Amplified DNA was then digested with the same enzymes and ligated to the vector. The recombinant plasmids were introduced into E. coli strain JM109 by electroporation, and were selected on plates containing 100 μg/ml ampicillin supplemented with 0.2% dextrose. After purification by restreaking, colonies were screened for the presence of insert DNA by PCR using primers oMal01 and oMal02 (Table 2). Clones with the appropriate sized inserts were sequenced by the Tufts University Core Facility. Clones with the correct sequence that were selected for further evaluation were: BB0108 clone O1, BB0463 clone 3, BB0844 clone 11, and BBB07 clone J1. Plasmids from these clones were purified using the Wizard Mini-Prep Kit (Promega) and introduced into E. coli strain KS330 by electroporation, and the sequences of the recombinant plasmids were again verified. We were never able to obtain an intact version of BBJ36, as all clones sequenced, derived from multiple independent PCR reactions, contained the same stop codon at position 199 (Q199*) before the RGD sequence. We therefore believe that in B. burgdorferi strain N40 D10E9 the bbj36 gene is non-functional with regard to integrin binding. Similarly, we were unable to obtain clones encoding an intact copy of BB0058, so these two proteins were not further pursued as candidate integrin ligands. Generation of clones containing the integrin binding domains of P66 and invasin, as well as P93 and BapA, were described previously (Coburn et al., 1999; Leong et al., 1990), and followed very similar protocols. MBP-βgal (malE-lacZα) is encoded by the vector pMalC2 and was therefore purified from cells harboring the unmodified vector.
Table 2.
PCR Primers and Conditions
| name | sequence, 5′ ⇒ 3′1 | amino acids encoded, changes from B31 M1 sequence2 | PCR conditions: annealing temp(Ta), [MgCl2]3 |
|---|---|---|---|
| oMal 01 | cgctttctggtatgccgtgcgta | NA | 48°C, 4 mM |
| oMal 02 | tctcatccgccaaaacagccaag | NA | |
| BB0108F1 | gagaggatccactggttttgattctaaggttgata | 38–333, I46L | 54°C, 3.5 mM |
| BB0108R2 | gagagtcgacttaggaatccaagatttgtatatttgc | ||
| BB0463F (NDPKF) | gagaggatccactttatgtattgttaagccagatgga | 8–169 | 49°C, 2 mM |
| BB0463R (NDPKR) | gagagtcgacttaacaataataatgctcacattcatcatc | ||
| BB0844F | gagaggatccatgcaagacaagaacgtgaaa | 32–314 | 58°C, 2 mM |
| BB0844R3 | ggaggtcgacttatctatcgccactagaaaagattttgct | ||
| BBB07F2 | gagaggatccagtgctcattttggatttactca | 25–357, N257D | 52°C, 3.5 mM |
| BBB07R1 | agagtcgacttattcccaaggttctattttttcaa |
Restriction endonuclease sites used for cloning are underlined.
Amino acids that were changed from the B31 clone M1 sequence (http://cmr.tigr.org/tigr-scripts/CMR/GenomePage.cgi?database=gbb) in all clones sequenced and therefore likely to represent polymorphisms between B. burgdorferi strains B31 M1 and N40 D10E9.
All reactions were conducted as follows: 95°C 15 min, 92° 30 sec., Ta 1 min, 72° 1 min, cycle 40 x, 72°C 7 min. The [MgCl2] listed is the final concentration and includes both that present in the manufacturer’s buffer and that added according to optimization for each primer set. The conditions listed for the forward (F) primer were used for amplifications with the reverse (R) primer listed immediately below.
Recombinant MBP fusion proteins were purified as described previously (Coburn et al., 1999; Leong et al., 1990). Briefly, cells were grown at 30° C in 2xYT medium to OD600 of 0.4 to 0.6, and expression was induced by the addition of IPTG (isopropylthiogalactoside) to 1 mM, followed by further incubation for 2 hours. The bacteria were harvested by centrifugation, washed once in HBS, and lysed in a French pressure cell in the presence of 1 mM benzamidine HCl, 0.01 TIU/ml aprotinin, and 1 mM PMSF (protease inhibitors). After clarification by centrifugation, the supernatant was applied to a cross-linked amylose column (New England Biolabs, Beverly, MA). The column was washed extensively with HBS to remove unbound proteins, then the fusion proteins (and native E. coli MBP) were eluted with HBS containing the protease inhibitors and 10 mM maltose. Protein concentrations were determined using the BCA kit (Pierce Chemical Company, Rockford, IL), and the purity was checked by gel electrophoresis using standard protocols (Laemmli, 1970). The proteins were stored in small aliquots at −70°C. Control proteins included purified MBP (New England Biolabs) and MBP-β-galactosidase (MBP-LacZα), which was purified in our laboratory after induction of expression from the plasmid without any foreign DNA inserted, to control for the effects of possible endotoxin contamination of the protein preparations.
Assessment of MBP fusion protein interactions with integrins
Binding of the MBP fusion proteins to purified integrins was assayed essentially as previously described (Coburn et al., 1999). Briefly, integrins were coated onto 96 well plates at 1, 3, or 10 μg/ml overnight at 4°C. After washing with HBS and blocking with HBSBD, the wells were probed in quadruplicate with 50 μl of the MBP fusion protein diluted in HBSBD to 1, 3, or 10 μg/ml. After incubation for 2–3 h at ambient temperature, the wells were washed with HBS and fixed with 3% paraformaldehyde in PBS. Fusion protein binding was quantified by ELISA using anti-MBP rabbit antiserum (New England Biolabs) diluted 1:5,000, followed by goat anti-rabbit IgG conjugated to alkaline phosphatase, diluted 1:10,000. For experiments in which peptides were used to assess specific integrin binding, the peptides were diluted in HBSBD and incubated at the specified concentrations for 30 min at ambient temperature with the plated integrins prior to the addition of the MBP fusion proteins. For competition with B. burgdorferi, integrin α3β1 was plated at 3 μg/ml and, after blocking non-specific binding sites, was incubated with varying concentrations of MBP fusion proteins or MBP alone. B. burgdorferi strain N40 D10E9 was then added to the wells, and attachment of the bacteria was quantified by ELISA using a rabbit polyclonal antiserum diluted 1:5000, followed by an alkaline phosphatase-conjugated anti-rabbit IgG diluted 1:5000, essentially as described previously (Coburn et al., 1999).
Primary cultures of human chondrocyte cells and infection with B. burgdorferi
Primary Human chondrocyte cells (HCs) from a healthy donor were purchased from Cambrex Corporation and maintained in chondrocyte growth medium (Cambrex) containing 10% fetal calf serum (FCS) at 37°C with 5% CO2. Low passage (passages 2 to 6) B. burgdorferi strain N40 (clone D10E9) was cultured in Barbour-Stoenner-Kelly (BSK-H) medium (Sigma) as previously described (Hu et al., 1995; Barbour, 1984). Spirochetes were washed three times and resuspended in chondrocyte growth medium. Cell cultures at 70 to 85% confluence were infected with B. burgdorferi at multiplicity of infection (MOI) of 10, or incubated with 50 μM MBP-BBB07, MBP-P66, or MBP-β-galactosidase in the presence of 50 μg/ml polymyxin B (to inactivate any possible endotoxin in the protein preparations) for various time periods, and cells were harvested in Trizol Reagent (Invitrogen) for total RNA isolation.
siRNA transfection
Primary human chondrocyte cells were transfected with 100nM integrin α3 siRNA (ITGA3, Dharmacon) or with 100nM control siRNA (Dharmacon) using Lipofectamine 2000 (Invitrogen) following the manufacturer’s protocol. Integrin α3 mRNA levels were decreased approximately 82% after transfection with the ITGA3 siRNA as compared to either the siControl or the lipofectamine controls. The cells were then treated with 50 μM purified proteins (BBB07 or P66 or MBP-LacZα) 24h after siRNA transfection and were harvested at 8h post treatment. Transfection of siRNA had no cytotoxic effect on primary chondrocytes as determined by trypan blue exclusion.
Quantitative real time reverse transcriptase PCR
Total RNA was isolated using Trizol (Invitrogen) and then treated with DNasel (Ambion) according to the manufacturer’s instructions. First-strand synthesis of cDNA from DNase treated total RNA was performed by using Improm II reverse transcriptase (Promega, Madison, Wis.) according to the manufacturer’s instructions. Reactions performed in the absence of reverse transcriptase were used to control for contamination by genomic DNA. cDNA samples contaminated by genomic DNA were discarded, and the original RNA was again treated with DNase before the reverse transcriptase reaction was repeated. Quantification of cDNA from specific mRNA transcripts was accomplished by real-time quantitative reverse transcriptase PCR (rt-RT-PCR) (BioRad) by using SYBR Green technology (Quantitect Sybr Green PCR kit; QIAGEN) as previously described (13, 46). β-Actin was used as an internal control.
Statistical analysis
The statistical significance between groups was analyzed by using the non-parametric Mann-Whitney U test. Differences were considered statistically significant when the p value was ≤0.05.
Acknowledgments
We thank Allen Steere for anti-Lyme spirochete antiserum, and the GRASP Intestinal Microbiology Core (Center for Gastroenterology Research on Absorptive and Secretory Processes at Tufts-New England Medical Center, PHS grant 1 P30DK39428 awarded by NIDDK) for help with all work with E. coli. This work was funded by NIAID grant R01 AI-051407 to J.C., R01 AI-050043 to L.T.H., and awards from the American Lung Association and the Earl P. Charlton Research Fund to A.K.B. E.D. and L.B were supported byT32-GM007310.
References
- Barbour AG. Isolation and cultivation of Lyme disease spirochetes. Yale Journal of Biology & Medicine. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- Behera AK, Hildebrand E, Scagliotti J, Steere AC, Hu LT. Induction of host matrix metalloproteinases by Borrelia burgdorferi differs in human and murine lyme arthritis. Infect Immun. 2005;73:126–134. doi: 10.1128/IAI.73.1.126-134.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behera AK, Thorpe CM, Kidder JM, Smith W, Hildebrand E, Hu LT. Borrelia burgdorferi-induced expression of matrix metalloproteinases from human chondrocytes requires mitogen-activated protein kinase and Janus kinase/signal transducer and activator of transcription signaling pathways. Infect Immun. 2004;72:2864–2871. doi: 10.1128/IAI.72.5.2864-2871.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behera AK, Hildebrand E, Uematsu S, Akira S, Coburn J, Hu LT. Identification of a TLR-independent pathway for Borrelia burgdorferi-induced expression of matrix metalloproteinases and inflammatory mediators through binding to integrin α3β1. J Immunol. 2006;177:657–664. doi: 10.4049/jimmunol.177.1.657. [DOI] [PubMed] [Google Scholar]
- Carter WG, Ryan MC, Gahr PJ. Epiligrin, a new cell adhesion ligand for integrin alpha 3 beta 1 in epithelial basement membranes. Cell. 1991;65:599–610. doi: 10.1016/0092-8674(91)90092-d. [DOI] [PubMed] [Google Scholar]
- Casjens S, Palmer N, van Vugt R, Huang WM, Stevenson B, Rosa P, et al. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Molecular Microbiology. 2000;35:490–516. doi: 10.1046/j.1365-2958.2000.01698.x. [DOI] [PubMed] [Google Scholar]
- CDC. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. Morb Mortal Wkly Rep. 1995;44:590–591. [PubMed] [Google Scholar]
- Coburn J, Cugini C. Targeted mutation of the outer membrane protein P66 disrupts attachment of the Lyme disease spirochete, Borrelia burgdorferi, to integrin αvβ3. Proc Natl Acad Sci. 2003;100:7301–7306. doi: 10.1073/pnas.1131117100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn J, Leong J, Erban J. Integrin αIIbβ3 mediates binding of the Lyme disease agent, Borrelia burgdorferi, to human platelets. Proc Natl Acad Sci USA. 1993;90:7058–7063. doi: 10.1073/pnas.90.15.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn J, Barthold SW, Leong JM. Diverse Lyme disease spirochetes bind integrin αIIbβ3 on human platelets. Infect Immunity. 1994;62:5559–5567. doi: 10.1128/iai.62.12.5559-5567.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn J, Magoun L, Bodary SC, Leong JM. Integrins αvβ3 and α5β1 mediate attachment of lyme disease spirochetes to human cells. Infect Immun. 1998;66:1946–1952. doi: 10.1128/iai.66.5.1946-1952.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn J, Chege W, Magoun L, Bodary SC, Leong JM. Characterization of a candidate Borrelia burgdorferi β3-chain integrin ligand identified using a phage display library. Mol Microbiol. 1999;34:926–940. doi: 10.1046/j.1365-2958.1999.01654.x. [DOI] [PubMed] [Google Scholar]
- D’Souza SE, Ginsberg MH, Plow EF. Arginyl-glycyl-aspartic acid (RGD): a cell adhesion motif. TIBS. 1991;16:246–250. doi: 10.1016/0968-0004(91)90096-e. [DOI] [PubMed] [Google Scholar]
- Dedhar S, Jewell K, Rojiani M, Gray V. The receptor for the basement membrane glycoprotein entactin is the integrin alpha 3/beta 1. J Biol Chem. 1992;267:18908–18914. [PubMed] [Google Scholar]
- Defoe G, Coburn J. Delineation of Borrelia burgdorferi p66 sequences required for integrin alphaIIb betaIII recognition. Infection & Immunity. 2001;69:3455–3459. doi: 10.1128/IAI.69.5.3455-3459.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwel GO, de Melker AA, Hogervorst F, Jaspars LH, Fles DL, Kuikman I, et al. Distinct and overlapping ligand specificities of the alpha 3A beta 1 and alpha 6A beta 1 integrins: recognition of laminin isoforms. Mol Biol Cell. 1994;5:203–215. doi: 10.1091/mbc.5.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler F, Whalen JA, Reinhardt BN, Steere AC. Western blotting in the serodiagnosis of Lyme disease [see comments] Journal of Infectious Diseases. 1993;167:392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- Elices MJ, Urry LA, Hemler ME. Receptor functions for the integrin VLA-3: fibronectin, collagen, and laminin binding are differentially influenced by Arg-Gly-Asp peptide and by divalent cations. Journal of Cell Biology. 1991;112:169–181. doi: 10.1083/jcb.112.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- Guo N, Templeton NS, Al-Barazi H, Cashel JA, Sipes JM, Krutzsch HC, Roberts DD. Thrombospondin-1 promotes alpha3beta1 integrin-mediated adhesion and neurite-like outgrowth and inhibits proliferation of small cell lung carcinoma cells. Cancer Res. 2000;60:457–466. [PubMed] [Google Scholar]
- Hemler ME. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Ann Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Hirschfeld M, Kirschning CJ, Schwandner R, Wesche H, Weis JH, Wooten RM, Weis JJ. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J Immunol. 1999;163:2382–2386. [PubMed] [Google Scholar]
- Hu LT, Perides G, Noring R, Klempner MS. Binding of human plasminogen to Borrelia burgdorferi. Infection & Immunity. 1995;63:3491–3496. doi: 10.1128/iai.63.9.3491-3496.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu LT, Eskildsen MA, Masgala C, Steere AC, Arner EC, Pratta MA, et al. Host metalloproteinases in Lyme arthritis. Arthritis Rheum. 2001;44:1401–1410. doi: 10.1002/1529-0131(200106)44:6<1401::AID-ART234>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Isberg RR, Leong JM. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60:861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- Kikkawa Y, Sanzen N, Sekiguchi K. Isolation and characterization of laminin-10/11 secreted by human lung carcinoma cells, laminin-10/11 mediates cell adhesion through integrin alpha3 beta1. J Biol Chem. 1998;273:15854–15859. doi: 10.1074/jbc.273.25.15854. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leininger E, Ewanowich CA, Bhargava A, Peppler MS, Kenimer JG, Brennan MJ. Comparative roles of the Arg-Gly-Asp sequence present in the Bordetella pertussis adhesins pertactin and filamentous hemagglutinin. Infection & Immunity. 1992;60:2380–2385. doi: 10.1128/iai.60.6.2380-2385.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leininger E, Roberts M, Kenimer JG, Charles IG, Fairweather N, Novotny P, Brennan MJ. Pertactin, an Arg-Gly-Asp-containing Bordetella pertussis surface protein that promotes adherence of mammalian cells. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:345–349. doi: 10.1073/pnas.88.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong JM, Fournier RS, Isberg RR. Identification of the integrin binding domain of the Yersinia pseudotuberculosis invasin protein. EMBO Journal. 1990;9:1979–1989. doi: 10.1002/j.1460-2075.1990.tb08326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong JM, Morrissey PE, Marra A, Isberg RR. An aspartate residue of the Yersinia pseudotuberculosis invasin protein that is critical for integrin binding. EMBO Journal. 1995a;14:422–431. doi: 10.1002/j.1460-2075.1995.tb07018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong JM, Morrissey PE, Ortega-Barria E, Pereira ME, Coburn J. Hemagglutination and proteoglycan binding by the Lyme disease spirochete, Borrelia burgdorferi. Infection & Immunity. 1995b;63:874–883. doi: 10.1128/iai.63.3.874-883.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T, Fritsch EF, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory; New York: 1982. [Google Scholar]
- Miller JC, Stevenson B. Immunological and genetic characterization of Borrelia burgdorferi BapA and EppA proteins. Microbiology. 2003;149:1113–1125. doi: 10.1099/mic.0.26120-0. [DOI] [PubMed] [Google Scholar]
- Ntchobo H, Rothermel H, Chege W, Steere AC, Coburn J. Recognition of Multiple Antibody Epitopes throughout Borrelia burgdorferi p66, a Candidate Adhesin, in Patients with Early or Late Manifestations of Lyme Disease. Infection & Immunity. 2001;69:1953–1956. doi: 10.1128/IAI.69.3.1953-1956.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preac-Mursic V, Wilske B, Schierz G. European Borrelia burgdorferi isolated from humans and ticks: culture conditions and antibiotic susceptibility. Zbl Bakt Hyg (A) 1986;263:112–118. doi: 10.1016/s0176-6724(86)80110-9. [DOI] [PubMed] [Google Scholar]
- Pugsley A. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin S, Isberg RR, Leong JM. The integrin-binding domain of invasin is sufficient to allow bacterial entry into mammalian cells. Infection & Immunity. 1992;60:3909–3912. doi: 10.1128/iai.60.9.3909-3912.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relman D, Tuomanen E, Falkow S, Golenbock DT, Saukkonen K, Wright SD. Recognition of a bacterial adhesion by an integrin: macrophage CR3 (alpha M beta 2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell. 1990;61:1375–1382. doi: 10.1016/0092-8674(90)90701-f. [DOI] [PubMed] [Google Scholar]
- Sadziene A, Thomas DD, Bundoc VG, Holt SC, Barbour AG. A flagella-less mutant of Borrelia burgdorferi. Structural, molecular, and in vitro functional characterization. J Clin Invest. 1991;88:82–92. doi: 10.1172/JCI115308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin OS, Behera AK, Bronson RT, Hu LT. Role of novel protein kinase C isoforms in Lyme arthritis. Cell Microbiol. 2007 doi: 10.1111/j.1462-5822.2007.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobek V, Birkner N, Falk I, Wurch A, Kirschning CJ, Wagner H, et al. Direct Toll-like receptor 2 mediated co-stimulation of T cells in the mouse system as a basis for chronic inflammatory joint disease. Arthritis Res Ther. 2004;6:R433–446. doi: 10.1186/ar1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanek NN, Simon SI, Jacques-Palaz K, Mariscalco MM, Dunny GM, Rakita RM. Enterococcus faecalis aggregation substance promotes opsonin-independent binding to human neutrophils via a complement receptor type 3-mediated mechanism. FEMS Immunol Med Microbiol. 1999;26:49–60. doi: 10.1111/j.1574-695X.1999.tb01371.x. [DOI] [PubMed] [Google Scholar]
- Weitzman JB, Pasqualini R, Takada Y, Hemler ME. The function and distinctive regulation of the integrin VLA-3 in cell adhesion, spreading, and homotypic cell aggregation. J Biol Chem. 1993;268:8651–8657. [PubMed] [Google Scholar]
- Wu C, Chung AE, McDonald JA. A novel role for alpha 3 beta 1 integrins in extracellular matrix assembly. J Cell Sci. 1995;108(Pt 6):2511–2523. doi: 10.1242/jcs.108.6.2511. [DOI] [PubMed] [Google Scholar]